Abstract
The dopamine D2 receptor (DRD2) is a G protein-coupled receptor (GPCR) that is generally considered to be a primary target in the treatment of schizophrenia. First generation antipsychotic drugs (e.g. haloperidol) are antagonists of the DRD2, while second generation antipsychotic drugs (e.g. olanzapine) antagonize DRD2 and 5HT2A receptors. Notably, both these classes of drugs may cause side effects associated with D2 receptor antagonism (e.g. hyperprolactemia and extrapyramidal symptoms). The novel, “third generation” antipsychotic drug, aripiprazole is also used to treat schizophrenia, with the remarkable advantage that its tendency to cause extrapyramidal symptoms is minimal. Aripiprazole is considered a partial agonist of the DRD2, but it also has partial agonist/antagonist activity for other GPCRs. Further, aripiprazole has been reported to have a unique activity profile in functional assays with the DRD2. In the present study the molecular pharmacology of aripiprazole was further examined in HEK cell models stably expressing the DRD2 and specific isoforms of adenylyl cyclase to assess functional responses of Gα and Gβγ subunits. Additional studies examined the activity of aripiprazole in DRD2-mediated heterologous sensitization of adenylyl cyclase and cell-based dynamic mass redistribution (DMR). Aripiprazole displayed a unique functional profile for modulation of G proteins, being a partial agonist for Gαi/o and a robust antagonist for Gβγ signaling. Additionally, aripiprazole was a weak partial agonist for both heterologous sensitization and dynamic mass redistribution.
Keywords: Aripiprazole, dopamine, Gbetagamma, GPCR, functional selectivity
1. Introduction
Functionally-selective or biased ligands distinctly activate different signaling pathways through a single G protein-coupled receptor (GPCR) [1]. Although classically, ligands of GPCRs have been classified as full, partial, or inverse agonists, or antagonists; it is now accepted that the same ligand can display different pharmacological profiles at different signaling pathways through the same GPCR [2]. Increasing evidence suggesting that biased ligands could provide safer and more effective drug therapies [3].
The dopamine D2 receptor (DRD2) is a GPCR that is targeted in the therapies of Parkinson’s disease and schizophrenia. The DRD2 couples to inhibitory heterotrimeric G proteins leading to activation of Gα subunits that inhibit adenylyl cyclases, as well as promoting Gβγ signaling. Additional adaptive signaling responses of the DRD2 include β-arrestin recruitment and heterologous sensitization of adenylyl cyclase [4, 5]. The drugs used in the therapies for Parkinson’s disease (e.g. pramipexole, ropinirole) are agonists of the Gα response of the DRD2, whereas those used for schizophrenia are either antagonists or partial agonists (e.g. haloperidol, aripiprazole, respectively) [6].
The dopamine hypothesis of schizophrenia suggests that antagonists of dopamine receptors would decrease the positive symptoms of schizophrenia [7]. Accordingly, the first generation of antipsychotic drugs antagonizes the DRD2, while second generation antipsychotic drugs have more complex pharmacology, antagonizing both the DRD2 and 5HT2A for their therapeutic effects. The first generation agents, and to some extent the second generation antipsychotics, also antagonize other GPCRs in the central nervous system (e.g. muscarinic and histamine as well as other dopamine and serotonin receptors) resulting in a number of side effects [8]. Aripiprazole has been suggested to be a potential prototype for third generation antipsychotic drugs [9, 10]. It has also been hypothesized that the unique actions of aripiprazole involve functional selectivity or its partial agonist activity that may stabilize the dopaminergic signaling through the DRD2 [9, 11–15]. Previous studies have demonstrated that aripiprazole is a partial agonist for inhibition of cAMP accumulation through the DRD2 (i.e. Gα signaling) [12, 14, 16]. In contrast, it has also been reported that aripiprazole is an antagonist in GTPγS binding assays with the DRD2 [13, 14]. It was also revealed that aripiprazole failed to activate outward potassium currents following activation of the DRD2 in MES-23.5 cells, indirectly suggesting that it was inactive or possibly an antagonist for Gβγ signaling through the DRD2 [14].
The observations highlighted above prompted us to further explore the molecular pharmacology of aripiprazole at the DRD2 in comparison to a small subset of clinically-relevant DRD2 ligands. Specifically, we measured Gα signaling, Gβγ signaling, heterologous sensitization of adenylyl cyclase, and dynamic mass redistribution (DMR). Aripiprazole displayed a unique profile for activation of G protein signaling, behaving as a robust antagonist of Gβγ subunits, while partially activating Gα. Additionally, aripiprazole was a weak partial agonist/antagonist for heterologous sensitization of adenylyl cyclase and in cell-based DMR experiments. The unique antagonist profile of aripiprazole may provide additional opportunities for developing targeted antipsychotic agents.
2. Materials and Methods
2.1 Compounds used
The following compounds were purchased from Sigma-Aldrich (St. Louis, MO): dopamine hydrochloride, (±) quinpirole dihydrochloride, pramipexole dihydrochloride, ropinirole hydrochloride, rotigotine hydrochloride, clozapine, spiperone and 3-isobutyl-1-methylxanthine (IBMX). Aripiprazole was purchased from Santa Cruz Biotechnology (Dallas, TX). Forskolin was purchased from Tocris (Ellisville, MO). Haloperidol was a gift from D. Nichols, Purdue University.
2.2 Cell culture
Human embryonic kidney (HEK) cells stably expressing the long isoform of the DRD2 (HEK-D2) and adenylyl cyclase 2 (HEK-AC2/D2) or adenylyl cyclase 5 (HEK-AC5/D2) were cultured in Dubelcco’s Modified Eagle Medium (Life Technologies, Grand Island, NY) supplemented with 5% bovine calf serum (Hyclone, Logan, UT), 5% fetal clone I (Hyclone, Logan, UT), and Antibiotic-Antimycotic (Life Technologies, Grand Island, NY), and puromycin (Sigma-Aldrich, St. Louis, MO) (HEK-D2), or zeocin (Life Technologies, Grand Island, NY) and G418 (Invivogen, San Diego, CA) (HEK-AC2/D2), or puromycin and G418 (HEK-AC5/ D2). Cells were grown to confluency in 15 cm disses, harvested with Cell Dissociation Buffer (Life Technologies, Grand Island, NY), and resuspended in 5 ml of fetal bovine serum (Hyclone, Logan, UT) containing 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO), 1 ml was added to cryovials that were incubated overnight at −80°C in a CoolCell device (BioCision, Larkspur, CA) for cryopreservation. On the following day, cryovials were stored in liquid N2 until the assay day.
