Abstract
Background:
The prevalence and clinical significance of right ventricular (RV) systolic dysfunction (RVD) in patients with heart failure and preserved EF (HFpEF) are not well characterized.
Methods and Results:
Consecutive, prospectively identified HFpEF (Framingham HF criteria, EF ≥50%) patients (N=562) from Olmsted County, Minnesota underwent echocardiography at HF diagnosis and follow-up for cause specific mortality and HF hospitalization. RV function was categorized by tertiles of tricuspid annular plane systolic excursion (TAPSE) and by semi-quantitative (normal, mild RVD or moderate-severe RVD) 2D assessment. Whether RVD was defined by semi-quantitative assessment or TAPSE ≤ 15 mm, HFpEF patients with RVD were more likely to have atrial fibrillation, pacemakers and chronic diuretic therapy. At echo, patients with RVD had slightly lower LVEF, worse diastolic dysfunction, lower blood pressure and cardiac output, higher pulmonary artery systolic pressure (PASP), and more severe RV enlargement and tricuspid valve regurgitation. Adjusting for age, sex, PASP and comorbidities, the presence of any RVD by semi-quantitative assessment was associated with higher all-cause (hazard ratio (HR) = 1.35 (1.03-1.77; p=0.03)) and cardiovascular (HR=1.85 (1.20-2.80; p=0.006)) mortality and higher first (HR=1.99 (1.35-2.90; p=0.0006) and multiple (HR=1.81 (1.18-2.78; p=0.007) HF hospitalization rates. RVD defined by TAPSE values showed similar but weaker associations with mortality and HF hospitalizations.
Conclusions:
In the community, RVD is common in HFpEF patients, associated with clinical and echocardiographic evidence of more advanced HF and predictive of poorer outcomes.
Keywords: Diastole, Heart failure with preserved ejection fraction, Hypertension, Pulmonary hypertension, Right ventricle, TAPSE
BACKGROUND
In heart failure (HF) with reduced ejection fraction (HFrEF), right ventricular (RV) systolic dysfunction (RVD) is common,1 associated with impaired functional capacity and portends a poor prognosis.2-7 In HFrEF, ischemic or myopathic processes may directly involve the RV and lead to RVD. Isolated insults to the left ventricle (LV) can lead to pulmonary hypertension (PH) and neurohumoral and cytokine activation. The resulting RV pressure overload, inflammation and altered RV myocardial gene expression promote RVD in the absence of primary RV myocardial injury.8
The prevalence and functional and prognostic implications of RVD in HF with preserved ejection fraction (HFpEF) are less clear. While infarction or myopathic processes isolated to the RV are uncommon, PH is equally prevalent in HF with reduced or preserved LV ejection fraction (LVEF),9-11 neurohumoral activation occurs in HFpEF12 and comorbidities, which are highly prevalent in HFpEF, may play a fundamental role in the pathogenesis of altered myocardial function in HFpEF.13 Thus, HFpEF patients may be at risk for RVD.
Understanding the prevalence and clinical implications of altered RV function in large HF cohorts is hindered by the challenges to quantitative assessment of RV structure and function.14,15 While a growing number of RV functional indices have been proposed, feasibility, concordance, sensitivity and specificity for RVD and clinical implications of these parameters are poorly described, particularly in HF.16 In the limited studies to date, estimates of RVD prevalence in HFpEF vary widely with the cohort studied, RV functional measure utilized and partition values used to define RVD.17-19
Recognition of the prevalence and clinical implications of RVD and its relation to PH in HFpEF patients is important to better understand HFpEF pathophysiology, facilitate accurate diagnosis and prognostication and identify potential therapeutic targets.20,21 Accordingly, the objective of the current study was to characterize RV function using two highly feasible and widely available measures in a large, community based cohort of HFpEF patients. Clinical and echocardiographic features and outcomes associated with differences in RV function (as assessed by tricuspid annular plane systolic excursion (TAPSE) and semi-quantitative assessment of RV function) were studied.
METHODS
The study was approved by the Mayo Clinic institutional review board. All subjects provided written consent for inclusion in this study.
Study subjects
This Olmsted county HFpEF cohort has been previously described.22 Briefly, consecutive adult patients with HFpEF (Framingham criteria for HF diagnosis and LVEF≥50%) were identified by real-time interrogation of electronic medical records using natural language processing techniques and prospectively enrolled between September, 2003, and August, 2009. Exclusion criteria were: Significant left-sided valve disease, known cardiomyopathies, congenital heart disease or pericardial disease. Clinical characteristics and comorbidities including chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) were defined as previously described.22
Echocardiography
Body size, blood pressure and heart rate were measured at the time of echocardiography.
Tricuspid annular plane systolic excursion (TAPSE)
As m-mode TAPSE was not routinely measured in our echocardiography laboratory during the enrollment period, TAPSE was measured on previous studies using two dimensional (2D) images from the apical four chamber view. 2 D TAPSE was measured by subtracting the distance between the lateral tricuspid leaflet insertion to the tricuspid annulus and sector apex in systole from the distance between the two in diastole. Three measurements in sinus rhythm (five in atrial fibrillation) were averaged. 2 D TAPSE measurement was feasible in 500 (89%) subjects.
Correlation of off line 2D and m mode derived TAPSE was examined in subjects undergoing echocardiography for other indications (n=15) who had m-mode TAPSE measured. We also measured 2D TAPSE in an age and sex matched normal cohort without HFpEF, CAD, DM or hypertension (n=89).
Semi-quantitative RV systolic function and RV size assessment
Per local echocardiography protocol, RV systolic function was assessed by integrating visual assessment of contractility of the RV outflow tract, RV apex and interventricular septum from different views and characterized on an ordinal scale. RV size was assessed semi-quantitatively as normal size (≤ ⅔ of the LV size) or as mildly (RV similar to the LV size), moderately (RV larger than the LV) or severely (RV much larger than the LV) enlarged.
When semi-quantitative RV enlargement (n=7, 1.2%) or dysfunction (n=8, 1.4%) were described without a quantifier, the severity was assumed to be mild. When RV function was not specifically commented on (n=21; 4%), the following methods were preferentially used to approximate RV function in the following order: linear interpolation (closest echocardiograms before and after; n=14), last observation carried forward (n= 4) or next observation carried backward (n=3). For the carried observations the median time from index echocardiograms was 2 years.
Throughout the manuscript the term RVD is used to denote RV systolic dysfunction.
