Abstract
Background
The progressive resistance exercise (PRE) in Parkinson’s disease trial (PRET-PD) showed that PRE improved the motor signs of PD compared to a modified Fitness Counts (mFC) program. It is unclear how long-term exercise affects physical function in these individuals.
Objective
To examine the effects of long-term PRE and mFC on physical function outcome measures in individuals with PD.
Methods
A preplanned secondary analysis was conducted using data from the 38 patients with idiopathic PD who completed the PRET-PD trial. Participants were randomized into PRE or mFC groups and exercised 2 days/week up to 24 months. Blinded assessors obtained functional outcomes on and off medication at baseline, 6 and 24 months with the Modified Physical Performance Test (mPPT), five times sit to stand test (STS), Functional Reach Test (FRT), Timed Up and Go (TUG), Berg Balance Scale (BBS), 6 minute walk test (6MWT), and 50ft walking speed (walk speed).
Results
The groups did not differ on any physical function measure at 6 or 24 months (p’s > 0.1). Across time, all physical function measures improved from baseline to 24 months when tested on medication (p’s < .0001), except for 6MWT(p = .068). Off medication results were similar except that the 6MWT was now significant.
Conclusions
24 months of supervised and structured exercise (either PRE or mFC) is effective at improving functional performance outcomes in individuals with moderate PD. Clinicians should strive to include structured and supervised exercise in the long-term plan of care for individuals with PD.
Keywords: Parkinson’s, exercise-randomized clinical trial, function, rehabilitation, outcome assessment
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder affecting approximately 1% of people over age 60 and 4% over aged 80.1 The clinical progression of PD is multidimensional and leads to a wide spectrum of impairments across gait, balance, strength, and cognition which, in turn, contribute to diminished physical function and disability. Standard medical treatment for the management of the motor and non-motor symptoms of PD has typically been pharmacological (e.g. dopamine replacement therapy)2, 3 and surgical (e.g. deep brain stimulation).3, 4 However, the progressive nature of the disease along with the changes in mobility and physical function associated with aging require management strategies to delay symptom onset or slow disease progression to prolong functional independence in individuals with PD.5 Rehabilitation therapy may be recommended in the management of PD to maximize functional ability and minimize secondary complications. Mounting evidence shows that exercise may be therapeutically beneficial for individuals with PD.5-11
The types of exercise that have been studied to date in PD include aerobic exercise and treadmill training,12-15 tai chi,7, 16 balance training,17, 18 dancing,19, 20 and progressive resistance exercise.17, 21 A recent Cochrane review reported on the short term effects of physical therapy in PD and concluded that physical therapy utilizing various exercise types can provide small but important changes in walking speed, balance, and clinician rated disability measured by the Unified Parkinson’s Disease Rating Scale (UPDRS).22 One limitation noted by the review however was that the trials included were relatively small, usually comparing physical therapy to no physical therapy over short periods of time. It is not clear if exercise over long periods conveys continued improvements in patient function or whether the progressive nature of the disease overtakes the benefits of exercise in the long term. It is also not clear whether different modes of exercise convey different benefits in PD. For example, aerobic treadmill training was shown to be superior to general strength and flexibility exercises on improving walking speed in PD, while general strength and flexibility exercises have been shown to be superior to treadmill training on improving muscle strength.13 Similarly, progressive resistance exercise had a greater benefit on strength and the motor signs of PD as measured by the motor subscale of the UPDRS (UPDRS-III) at 24 months compared to a low intensity stretching, balance, and strengthening program.21 Therefore, specific exercise prescriptions may be necessary to target specific impairments in individuals with PD. A clearer understanding of the benefits that each type of exercise conveys for individuals with PD will assist in the development of patient specific exercise prescription.