2.3 Gαi/o assay
Cryopreserved HEK-AC5/D2 cells were thawed in a 37°C water-bath, resuspended in 10 ml optiMEM (Life Technologies, Grand Island, NY) and centrifuged at 500 × g for 5 min. The cells were resuspended in 1 ml optiMEM, and counted using a Countess automated cell counter (Life Technologies, Grand Island, NY). Cells were plated in a white, flat bottom, low-volume, tissue culture-treated 384 well plate (PerkinElmer, Shelton, CT) at a final density of 2 000 cells/well. The plate was centrifuged for 1 min at 100 × g and incubated in a 37°C humidified incubator for 1 h. The DRD2 ligand was added followed by the addition of forskolin (3 µM final concentration) containing IBMX (0.5 mM final concentration). Cells were incubated at room temperature for 1 h and cAMP accumulation was measured using Cisbio’s dynamic 2 kit (Cisbio Bioassays, Bedford, MA) according to the manufacturer’s instructions. Plates were analyzed for fluorescent emissions at 620 nm and 665 nm using 330 nm as the excitation wavelength in a Synergy 4 (Biotek, Winooski, VT), and ratiometric analysis was carried out by dividing the 665 nm emissions by the 620 nm emissions to extrapolate the cAMP concentrations from a cAMP standard curve.
2.4 Gβγ assay
The assays used to measure Gβγ activation by the DRD2 were done using the specific properties of AC2, AC4, and AC7 [17, 18]. These AC isoforms are insensitive to inhibition by Gαi/o and potentiated by Gβγ subunits from Gαi/o-coupled receptors in the presence of direct AC-activators [19]. HEK-AC2/D2 cells were thawed in a 37°C water-bath, resuspended in 10 ml optiMEM and centrifuged at 500 × g for 5 min. The resuspension and centrifugation steps were repeated. The cells were resuspended in 1 ml optiMEM and counted. Cells were diluted to a concentration of 5 × 105 cells/ml and the cell suspension was added in a white, low-volume, flat bottom, tissue culture-treated 384 well plate resulting in a final density of 2 500 cells/well. The plate was centrifuged for 1 min at 100 × g and incubated in a 37°C humidified incubator for 1 h. The DRD2 ligand was added and cAMP accumulation was initiated by the addition of phorbol 12-myristate 13-acetate (PMA) (Tocris, Ellisville, MO) (final concentration of 1 µM) in the presence of 0.5 mM IBMX, to specifically stimulate AC2. Cells were incubated at room temperature for 1 h and cAMP accumulation was measured as described above.
2.5 Heterologous sensitization of adenylyl cyclase
Heterologous sensitization is a cellular adaptive response that occurs after prolonged periods of stimulation of Gαi/o-coupled receptors [5]. For the sensitization assays, HEKAC5/ D2 cells were thawed in a 37°C water-bath, resuspended in 10 ml optiMEM and centrifuged at 500 × g for 5 min. The cells were resuspended in 1 ml optiMEM and counted. Cells were diluted and added to a white, flat-bottom, tissue culture-treated 384 well plate (PerkinElmer, Shelton, CT) resulting in a final density of 2 000 cells/well. The plate was centrifuged for 1 min at 100 × g and incubated in a 37°C humidified incubator for 1 h. To induce sensitization, the DRD2 ligand was added and cells were pre-incubated in a 37°C humidified incubator for 2 h. Following the 2 h incubation (i.e. sensitization period), forskolin (300 nM final concentration) containing 0.5 mM IBMX was added in the presence of 1 µM spiperone (to inhibit acute ligand activation of the DRD2). Cells were incubated at room temperature for 1 h and cAMP accumulation was measured as described above.
2.6 Dynamic Mass Redistribution
For DMR assays, 20 µl per well of HEK-D2 growth medium was added to an EnSpire- LFC 384 fibronectin coated plate (PerkinElmer, Waltham, MA). Plate was centrifuged for 1 min at 500 × g and placed in 37°C humidified incubator while cell suspension was prepared. HEKD2 cells were dissociated and collected using 0.25% trypsin-EDTA (Life Technologies, Grand Island, NY), diluted to 10 ml with growth media, centrifuged at 500 × g for 5 min, and resuspended in 10 ml growth medium. Cells were counted and diluted to 5 × 105 cells/ml in growth medium, and 30 µl of this suspension was added to wells of EnSpire plate to achieve a final density of 15 000 cells/well and a total volume of 50 µl/well. Cells were incubated in a humidified incubator until 95% confluent, typically 16–24 h. One hour prior to assay, cell-containing wells were washed twice with room temperature assay buffer, 20 mM HEPES (Fisher Scientific, Pittsburg, PA) in HBSS (Life Technologies, Grand Island, NY) using a JANUS MDT Mini liquid handling robot (PerkinElmer, Shelton, CT). Cells were then incubated for 1 h at ambient temperature to allow development of stable DMR baseline. In agonist-mode, cells were incubated for 1 h in 40 µl assay buffer, and in antagonist mode cells were incubated for 1 h in 30 µl assay buffer supplemented with 10 µl of 5× concentrated antagonist. After 1 h, 10 baseline reads were generated, 10 µl of 5× concentrated agonist was added, and final DMR was measured for 200 reads using a PerkinElmer EnSpire Multimode Plate Reader equipped with Corning EPIC label-free technology (Waltham, MA). DMR responses were quantified by measuring both the total area under the curve (AUC) as well as the maximal DMR peak amplitude using GraphPad Prism (GraphPad Software Inc., San Diego, CA).
2.7 Data collection and analysis
All data reported represent the average of at least three independent experiments conducted in duplicate. Analysis for EC50/IC50 values and 95% confidence intervals (C.I.) were completed using GraphPad Prism.