Pulmonary artery systolic pressure (PASP)
PASP was measurable in 496 (88%) patients and was estimated as the RV systolic pressure (RVSP), as pulmonary valve stenosis was excluded in all patients. RVSP was calculated from the continuous wave Doppler tricuspid valve regurgitant velocity using the simplified Bernoulli equation and right atrial pressure estimated in 5 mmHg increments between 5 to 20 mmHg, based on the size and collapsibility of the inferior vena cava.23
Left ventricular structure and function
LVEF assessment was based on the echocardiographer’s collation of multiple assessments as previously described.24,25 Other standard LV structural and functional indices were calculated from 2D, m mode and Doppler measurements according to ASE guidelines as previously described.22 Left atrial (LA) volume was measured with the area-length method.22 Characterization of LV diastolic function was performed as previously described.24 Briefly, the speed of LV relaxation was estimated by the early diastolic medial LV septal tissue velocity (e’). The early transmitral flow velocity (E) to e’ (E/e’) ratio was used as an estimate of LV filling pressure. The early diastolic transmitral flow deceleration time (DT) was used to assess restriction to LV filling as it reflects rapid elevation of LV diastolic pressures with filling in the setting of impaired relaxation.26
Tricuspid valve regurgitation (TR)
Routine assessment of TR in our echocardiographic laboratory incorporates semi-quantitative methods (color flow imaging and hepatic vein flow pulsed wave Doppler integration) and is graded on a six-point (trivial to severe) ordinal scale as described previously.27 For the current analysis, patients with moderate, moderate-severe or severe TR were characterized as having “Mod-Severe” TR; patients with mild or mild-moderate TR were characterized as having “Mild-Mod” TR and patients with trivial or no TR were characterized as having no TR.
Laboratory data
Glomerular filtration rate (GFR) was estimated using the modification of diet in renal disease (MDRD) formula.
Follow up and outcomes
Subjects were followed for up to 10 years (through October/November, 2013). HF hospitalization was defined as a primary dismissal diagnosis of HF (ICD9CM code 428.xx).28 Death was ascertained from the Mayo electronic records and the Rochester epidemiology project as described previously. Cause of death (immediate) was obtained from the death certificate or autopsy report as documented by a pathologist as described previously.29 Cardiovascular death was defined as death due to HF, arrhythmia, ischemic heart disease, valvular heart disease, stroke, vascular disease or pulmonary embolism.
Statistical Analysis
Data are presented as medians (25th, 75th percentile) or % frequency. Nonparametric rank tests and chi square test for independence/Fisher’s exact test were used for across group comparison of continuous and categorical variables, respectively. We did not adjust for multiple comparisons.
Kaplan-Meier analysis and log-rank statistic were used to compare survival and event free survival between groups. Cox proportional-hazards regression was used to adjust for pertinent covariates. Stepwise linear regression model with backward elimination was constructed by forcing age and sex into the model and entering all potential explanatory variables: comorbidities (atrial fibrillation, diabetes mellitus, COPD and OSA) in addition to PASP, TR and RV function. Variables with a p value ≥0.10 were eliminated.
Andersen and Gill formulation of the Cox proportional-hazards regression was used to model time to multiple HF hospitalizations, whereby subjects were allowed to experience multiple events with risk discontinuation during a hospitalization episode. The hazard ratio (95% confidence interval) associated with dichotomous variables or a one standard deviation change in continuous variables was provided. All analyses were 2 tailed, and a p value <0.05 was considered statistically significant. Analysis was performed using the JMP® and SAS® statistical softwares (SAS Corporation).
RESULTS
2D TAPSE
Values for TAPSE derived by 2D or m mode methods showed good correlation and agreement (Supplemental Figure 1A and B). In an age (78 (72-85) years) and sex (56% women) matched normal community cohort without HFpEF or cardiovascular disease (n=89), the median 2D TAPSE was 19.0 (16.8 – 21.7) mm and the mean 2D TAPSE was 19.5 ± 3.8 mm; similar to values for m mode TAPSE previously reported in healthy persons over 70 years of age (18.0 ± 3.0 mm).30
RV Function in HFpEF
In HFpEF patients, the median TAPSE was 17 (14-21) mm and 177 (35%) of 500 patients with measurable TAPSE had a value below the ASE specified lower limit of normal (16 mm; Figure 1A).15 By semi-quantitative assessment, 118 (21%) of 562 patients with semi-quantitative assessment had some degree (mild or moderate-severe) of RVD and TAPSE was lower in these patients (13 (10-16) mm) than in patients with normal RV function (19 (15-22) mm; p<0.0001) by semi-quantitative assessment. TAPSE values declined with increasing severity of semi-quantitative RVD (Figure 1B). The distribution of TAPSE differed from that observed in the age and sex matched control population (Figure 1C). Agreement (kappa (confidence interval)) between categorical designation of RVD (TAPSE < 16 mm or any RVD by semi-quantitative) was not strong (kappa 0.30 (0.21-0.38), Supplemental Table 1.
Figure 1.
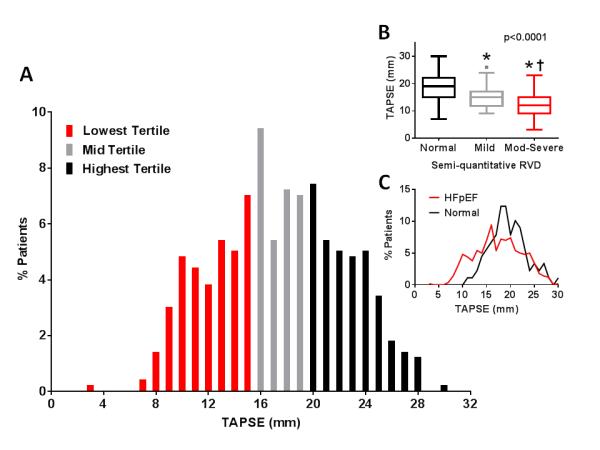
Distribution of tricuspid annular plane systolic excursion (TAPSE) in HFpEF patients (A; red, lowest; grey, middle and black, highest tertile). Insert (B) shows Tukey box and whisker plots of TAPSE values in patients with normal, mildly or moderate-severely depressed RV systolic function by semi-quantitative assessment. * p<0.05 vs normal RV function; † p<0.05 vs mildly depressed RV systolic function. Insert (C) shows the distribution of TAPSE in HFpEF and in an age and sex matched healthy control population without cardiovascular disease.
Clinical characteristics of subjects according to RV function
The clinical characteristics of patients with TAPSE values in the highest and mid tertiles did not differ from each other (Table 1). As compared with patients in the highest/mid tertiles (combined), patients in the lowest TAPSE tertile were more likely to have coronary artery disease, atrial fibrillation, permanent pacing and treatment with ACE/ARB and diuretics.
Table 1.
