Abstract
Introduction
Isolated nuclei of sheep proximal tubules express angiotensin receptors as well as angiotensinogen (AGT) and renin. The present study characterized the NRK-52E tubular epithelial cell line for the intracellular expression of renin-angiotensin system (RAS) components.
Methods
RAS components were visualized by immunofluorescent staining in intact cells and protein expression in isolate nuclei.
Results
An antibody to the Ang I sequence of AGT (AI-AGT) revealed only cytosolic staining, while an antibody to an internal epitope of AGT (Int-AGT) revealed primarily nuclear staining. Immunoblots of nuclear and cytosolic fractions confirmed the differential cell staining of AGT. Immunostaining for renin was present on nuclei of intact cells. Nuclear renin activity averaged 0.77 ± 0.05 nmol/mg protein/hr that was reduced by aliskiren (0.13 ± 0.01 nmol/mg/hr, n=3, p<0.01); trypsin activation increased activity 3-fold. Peptide staining localized Ang II and Ang-(1–7) to the nucleus and peptide content averaged 59 ± 2 and 57 ± 22 fmol/mg (n=4), respectively. Peptide metabolism in isolated nuclei revealed the processing of Ang I to Ang-(1–7) by thimet oligopeptidase.
Conclusion
We conclude that the NRK-52E cells express an intracellular RAS localized to the nucleus and may be an appropriate cell model to elucidate the functional relevance of this system.
Keywords: NRK-52E, angiotensin, renin, thimet oligopeptidase, angiotensinogen
INTRODUCTION
The renin angiotensin system (RAS) is an endocrine system that plays a major role in the physiological regulation of blood pressure and fluid homeostasis. Dysregulation of the RAS is also thought to contribute to the development and progression of cardiovascular and renal injuries. Moreover, the pharmacological agents that block various components of the RAS now encompass the primary therapies for treatment of hypertension, heart failure and diabetic renal injury. Although originally identified as a classic endocrine system, the evidence clearly reveals a local or tissue RAS in various organs including the kidney, heart, adrenals and brain (1–3). In this regard, an intracellular system localized to cellular organelle including the nucleus and mitochondria have been described in the both tubular epithelial and mesangial cells of the kidney, as well as the myocytes and fibroblasts of the heart (4–11). The physiological relevance and the regulation of this intracellular RAS have not been established. Indeed, it is not clear precisely how the intracellular system functions at the cellular level to synthesize the active RAS peptides angiotensin II (Ang II) and Ang-(1–7), nor the contribution of these peptides to intracellular signaling and cell function. Moreover, there is now compelling evidence for a functional renin receptor that binds prorenin to non-proteolytically activate the enzyme, as well as to mediate the functional signaling of the receptor that is not dependent on Ang II generation (12–14). Interestingly, the prorenin receptor (PRR) is primarily localized intracellularly rather than on the cell membrane suggesting that the receptor may also contribute to a functional intracellular RAS (15).
Elucidation of the physiological relevance of an intracellular RAS is important clinically as well. The current therapeutic regimens to treat high blood pressure and attenuate renal and cardiovascular injury include AT1 receptor antagonists (ARBs), angiotensin converting enzyme (ACE) and renin inhibitors; however, the therapeutic benefit of these agents to target the intracellular system is not known. Previous studies in our laboratory and others have demonstrated a high density of Ang II receptors on isolated nuclei from the kidney (16;17). In the rat kidney, the majority of these nuclear binding sites are the AT1 subtype that is functionally linked to Ang II-dependent increases in oxidative stress and calcium (18;19). AT1 receptor-dependent formation of reactive oxygen species (ROS) was also demonstrated in isolated nuclei from the sheep kidney; however, both AT2 and Ang-(1–7) (AT7) sties were functionally coupled to nitric oxide formation and may antagonize the actions of the nuclear AT1 receptor (20–22). Additional studies revealed the precursor components angiotensinogen and renin in isolated nuclei from proximal tubules of the sheep kidney that may portend for the intracellular or nuclear formation of Ang II and Ang-(1–7) (21). Moreover, we detected the peptidase activities for ACE and ACE2 in intact nuclei that processed exogenous Ang I to Ang II and Ang II to Ang-(1–7), respectively (20). To facilitate our understanding of the tubular RAS within the kidney, the current studies sought to identify a renal cell line that express the components of this system and determine their intracellular localization.
MATERIALS AND METHODS
Cell Culture
Normal kidney proximal epithelial cells (NRK-52E) cells were obtained from American Tissue Type Culture (Arlington VA; passage 8) and maintained at 37°C in plastic 75 cm2 flasks in Dulbecco’s modified Eagle’s medium (DMEM/F12, Invitrogen) containing 5% fetal bovine serum (FBS), L-Glutamine and 15 mM HEPES buffer. The culture flasks were kept in a 95% air and 5% CO2 humidified environment at 37°C. At confluence, the cells were washed and maintained in serum free DMEM/F12 without supplements for 48 hours prior to the biochemical studies.
Nuclear and cytosolic fractions
Confluent cells were washed twice with PBS and harvested. Harvested cells were centrifuged at 1000 g for 10 minutes and the resulting cells pellet was homogenized on ice with a glass pestle in 20 mM Tris buffer containing 5mM MgCl2 and 25 mM KCl at pH 7.8. Homogenates were centrifuged at 1000 g to obtain the crude nuclear pellet and further purified in a high sucrose buffer. The crude pellet was reconstituted with 0.3 M sucrose in 10 mM HEPES buffer pH 7.4, layered over the 0.88 M sucrose buffer and centrifuged at 1200 g for 10 minutes at 4°C to obtain the nuclear pellet (23). The cell supernatant was centrifuged at 100,000 g for 60 min at 4°C to obtain the cytosolic fraction.
Western blotting
Nuclear and cytosolic pellets (~50 μg) were boiled in PBS (pH 7.4), diluted in Laemmli buffer with β-mercaptoethanol, separated on 10% SDS polyacrylamide gels for 1 h at 120V in Tris-glycine SDS and transferred to a polyvinylidene difluoride membranes (PVDF). Blots were blocked with 5% Bio-Rad Dry Milk and TBS with Tween and probed overnight at 4°C with primary antibodies against the following: rat AGT residues 42–57 (internal sequence, Int-AGT, 1:1000), Ang I sequence (residues 25–34) of AGT (AI-AGT, 1:1000) and renin (1:5000; Inagami antibody no. 826). Membranes were treated with HRP-labeled polyclonal anti rabbit secondary antibodies (1:5000) for 1 hour and detected with chemiluminescent substrates (Pierce Biotechnology, Rockford, IL). Membranes were probed with mouse monoclonal anti-β-actin (Sigma, St. Louis, MO, 1:5000) antibody as a loading control and bands were quantified using MCID densitometry software (InterFocus Imaging, Linton, England).
Immunofluorescent microscopy
NRK-52E cells were grown in 8 chamber slides for two days in DMEM/F12 5% FBS which was replaced with serum free media for one day. Cells were washed with PBS and fixed with 2% paraformaldehyde for 15 minutes. Following a PBS rinse, cells were permeabilized with 0.2% Triton and then blocked with 3% BSA (Sigma #A-8022). The fixed cells were probed with primary antibodies for AGT and renin previously described for western blot analysis. Cells were also probed with affinity purified antibodies to Ang II (Phoenix Pharmaceutical) and Ang-(1–7) (custom antibody), PRR (Abcam ab64957) antibodies. Antibodies were diluted in 3% normal donkey serum at the following: Int-AGT (1:100), AI-AGT (1:100), renin (1:500), PRR (1:500), Ang II (1:500) Ang-(1–7) (1:25), calnexin (1:100). After overnight incubation with the primary antibody at 4°C, cells were rinsed with PBS, incubated with fluorescent anti-rabbit Alexa Fluor 488 secondary antibody (Invitrogen, Carlsbad, CA), and the slides mounted with Molecular Probes ProLong mounting media with DAPI (Invitrogen) to stain the nuclei.