3. Results
3.1 Aripiprazole is a partial agonist for Gα activation through the DRD2
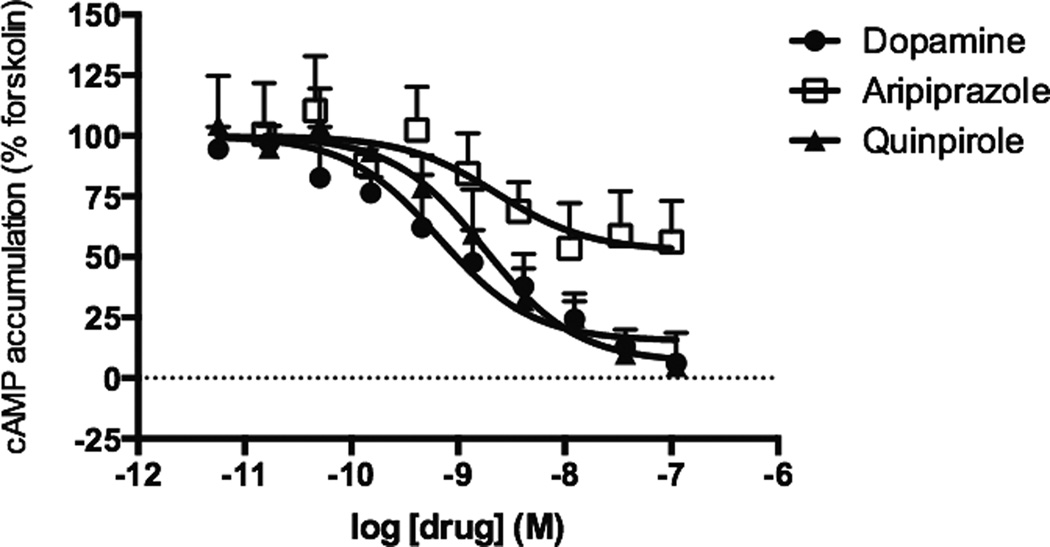
Previous studies have reported that aripiprazole is a partial agonist for inhibition of cAMP and an antagonist in GTPγS binding assays through the DRD2 [12–14, 16]. The work presented here examined DRD2-mediated inhibition of cAMP production in HEK cells stably expressing the DRD2 and adenylyl cyclase 5 (AC5). AC5 is abundantly expressed in the striatum, a region where the DRD2 is highly expressed and is thought to be essential for motor effects associated with DRD2 antagonism [20, 21]. In agreement to what was previously observed, aripiprazole was a partial agonist for inhibiting forskolin-mediated cAMP production displaying 48% of dopamine’s response (Figure 1). The prototypical DRD2 agonist, quinpirole resulted in 98% of dopamine’s response, and clinically used DRD2 agonists pramipexole, ropinirole, and rotigotine also had agonist responses with > 80% of dopamine’s response (Table 1). The potency of aripiprazole (ca. 4 nM) was comparable to that of dopamine and quinpirole (Table 1).
Figure 1.
Inhibition of cAMP accumulation by aripiprazole and reference DRD2 ligands. Activation of Gαi/o was assessed by measuring inhibition of forskolin-stimulated cAMP accumulation in HEK-AC5/D2 cells. Data shown represent the average and S.E.M. of at least three independent experiments conducted in duplicate.
Table 1.
Activation of Gαi/o, Gβγ, heterologous sensitization, and DMR downstream of the DRD2. Gαi/o signaling and heterologous sensitization were measured in HEK-AC5/D2 cells. Activation of Gβγ signaling was measured in HEK-AC2/D2 cells and DMR was measured in HEK-D2 cells. EC50 values are shown in nM with the 95% confidence interval. The maximal effects are shown as percentages of dopamine’s response with standard errors. Data in the table represent the average of at least three independent experiments. ND = not determined.
| Gαi/o activation | Gβγ activation | Sensitization | DMR (AUC) | |||||
|---|---|---|---|---|---|---|---|---|
| Compound | EC50 (nM) | Max (%) | EC50 (nM) | Max (%) | EC50 (nM) | Max (%) | EC50 (nM) | Max (%) |
| Dopamine | 1.5 (0.6–3.7) | 100 (±3) | 46 (28–75) | 100 (±4) | 8.2 (5.5–12.2) | 100 (±3) | 7.6 (6.7–8.6) | 100 (±1) |
| Quinpirole | 2.1 (1.0–4.5) | 98 (±3) | 74 (50–109) | 99 (±4) | 8.2 (6.1–11.0) | 124 (±3) | 37 (32–44) | 104 (±1) |
| Aripiprazole | 4.1 (0.6–30) | 48 (±9) | ND | - | 1.6 (0.7–3.9) | 18 (±1) | 730 (433–1231) | 32 (±1) |
| Pramipexole | 0.9 (0.4–1.9) | 87 (±4) | 17 (7.7–36) | 98 (±5) | 1.9 (1.2–3.0) | 119 (±4) | 8.3 (5.8–12) | 98 (±2) |
| Ropinirole | 2.6 (1.1–6.1) | 81 (±6) | 47 (25–89) | 70 (±4) | 8.4 (6.1–11.5) | 126 (±3) | 33 (26–41) | 100 (±2) |
| Rotigotine | 0.05 (0.01–0.11) | 88 (±4) | 0.7 (0.3–1.4) | 79 (±4) | 0.5 (0.3–0.7) | 116 (±5) | ND | - |
3.2 Aripiprazole is an antagonist of Gβγ signaling through the DRD2
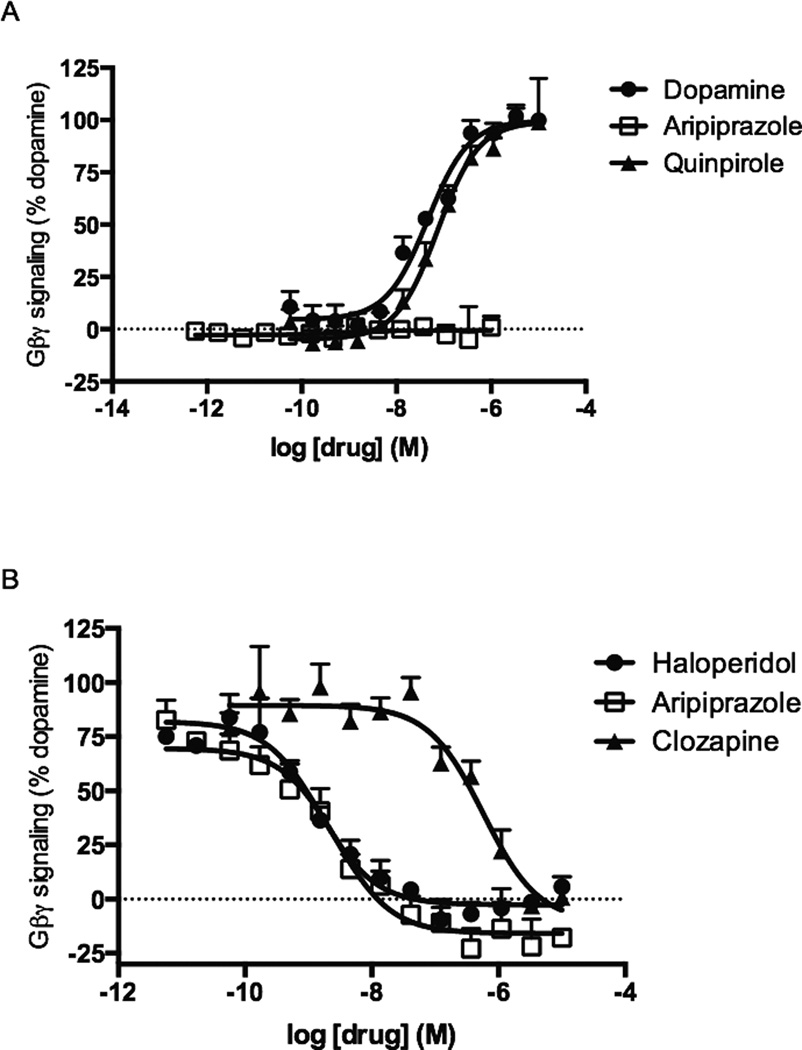
To explore the ability of aripiprazole to modulate Gβγ signaling through the DRD2 we measured Gβγ-mediated potentiation of AC2. AC2 is insensitive to direct Gαi/o regulation, but is conditionally activated by Gβγ subunits [19]. Thus, the cAMP response of AC2 to stimulators such as Gαs or PKC can be potentiated by the release of Gβγ subunits following DRD2 activation [18]. HEK cells stably expressing the DRD2 and AC2 were used, and DRD2-mediated potentiation of PKC-stimulated AC2 activity was assessed. As expected, dopamine and quinpirole elicited dose-dependent enhancements in PMA-stimulated cAMP accumulation in HEK-AC2/D2 cells with EC50 values of 46 nM (95% CI [28 – 75 nM]) and 74 nM (95% CI [50 – 109 nM]), respectively (Figure 2A). Pramipexole, ropinirole, and rotigotine also led to dosedependent enhancements of the cAMP response, resulting in 98%, 70%, and 79% of dopamine’s response, respectively (Table 1). In contrast, aripiprazole failed to enhance PMA-stimulated AC2 activity in this assay (Figure 2A).