Despite an increasing awareness of the role of exercise in effecting short term changes, the benefits of longer term exercise on functional limitations in PD are not as well understood. The natural progression of PD entails more impaired functional performance and an increased level of disability. Exercise has been shown to have either no improvement13, 23, 24 or improvements after 4 months of exercise that were not maintained after 16 months of exercise9 on overall physical function in individuals with PD. Corcos et al.21 was the first randomized controlled trial, the Progressive Resistance Exercise Training in Parkinson’s Disease (PRET-PD) trial, to show the longer term effects of progressive resistance exercise up to 24 months on the motor signs (UPDRS-III), physical function and quality of life in individuals with PD. Patients with moderate PD participated in either a progressive resistance exercise (PRE) strengthening program or a modified Fitness Counts (mFC) exercise program consisting of low intensity stretching, non-progressive strengthening, breathing, and balance exercises recommended by the National Parkinson Foundation.25 While both programs had similar effects on the motor rating of the UPDRS at 6 months, only the PRE group maintained the improvements in UPDRS-III scores at 12, 18 and 24 months.21 Functional performance was examined using only one measure of function, the modified Physical Performance Test (mPPT) collected in the off medicated state. Both PRE and mFC groups showed improved functional performance on the mPPT across 24 months. The PRET-PD trial provided the first evidence that long-term, supervised exercise could affect general functional performance in individuals with PD.
The mPPT is a general test of diminished functional capacity.26 Physical function in PD is multi-faceted and in addition to general functional performance includes aspects of balance and functional mobility. It is not clear whether, in the face of disease progression, longer term exercise here defined as greater than 6 months, can positively affect general functional performance as well as specific measures of balance and functional mobility in individuals with PD. It is also not clear whether a specific type of therapeutic exercise conveys greater benefit on different aspects of physical function in PD than another type of exercise. This study reports a secondary analysis of functional outcomes data from the PRET-PD trial.21 The purpose was to: 1) investigate whether long-term exercise has similar effects across multiple measures of physical function including specific measures of balance and functional mobility in individuals with PD and 2), examine whether long-term PRE is better than mFC at affecting physical function across multiple measures of function in individuals with PD. Two exercise approaches were compared at 6 months and at 24 months: a progressive resistance exercise (PRE) program and a non-progressive modified Fitness Counts (mFC) exercise program. The hypotheses were that (1) regardless of whether individuals were assigned to the PRE or mFC group, 24 months of exercise would lead to improvement in all aspects of functional ability compared to baseline, and, (2) comparing between groups, that at both short term (i.e. 6 months) and long-term (i.e. 24 month) time points, the PRE group would exhibit significantly more improvement in all aspects of physical function compared to the mFC group. This hypothesis was based on the maintained improvement of UPDRS-III scores in the PRE group over 24 months compared to the mFC group in the primary study.21 While the primary study did not show between groups differences on mPPT scores at either 6 or 24 months we hypothesized that an intervention specific effect would be found for these more specific functional measures. Participants were studied in both the on and off medication state to assess the most broad effect of exercise on physical performance in patients with PD.
Method
This secondary data analysis used a subset of data from the PRET-PD trial, a prospective, parallel-group, single center, randomized controlled trial.21 Physical function outcomes were one of multiple outcome domains tested in the PRET-PD trial which included other secondary outcomes of bradykinesia and strength, tremor, quality of life, and cognition. This paper will report findings related to the physical function outcomes only. The order of testing was pseudo randomized between outcome domains and between the different cognitive outcomes. In the PRET-PD study, patients with moderate stage, idiopathic PD, confirmed by a Movement Disorders specialist as outlined by the Parkinson’s Disease Society Brain Bank criteria were self-referred or recruited from Rush University Medical Center. Eligibility criteria were aged 50 to 67 years, on stable dopaminergic therapy, and able to walk for 6 minutes. Patients with moderate disease severity were studied because these patients are sufficiently impaired so that a treatment effect can be observed, but not so impaired that there are major safety issues. Patients were screened using the Physical Activity Readiness Questionnaire27 which is valid to use for ages 15-69. Since the study duration was 2 years, the upper age limit was set at 67. Exclusion criteria were no significant neurological history other than PD; significant arthritis; failed Physical Activity Readiness Questionnaire;27 cognitive impairment as indicated by a Mini-Mental State Examination score < 23;28 already exercising; and history of surgery for PD. Patients were followed for 24 months or until they withdrew from the study. The institutional review boards at Rush University Medical Center and the University of Illinois at Chicago approved the study. Patients provided written informed consent. Race and ethnicity data were recorded as required by National Institutes of Health policy (PHS 398).