Peptide Assays
Cell nuclei were isolated as described above and stored at −80°C. The nuclear pellet was reconstituted in MilliQ water and placed in a boiling water bath for 15 minutes. The nuclear fraction was acidified with trifluoroacetic acid (TFA) to a final concentration of 0.2%, sonicated and centrifuged at 20,000g for 20 min at 4°C. The resultant supernatant was applied to an activated Sep-Pak C18 extraction column, washed with 0.2% TFA, and the peptide fraction eluted with 3 ml 80% methanol in 0.2% TFA. Measurement of immunoreactive Ang II and Ang-(1–7) in the extracted nuclei was performed using two distinct RIAs (24). The Ang-(1–7) RIA fully recognizes Ang-(1–7) and Ang-(2–7), but cross-reacts less than 0.01% with Ang-(3–7), Ang II, Ang I, and their fragments. The Ang II RIA equally recognizes Ang III, Ang-(3–8), and Ang-(4–8), but cross-reacts less than 0.01% with Ang I and Ang-(1–7). The limit of detection was 4 fmol/tube for Ang-(1–7) and 0.5 fmol/tube for Ang II.
Renin assay
The cell nuclei were isolated as described above and stored at −80°C. The nuclear pellet was reconstituted in 2 ml of 25 mM HEPES, 125 mM NaCl at pH 7.4 and left for 60 min on ice. A sample from the lysate was taken for protein content. For basal renin activity assay, 0.4 ml of nuclear lysate was added to 0.2 ml of nephrectomized rat plasma (NRP) as the source of exogenous AGT substrate. The following protease inhibitors were added to prevent the metabolism of Ang I: amastatin (AM; 2 μM), bestatin (BS; 10 μM), chymostatin (CHYM; 10 μM), benzyl succinate (BSC; 10 μM), para-chloromercuribenzoic acid (PCMB; 0.5 mM) and EDTA (2.5 mM) (all at final concentrations). The assay was performed at 37°C for 90 minutes alone or with the renin inhibitor aliskiren (1 μM, final concentration) (24). Activation of prorenin was performed by addition of 0.1 mg trypsin (Sigma, St. Louis, MO) to the nuclear sample with the peptidase inhibitors for 60 minutes on ice (24). Trypsin was inactivated using by incubation with an excess of soybean trypsin inhibitor (SBTI; 1 mg/ml, Sigma) for 15 min at room temperature. SBTI is a potent serine protease inhibitor that does not attenuate renin, an aspartyl protease. Following SBTI, the renin assay was performed as described above. All renin and prorenin samples were extracted on Sep-Pak C18 columns prior to the assessment of the Ang I by RIA as described (24). Blank values for the assay (aliskerin with and without lysates) were subtracted from the sample values; renin activity was expressed as nmol Ang I generated per hour per mg protein.
Angiotensinogen assay for renin
A rat kidney cortical homogenate as a source for renin was added to a cocktail of protease inhibitors (Sigma) (1/100) and SBTI (1mg) in the presence or absence of aliskiren (10 μM) in a final volume of 1 ml buffer (10 mM HEPES, 125 mM NaCl, 10 μM ZnCl2, pH 7.4). NRP as the source of intact AGT was then added and incubated at 37°C for 60 minutes. Aliquots were removed at 5, 15, 30 and 60 minutes and analyzed by western blot using the Ang I-intact AGT (AI-AGT) antibody. The membrane was then stripped and probed with the second AGT antibody (Int-AGT).
Peptide metabolism
To characterize the processing of the peptides in vitro, 125I-Ang-I or Ang II (0.5 nM) were incubated with the solubilized nuclear fractions as previously described (25). Metabolism assays were conducted at 37°C in 10 mM HEPES, 125 mM NaCl, 10 μM ZnCl2, pH 7.4, with 2–3 μg protein of the nuclear fraction in a final volume of 0.5 ml with or without the indicated inhibitors. The reaction was stopped by addition of ice-cold 1.0% phosphoric acid, centrifuged at 16,000 g, and the supernatants stored at −20°C. Samples were separated by reverse-phase high-performance liquid chromatography (HPLC) and the 125I-products were detected by a Bioscan flow-through γ detector (25). Products were identified by comparison of their retention times to 125I-angiotensn standards. Peptides were iodinated by the chloramine T method and purified by HPLC (specific activity > 2000 Ci/mmol). The aminopeptidase inhibitors amastatin AM (2 μM) and BS (10 μM) were included in the basal assay conditions. We subsequently the following inhibitors to block thiol peptidases (PCMB; 0.5 mM); neprilysin (SCH39370 or SCH, 10 μM), prolyl oligopeptidase (Z-prolyl prolinal or ZPP, 50 μM) and thimet oligopeptidase (c-phenylpropyl-alanine-alanine-phenylalanine-p-aminobenzoate or CPP, 50 μM). Enzyme activities were expressed as fmol product per mg protein per minute (fmol/mg/min).
Statistical analysis
All measurements are expressed as mean ± standard error (SEM). Differences between the groups were analyzed by one-way ANOVA and Newman-Keuls multiple comparison analysis. All figures were constructed with GraphPad Prism V plotting and statistical software. A probability value of < 0.05 was required for statistical significance.