Figure 2.
Modulation of Gβγ subunit signaling by aripiprazole and reference DRD2 ligands in HEK-AC2/D2 cells. A. Activation of Gβγ signaling was assessed by measuring DRD2 potentiation of the AC2-stimulated cAMP accumulation in HEK-AC2/D2 cells. B. Antagonism of dopamine’s Gβγ response through the DRD2. Cells were treated with antagonists for 30 min, followed by a 1 h incubation with 300 nM dopamine in the presence of 1 µM PMA and 0.5 mM IBMX. Data shown represent the average and S.E.M. of at least three independent experiments conducted in duplicate.
Because aripiprazole did not activate Gβγ subunit signaling in our assay, we explored its ability to antagonize dopamine’s Gβγ response. As shown in Figure 2B, aripiprazole fully inhibited dopamine’s response in a dose-dependent manner, with an IC50 value of 2.8 nM (95% CI [1.3 – 5.9 nM]). These results are consistent with the lack of GIRK channel activation observed by Shapiro et al. (2003) and demonstrate for the first time that aripiprazole is a potent antagonist of Gβγ signaling through the DRD2. The first generation antipsychotic drug haloperidol and the second generation antipsychotic drug clozapine were also tested for antagonism of Gβγ signaling. Both compounds fully inhibited dopamine’s response, with IC50 values of 1.5 nM (95% CI [0.8 – 2.8 nM) and 575 nM (95% CI [270 – 1 226 nM]), respectively (Figure 2B).
3.3 Aripiprazole’s effects on heterologous sensitization of AC5
Because aripiprazole was a partial agonist for Gαi/o activation and an antagonist for Gβγ subunits, a more complex G protein-dependent signaling pathway was analyzed. Heterologous sensitization of adenylyl cyclase is a cellular adaptive response that occurs following chronic activation of Gαi/o-coupled receptors [5]. In this phenomenon, prolonged Gαi/o agonist exposure results in a marked enhancement of subsequent drug-stimulated cAMP accumulation. Mechanistically, it has been shown that both Gα and Gβγ subunits of G proteins are involved in this cellular response [5].
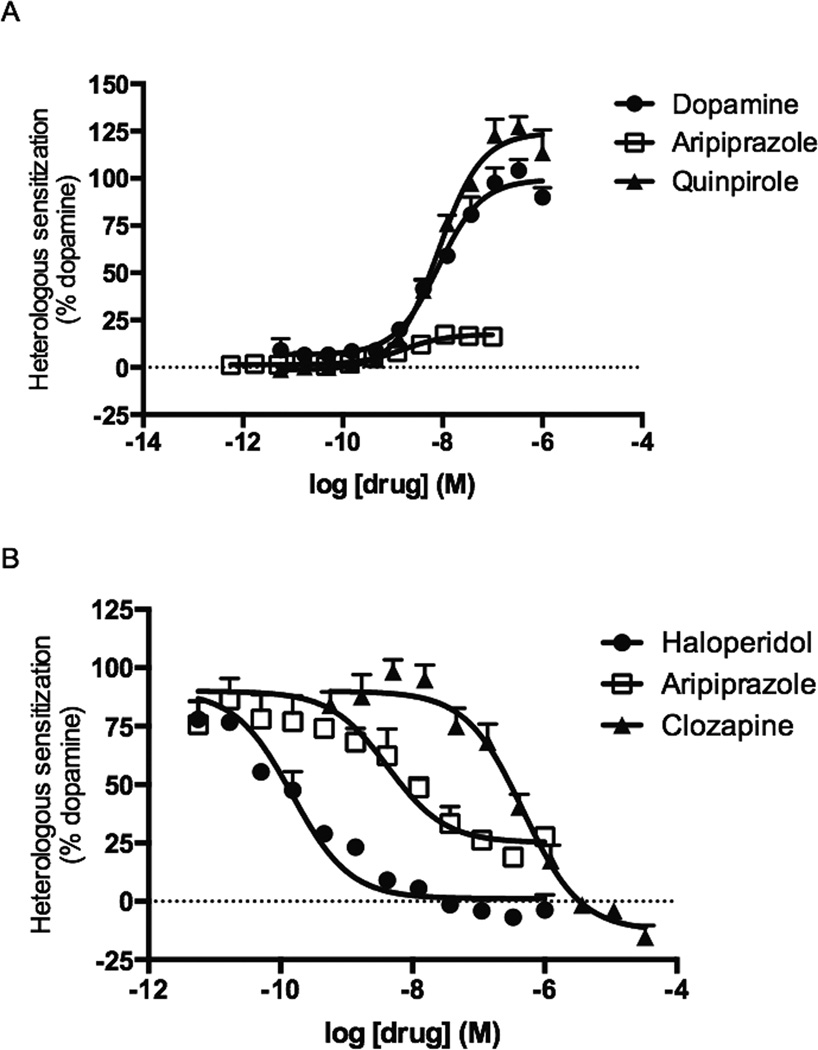
Heterologous sensitization of AC5 was measured in HEK cells stably expressing AC5 and the DRD2 by pre-incubating the cells with the DRD2 ligands for 2 h before stimulating cAMP accumulation with forskolin. Pretreatment with dopamine or quinpirole resulted in dose-dependent enhancements in the forskolin-stimulated cAMP accumulation with EC50 values of approximately 8 nM (Figure 3A). Pramipexole, ropinirole, and rotigotine also led to heterologous sensitization of AC5 and had maximal responses greater than 100% (Table 1). In contrast, aripiprazole displayed a weak partial agonist response in this assay, resulting in only 18% of dopamine’s response (Table 1). Subsequent antagonist-mode studies revealed that aripiprazole was a potent partial antagonist of dopamine-mediated heterologous sensitization of AC5 with an IC50 value of 4.2 nM (95% CI [2.0 – 8.9 nM]) (Figure 3B). Additional studies revealed that haloperidol and clozapine were also antagonists of heterologous sensitization with IC50 values of 0.2 nM (95% CI [0.1 – 0.2 nM]) and 473 nM (95% CI [320 – 698 nM]), respectively (Figure 3B).