Participant characteristics and the complete trial profile for the PRET-PD trial have been previously published.21 Two exercise interventions were studied, Modified Fitness Counts (mFC) and Progressive Resistance Exercise (PRE). Subjects in both groups performed one-on-one training twice a week for the first 6 months with a certified personal trainer. For the remaining 18 months, one-on-one training was provided once a week and subjects performed the second session each week on their own. If a subject missed a session, they were instructed to make it up. Thus, subjects were expected to have completed a total of 208 exercise sessions at the end of 2 years. Subjects performed the exercise programs while on antiparkinsonian medication. In order to maximize compliance: 1) exercise sessions were held at a gym facility close to the subject’s home which was paid for by the study, 2) exercise sessions were scheduled at the subject’s convenience, 3) subjects were asked to exercise only twice a week, 4) if subjects missed 2 consecutive sessions, immediate action was taken by the exercise study coordinator to resolve any issues, and 5) the exercise study coordinator contacted the subject’s trainers every 2-3 months to check on the subject’s well-being. Both exercise programs had the same warm up and cool down phases that each lasted up to 10 minutes. These exercises included 3 minutes of walking followed by 5 repetitions of the following 5 stretching exercises: 1) neck circles to both directions, 2) trunk rotation while lying down to both directions, 3) arm circles in both directions, 4) hamstring stretches while sitting and 5) ankle stretches while standing. The stretches were performed dynamically but slowly and under control. The total duration for each exercise session in both programs was approximately 60-90 minutes. Exercise sessions were separated by at least 48 hours.29 As such, the programs were identical in all aspects (duration of exercise, number of exercise sessions, and time with the personal trainer) except for the specific exercises.
Modified Fitness Counts (mFC) is an exercise program recommended by the National Parkinson Foundation.30 The program focuses on stretching, non-progressive strengthening, balance exercises, and breathing. Modifications to the program were to exclude aerobic conditioning and the addition of weighted exercise thus allowing us to directly compare progressive resistance exercise in which load was systematically increased with an exercise program that did not utilize weighted exercise. There were 12 stretching exercises in mFC: standing chest stretch, seated neck and chest stretch, seated rotation stretch, overhead stretch, standing back stretch, hamstring stretch, lying shoulder stretch, seated side stretch, standing shoulder stretch, rotation stretch, calf stretch and ankle circles. All stretching exercises were performed 3 times for 3-5 breath counts. The 7 strengthening exercises consisted of: wall slides, bridging, shoulder blade squeeze (sitting without resistance and standing with a resistance tube), quadriceps strengthening (long arc quad), quadruped trunk strengthening (opposite arm/leg lifts), and prone on elbows. Three sets of 10 repetitions were performed for all strengthening exercises. There was also a 2 to 3 seconds of rest (or more if needed) between all stretching and strengthening exercises. The last set of exercises, balance exercises consisted of 2 exercises: weight shifts forward and backward 10 to 20 times while standing with feet placed hip width apart and single leg stance on each leg for 5-10 seconds.
The PRE program consisted of 11 strengthening exercises: chest press, latissimus pull downs, reverse flys, double leg press, hip extension, shoulder press, biceps curl, rotary calf (ankle plantar flexion), triceps extension, seated quadriceps extension, and back extension.31 At the first visit with the personal trainer, a one-repetition maximum (1RM) was established for each exercise. Resistance was set at approximately 30-40% of the 1RM for upper body exercises and 50-60% of 1RM for lower body exercises during the first week of training. As soon as the subject was able to perform a set of the exercises using good form and perceive the exercise to be somewhat easy, the resistance was increased by at least 5%31 or as allowed by the equipment. Each repetition lasted 6-9 seconds. Subjects raised the weight over 2-3 seconds, paused briefly (2-3 seconds), and slowly lowered the weight (3-4 seconds).32 Subjects performed 3 sets of 8 repetitions for each exercise starting with just 1 set and working up to 3 sets as they progressed. After 8 weeks on the strength program, subjects switched to a strength plus speed program. Here the emphasis was on the speed with which each repetition was completed. The resistance was set at 70-80% of their 1RM and each subject performed 2 sets of 12 repetitions. Every 8 weeks subjects alternated between the strength and strength plus speed training programs. Full details regarding the set-up and management of both exercise programs has previously been described.21