RESULTS
Angiotensinogen (AGT) Antibodies
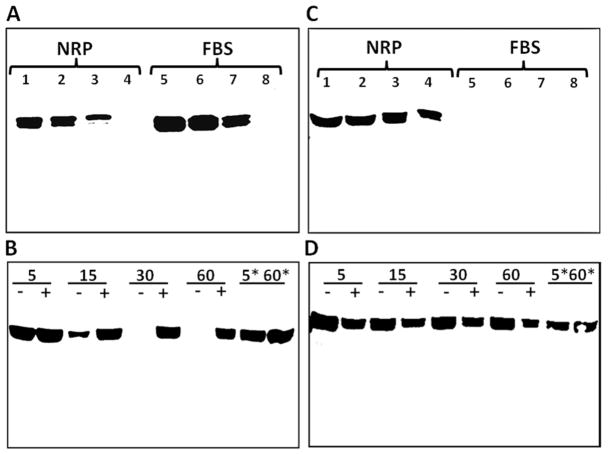
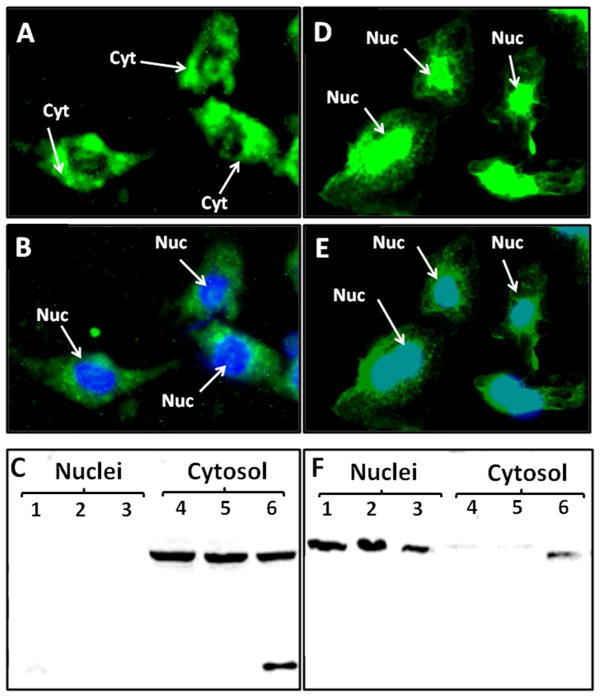
The present study utilized antibodies raised against two different epitopes of the AGT protein. The AI-AGT antibody recognized the N terminus or Ang I sequence of rat AGT while the Int-AGT antibody was directed against an internal sequence distal to Ang I (17). We characterized both antibodies by protein immunoblots using 0.5 to 5.0 μl of either nephrectomized rat plasma (NRP) as a source of intact AGT or commercial fetal bovine serum (FBS) in which the NRK-E52 cells were routinely maintained (Figure 1). As shown in Figure 1A, the AI-AGT antibody recognized two protein bands of approximately 60 and 55 kDa from NRP, as well as two similar sized bands in the FBS. The NRP samples were then exposed to renin from a rat cortical homogenate at 37°C or 4°C with or without the renin inhibitor aliskiren and the immunoblot probed with the AI-AGT antibody. In the absence of aliskiren, the AGT band was reduced at 15 min and essentially abolished by 30 min at 37°C (Figure 1B). Addition of aliskiren or incubation at 4°C prevented the disappearance of the protein up to 60 min. In contrast, the Int-AGT antibody clearly recognized AGT in the NRP samples (Figure 1C, 0.5–5.0 μl serum), but did not detect AGT in FBS (Figure 1C). The Int-AGT antibody appeared to exhibit greater sensitivity to rat AGT; the antibody detected 0.5 μl of NRP while the AI-AGT antibody required a sample volume 2 to 5-fold higher (lane 4, Figure 1C versus 1A). Moreover, re-probing the immunoblot from Figure 1B with the Int-AGT antibody revealed the AGT bands in the samples incubated with renin (Figure 1D). These data confirm that the Int-AGT antibody recognized residues distal to the Ang I sequence of AGT in rat plasma, but not FBS, and that the AI-AGT antibody does not detect the renin-processed or des-Ang I form of rat AGT (and likely FBS). Utilizing the two AGT antibodies, we assessed expression of the protein by immunofluorescent (IMF) staining and protein immunoblots of isolated nuclear and cytosolic fractions of the NRK-52E cells (Figure 2). As shown in Figure 2A and 2B, IMF staining with the AI-AGT antibody revealed primarily cytosolic staining of the protein that was apparently exclusive of the nucleus. Moreover, the immunoblot showed a 55 kDa band in the cytosolic fraction (lanes 4–6), but no immunoreactive band in the nuclear fraction of the NRK-52E cells (lanes 1–3, Figure 2C). In contrast, IMF staining with the Int-AGT antibody revealed the predominant localization of AGT to the area of the cell nucleus (Figure 2D and 2E). The immunoblot with the Int-AGT antibody also revealed a prominent protein band (55 kDa) in the nuclear fraction (lanes 1–3), as well as a faint band in the cytosol (lanes 4–6, Figure 2F).
Figure 1.
Characterization of antibodies to rat and bovine angiotensinogen (AGT). Panel A: Immunoblot of nephrectomized rat plasma (NRP) and fetal bovine serum (FBS) probed with Ang I-AGT (AI-AGT) antibody. The AI-AGT antibody detected AGT bands at 55 and 60 kDa in both NRP and FBS samples. Panel B: incubation of renin with AGT in NRP for 5 to 60 minutes at 37°C or on ice (*5 & *60 minutes) in the absence (−) or presence (+) of the renin inhibitor aliskerin (10 μM) and probed with AI-AGT antibody. Panel C: Immunoblot of NRP and FBS samples with the AGT antibody directed to internal sequence distal to Ang I (Int-AGT) that recognized rat but not bovine AGT. Panel D: the immunoblot from B was stripped and reprobed with the Int-AGT antibody revealing no effect of renin exposure on AGT expression.
Figure 2.
Angiotensinogen (AGT) expression in the NRK-52E cells. Panel A: Immunofluorescent (IMF) staining of NRK-52E cells with the Ang I-intact AGT (AI-AGT) antibody reveals predominantly cytosolic (Cyt) staining. Panel B: Identical image of panel A with DAPI nuclear (Nuc) staining in blue. Panel C: Immunoblot of nuclear and cytosolic fractions with AI-AGT antibody reveals a predominant 55 kDa band in cytosolic fractions. Panel D: IMF staining with Int-AGT antibody reveals nuclear associated staining. Panel E: Identical image of panel D with DAPI nuclear (Nuc) staining in blue. Panel F: Immunoblot of nuclear and cytosolic fractions reveals predominant 55 kDa band in the nuclear fraction. IMF staining was representative of 3 different cell passages. Immunoblot fractions were from 3 different cell passages.
Renin and (Pro) renin receptor
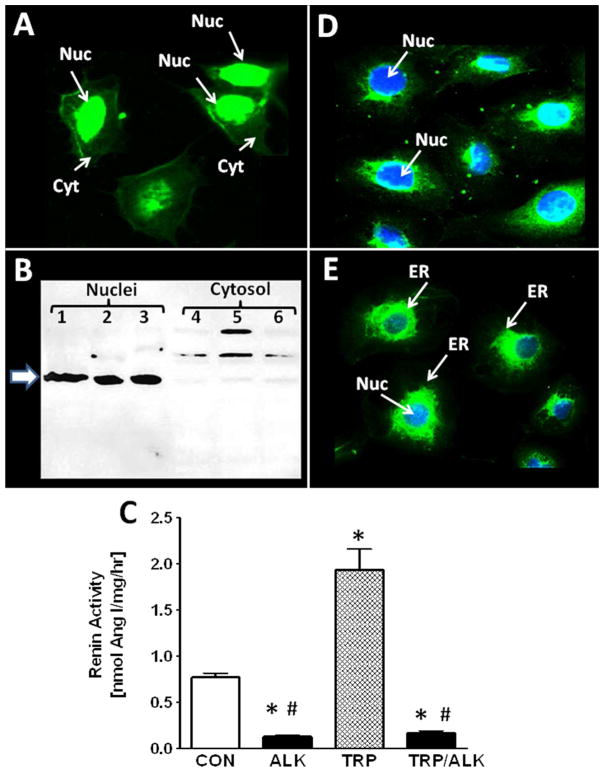
The apparent absence of intact AGT in the nucleus suggested that renin or a renin-like enzyme cleaved AGT either within the nucleus or outside the organelle with subsequent nuclear transport of the precursor. As shown in Fig. 3A, IMF staining for renin revealed the predominant nuclear localization of the enzyme in the cells. Protein immunoblots of the nuclear and cytosolic fractions also revealed a predominant immunoreactive band at ~ 55 kDa in the nuclear fraction and a faint band in the cytosol (Figure 3B). We next assessed renin activity in isolated nuclei of the NRK-52E cells by addition of exogenous NRP AGT in the absence or presence of aliskiren. Renin activity averaged 0.77 ± 0.05 nmol Ang I/mg/hr and aliskiren reduced activity by 83% (Figure 3C). To determine the prorenin levels, nuclear extracts were pretreated with trypsin to convert prorenin to renin and the generation of Ang I from NRP AGT was determined in the presence of the trypsin inhibitor SBTI. Trypsin activation of nuclear prorenin significantly increased enzyme activity approximately 3-fold to 1.93 ± 0.23 nmol Ang I/mg protein/hr and aliskiren inhibited 93% of the Ang I-forming activity (Figure 3C). These findings suggest the presence of both renin and prorenin in the NRK-52E cell nuclei. Since prorenin may preferentially bind to the prorenin receptor to increase catalytic activity and/or induce cell signaling, we stained the cells with an antibody against the prorenin receptor. As shown in Figure 3D, IMF staining revealed primarily perinuclear staining of the prorenin receptor in cells counter-stained with the nuclear marker DAPI shown in blue. In comparison, cells were also stained with an antibody to calnexin, a protein intrinsic to the perinuclear/ER membrane and the DAPI marker (Figure 3E).