Figure 3.
Heterologous sensitization by aripiprazole and reference DRD2 ligands in HEKAC5/ D2 cells. A. Heterologous sensitization of AC5 was measured by pre-treating cells with the DRD2 ligand for 2 h, followed by the addition of forskolin in the presence of IBMX and spiperone. B. Antagonism of heterologous sensitization of AC5 was measured by pre-incubating the cells with antagonists for 30 min, followed by a 2 h pre-treatment with 100 nM dopamine. Cyclic AMP accumulation was then stimulated by forskolin in the presence of IBMX and spiperone. Data shown represent the average and S.E.M. of three independent experiments conducted in duplicate.
3.4 Aripiprazole’s effects on dynamic mass redistribution (DMR)
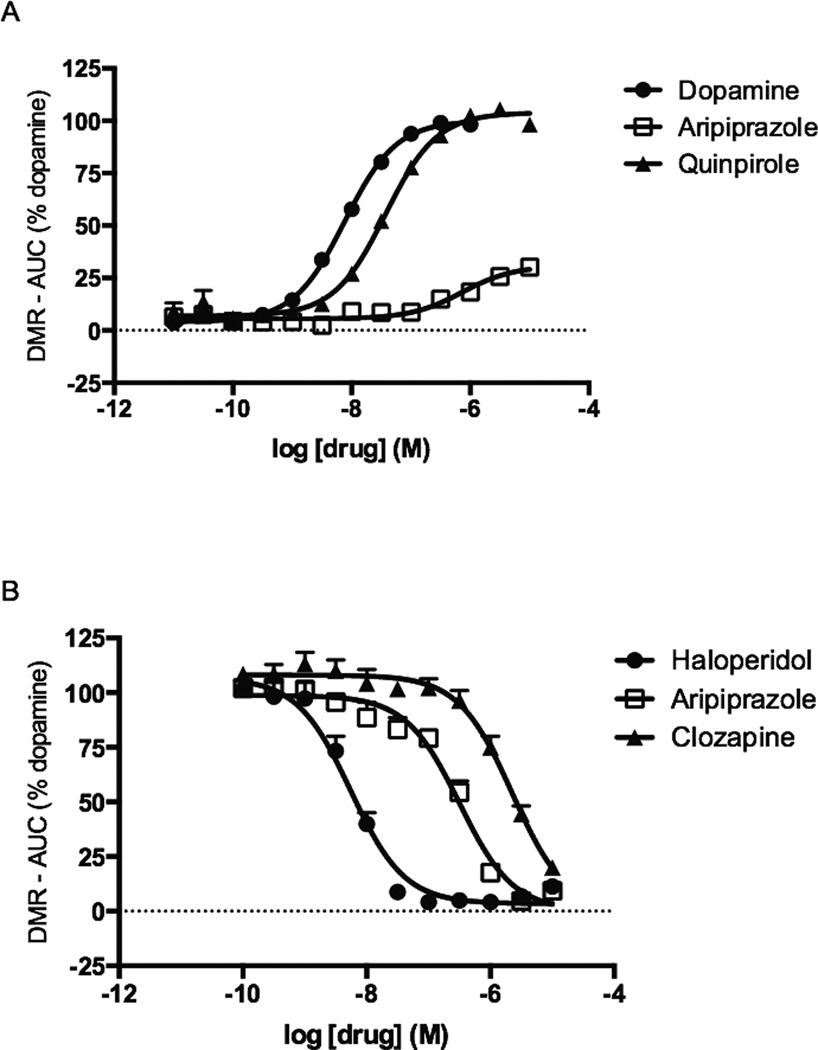
Another approach used to measure aripiprazole’s effects on the DRD2 employed a labelfree holistic approach. DMR responses reflect changes in cellular shape, which are hypothesized to be caused by the intracellular movement of biomolecules due to ligand-mediated signaling events [22]. These assays were conducted in HEK cells stably expressing the DRD2. Dopamine, quinpirole, pramipexole, and ropinirole displayed dose-dependent positive DMR responses with EC50 values ranging from approximately 8 nM to 40 nM (Table 1). All of these compounds elicited similar maximal responses, ranging from 98% to 104% of dopamine’s response (Table 1). In contrast, aripiprazole was only a partial agonist, with a maximal effect of 32% (Figure 4A). Aripiprazole was also the least potent compound tested with an EC50 value of 730 nM (95% CI [433 – 1 231 nM]), whereas dopamine was the most potent (Table 1).
Figure 4.