Clinical characteristics in subjects according to TAPSE assessed RV function.
| Upper and middle combined |
Upper tertile TAPSE ≥ 20 mm |
Middle tertile TAPSE 16-19 mm |
Lower tertile TAPSE ≤ 15 mm |
Across group p value |
|
|---|---|---|---|---|---|
| N | 323 | 178 | 145 | 177 | |
| Age, years | 78 (70-85) | 77 (68-85) | 79 (72-87) | 80 (73-86) | 0.10 |
| Women, n (%) | 186 (58) | 102 (57) | 84 (58) | 100 (57) | 0.97 |
| BSA, m2 | 1.94 (1.75-2.14) | 1.97 (1.77-2.15) | 1.89 (1.72-2.08) | 1.88 (1.71-2.16) | 0.14 |
| BMI, Kg/m2 | 28.9 (25.1-34.4) | 29.7 (25.3-35.1) | 27.7 (24.4-32.7) | 27.8 (24.2-33.1) | 0.08 |
| Comorbidities | |||||
| Hypertension, n (%) | 273 (85) | 153 (86) | 120 (83) | 152 (86) | 0.67 |
| Diabetes, n (%) | 113 (35) | 69 (39) | 44 (30) | 63 (36) | 0.29 |
| Ever smoker, n (%) | 169 (52) | 94 (53) | 75 (52) | 101 (57) | 0.59 |
| CAD, n (%) | 161 (50) | 87 (49) | 74 (51) | 116 (66) ‡ | 0.003 |
| Atrial fibrillation, n (%) | 113 (35) | 64 (36) | 49 (34) | 114 (64) ‡ | <0.001 |
| COPD, n (%) | 91 (28) | 50 (28) | 41 (28) | 65 (37) | 0.14 |
| OSA, n (%) | 79 (24) | 47 (26) | 32 (22) | 43 (24) | 0.67 |
| Permanent pacemaker, n (%) | 69 (21) | 32 (18) | 37 (26) | 60 (34) ‡ | 0.003 |
| GFR, ml/min/1.73m2 (n=333) | 56 (42-69) | 55 (42-69) | 57 (43-70) | 53 (41-63) | 0.52 |
| Hemoglobin, g/dl (n=333) | 12.0 (10.5-13.6) | 12.2 (10.7-13.4) | 11.8 (10.4-13.6) | 12.2 (10.8-13.5) | 0.85 |
| Medications | |||||
| ACE/ARB, n (%) | 149 (46) | 81 (46) | 68 (47) | 101 (57) ‡ | 0.06 |
| Beta blocker, n (%) | 203 (63) | 112 (63) | 91 (63) | 117 (66) | 0.77 |
| Diuretics, n (%) | 194 (60) | 105 (59) | 89 (61) | 124 (70) ‡ | 0.08 |
| Statins, n (%) | 142 (44) | 73 (41) | 69 (48) | 89 (50) | 0.20 |
Abbreviations: BSA, body surface area; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive lung disease, GFR, glomerular filtration rate; ACE/ARB, angiotensin converting enzyme inhibitor or angiotensin receptor blocker
p<0.05 mid vs highest tertile
p< 0.05 lowest tertile versus highest and mid TAPSE tertile combined
Across group p value: Difference across the tertiles of TAPSE (lower, middle and upper tertiles), Kruskal Wallis test
As compared with patients with normal RV function, those with mild RVD by semi-quantitative analysis were more likely to have atrial fibrillation and permanent pacing. Patients with moderate-severe RVD by semi-quantitative assessment were slightly younger but otherwise similar to those with mild RVD (Table 2). As compared with patients with normal RV function, patients with any RVD (mild and moderate-severe combined) were more likely to have atrial fibrillation, permanent pacing and treatment with diuretics.
Table 2.
Clinical characteristics in subjects according to semi-quantitatively assessed RV function.
| Normal RV function | Mild RVD | Moderate to severe RVD |
Across group p value |
Any RVD | |
|---|---|---|---|---|---|
| N | 444 | 65 | 53 | 118 | |
| Age, years | 79 (62-86) | 82 (74-85) | 74 (66-82) † | 0.01 | 79 (69-84) |
| Women, n (%) | 258 (58) | 32 (49) | 30 (57) | 0.40 | 62 (53) |
| BSA, m2 | 1.94 (1.71-2.15) | 1.94 (1.79-2.18) | 1.86 (1.73-2.19) | 0.73 | 1.90 (1.74-2.20) |
| BMI, Kg/m2 | 28.86 (24.7-34.5) | 28.4 (25.6-34.2) | 26.2 (23.5-36.7) | 0.59 | 28.2 (24.1-34.6) |
| Comorbidities | |||||
| Hypertension, n (%) | 379 (85) | 57 (88) | 43 (81) | 0.60 | 100 (85) |
| Diabetes, n (%) | 161 (36) | 19 (29) | 16 (30) | 0.41 | 35 (30) |
| Ever smoker, n (%) | 233 (52) | 40 (62) | 28 (53) | 0.39 | 68 (58) |
| CAD, n (%) | 243 (55) | 40 (62) | 28 (53) | 0.55 | 68 (58) |
| Atrial fibrillation, n (%) | 180 (41) | 43 (66)* | 32 (60) | <0.0001 | 75 (64) † |
| COPD, n (%) | 132 (30) | 24 (37) | 18 (34) | 0.45 | 42 (36) |
| OSA, n (%) | 107 (24) | 20 (31) | 17 (32) | 0.27 | 37 (31) |
| Pacemaker, n (%) | 112 (25) | 25 (38)* | 13 (25) | 0.07 | 38 (32) ‡ |
| GFR, ml/min/1.73m2 (n=333) | 56 (43-68) | 56 (39-66) | 54 (40-82) | 0.84 | 56 (40-70) |
| Hemoglobin, g/dl (n=333) | 12.1 (10.7-13.5) | 11.4 (10.3-12.8) | 12.3 (10.7-13.9) | 0.23 | 11.9 (10.4-13.2) |
| Medications | |||||
| ACE/ARB, n (%) | 217 (49) | 35 (54) | 28 (53) | 0.68 | 63 (53) |
| Beta blocker, n (%) | 284 (64) | 39 (60) | 31 (58) | 0.64 | 70 (59) |
| Diuretics, n (%) | 276 (62) | 47 (72) | 40 (75) | 0.06 | 87 (74) ‡ |
| Statins, n (%) | 215 (48) | 25 (38) | 21 (40) | 0.19 | 46 (39) |
Abbreviations as in Table 1.
Mild RVD vs Normal RV function;
p< 0.05 Moderate-severe vs mild RVD;
p<0.05 Any RVD vs normal RV function
Across group p value: Difference across the 3 groups of RV function (normal, mild and moderate to severe RVD), Kruskal Wallis test
Cardiovascular structure and function according to RV function assessed with TAPSE
Among HFpEF patients, LV structure, LV systolic and diastolic function, systemic arterial function and right heart function did not differ in patients with TAPSE values in the highest vs middle tertiles, except for the LV diastolic dimension which was slightly larger in the middle vs the highest TAPSE tertile (Table 3).
Table 3.