Figure 3.
Renin and prorenin receptor (PRR) expression in NRK-52E cells. Panel A: Immunofluorescent (IMF) staining of NRK-52E cells with the renin antibody reveals nuclear-associated staining. Panel B: Immunoblot of nuclear and cytosolic fractions with renin antibody reveals a 55 kDa (arrow) band in the nuclear fraction from cells of 3 different passages. Higher molecular weight bands were evident in the cytosolic fractions. Panel C: Renin activity in the nuclear fraction of control (CON) or trypsin-treated (TRP) nuclear lysates treated with or without the renin inhibitor aliskerin (ALK). Values are means ± SEM, *p< 0.01 vs. CON, n = 3 different passages. Panel D: IMF staining of PRR reveals perinuclear staining and DAPI nuclear staining (Nuc) in blue. Panel E: IMF staining of the endoplasmic reticulum (ER) marker calnexin and DAPI nuclear staining (Nuc) in blue.
Angiotensin Peptides
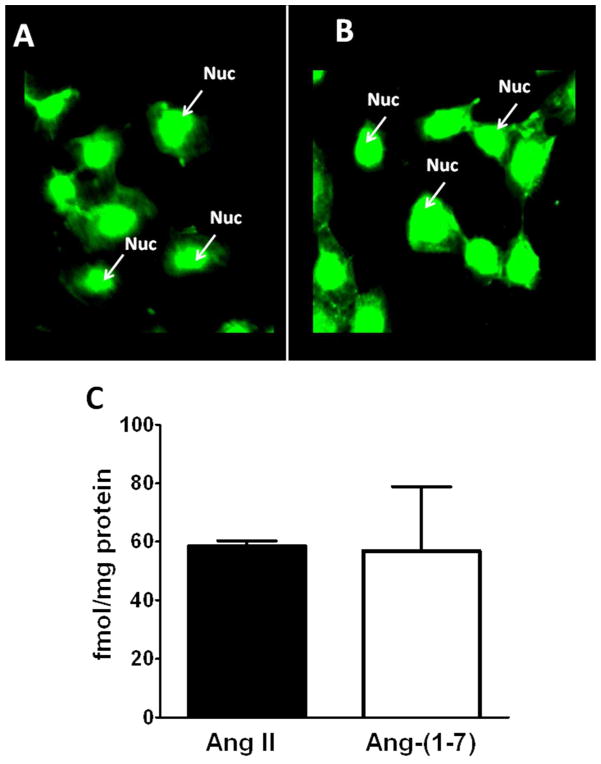
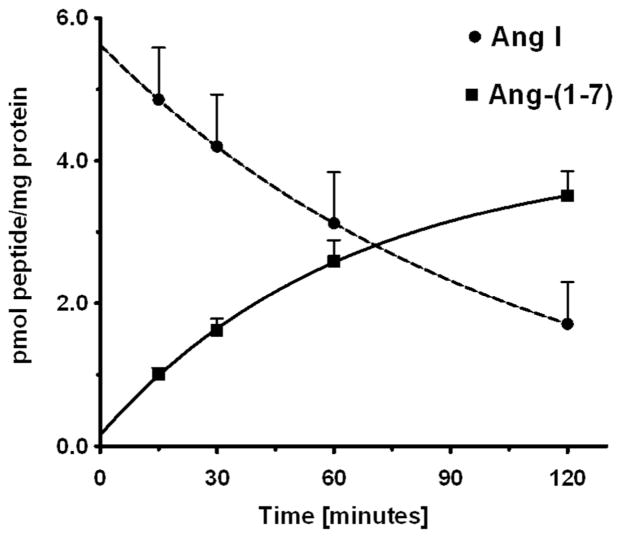
The expression of both AGT and renin within the NRK-52E cell nuclei may suggest a pathway for the intracellular synthesis of angiotensin peptides. Therefore, we assessed the cell staining and content of Ang II and Ang-(1–7) by two distinct antibodies specific to the C-terminus of each peptide. As shown in Figures 4A and 4B, the affinity-purified antibodies to Ang II and Ang-(1–7) revealed predominantly nuclear staining. Consistent with this pattern of staining, nuclear extracts from four different cell passages contained 59 ± 2 fmol/mg protein of Ang II and 57 ± 22 fmol/mg protein of Ang-(1–7) (Figure 4C). We then examined the potential processing enzymes that may contribute to Ang II or Ang-(1–7) within the nucleus. Solubilized nuclear fractions were incubated with 125I-Ang I or 125I-Ang II for 15 to 120 mins at 37°C and the products analyzed by a HPLC-coupled γ-detector (16). As shown in the chromatographic panels of Figure 5, Ang-(1–7) was the major peak of Ang I metabolism at 15 and 60 mins (Figure 5A and 5B, respectively). Ang I exhibited a half-life of 73 minutes in the nuclear cell extracts and Ang-(1–7) was the primary product over the 120 min time course (Figure 7). In contrast, there was minimal metabolism of the Ang II in the nuclear extracts with >70% of the peptide intact at 120 min (data not shown).
Figure 4.
Expression of Ang II and Ang-(1–7) in NRK-52E cells. Immunofluorescent staining using affinity-purified antibodies for Ang II (A) and Ang-(1–7) (B) reveal nuclear-associated staining. Panel C: Immunoreactive concentrations of Ang II and Ang-(1–7) in the nuclear fractions of NRK-52E cells. Values are means ± SEM; n=4.
Figure 5.
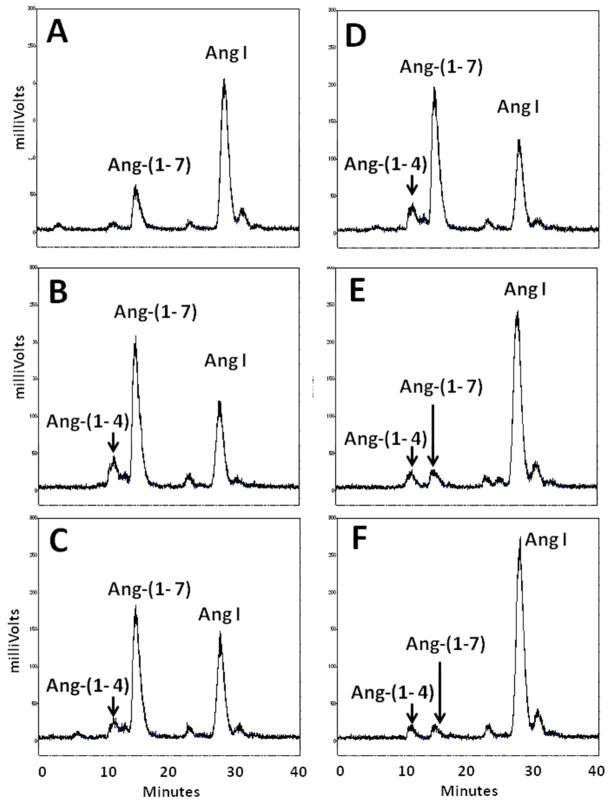
Conversion of Ang I to Ang-(1–7) in isolated nuclei from NRK-52E cells. HPLC analysis of 125I-Ang I metabolism at 37°C revealed primarily Ang-(1–7) (Ang 7) at 15 minutes (A) and 60 minutes (B). The neprilysin inhibitor SCH39370 (SCH; 10 μM) or prolyl oligopeptidase inhibitor z-prolyl-prolinal (ZPP; 50 μM) did not inhibit Ang-(1–7) generation (C and D, respectively). The thimet oligopeptidase inhibitor CPP (50 μM) and the thiol inhibitor PCMB (500 μM) essentially abolished Ang-(1–7) production (E and F, respectively). A minor peak of Ang-(1–4) (Ang 4) was detected in panels B–F.