Modulation of DMR by aripiprazole and reference DRD2 ligands in HEK-D2 cells. A. Dynamic mass redistribution was measured during stimulation with DRD2 ligands, and the AUC at each drug concentration was determined. B. Antagonism of DMR was measured by pre-treating cells with antagonists for 1 h, followed by incubation with 100 nM dopamine. Data shown represent the average and S.E.M. of at least three independent experiments conducted in duplicate.
Subsequent studies evaluated aripiprazole in antagonist mode. After pretreatment with aripiprazole for 1 h, the DMR response of HEK-D2 cells to an EC90 concentration of dopamine (100 nM) was completely abrogated (Figure 4B). This also held true for the DRD2 antagonists haloperidol and clozapine. Haloperidol was the most potent inhibitor tested, possessing an IC50 of 5.5 nM (95% CI [4.0 – 7.7 nM]). Aripiprazole and clozapine exhibited IC50s of 316 nM (95% CI [230 – 434 nM]) and 2.2 µM (95% CI [1.4 – 3.6 µM]), respectively (Figure 4B).
4. Discussion
Aripiprazole is considered to be the prototypical third generation antipsychotic drug. Clinical studies showed that aripiprazole was efficacious in acute and maintenance treatments of schizophrenia [23]. Aripiprazole was also superior than the second generation antipsychotic drug olanzapine in improving neurocognitive symptoms. Additionally, aripiprazole was effective as an adjunctive therapy for major depressive disorder and in treating other behavioral and mental illnesses, such as bipolar disorder and treatment-resistant mood and anxiety disorders [23, 24]. Remarkably, in comparison to other antipsychotic drugs, aripiprazole displays a significantly lower tendency to cause commonly observed side effects including extrapyramidal symptoms [25].
One of the hypotheses for the improved clinical profile of aripiprazole is that it acts as a “dopamine stabilizer”. This hypothesis proposes that because of aripiprazole’s partial agonist activity, when there are high levels of dopamine, aripiprazole behaves as a partial antagonist, inhibiting dopamine’s actions; however, in low levels of dopamine, aripiprazole behaves as a partial agonist, increasing or normalizing dopaminergic signaling [26]. Though this seems to be a simple and logical explanation, the unique pharmacological profile of aripiprazole in a variety of assays suggests that additional molecular mechanisms may underlie aripiprazole’s improved clinical effects.
For example, aripiprazole also has high affinity for other GPCRs, such as the dopamine D3 receptor, the 5HT2A 5HT1A, 5HT2B, and 5HT7 serotonin receptors, the H1 histamine receptor, and the α1 adrenergic receptor [14]. The functional effects of aripiprazole on serotonin receptors have been studied revealing partial agonist activity in assays with 5HT1A, 5HT2A, 5HT3C, 5HT7 serotonin receptors, inverse agonism of the 5HT2B serotonin receptor, and antagonism of the 5HT6 serotonin receptor [14]. These data suggest that the unique clinical profile of aripiprazole could be due to its interactions with other GPCRs.
Aripiprazole also displays a somewhat unique pharmacological profile in functional assays with the DRD2. Consistent with our results, aripiprazole partially inhibited forskolin-mediated cAMP accumulation in multiple cell lines [12, 14, 16]. However, in GTPγS binding assays with the DRD2 aripiprazole fully antagonized the responses to both dopamine and quinpirole [13, 14]. These data examining Gα responses are seemingly at odds and may be explained by some degree of functional selectivity for downstream Gα responses or alternatively, signal sensitivity or the lack of amplification in the GTPγS binding assays [27]. In our model of Gαi/o activation, the DRD2 was co-expressed with AC5 in HEK cells. AC5 was used because it is the most abundant adenylyl cyclase in the striatum, a brain region in which the DRD2 is highly expressed [20, 21]. Another unique finding from Shapiro et al. was that aripiprazole failed to increase the activity of GIRK channels [14]. Activation of GIRK channels by the DRD2 is a signaling event that is mediated by Gβγ subunits and has been associated with decreased synaptic activity in the basal ganglia [28]. The lack of a GIRK response was not explored further, however, we hypothesized that aripiprazole could potentially act as an antagonist for this downstream Gβγ response. HEK cells stably expressing AC2 and the DRD2 were employed to measure Gβγ activation in response to aripiprazole and control ligands. Although AC2 was used as a reporter of Gβγ signaling in our model, AC2 is widely expressed in the central nervous system [20]. Consistent with our hypothesis, aripiprazole dose-dependently antagonized dopamine’s ability to potentiate AC2 activity. The potency for blocking this Gβγ response was similar to that observed with the first generation antipsychotic drug, haloperidol. The results described above may be explained by transducer-effector coupling efficiency (i.e. agonists showed greater potency for Gα versus Gβγ), but also may suggest some level of pathway functional selectivity at divergent signaling pathways downstream of the DRD2.
Heterologous sensitization of adenylyl cyclase is a cellular adaptive response that occurs following prolonged periods of Gαi/o-coupled receptor activation [5]. DRD2-induced sensitization results in a marked enhancement of subsequent cAMP signaling in both cellular and animal models [5, 29, 30]. Enhanced or persistent DRD2 activation has also been implicated in schizophrenia [31–33]. Further, elevated brain adenylyl cyclase activity and cAMP levels in the cerebrospinal fluid have been reported in schizophrenics [34, 35]. The weak partial agonist response as well as antagonist activities observed for aripiprazole in our heterologous sensitization assays suggest that prolonged treatments with similar drugs will produce only modest adaptive effects on cAMP accumulation. This outcome may be a beneficial feature for antipsychotic drugs like aripiprazole that appear to stabilize or normalize DRD2 effects on intracellular second messengers both acutely and chronically.
The novel responses we observed with aripiprazole in the G protein-mediated assays prompted us to explore DMR as an unbiased readout for ligand-receptor signaling. In DMR experiments, plane polarized light is passed through specialized biosensor microtiter plates containing cells in the absence and presence of receptor ligand [37]. Mass movement within the cell causes changes in the cellular index of refraction, leading to altered resonance of polarized light. As mass moves towards the bottom of the plate, longer wavelengths resonate, producing positive DMR signals. Alternatively, as cellular mass moves away from the plate, shorter wavelengths resonate and a negative DMR signal is observed. DMR is a label-free approach for measuring integrated receptor responses in real time and has been used with a variety of G protein-coupled receptors. Previous work has characterized DMR signals mediated through Gαs, Gαi/o-, Gαq/11-, and Gα12/13-coupled receptors including muscarinic M2 and M3 receptors, α2 and β2 adrenergic receptors, and GPR55 [36, 37]. The DMR measurement profile of Gαi/ocoupled receptors presumably involves Gα subunit activation because it is prevented by pertussis toxin [37]. Consistent with this idea and similar to the heterologous sensitization results, aripiprazole elicited a weak partial agonist response. Somewhat surprisingly, aripiprazole pretreatment prevented the cellular DMR responses to an EC90 concentration of dopamine. This lack of observed partial agonist activity may reflect receptor desensitization or more speculative, altered receptor conformations leading to altered functional affinity in the DMR experiments [38].