Cardiovascular structure and function in subjects according to TAPSE assessed RV function.
| Upper and middle combined |
Upper tertile TAPSE ≥ 20 mm |
Middle tertile TAPSE 16-19 mm |
Lower tertile TAPSE ≤ 15 mm |
Across group p value |
|
|---|---|---|---|---|---|
| N | 323 | 178 | 145 | 177 | |
| LV structure | |||||
| LVEDd/BSA, mm/m2 | 25.4 (23.3-27.7) | 25.2 (23.1-27.0) | 26.4 (23.6-28.3) † | 25.3 (22.6-28.1) | 0.10 |
| LV mass/BSA | 98 (84-118) | 94 (83-112) | 101 (84-123) | 96 (82-122) | 0.32 |
| LV mass/height1.4 | 94 (77-115) | 91 (75-113) | 96 (80-117) | 91 (77-115) | 0.48 |
| Relative wall thickness | 0.44 (0.39-0.51) | 0.44 (0.39-0.52) | 0.44 (0.39-0.50) | 0.45 (0.40-0.50) | 0.67 |
| LV systolic function | |||||
| Ejection fraction, % | 61 (56-66) | 62 (58-66) | 60 (55-66) | 60 (55-65) | 0.05 |
| Stroke volume/BSA, ml/m | 45 (39-51) | 45 (40-51) | 44 (37-51) | 40 (33-48) ‡ | <0.0001 |
| Cardiac index, L/min/m2 | 3.0 (2.6-3.7) | 3.0 (2.6-3.7) | 3.0 (2.6-3.6) | 2.9 (2.4-3.4) ‡ | 0.10 |
| Heart rate, bpm | 69 (60-78) | 67 (59-77) | 68 (60-80) | 71 (62-82) ‡ | 0.02 |
| LV diastolic function | |||||
| Left atrial volume/BSA, ml/m2 | 44 (34-53) | 44 (35-52) | 44 (34-54) | 48 (38-57) ‡ | 0.03 |
| Medial e’, m/sec | 0.06 (0.04-0.07) | 0.06 (0.05-0.08) | 0.05 (0.04-0.07) | 0.06 (0.05-0.07) | 0.27 |
| E/e’ (medial e’) | 16 (11-20) | 15 (11-20) | 16 (11-23) | 18 (12-25) | 0.16 |
| Deceleration time, ms | 198 (173-232) | 203 (176-239) | 190 (169-227) | 185 (157-225) ‡ | 0.006 |
| Systemic arterial function | |||||
| Systolic BP, mmHg | 135 (120-150) | 135 (120-150) | 135 (118-150) | 124 (110-137) ‡ | <0.0001 |
| Pulse pressure, mmHg | 66 (52-80) | 68 (53-80) | 65 (48-81) | 59 (47-72) ‡ | 0.0004 |
| Ea, mmHg/ml | 1.41 (1.17-1.65) | 1.36 (1.16-1.63) | 1.49 (1.21-1.70) | 1.50 (1.16-1.81) ‡ | 0.05 |
| SVR dyne*s*cm−5 | 1213 (1001-1485) | 1195 (997-1385) | 1247 (1010-1604) | 1246 (995-1543) | 0.14 |
| SAC, ml/mmHg | 1.33 (0.99-1.62) | 1.35 (0.99-1.61) | 1.30 (0.96-1.64) | 1.30 (0.99-1.77) | 0.78 |
| Right heart | |||||
| RV enlargement, n (%) | 84 (26) | 43 (25) | 41 (28) | 90 (52) ‡ | <0.0001 |
| Mod-Severe TR, n (%) | 52 (16) | 33 (19) | 19 (13) | 75 (42) ‡ | <0.0001 |
| PASP, mmHg | 46 (36-56) | 47 (36-56) | 45 (36-55) | 49 (39-60) ‡ | 0.04 |
| TAPSE, mm | 20 (18-23) | 23 (21-24) | 17 (16-18) | 12 (10-14) | NA |
| Semi-quantitative RVD, n (%) | 38 | 14 (8) | 24 (17) | 69 (39) ‡ | <0.001 |
Abbreviations: LV, left ventricular; LVEDd, LV end-diastolic dimension; BSA, body surface area, BP, blood pressures; Ea, arterial elastance, SVR, systemic vascular resistance; SAC, systemic arterial compliance, RV, right ventricular, TR, tricuspid valve regurgitation; PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion: RVD, RV dysfunction
p<0.05 mid vs highest tertile
p< 0.05 lowest vs highest and mid TAPSE tertile combined
Across group p value: Difference across the tertiles of TAPSE (lower, middle and upper tertiles), Kruskal Wallis test
As compared with patients in the highest and middle TAPSE tertiles (combined), patients in the lowest tertile had similar LV structure (diastolic dimension, mass and relative wall thickness) and similar LVEF, but lower stroke volume and cardiac index despite higher heart rate (Table 3). Patients in the lowest TAPSE tertile also had worse LV diastolic function as evidenced by larger LA volume and shorter deceleration time, although relaxation (e’) and filling pressure (E/e’) were not different than in patients with higher TAPSE. Patients in the lowest TAPSE tertile had lower systemic systolic blood pressure and pulse pressure despite higher arterial elastance and similar systemic vascular resistance and arterial compliance. Patients in the lowest TAPSE tertile had a higher prevalence of RV enlargement (Table 3 and Supplemental Figure 2), Mod-Severe TR and semi-quantitative RVD and had higher PASP.
Cardiovascular structure and function according to RV function by semi-quantitative assessment
By semi-quantitative analysis, patients with moderate-severe RVD had smaller LV dimensions, lower systolic blood pressure and pulse pressure, higher prevalence of RV enlargement and lower TAPSE than patients with mild RVD (Table 4). However, in general, findings were similar when comparing patients with mild RVD or any RVD (mild or moderate-severe combined) to those with normal RV function.
Table 4.