Figure 7.
Time course for the disappearance of Ang I and formation of Ang-(1–7) in isolated nuclei from NRK-52E cells. The conversion of 125I-Ang I to 125I-Ang-(1–7) in nuclei at 37°C was quantitated by HPLC separation coupled to a γ-counter. Values are means ± SEM, n=4 of different cell passages. Non-linear decay curve for Ang I and exponential one-phase association of Ang-(1–7) were constructed in GraphPad Prism V.
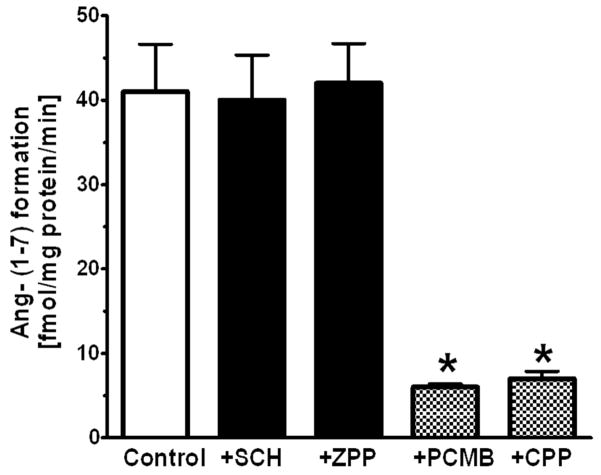
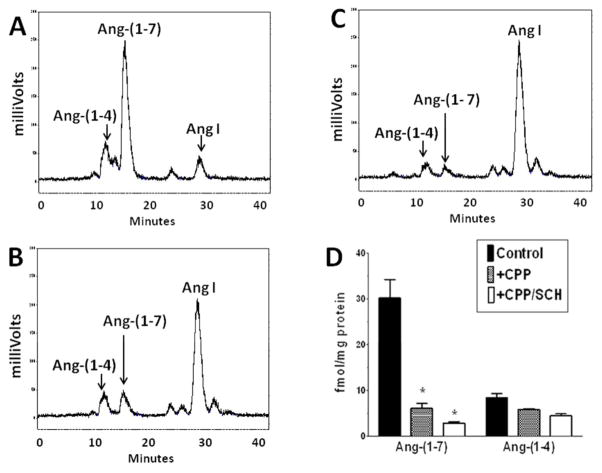
There are several endopeptidases including neprilysin, prolyl oligopeptidase and thimet oligopeptidase that directly process Ang I to Ang-(1–7) (26) The neprilysin agent SCH39370 and the prolyl oligopeptidase inhibitor ZPP did not block the generation of Ang-(1–7) in the nuclear extract (Figure 5C and 5D, respectively). Both the thimet oligopeptidase inhibitor CPP and the thiol protease inhibitor PCMB markedly reduced the peak of Ang-(1–7) and preserved the peak of Ang I (Figure 5E and 5F, respectively). Evaluation of these inhibitors at the 60 min incubation period revealed that CPP and PCMB inhibited Ang-(1–7) formation by over 80% and suggests that thimet oligopeptidase converts Ang I to Ang-(1–7) in isolated nuclei (Figure 6). We next assessed the Ang-(1–7) and Ang-(1–4)-forming activities at 120 minutes in the presence of CPP and SCH39370. Again, thimet oligopeptidase accounted for the majority of Ang-(1–7) at this time (Figure 8); however, there remained small peaks of Ang-(1–7) and Ang-(1–4) with CPP (Figure 8B). Addition of SCH39370 to CPP appeared to further reduce both Ang-(1–7) and Ang-(1–4) (Figure 8C), but the response did not reach statistical significance (Figure 8D).
Figure 6.
Influence of peptidase inhibitors on the generation of Ang-(1–7) from Ang I in the isolated nuclear fraction of NRK-52E cells. Peptidase inhibitors were neprilysin inhibitor SCH, prolyl oligopeptidase inhibitor ZPP, thimet oligopeptidase inhibitor CPP and thiol protease inhibitor PCMB. All values represent mean ± SEM, n=4 from different cell passages; P < 0.01 versus Control (CON).
Figure 8.
Influence of combined thimet oligopeptidase and neprilysin inhibition on Ang-(1–7) and Ang-(1–4) formation in isolated nuclei NRK-52E cells. Panel A: Metabolism of 125I-Ang I to Ang-(1–7) and Ang-(1–4) following 120 minute incubation with nuclei at 37°C. Panel B: Addition of thimet oligopeptidase inhibitor CPP (50 ZM) blocked the majority of Ang-(1–7) formation. Panel C: Addition of CPP and neprilysin inhibitor SCH39370 (SCH, 10 ZM). Panel D: Influence of CPP or CPP/SCH on the formation of Ang-(1–7) and Ang-(1–4) in nuclei at 120 minutes. All values represent mean ± SEM, n=3 from different cell passages; *P < 0.05 versus Control.
DISCUSSION
In the present study we characterized the RAS in the NRK-52E epithelial cell line to facilitate our understanding of this intracellular system. The NRK-52E cells are widely utilized to examine the functional aspects of the Ang II-AT1 receptor pathway, as well as the counter-regulatory actions of the Ang-(1–7)-AT7/Mas axis (27–32). The current findings revealed that NRK-52E cells express multiple components of an intracellular RAS and parallel our previous findings in the isolated tubules of the sheep kidney (9). In this case, both immunofluorescent staining and western blots demonstrated that NRK-52E cells express AGT, (pro)renin, (pro)renin receptor and the peptides Ang II and Ang-(1–7) in the nuclear or perinuclear compartments. Activity studies revealed renin-dependent conversion to Ang I; however, prorenin was the more abundant form of the enzyme in a 3:1 ratio in the nuclear fraction. Finally, we demonstrate endopeptidase activity in the nuclear fraction that converts Ang I directly to Ang-(1–7) without the prerequisite formation of Ang II. The identity of the nuclear endopeptidase is likely thimet oligopeptidase based on the sensitivity to both the selective inhibitor CPP and the thiol agent PCMB.