Previous functional selectivity studies with aripiprazole-like drugs propose somewhat opposing hypotheses. It has been posited that aripiprazole’s ability to antagonize β-arrestin signaling through the DRD2 is key for antipsychotic activity [39–41]. Antagonism of β-arrestin leads to higher Akt activity and, thus, glycogen synthase kinase 3 inhibition potentially yielding antipsychotic activity [42–44]. In contrast, another team has shown that aripiprazole is a partial agonist of β-arrestin through the DRD2 [11]. They reported that aripiprazole analogs that selectively activate β-arrestin display behaviors consistent with antipsychotic activity with reduced extrapyramidal symptoms in mice. These apparently opposing hypotheses can be reconciled by assuming that some degree of β-arrestin agonism/antagonism (i.e. partial agonism) or DRD2 receptor stabilization of that pathway is important for antipsychotic efficacy.
The present results demonstrate a novel DRD2 modulation profile for aripiprazole (i.e. antagonist for Gβγ signaling) that may suggest some level of functional selectivity or signaling pathway specificity. In addition to aripiprazole, we showed that other clinically used antipsychotic drugs (i.e. haloperidol and clozapine) also antagonized Gβγ signaling through the DRD2. These results suggest that inhibition of Gβγ signaling through the DRD2 may be a shared feature of clinically used antipsychotic drugs. Two recent studies suggest that partial agonism of β-arrestin and inhibition of Gαi/o through the DRD2 may provide antipsychotic activity with reduced motor side effects [11, 45]. Unfortunately, the activity of those ligands at Gβγ signaling effectors (e.g. AC2 or GIRK) was not examined. It would be interesting to explore the activity of such ligands for Gβγ-dependent pathways. The physiological responses to antagonism of DRD2 Gβγ signaling are anticipated to be diverse and involve multiple effectors expressed throughout the central nervous system including multiple AC isoforms (i.e. AC2, AC4, and AC7), GIRK and N-type calcium channels, phospholipase C isoforms, phosphoinositide 3 kinase, glycine receptors, phosducin, tubulin, and ERK [46, 47]. The potential number of pathways involving the DRD2 Gβγ effectors may represent a new muti-pathway approach versus a multi-receptor strategy in the “War on Mental Illness.”
Acknowledgements
Special thanks to the University of Iowa High-Throughput Screening (UIHTS) center director Dr. Meng Wu. Support for the UIHTS facility was provided by NIH S10 RR029274. The authors also wish to acknowledge Heidi Morgan for technical assistance in establishing the DMR assay and Isabelle Verona Brust for the graphical abstract.
Funding:
This research was supported by R01 MH060397 and R21 MH101673 from the National Institute of Mental Health as well as by Purdue University. This research was also supported by an American Foundation for Pharmaceutical Education fellowship and the Predoctoral Training Program in Pharmacological Sciences T32 GM067795 (MPH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds studied in this article:
Aripiprazole (PubChem CID: 60795), dopamine hydrochloride (PubChem CID: 65340), pramipexole dihydrochloride (PubChem CID: 166589), ropinirole hydrochloride (PubChem CID: 68727), rotigotine hydrochloride (PubChem CID: 180335), clozapine (PubChem CID: 2818), haloperidol (PubChem CID: 3559).
Disclosures:
TFB, MPH, DLR, and VJW report no financial relationships with commercial interests.
References
- 1.Urban J, Clarke W, von Zastrow M, Nichols D, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 2.Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- 3.Whalen E, Rajagopal S, Lefkowitz R. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaulieu J, Gainetdinov R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 5.Watts V, Neve K. Sensitization of adenylate cyclase by Galphai/o-coupled receptors. Pharmacol Ther. 2005;106(3):405–421. doi: 10.1016/j.pharmthera.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Meissner W, Frasier M, Gasser T, Goetz C, Lozano A, Piccini P, et al. Priorities in Parkinson's disease research. Nat Rev Drug Discov. 2011;10:377–393. doi: 10.1038/nrd3430. [DOI] [PubMed] [Google Scholar]
- 7.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE, et al. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60:358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mailman R, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16:488–501. doi: 10.2174/138161210790361461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones M, White J, Gray R. Partial agonists and schizophrenia: theoretical developments for the development of mental health nursing. J Psychiatr Ment Health Nurs. 2009;16:409–415. doi: 10.1111/j.1365-2850.2009.01373.x. [DOI] [PubMed] [Google Scholar]
- 11.Allen J, Yost J, Setola V, Chen X, Sassano M, Chen M, et al. Discovery of beta-Arrestin-Biased Dopamine D2 Ligands for Probing Signal Transduction Pathways Essential for Antipsychotic Efficacy. Proc Natl Acad Sci U S A. 2011;108:18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- 13.Fell MJ, Perry KW, Falcone JF, Johnson BG, Barth VN, Rash KS, et al. In vitro and in vivo evidence for a lack of interaction with dopamine D2 receptors by the metabotropic glutamate 2/3 receptor agonists 1S,2S,5R,6S-2-aminobicyclo[3.1.0]hexane-2,6-bicaroxylate monohydrate (LY354740) and (−)-2-oxa-4-aminobicyclo[3.1.0] Hexane-4,6-dicarboxylic acid (LY379268) J Pharmacol Exp Ther. 2009;331:1126–1136. doi: 10.1124/jpet.109.160598. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 15.Urban JD, Vargas GA, von Zastrow M, Mailman RB. Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology. 2007;32:67–77. doi: 10.1038/sj.npp.1301071. [DOI] [PubMed] [Google Scholar]
- 16.Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, et al. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20:612–627. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 17.Cooper D, Crossthwaite A. Higher-order organization and regulation of adenylyl cyclases. Trends Pharmacol Sci. 2006;27:426–431. doi: 10.1016/j.tips.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Watts VJ, Neve KA. Activation of type II adenylate cyclase by D2 and D4 but not D3 dopamine receptors. Mol Pharmacol. 1997;52:181–186. doi: 10.1124/mol.52.2.181. [DOI] [PubMed] [Google Scholar]