Cardiovascular structure and function in subjects according to semi-quantitatively assessed RV function
| Normal RV function |
Mild RVD | Moderate to severe RVD |
Across group p value |
Any RVD | |
|---|---|---|---|---|---|
| N | 444 | 65 | 53 | 118 | |
| LV structure | |||||
| LVEDd/BSA, mm/m2 | 25.4 (23.2-27.9) | 25.6 (23.7-27.7) | 23.9 (21.6-26.9) † | 0.06 | 25.1 (22.3-27.3) |
| LV mass/BSA | 98 (84-122) | 100 (87-127) | 87 (74-107) | 0.008 | 94 (78-118) |
| LV mass/height1.4 | 94 (79-113) | 96 (81-126) | 85 (67-112) | 0.06 | 91 (72-117) |
| Relative wall thickness | 0.44 (0.40-0.51) | 0.47 (0.42-0.54)* | 0.44 (0.40-0.52) | 0.13 | 0.45 (0.40-0.53) |
| LV systolic function | |||||
| Ejection fraction, % | 61 (56-66) | 58 (54-64)* | 61 (56-66) | 0.007 | 59 (54-65) ‡ |
| Stroke volume/BSA, ml/m2 | 44 (38-51) | 39 (34-48)* | 40 (32-45) | 0.0004 | 39 (33-45) ‡ |
| Cardiac index, L/min/m2 | 3.0 (2.6-3.7) | 3.0 (2.4-3.3) | 2.8 (2.3-3.4) | 0.11 | 2.9 (2.4-3.4) ‡ |
| Heart rate, bpm | 69 (60-80) | 72 (61-82) | 71 (60-83) | 0.47 | 71 (61-82) |
| LV diastolic function | |||||
| Left atrial volume/BSA, ml/m2 | 44 (35-54) | 49 (42-58)* | 47 (4-57) | 0.05 | 48 (40-57) ‡ |
| Medial e’, m/sec | 0.06 (0.04-0.07) | 0.05 (0.05-0.08) | 0.06 (0.05-0.07) | 0.56 | 0.06 (0.05-0.07) |
| E/e’ (medial e’) | 16 (11-23) | 18 (13-25) | 16 (10-21) | 0.28 | 17 (12-22) |
| Deceleration time, ms | 198 (169-235) | 176 (157-205)* | 183 (156-213) | 0.0025 | 182 (157-210) ‡ |
| Systemic arterial function | |||||
| Systolic BP, mmHg | 132 (118-148) | 131 (110-142) | 118 (108-130) † | 0.001 | 128 (110-142) ‡ |
| Pulse pressure, mmHg | 64 (50-80) | 60 (48-78) | 55 (42-66) † | 0.002 | 58 (47-72) ‡ |
| Ea, mmHg/ml | 1.45 (1.18-1.72) | 1.42 (1.19-1.95) | 1.53 (1.12-1.88) | 0.84 | 1.48 (1.18-1.89) |
| SVR, dyne*s*cm−5 | 1237 (1013-1501) | 1212 (985-1653) | 1277 (991-1568) | 0.96 | 1231 (994-1605) |
| SAC, ml/mmHg | 1.31 (0.99-1.65) | 1.30 (0.89-1.66) | 1.30 (1.00-1.89) | 0.58 | 1.30 (0.97-1.74) |
| Right heart | |||||
| RV enlargement, n (%) | 85 (20) | 54 (84)* | 52 (98) ‡ | <0.0001 | 106 (91) ‡ |
| Mod-Severe TR, n (%) | 78 (18) | 33 (51)* | 31 (58) | <0.0001 | 64 (54) ‡ |
| PASP, mmHg | 44 (34-54) | 55 (48-64)* | 58 (49-75) | <0.0001 | 56 (48-69) ‡ |
| TAPSE, mm | 19 (15-22) | 15 (12-17)* | 12 (9-15) † | <0.0001 | 13 (10-16) ‡ |
Abbreviations as in Table 3.
Mild RVD vs Normal;
P< 0.05 Moderate-severe vs mild RVD;
p<0.05 Any RVD vs normal RV function
Across group p value: Difference across the 3 groups of RV function (normal, mild and moderate to severe RVD), Kruskal Wallis test
As compared with patients with normal RV function by semi-quantitative assessment, patients with any RVD (mild and moderate-severe combined) had similar LV dimension, LV mass and relative wall thickness, but lower LVEF, stroke volume and cardiac index (Table 4). Patients with RVD also had worse LV diastolic function as evidenced by larger LA volume and shorter deceleration time although relaxation (e’) and filling pressure (E/e’) were not different than patients with normal RV function. Patients with RVD had lower systolic blood pressure and pulse pressure but arterial elastance, systemic vascular resistance and arterial compliance were similar to that observed in patients with normal RV function. Patients with RVD had a higher prevalence of RV enlargement and Mod-Severe TR, lower TAPSE and higher PASP.
Pulmonary hypertension and RV function in HFpEF
In this HFpEF cohort, age increased across tertiles (≤ 39, 40-52 and ≥ 53 mmHg) of PASP but the prevalence of other comorbidities, including COPD and OSA was not different across tertiles of PASP in all patients (Supplemental Table 2) and in those with evidence of RV dysfunction by TAPSE or semi-quantitative assessment (data not shown).
The distribution of PASP tertiles was not different across TAPSE tertiles (p value = 0.17), but more patients with RVD by semi-quantitative analysis had PASP in the highest tertile (p <0.0001, Supplemental Figure 3).
Prognostic significance of RV function and pulmonary hypertension in HFpEF
All-cause mortality
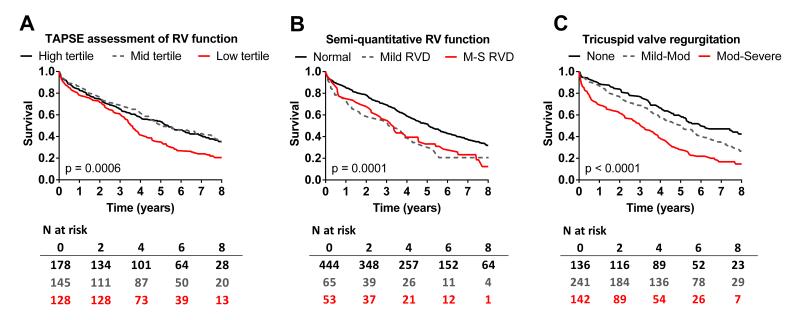
At eight years, of the 562 subjects 367 had died and 195 were censored (median follow up of 4.6 years). Survival varied by TAPSE tertiles but mortality risk appeared confined to the lowest tertile and survival curves did not diverge until approximately three years after assessment (Figure 2A and Table 5). Survival varied by semi-quantitative characterization of RVD where mild or moderate-severe RVD were associated with similar reduction in survival with early and progressive divergence of survival curves (Figure 2B and Table 5).
Figure 2.
Kaplan Meier survival curves for HFpEF patients according to the level of right ventricular (RV) function or tricuspid regurgitation: In A, survival by tertiles of tricuspid annular plane systolic excursion (TAPSE); In B, survival according to RV function assessed by semiquantitative assessment (Normal, mild or moderate-severe (M-S) RV dysfunction (RVD)). In C, survival according to the severity of tricuspid regurgitation (None, Mild-Mod or Mod-Severe).
Table 5.
Association of RV function, PASP and TR with adverse outcomes in HfpEF.
| All-cause mortality | CV mortality | First HF hospitalization | All HF hospitalizations | |||||
|---|---|---|---|---|---|---|---|---|
| HR (CI) | P value | HR (CI) | P value | HR (CI) | P value | HR (CI) | P value | |
| Univariate Analysis | ||||||||
| PASP (per SD) | 1.53 (1.37-1.69) | <0.0001 | 1.67 (1.40-1.96) | <0.0001 | 1.47 (1.25-1.71) | <0.0001 | 1.48 (1.26-1.74) | <0.0001 |
| TAPSE (per SD) | 0.82 (0.73-091) | 0.0003 | 0.73 (0.60-0.87) | 0.0005 | 0.72 (0.61-0.85) | <0.0001 | 0.71 (0.60-0.86) | 0.002 |
| Semi-Quant RVD (any) | 1.68 (1.32-2.12) | <0.0001 | 2.12 (1.45-3.05) | 0.0002 | 2.42 (1.73-3.35) | <0.0001 | 2.59 (1.81-3.70) | <0.0001 |
| Tricuspid Regurgitation: | <0.0001 | <0.0001 | 0.003 | 0.001 | ||||
| Mild-Mod | 1.41 (1.07-1.87) | 0.01 | 1.41 (0.88-2.32) | 0.16 | 1.43 (0.95-2.2) | 0.08 | 1.34 (0.84-2.12) | 0.22 |
| Mod-Severe | 2.45 (1.83-3.30) | <0.0001 | 2.77 (1.70-4.62) | <0.0001 | 2.14 (1.38-3.38) | 0.0007 | 2.31 (1.43-3.73) | 0.0006 |
|
| ||||||||
| Multivariable Analysis | ||||||||
| Model 1: PASP, TAPSE and Comorbidities † | ||||||||
| PASP (per SD) | 1.50 (1.33-1.68) | <0.0001 | 1.57 (1.29-1.90) | <0.0001 | 1.44 (1.21-1.71) | <0.0001 | 1.50 (1.27-1.76) | <0.0001 |
| TAPSE (per SD) | 0.99 (0.79-1.01) | 0.08 | 0.77 (0.64-0.94) | 0.01 | 0.82 (0.68-0.99) | 0.03 | 0.83 (0.70-0.98) | 0.03 |
| Model 2: PASP, Semi-Quantitative Assessment of RV Function and Comorbidities † | ||||||||
| PASP (per SD) | 1.42 (1.26-1.60) | <0.0001 | 1.48 (1.21-1.78) | 0.0001 | 1.27 (1.06-1.51) | 0.01 | 1.30 (1.08-1.57) | 0.005 |
| RVD (any) | 1.35 (1.03-1.77) | 0.03 | 1.85 (1.20-2.80) | 0.006 | 1.99 (1.35-2.90) | 0.0006 | 1.81 (1.18-2.78) | 0.007 |
Abbreviations as in Table 3.