Utilizing two different antibodies to rat AGT, we primarily detected the processed form of AGT (des-Ang I Aogen) in the nucleus. Lavoie et al. detected AGT primarily in the nucleus of human astrocytes and identified a nuclear localization signal in the C-terminal portion of the protein; however, these studies did not determine whether the precursor was processed or intact (7). In the current study, the presence of the cleaved AGT in the nucleus suggests proteolytic processing potentially by renin which may contribute to local levels of Ang II and Ang-(1–7) detected in the nuclear fraction. Indeed, this may constitute a pathway for the generation and delivery of peptide ligands to nuclear angiotensin receptors evident in the tubule epithelium, as well as in other tissues. Alternatively, AGT may be processed outside of the nucleus and the des-Ang I form subsequently transported to the nucleus. Apart from its role as the precursor to Ang II or Ang-(1–7), nuclear AGT may potentially contribute to cell signaling and function. Corvol and colleagues demonstrated that both intact and the Ang I-cleaved form of AGT attenuated angiogenesis (33;34). In this regard, Pan et al reported high affinity binding sites for AGT that internalized following binding in primary cultures of human proximal tubules (35). In contrast to the nuclear localization of des-Ang I AGT, the Ang I-directed antibody revealed the protein in both the cytosolic compartment of the NRK-52E cells, as well as in FBS. The circulating RAS is upregulated during pregnancy and the presence of AGT in FBS is not surprising. Since the Int-AGT antibody failed to recognize FBS AGT or detect AGT to the same extent as the AI-AGT antibody in the cell cytosol, it is possible that cytosolic AGT may primarily originate from uptake of AGT in the cell media. Recent studies have questioned the prevailing tenet that tubular AGT solely reflects local synthesis of the protein (36;37). Indeed, the protein transporter megalin may mediate the cellular uptake and stable sequestration of AGT within a subset of renal proximal tubules (37). However, it is not clear as to the cellular fate of AGT taken up from the tubular fluid and whether this pathway contributes to the generation of angiotensin peptides. Based on the present findings, the intact form of AGT in the NRK-52E cytosol may localize to a subcellular compartment separate from rat renin. Alternatively, provided the cytosolic form of AGT arises primarily from FBS, the presence of intact AGT may reflect inefficient processing by rat renin due to species differences in the recognition of AGT by renin. Clearly, additional studies are required to elucidate whether the cytosolic from of AGT in the NRK-52E cells reflects the uptake of FBS AGT and the extent that megalin contributes to this process.
The present characterization of the RAS in the NRK-52E cells arose from the demonstration of nuclear renin and AGT in isolated sheep proximal tubules, as well as the expression of Ang receptors on isolated nuclei (9). The localization of renin to the non-JG compartments of the kidney is somewhat controversial. Lalouel and colleagues originally demonstrated renin in the principal cells of the collecting duct in both the mouse and rat kidney (38;39). Subsequent studies suggest that renin regulation in the collecting duct of the rat kidney differs from that of JG-derived renin (40;41). Immunoreactive renin and mRNA expression were also reported in the proximal tubules; however, the intracellular localization of the protease was not determined (42–44). In this regard, an intracellular or truncated form of renin was identified in the mitochondria which may contribute to local Ang II generation (45–47). The isolated nuclei of the NRK-52E cells contained both active renin and prorenin that converted exogenous AGT to Ang I; this activity was essentially abolished by the renin inhibitor aliskerin. IMF staining also revealed that the prorenin receptor localized to the perinuclear compartment of the NRK-52E cells which is consistent with the findings of Schefe and colleagues (15). Apart from contributing to activation of the enzyme, the receptor may bind prorenin to stimulate cellular pathways including MAP kinase and TGF-β release that are not dependent on the Ang II-AT1 receptor axis (13;14;48;49). The extent that renin or activated prorenin contributes to the intracellular formation of Ang II or Ang-(1–7) in the NRK-52E cells awaits further study. Furthermore, studies are necessary to elucidate whether the prorenin receptor contributes to functional signaling pathways in these cells through an interaction with intracellular (pro)renin.
In addition to AGT and renin, both immunoreactive Ang II and Ang-(1–7) were identified in the isolated nuclear fractions of the NRK-52E cells. Casarini and colleagues initially reported IMF staining for both Ang II and Ang-(1–7) in the nucleus of isolated mesangial cells (10). Consistent with presence of Ang-(1–7) in the nuclei, we find an endopeptidase-like activity that processed Ang I predominantly to Ang-(1–7). The inhibitor profile suggested that the nuclear peptidase is likely thimet oligopeptidase (EC 3.4.24.15). Inhibitors selective for other endopeptidases that form Ang-(1–7) including neprilysin (EC 3.4.24.11) and prolyl-oligopeptidase (EC 3.4.21.26) did not significantly reduce the conversion of Ang I to Ang-(1–7). Thimet oligopeptidase is a zinc-dependent member of the metallopeptidase M3 family, but is sensitive to thiol or cystiene inhibitors such as PCMB and the selective inhibitor CPP (50–52). Interestingly, thimet oligopeptidase was localized to the nucleus of neurons in the rat brain by immunocytochemical staining (53;54). In this regard, Thompson et al. identified the nuclear localization signal sequence for human thimet oligopeptidase (55). Moreover, the overexpression of intracellular thimet oligopeptidase in HEK cells attenuated Ang II-AT1 receptor signaling as well as the actions of other G protein-coupled receptors (56;57). Blockade of Ang-(1–7) formation from Ang I did not result in the generation of Ang II in the nuclear fraction nor did we find extensive metabolism of Ang II to Ang-(1–7) or any other products. Although these findings suggest that Ang II is stable in the nuclear fraction, they do not identify the pathway for the nuclear formation or sequestration of Ang II and we can only suggest several possibilities for the nuclear expression of Ang II at this time. The peptide may arise from the direct processing of AGT through a non-renin mechanism or an intermediate peptide substrate such as Ang-(1–12) (2;26). Alternatively, nuclear Ang II may reflect the trafficking of the Ang II-AT1 receptor complex from the plasma membrane to the nucleus (58–62). Finally, Gonzalez-Villalobos et al. report that uptake of both Ang II and Ang-(1–7) was facilitated by the protein transporter megalin in the OK epithelial cell line, an accepted cell model for megalin-dependent uptake of albumin; however, the intracellular fate of the internalized peptides was not evaluated in these studies (63;64).
In conclusion, the present study characterized the NRK-52E epithelial cell line as tubule model for the expression of the intracellular RAS components within the kidney. The kidney is one of the primary targets to specifically block the ACE-Ang II-AT1 receptor axis by various therapeutic approaches including ACE inhibition, AT1 receptor antagonism, and more recently, renin blockade to attenuate blood pressure and renal injury. However, these regimens may not completely prevent the pathological consequences of an activated RAS. This may result from either insufficient drug doses to effectively block the intracellular RAS, low lipophilicity of certain agents that leads to inadequate intracellular concentrations, or an inability to inhibit downstream signaling pathways independent of Ang II generation (65). Indeed, increasing the dose of candesartan to a level higher than the maximal recommended dose for heart failure and hypertension significantly reduced the persistent proteinuria without a further decline in blood pressure (66). Renin inhibitors such as aliskiren do not block the binding of (pro)renin to the receptor (67). Therefore, the proinflammatory and profibrotic responses of an activated RAS may not be completely attenuated by renin inhibitors that would effectively reduce blood pressure. In this regard, introducing membrane permeable nonpeptide agonists for the AT7 and AT2 receptors along with renin inhibition and ARBs may overcome the loss of either AT7 or AT2 receptor activation by renin inhibitors, as well as achieve maximal blockade of the deleterious effects of the AngII-AT1 receptor axis.