- 19.Federman AD, Conklin BR, Schrader KA, Reed RR, Bourne HR. Hormonal-Stimulation of Adenylyl Cyclase through Gi-Protein Beta-Gamma-Subunits. Nature. 1992;356:159–161. doi: 10.1038/356159a0. [DOI] [PubMed] [Google Scholar]
- 20.Chern Y. Regulation of adenylyl cyclase in the central nervous system. Cell Signal. 2000;12:195–204. doi: 10.1016/s0898-6568(99)00084-4. [DOI] [PubMed] [Google Scholar]
- 21.Lee KW, Hong JH, Choi IY, Che Y, Lee JK, Yang SD, et al. Impaired D2 dopamine receptor function in mice lacking type 5 adenylyl cyclase. J Neurosci. 2002;22:7931–7940. doi: 10.1523/JNEUROSCI.22-18-07931.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder R, Schmidt J, Blattermann S, Peters L, Janssen N, Grundmann M, et al. Applying label-free dynamic mass redistribution technology to frame signaling of G protein-coupled receptors noninvasively in living cells. Nat Protoc. 2011;6:1748–1760. doi: 10.1038/nprot.2011.386. [DOI] [PubMed] [Google Scholar]
- 23.DeLeon A, Patel NC, Crismon ML. Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clinical therapeutics. 2004;26:649–666. doi: 10.1016/s0149-2918(04)90066-5. [DOI] [PubMed] [Google Scholar]
- 24.Pae CU, Forbes A, Patkar AA. Aripiprazole as adjunctive therapy for patients with major depressive disorder: overview and implications of clinical trial data. CNS drugs. 2011;25:109–127. doi: 10.2165/11538980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Marder SR, McQuade RD, Stock E, Kaplita S, Marcus R, Safferman AZ, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res. 2003;61:123–136. doi: 10.1016/s0920-9964(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 26.Tamminga CA, Carlsson A. Partial dopamine agonists and dopaminergic stabilizers, in the treatment of psychosis. Curr Drug Targets CNS Neurol Disord. 2002;1:141–147. doi: 10.2174/1568007024606195. [DOI] [PubMed] [Google Scholar]
- 27.Harrison C, Traynor JR. The [35S]GTPgammaS binding assay: approaches and applications in pharmacology. Life Sci. 2003;74:489–508. doi: 10.1016/j.lfs.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chester JA, Mullins AJ, Nguyen CH, Watts VJ, Meisel RL. Repeated quinpirole treatments produce neurochemical sensitization and associated behavioral changes in female hamsters. Psychopharmacology. 2006;188:53–62. doi: 10.1007/s00213-006-0468-2. [DOI] [PubMed] [Google Scholar]
- 30.Culm KE, Lugo-Escobar N, Hope BT, Hammer RP., Jr Repeated quinpirole treatment increases cAMP-dependent protein kinase activity and CREB phosphorylation in nucleus accumbens and reverses quinpirole-induced sensorimotor gating deficits in rats. Neuropsychopharmacology. 2004;29:1823–1830. doi: 10.1038/sj.npp.1300483. [DOI] [PubMed] [Google Scholar]
- 31.Bach ME, Simpson EH, Kahn L, Marshall JJ, Kandel ER, Kellendonk C. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proc Natl Acad Sci U S A. 2008;105:16027–16032. doi: 10.1073/pnas.0807746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, et al. Psychosis pathways converge via D2high dopamine receptors. Synapse. 2006;60:319–346. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- 34.Kerwin RW, Beats BC. Increased forskolin binding in the left parahippocampal gyrus and CA1 region in post mortem schizophrenic brain determined by quantitative autoradiography. Neurosci Lett. 1990;118:164–168. doi: 10.1016/0304-3940(90)90617-i. [DOI] [PubMed] [Google Scholar]
- 35.Muly C. Signal transduction abnormalities in schizophrenia: the cAMP system. Psychopharmacol Bull. 2002;36:92–105. [PubMed] [Google Scholar]
- 36.Ferrie AM, Sun H, Zaytseva N, Fang Y. Divergent label-free cell phenotypic pharmacology of ligands at the overexpressed beta(2)-adrenergic receptors. Sci Rep. 2014;4:3828. doi: 10.1038/srep03828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroder R, Janssen N, Schmidt J, Kebig A, Merten N, Hennen S, et al. Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat Biotechnol. 2010;28:943–949. doi: 10.1038/nbt.1671. [DOI] [PubMed] [Google Scholar]
- 38.Kenakin T. What is pharmacological 'affinity'? Relevance to biased agonism and antagonism. Trends Pharmacol Sci. 2014 doi: 10.1016/j.tips.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Beaulieu J, Tirotta E, Sotnikova T, Masri B, Salahpour A, Gainetdinov R, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del'guidice T, Lemasson M, Beaulieu J. Role of Beta-arrestin 2 downstream of dopamine receptors in the Basal Ganglia. Front Neuroanat. 2011;5:58. doi: 10.3389/fnana.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu J, Gainetdinov R, et al. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci U S A. 2008;105:13656–13661. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaulieu J, Marion S, Rodriguiz R, Medvedev I, Sotnikova T, Ghisi V, et al. A beta-arrestin-2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 43.Beaulieu J, Sotnikova T, Marion S, Lefkowitz R, Gainetdinov R, Caron M. An Akt/betaarrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Urs NM, Snyder JC, Jacobsen JP, Peterson SM, Caron MG. Deletion of GSK3beta in D2Rexpressing neurons reveals distinct roles for beta-arrestin signaling in antipsychotic and lithium action. Proc Natl Acad Sci U S A. 2012;109:20732–20737. doi: 10.1073/pnas.1215489109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Sassano MF, Zheng L, Setola V, Chen M, Bai X, et al. Structure-functional selectivity relationship studies of beta-arrestin-biased dopamine D(2) receptor agonists. J Med Chem. 2012;55:7141–7153. doi: 10.1021/jm300603y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbe JC, et al. The expanding roles of Gbetagamma subunits in G protein-coupled receptor signaling and drug action. Pharmacol Rev. 2013;65:545–577. doi: 10.1124/pr.111.005603. [DOI] [PubMed] [Google Scholar]
- 47.Lin Y, Smrcka AV. Understanding molecular recognition by G protein betagamma subunits on the path to pharmacological targeting. Mol Pharmacol. 2011;80:551–557. doi: 10.1124/mol.111.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]