Unadjusted HR (95% CI). Reference group for tricuspid regurgitation is no tricuspid regurgitation
Hazard ratios adjusted for age, sex, atrial fibrillation, diabetes, COPD and OSA
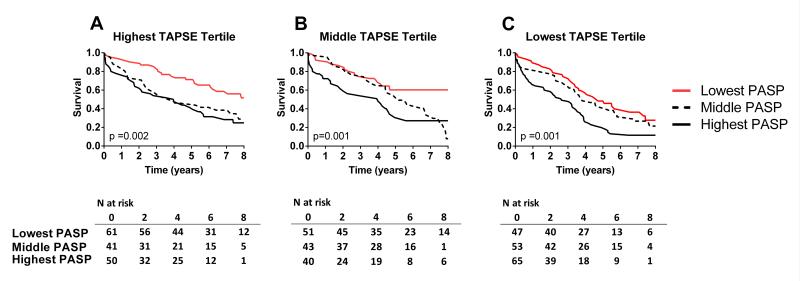
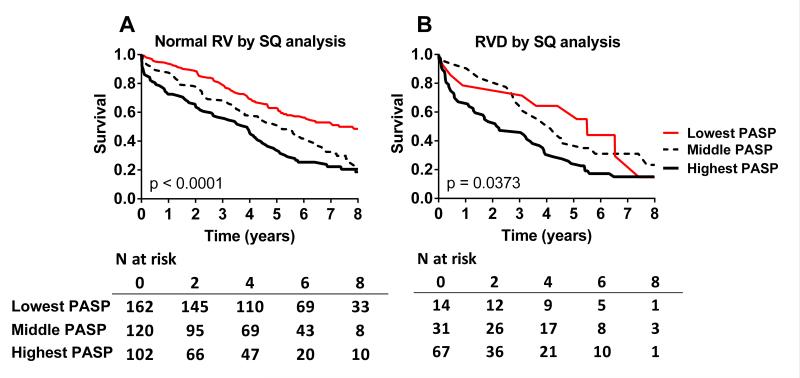
Survival varied across PASP tertiles among patients in the highest, middle or lowest TAPSE tertiles (Figure 3A-C). Survival varied across PASP tertiles in patients with normal RV function and in patients with any RVD by semi-quantitative assessment (Figure 4A and B). Adjusting for pertinent covariates, PASP but not TAPSE was independently associated with decreased survival (Table 5). Both PASP and semi-quantitative evidence of RVD (any RVD) were independently associated with decreased survival (Table 5).
Figure 3.
Kaplan Meier survival curves for HFpEF patients according to tertiles of pulmonary artery systolic pressure (PASP) among patients in highest (TAPSE≥20 mm; A), middle (TAPSE 16-19 mm; B) and lowest TAPSE tertile (TAPSE ≤ 15 mm; C),
Figure 4.
Kaplan Meier survival curves for HFpEF patients according to tertiles of pulmonary artery systolic pressure (PASP) among patients with normal RV function by semi-quantitative assessment (A), or RV dysfunction (mild or moderate-severe) by semi-quantitative assessment (B).
Cardiovascular mortality
In univariate analysis, each of TAPSE, semi-quantitative RVD and PASP were associated with cardiovascular mortality (Table 5). Adjusting for pertinent covariates, higher PASP and lower TAPSE were each independently associated with higher cardiovascular mortality (Table 5). Similarly, PASP and semi-quantitative evidence of RVD (any RVD) were each independently associated with higher cardiovascular mortality (Table 5).
HF hospitalizations
Among HFpEF patients with assessment of both PASP and TAPSE (n=451), during follow up, there were 340 HF hospitalizations among 164 unique subjects (range 1-10 hospitalizations). In univariate analysis, each of TAPSE, semi-quantitative RVD and PASP were associated with time to first or multiple HF hospitalizations (Table 5). Adjusting for pertinent covariates, higher PASP and lower TAPSE were each independently associated with time to first or multiple hospitalizations (Table 5). Higher PASP and semi-quantitative evidence of RVD (any RVD) were each independently associated with time to first or multiple HF hospitalizations (Table 5).
Tricuspid valve regurgitation in HFpEF
Tricuspid regurgitation was quantified in 519 (92%) of the HFpEF patients and was Mod-Severe in 142 (27%), Mild-Mod in 241 (47%) and absent in 136 (26%). Mod-Severe TR was more common in patients with RVD (Tables 3 and 4). In univariate analysis, the severity of TR was associated with higher all-cause (Figure 2C) and cardiovascular mortality (Table 5) as well as with time to first or multiple HF hospitalizations (Table 5). However, in models including TAPSE or semi-quantitative RVD, severity of TR was no longer significantly associated with any of the outcomes and was eliminated from the models.
DISCUSSION
In the community, whether assessed by TAPSE (35%) or semi-quantitative methods (21%), RVD was present in a significant subset of HFpEF patients. HFpEF patients with RVD were more likely to have atrial fibrillation, pacemakers and diuretic therapy. At echo, patients with RVD had slightly lower LVEF, worse diastolic dysfunction, lower blood pressure and cardiac output, and more significant PH, RV enlargement and TR. While those categorized as having RVD by either method shared similar clinical and echocardiographic characteristics on average, concordance for RVD designation by the two methods was not strong. The prognostic implications of semi-quantitative RVD were more striking with patients with any severity of RVD by semi-quantitative assessment having worse all-cause and cardiovascular mortality and risk of first and all HF hospitalizations after adjustment for level of PH and pertinent comorbidities. The prognostic implications of a low TAPSE were less striking. Patients with higher PASP and RVD had the worst outcomes. These data may assist in the recognition of HFpEF where it should be realized that RVD is common in HFpEF and is associated with clinical and echocardiographic evidence of more advanced HF and with poorer outcomes.