Acknowledgments
This present studies represent partial fulfillment of the requirements for the degree of Doctorate of Philosophy in the Department of Physiology and Pharmacology Wake Forest University School of Medicine for Ebaa Alzayadneh. The authors acknowledge Nancy Pirro and Brian Westwood for their technical contribution to these studies.
FUNDING
These studies were supported by the National Institutes of Health (HL-51952, HL-56973) and the Wake Forest Venture Fund.
Footnotes
CONFLICT OF INTERESTS
None declared
References
- 1.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–87. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 3.Velez JC. The importance of the intrarenal renin-angiotensin system. Nat Clin Pract Nephrol. 2009;5(2):89–100. doi: 10.1038/ncpneph1015. [DOI] [PubMed] [Google Scholar]
- 4.Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol. 2008;294(4):H1675–1684. doi: 10.1152/ajpheart.91493.2007. [DOI] [PubMed] [Google Scholar]
- 5.Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2007;293(2):H939–H948. doi: 10.1152/ajpheart.00391.2007. [DOI] [PubMed] [Google Scholar]
- 6.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011;108(36):14849–54. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherrod M, Liu X, Zhang X, Sigmund CD. Nuclear localization of angiotensinogen in astrocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R539–R546. doi: 10.1152/ajpregu.00594.2004. [DOI] [PubMed] [Google Scholar]
- 8.Ellis B, Li XC, Miguel-Qin E, Gu V, Zhuo JL. Evidence for a functional intracellular angiotensin system in the proximal tubule of the kidney. Am J Physiol Regul Integr Comp Physiol. 2012;302(5):R494–R509. doi: 10.1152/ajpregu.00487.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol. 2012;302(5):R518–R530. doi: 10.1152/ajpregu.00525.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camargo de Andrade MC, Di Marco GS, de Paulo CTV, Mortara RA, Sabatini RA, Pesquero JB, et al. Expression and localization of N-domain ANG I-converting enzymes in mesangial cells in culture from spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2006;290(2):F364–F375. doi: 10.1152/ajprenal.00110.2005. [DOI] [PubMed] [Google Scholar]
- 11.De Mello WC, Danser AH. Angiotensin II and the heart: On the intracrine renin-angiotensin system. Hypertension. 2000;35(6):1183–8. doi: 10.1161/01.hyp.35.6.1183. [DOI] [PubMed] [Google Scholar]
- 12.Batenburg WW, Krop M, Garrelds IM, de VR, de Bruin RJ, Burckle CA, et al. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens. 2007;25(12):2441–53. doi: 10.1097/HJH.0b013e3282f05bae. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109(11):1417–27. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, et al. Renin increases mesangial cell transforming growth factor-B1 matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kid Int. 2006;69:105–13. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- 15.Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, et al. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res. 2006;99(12):1355–66. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- 16.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2.Lewis rat. Am J Physiol Renal Physiol. 2006;290:F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 17.Li XC, Zhuo JL. Intracellular ANG II directly induces in vitro transcription of TGF-beta1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Cell Physiol. 2008;294(4):C1034–C1045. doi: 10.1152/ajpcell.00432.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol. 2006;290(6):F1382–F1390. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun. 2009;384(2):149–54. doi: 10.1016/j.bbrc.2009.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, Chappell MC. Angiotensin-(1–7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension. 2010;55(1):166–71. doi: 10.1161/HYPERTENSIONAHA.109.141622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, et al. Nuclear angiotensin-(1–7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol. 2010;299(5):F983–F990. doi: 10.1152/ajprenal.00371.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, et al. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol. 2009;296(6):F1484–F1493. doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hacot S, Coute Y, Belin S, Albaret MA, Mertani HC, Sanchez JC, et al. Isolation of nuclei. Curr Protoc Cell Biol. 2010;Chapter 3(Unit3) doi: 10.1002/0471143030.cb0336s47. [DOI] [PubMed] [Google Scholar]
- 24.Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol. 2008;295:H10–H20. doi: 10.1152/ajpheart.01277.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaltout HA, Westwood B, Averill DB, Ferrario CM, Figueroa J, Diz DI, et al. Angiotensin metabolism in renal proximal tubules, urine and serum of sheep: Evidence for ACE2-dependent processing of Angiotensin II. Am J Physiol Renal Physiol. 2006;292:F82–F91. doi: 10.1152/ajprenal.00139.2006. [DOI] [PubMed] [Google Scholar]
- 26.Chappell MC. Nonclassical Renin-Angiotensin system and renal function. Compr Physiol. 2012;2(4):2733–52. doi: 10.1002/cphy.c120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou CH, Chuang LY, Lu CY, Guh JY. Interaction between TGF-beta and ACE2-Ang-(1–7)-Mas pathway in high glucose-cultured NRK-52E cells. Mol Cell Endocrinol. 2013 Feb 5;366(1):21–30. doi: 10.1016/j.mce.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Cao W, Zhou QG, Nie J, Wang GB, Liu Y, Zhou ZM, et al. Albumin overload activates intrarenal renin-angiotensin system through protein kinase C and NADPH oxidase-dependent pathway. J Hypertens. 2011;29(7):1411–21. doi: 10.1097/HJH.0b013e32834786f0. [DOI] [PubMed] [Google Scholar]
- 29.Kim SM, Kim YG, Jeong KH, Lee SH, Lee TW, Ihm CG, et al. Angiotensin II-induced mitochondrial Nox4 is a major endogenous source of oxidative stress in kidney tubular cells. PLoS One. 2012;7(7):e39739. doi: 10.1371/journal.pone.0039739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koka V, Huang XR, Chung AC, Wang W, Truong LD, Lan HY. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol. 2008;172(5):1174–83. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang F, Huang XR, Chung AC, Hou CC, Lai KN, Lan HY. Essential role for Smad3 in angiotensin II-induced tubular epithelial-mesenchymal transition. J Pathol. 2010;221(4):390–401. doi: 10.1002/path.2721. [DOI] [PubMed] [Google Scholar]
- 32.Cao W, Xu J, Zhou ZM, Wang GB, Hou FF, Nie J. Advanced oxidation protein products activate intrarenal renin-angiotensin system via a CD36-mediated, redox-dependent pathway. Antioxid Redox Signal. 2013;18(1):19–35. doi: 10.1089/ars.2012.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brand M, Lamande N, Sigmund CD, Larger E, Corvol P, Gasc JM. Angiotensinogen modulates renal vasculature growth. Hypertension. 2006;47(6):1067–74. doi: 10.1161/01.HYP.0000221065.05670.23. [DOI] [PubMed] [Google Scholar]
- 34.Celerier J, Cruz A, Lamande N, Gasc JM, Corvol P. Angiotensinogen and its cleaved derivatives inhibit angiogenesis. Hypertension. 2002;39(2):224–8. doi: 10.1161/hy0202.103441. [DOI] [PubMed] [Google Scholar]