Prevalence of RV dysfunction in HFpEF
In HFrEF, the frequency of RVD has been assessed using a number of different RV function parameters in variable study populations. In HFrEF patients with ischemic or non-ischemic LV systolic dysfunction referred for transplantation or with moderate or severe symptoms, reduced RV ejection fraction (RVEF < 35-40%) measured by a thermodilution technique or radionuclide ventriculography was present in 50-75% of patients.4,31-33 A study of unselected HFrEF patients found evidence of RVD assessed by tricuspid annular S’ in 68% of patients.34 A meta-analysis including 4732 patients with HF and/or LV systolic dysfunction reported an overall prevalence of RVD of 47% but emphasized the high variability in prevalence, RV assessment techniques and study population characteristics.1
As the RV may be involved by an ischemic or myopathic process in patients with HFrEF, the prevalence of RVD may be lower in HFpEF, where underlying etiologies for HFpEF are thought to primarily affect the LV. Indeed, in the current study, using either TAPSE or semi-quantitative assessment of RV function, we found a lower prevalence of RVD than in HFrEF studies which used RVEF to assess RV function. The community based nature of the current study may also contribute to the lower prevalence as most studies in HFrEF were confined to referral cohorts with advanced HF.
Few studies have assessed the prevalence or prognostic implications of RVD in HFpEF. In a small cohort study, Puwanant et al reported that approximately 20% of HFpEF patients had a reduced (<45%) RV fractional area change.17 This quantitative measure is most analogous to the semi-quantitative estimation of RVD used here and the prevalence of RVD based on this measure is similar to that observed in our study. Consistent with our results, in the same cohort, the prevalence of RVD based on reduced (< 15 mm) TAPSE or on reduced (< 11.5 cm/s) tricuspid annulus peak systolic tissue velocity (S’) was higher(≈30-40%).17 These data suggest that TAPSE is either a more sensitive or less specific measure of RVD. In a much larger study, Morris et al reported that a variety of RV assessment parameters (RV longitudinal systolic strain, TAPSE, S’, RV fractional area change) were lower in patients with HFpEF than patients with Doppler evidence of diastolic dysfunction but no HF.18 However, the concordance and prognostic implications of these multiple parameters were not assessed and the majority of HFpEF patients (75%) had reduced RV S’, calling into question the discriminatory value of RVD so defined. In a large observational cohort of somewhat younger patients (mean age 65 years) with well-defined HFpEF, 28% had a TAPSE < 16 mm and 14% had a RV fractional area change < 35%.19 In the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial (TOPCAT) echocardiographic sub-study, TR velocity was measureable in 450 (48% of sub-study patients) subjects and was abnormal (> 2.9 m/s) in 162 (36%). RV fractional area change was abnormal (< 35%) in only 4% of patients.35 Whether this represents a difference in the technique or the type of patients enrolled in this particular clinical trial is unclear.
The different measures of RV function (TAPSE and semi-quantitative assessment) correlated only modestly with abnormal values for the two measures identifying slightly different groups of patients. Our findings suggest that semi-quantitative RVD carries more potent prognostic implications than TAPSE defined RVD.
While the prevalence of RVD in a community based cohort of HFrEF patients was not addressed in this study, interpreted in the context of available studies in HFrEF, our findings suggest that the prevalence of RVD in HFpEF is significant, but lower than in HFrEF.
Etiology of RV dysfunction in HFpEF
While cause and effect cannot be established from this study, the higher prevalence of atrial fibrillation and permanent pacing in those with RVD suggests a potential role for these factors in contributing to impaired RV function where the RV may display enhanced sensitivity to the negative inotropic effects of rhythm irregularity36 or pacing induced dyssynchrony. The severity of PH and diastolic dysfunction were both worse in HFpEF with RVD suggesting a role for chronic pressure overload in contributing to RVD. Other potential contributing comorbidities such as coronary disease and lung disease were not consistently associated with RVD but we cannot rule out a contribution of these factors in some patients.
Significance of RV dysfunction in HFpEF
In HFrEF, the presence of RVD is associated with worse clinical status, exercise capacity and prognosis.1-7,16 Here we find that RVD is also associated with higher all cause and cardiovascular mortality and HF hospitalization rates in HFpEF, even after adjustment for age, comorbidities and PH severity. While functional status was not assessed in this study, patients with RVD had lower resting cardiac output suggesting the potential for more impaired exercise capacity.
In this observational study, RVD predicted outcomes independently of PASP. This is in contradistinction to a study in advanced HFrEF31 where RVD (thermodilution derived RV ejection fraction) conferred poor prognosis only when (invasively measured) mean pulmonary artery pressure was > 20 mmHg. However, in the previous HFrEF study31, numbers in some subgroups were small, patients were young (51 years) and predominately had non-ischemic dilated cardiomyopathy.
While not shown to be effective in unselected HFpEF patients enrolled in the RELAX trial,37 the phosphodiesterase type 5 inhibitor sildenafil had favorable effects in a small study of HFpEF patients who had significant RVD and PH.38 Our findings suggest this HFpEF subgroup is significant and at high risk.
Tricuspid Valve Regurgitation in HFpEF
Mod-Severe TR, atrial fibrillation, PH and RV pacing were all more common in patients with HFpEF and RVD. Annular dilatation due to atrial enlargement in atrial fibrillation, RV failure and dilatation due to Group II PH or pacemaker lead impingement on the tricuspid valve leaflets could all cause or exacerbate TR in HFpEF. Once established, TR itself could contribute to progressive RV remodeling and RVD. While TR was associated with worse outcomes in HFpEF, these associations were no longer significant after adjusting for the severity of RVD.
Limitations
We cannot distinguish between isolated pre-capillary versus combined pre- and post-capillary PH. The prognostic significance of PH and its association with RVD may be different according to the duration and type of PH.39, 40 RV diastolic function was not assessed in this population and is likely more prevalent than systolic dysfunction. Data on NYHA functional class were not available. Assessment of TR severity was semi-quantitative. However, methods for quantitative assessment of TR are not as well established as for mitral regurgitation and less often performed in routine clinical practice.
Conclusion
In this community based HFpEF cohort, evidence of RVD was present in a significant subset of patients and was associated with more advanced clinical and echocardiographic characteristics and poorer outcomes. However, the prevalence of RVD depends on the method used to assess RV function with different methods identifying slightly different patient groups. The optimal technique to assess RVD remains to be defined. These data may assist in the recognition of HFpEF where it should be realized that RV systolic dysfunction may accompany HFpEF and portends a poorer prognosis, irrespective of severity of PH or comorbid conditions.
Supplementary Material
Acknowledgments
Funding Sources: This study (HL72435 and HL 55502) and/or the investigators (MMR: U01HL 84907 and PO1HL 76611; SFM: T32-HL007111 and U01 HL 084907; VLR: R01 HL72435) were supported by the National Institutes of Health and Mayo Clinic. Dr. Mohammed is a heart failure clinical research network skills development fellow (U10 HL 110262).