- 35.Pan N, Luo J, Kaiser SJ, Frome WL, Dart RA, Tewksbury DA. Specific receptor for angiotensinogen on human renal cells. Clin Chim Acta. 2006;373(1–2):32–6. doi: 10.1016/j.cca.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 36.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, et al. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23(7):1181–9. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, et al. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem. 2010;285(53):41935–46. doi: 10.1074/jbc.M110.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34(6):1265–74. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 39.Rohrwasser A, Ishigami T, Gociman B, Lantelme P, Morgan T, Cheng T, et al. Renin and kallikrein in connecting tubule of mouse. Kidney Int. 2003;64(6):2155–62. doi: 10.1046/j.1523-1755.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension. 2011;57(3):594–9. doi: 10.1161/HYPERTENSIONAHA.110.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, et al. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44(2):223–9. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimpelmann J, Kumar D, Levine DZ, Wehbi G, Imig JD, Navar LG, et al. Early diabetes mellitus stimulates proximal tubule renin mRNA expression in the rat. Kidney Int. 2000;58(6):2320–30. doi: 10.1046/j.1523-1755.2000.00416.x. [DOI] [PubMed] [Google Scholar]
- 43.Henrich WL, McAllister EA, Eskue A, Miller T, Moe OW. Renin regulation in cultured proximal tubular cells. Hypertension. 1996;27(6):1337–40. doi: 10.1161/01.hyp.27.6.1337. [DOI] [PubMed] [Google Scholar]
- 44.Moe OW, Ujiie K, Star RA, Miller T, Widell J, Alpein RJ. Renin expression in renal proxijmal tubule. J Clin Invest. 1993;91:774–9. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavoie JL, Liu X, Bianco RA, Beltz TG, Johnson AK, Sigmund CD. Evidence supporting a functional role for intracellular renin in the brain. Hypertension. 2006;47(3):461–6. doi: 10.1161/01.HYP.0000203308.52919.dc. [DOI] [PubMed] [Google Scholar]
- 46.Peters J, Clausmeyer S. Intracellular sorting of renin: cell type specific differences and their consequences. J Mol Cell Cardiol. 2002;34(12):1561–8. doi: 10.1006/jmcc.2002.2079. [DOI] [PubMed] [Google Scholar]
- 47.Clausmeyer S, Sturzebecher R, Peters J. An alternative transcript of the rat renin gene can result in a truncated prorenin that is transported into adrenal mitochondria. Circ Res. 1999;84(3):337–44. doi: 10.1161/01.res.84.3.337. [DOI] [PubMed] [Google Scholar]
- 48.Sakoda M, Ichihara A, Kaneshiro Y, Takemitsu T, Nakazato Y, Nabi AH, et al. (Pro)renin receptor-mediated activation of mitogen-activated protein kinases in human vascular smooth muscle cells. Hypertens Res. 2007;30(11):1139–46. doi: 10.1291/hypres.30.1139. [DOI] [PubMed] [Google Scholar]
- 49.Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, et al. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol. 2007;18(6):1789–95. doi: 10.1681/ASN.2006091062. [DOI] [PubMed] [Google Scholar]
- 50.Malvezzi A, Higa PM, do AA, Silva GM, Gozzo FC, Ferro ES, et al. The cysteine-rich protein thimet oligopeptidase as a model of the structural requirements for S-glutathiolation and oxidative oligomerization. PLoS One. 2012;7(6):e39408. doi: 10.1371/journal.pone.0039408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chappell MC, Chappell MC, Gomez MN, Pirro NT, Ferrario CM. Release of angiotensin-(1–7) from the rat hindlimb: Influence of angiotensin-converting enzyme inhibition. Hypertension. 2000;35:348–352. doi: 10.1161/01.hyp.35.1.348. [DOI] [PubMed] [Google Scholar]
- 52.Chappell MC, Chappell MC, Tallant EA, Brosnihan KB, Ferrario CM. Processing of angiotensin I to angiotensin-(1–7) by vascular smooth muscle cells. J Vascular Medicine and Biology. 1995;5:129–137. [Google Scholar]
- 53.Healy DP, Orlowski M. Immunocytochemical localization of endopeptidase 24. 15 in rat brain. Brain Res. 1992;571:121–8. doi: 10.1016/0006-8993(92)90517-d. [DOI] [PubMed] [Google Scholar]
- 54.Massarelli EE, Casatti CA, Kato A, Camargo AC, Bauer JA, Glucksman MJ, et al. Differential subcellular distribution of neurolysin (EC 3.4.24.16) and thimet oligopeptidase (EC 3.4.24. 15) in the rat brain. Brain Res. 1999;851(1–2):261–5. doi: 10.1016/s0006-8993(99)02135-6. [DOI] [PubMed] [Google Scholar]
- 55.Thompson A, Huber G, Malherbe P. Cloning and functional expression of a metalloendopeptidase from human brain with the ability to cleave a beta-APP substrate peptide. Biochem Biophys Res Commun. 1995;213(1):66–73. doi: 10.1006/bbrc.1995.2099. [DOI] [PubMed] [Google Scholar]
- 56.Berti DA, Morano C, Russo LC, Castro LM, Cunha FM, Zhang X, et al. Analysis of intracellular substrates and products of thimet oligopeptidase in human embryonic kidney 293 cells. J Biol Chem. 2009;284(21):14105–16. doi: 10.1074/jbc.M807916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russo LC, Castro LM, Gozzo FC, Ferro ES. Inhibition of thimet oligopeptidase by siRNA alters specific intracellular peptides and potentiates isoproterenol signal transduction. FEBS Lett. 2012;586(19):3287–92. doi: 10.1016/j.febslet.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Lee DK, Lanca AJ, Cheng R, Nguyen T, Ji XD, Gobeil F, Jr, et al. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem. 2004;279(9):7901–8. doi: 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- 59.Morinelli TA, Raymond JR, Baldys A, Yang Q, Lee MH, Luttrell L, et al. Identification of a putative nuclear localization sequence within ANG II AT(1A) receptor associated with nuclear activation. Am J Physiol Cell Physiol. 2007;292(4):C1398–C1408. doi: 10.1152/ajpcell.00337.2006. [DOI] [PubMed] [Google Scholar]
- 60.Li XC, Navar LG, Shao Y, Zhuo JL. Genetic deletion of AT1a receptors attenuates intracellular accumulation of ANG II in the kidney of AT1a receptor-deficient mice. Am J Physiol Renal Physiol. 2007;293(2):F586–F593. doi: 10.1152/ajprenal.00489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: Role of AT(1) receptor. Hypertension. 2002;39(1):116–21. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 62.Zhuo JL, Carretero OA, Li XC. Effects of AT1 receptor-mediated endocytosis of extracellular Ang II on activation of nuclear factor-kappa B in proximal tubule cells. Ann N Y Acad Sci. 2006;1091:336–45. doi: 10.1196/annals.1378.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez-Villalobos R, Klassen RB, Allen PL, Johanson K, Baker CB, Kobori H, et al. Megalin binds and internalizes angiotensin-(1–7) Am J Physiol Renal Physiol. 2006;290(5):F1270–F1275. doi: 10.1152/ajprenal.00164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez-Villalobos R, Klassen RB, Allen PL, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physiol. 2005 Feb;288(2):F420–F427. doi: 10.1152/ajprenal.00243.2004. [DOI] [PubMed] [Google Scholar]
- 65.Kurtz TW, Klein U. Next generation multifunctional angiotensin receptor blockers. Hypertens Res. 2009;32(10):826–34. doi: 10.1038/hr.2009.135. [DOI] [PubMed] [Google Scholar]
- 66.Burgess E, Muirhead N, Rene de CP, Chiu A, Pichette V, Tobe S. Supramaximal dose of candesartan in proteinuric renal disease. J Am Soc Nephrol. 2009;20(4):893–900. doi: 10.1681/ASN.2008040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schefe JH, Neumann C, Goebel M, Danser J, Kirsch S, Gust R, et al. Prorenin engages the (pro)renin receptor like renin and both ligand activities are unopposed by aliskiren. J Hypertens. 2008;26(9):1787–94. doi: 10.1097/HJH.0b013e3283060f2e. [DOI] [PubMed] [Google Scholar]