Footnotes
Disclosures: None.
References
- 1.Iglesias-Garriz I, Olalla-Gomez C, Garrote C, Lopez-Benito M, Martin J, Alonso D, Rodriguez MA. Contribution of right ventricular dysfunction to heart failure mortality: A meta-analysis. Rev Cardiovasc Med. 2012;13:e62–69. doi: 10.3909/ricm0602. [DOI] [PubMed] [Google Scholar]
- 2.Weiner BH, Alpert JS, Dalen JE, Ockene IS. Response of the right ventricle to exercise in patients with chronic heart disease. Am Heart J. 1983;105:386–393. doi: 10.1016/0002-8703(83)90354-x. [DOI] [PubMed] [Google Scholar]
- 3.Baker BJ, Wilen MM, Boyd CM, Dinh H, Franciosa JA. Relation of right ventricular ejection fraction to exercise capacity in chronic left ventricular failure. Am J Cardiol. 1984;54:596–599. doi: 10.1016/0002-9149(84)90256-x. [DOI] [PubMed] [Google Scholar]
- 4.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 5.Gorcsan J, 3rd, Murali S, Counihan PJ, Mandarino WA, Kormos RL. Right ventricular performance and contractile reserve in patients with severe heart failure. Assessment by pressure-area relations and association with outcome. Circulation. 1996;94:3190–3197. doi: 10.1161/01.cir.94.12.3190. [DOI] [PubMed] [Google Scholar]
- 6.Kjaergaard J, Akkan D, Iversen KK, Kober L, Torp-Pedersen C, Hassager C. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur J Heart Fail. 2007;9:610–616. doi: 10.1016/j.ejheart.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, White M, Aban IB, Mujib M, Dell'Italia LJ, Ahmed A. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121:252–258. doi: 10.1161/CIRCULATIONAHA.109.887570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part ii: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 9.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, Weston SA, Jiang R, Roger VL. Pulmonary pressures and death in heart failure: A community study. J Am Coll Cardiol. 2012;59:222–231. doi: 10.1016/j.jacc.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bishu K, Borlaug BA, Chen HH, LeWinter MM, Deswal A, Lewis GD, Semigran MJ, McNulty S, Hernandez AF, Braunwald E, Redfield MM. Humoral activiation in decompensated heart failure with preserved or reduced ejection fraction. Circulation. 2010;122:A12658. doi: 10.1016/j.ahj.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 14.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part i: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 15.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the american society of echocardiography endorsed by the european association of echocardiography, a registered branch of the european society of cardiology, and the canadian society of echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-688. [DOI] [PubMed] [Google Scholar]
- 16.Haddad F, Kudelko K, Mercier O, Vrtovec B, Zamanian RT, de Jesus Perez V. Pulmonary hypertension associated with left heart disease: Characteristics, emerging concepts, and treatment strategies. Prog Cardiovasc Dis. 2011;54:154–167. doi: 10.1016/j.pcad.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Puwanant S, Priester TC, Mookadam F, Bruce CJ, Redfield MM, Chandrasekaran K. Right ventricular function in patients with preserved and reduced ejection fraction heart failure. Eur J Echocardiogr. 2009;10:733–737. doi: 10.1093/ejechocard/jep052. [DOI] [PubMed] [Google Scholar]
- 18.Morris DA, Gailani M, Vaz Perez A, Blaschke F, Dietz R, Haverkamp W, Ozcelik C. Right ventricular myocardial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24:886–897. doi: 10.1016/j.echo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–99. doi: 10.1161/CIRCHEARTFAILURE.113.000854. doi: 10.1161/CIRCHEARTFAILURE.113.000854. Epub 2013 Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 21.Forfia PR, Borlaug BA. Letter by forfia and borlaug regarding article, "pulmonary hypertension in heart failure with preserved ejection fraction: A target of phosphodiesterase-5 inhibition in a 1-year study". Circulation. 2012;125:e408. doi: 10.1161/CIRCULATIONAHA.111.064584. author reply e409-410. [DOI] [PubMed] [Google Scholar]
- 22.Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: A community based study. Circ Heart Fail. 2012;5:710–9. doi: 10.1161/CIRCHEARTFAILURE.112.968594. doi: 10.1161/CIRCHEARTFAILURE.112.968594. Epub 2012 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh JK, Seward JB, Tajik AJ. The echo manual. Lippincott Williams & Wilkins; Philadelphia: 2006. [Google Scholar]
- 24.Redfield MM, Jacobsen SJ, Burnett JC, Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 25.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5:720–726. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little WC, Ohno M, Kitzman DW, Thomas JD, Cheng CP. Determination of left ventriuclar chamber stiffness from the time for deceleration of early left ventriuclar filling. Circulation. 1995;92:1933–1939. doi: 10.1161/01.cir.92.7.1933. [DOI] [PubMed] [Google Scholar]
- 27.Patel JB, Borgeson DD, Barnes ME, Rihal CS, Daly RC, Redfield MM. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail. 2004;10:285–291. doi: 10.1016/j.cardfail.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Secular trends in renal dysfunction and outcomes in hospitalized heart failure patients. J Card Fail. 2006;12:257–262. doi: 10.1016/j.cardfail.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: A community perspective. Circ Heart Fail. 2008;1:91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Innelli P, Esposito R, Olibet M, Nistri S, Galderisi M. The impact of ageing on right ventricular longitudinal function in healthy subjects: A pulsed tissue doppler study. Eur J Echocardiogr. 2009;10:491–498. doi: 10.1093/ejechocard/jen313. [DOI] [PubMed] [Google Scholar]
- 31.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 32.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 33.Polak JF, Holman BL, Wynne J, Colucci WS. Right ventricular ejection fraction: An indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol. 1983;2:217–224. doi: 10.1016/s0735-1097(83)80156-9. [DOI] [PubMed] [Google Scholar]
- 34.Adhyapak SM. Effect of right ventricular function and pulmonary pressures on heart failure prognosis. Prev Cardiol. 2010;13:72–77. doi: 10.1111/j.1751-7141.2009.00053.x. [DOI] [PubMed] [Google Scholar]
- 35.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function in heart failure with preserved ejection fraction: Baseline findings from the echocardiographic study of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2014;7:104–15. doi: 10.1161/CIRCHEARTFAILURE.113.000887. doi: 10.1161/CIRCHEARTFAILURE.113.000887. Epub 2013 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: A review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709–715. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 37.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: A target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 39.Khush KK, Tasissa G, Butler J, McGlothlin D, De Marco T. Effect of pulmonary hypertension on clinical outcomes in advanced heart failure: Analysis of the evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness (escape) database. Am Heart J. 2009;157:1026–1034. doi: 10.1016/j.ahj.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 40.Aronson D, Eitan A, Dragu R, Burger AJ. Relationship between reactive pulmonary hypertension and mortality in patients with acute decompensated heart failure. Circ Heart Fail. 2011;4:644–650. doi: 10.1161/CIRCHEARTFAILURE.110.960864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.