All assessments were performed at the Clinical Motor Control Laboratory at the University of Illinois at Chicago by raters blinded to group allocation. Baseline measures were collected prior to beginning exercise, and after 6, 12, 18 and 24 months of exercise. Off-medication assessment was completed in the morning prior to their first dose of medication following 12-hour overnight withdrawal of medication.33 Patients then took their prescribed medication, had lunch and repeated all the assessments approximately 60 minutes after taking their medication (on-medication). Physical function was examined using measures that spanned general functional performance, balance, and functional mobility. General functional performance was measured using the modified Physical Performance Test (mPPT)26. Balance and functional mobility were assessed using the five time sit to stand (STS) test34 performed as fast as possible, the Functional Reach Test (FRT)35, the Timed Up and Go (TUG)36 performed as fast as possible, the Berg Balance Scale (BBS), the 6-minute walk test (6MWT)37 and as fast as possible walking speed during a 50-ft walk distance (walk speed). Although there is some overlap in which aspect of function each measure captures, we strove to include measures that would span multiple aspects of function, regardless of overlap (e.g. endurance and mobility (6MWT), functional mobility (TUG), lower body strength and functional mobility (STT), balance (BBS), overall physical function (mPPT), walking speed (50 ft walk) and flexibility/dynamic postural control (FRT)). These measures have been widely reported in the literature, and have been shown to be reliable and valid in older adults and patients with PD.23, 26, 38-45 Statistical analyses were performed using the SAS software package (version 9.2; SAS institute, Inc., Cary, NC). The analysis did not impute missing data and assumed data were missing at random. The mPPT and BBS are measured on an ordinal scale, and therefore they were analyzed using non-parametric statistics. Although the 6MWT, TUG, FRT, STS, and walking speed are on ratio scales, these five were not normally distributed; therefore, non-parametric comparisons were used for all tests. Analyses were completed using the change score from baseline at 6 and 24 months. The Wilcoxon rank-sum test was used to test differences between groups at baseline, 6, and 24 months, while Friedman’s Chi Square was used to test for a main effect of time. The Wilcoxon signed-rank test was then used for planned post hoc pairwise comparisons to determine whether there was a significant effect of exercise at 6 months and at 24 months. The purpose of this study was to examine short and long term changes in function. Therefore, although testing was done every 6 months, analyses were done only at 6 and 24 months to reduce the number of comparisons. Analyses were performed separately for the on and off medication data. All statistical tests were 2-sided and we used a P value < 0.05 as statistical significance. Effect size was based on the Hodges-Lehman estimate of location shift of the median and 95% confidence intervals around the median.
Results
The change scores from baseline at 6 and 24 months of the 38 participants who completed the 24 month trial were used for this secondary analysis. Full details regarding dropout have previously been provided21, but in brief, dropout reasons were similar for both groups, and while baseline quality of life as measured by the PDQ-39 in the mFC group was worse for the noncompleters relative to the completers, there were no other differences between noncompleters and completers in either treatment group.
The treatment groups did not differ significantly at baseline on any physical function measure with the exception of the off medication FRT in which case the mFC group performed better than the PRE group at baseline (Table 1).
Table 1.
Characteristics of Patients at Baseline by Treatment Group.
| Variable | Treatment group
|
Difference Between Groups (95% Confidence Interval) | P† | |
|---|---|---|---|---|
| Modified Fitness Counts (n= 24) | Progressive Resistance Training(n = 24) | |||
| Demographic | ||||
| Age (y)s | 58.6 (5.6)a | 59.0 (4.6) | -0.4 (-2.6 to 3.4) | 0.78 |
| Sex - number | ||||
| Male | 14 | 14 | ||
| Female | 10 | 10 | ||
| Years since diagnosis | 6.5 (4.7)a | 6.5 (4.1) | 0.0 (-2.5 to 2.6) | 0.97 |
| Levodopa equivalent units in mg/day | 705 (405) | 598 (355) | -100 (-125 to 350)c | 0.37 |
| Motor Severity | ||||
| On medication Unified Parkinson Disease Rating Scale, part III, motor subscale score (range, 0-108) | 20.9 (8.0)a | 21.6 (10.1) | 0.7 (-4.4 to 6.2) | 0.74 |
| Off medication Unified Parkinson Disease Rating Scale, part III, motor subscale score (range, 0-108) | 34.7 (11.5)a | 34.5 (11.9) | -0.2 (-7.0 to 6.6) | 0.95 |
| Off medication Hoehn and Yahr staging scale, (range, 1-5) | 2.3 (0.53) | 2.2 (0.41) | -0.1 (-0.4 to 0.2) | 0.55 |
| On medication Hoehn and Yahr staging scale, (range, 1-5) | 1.9 (0.28) | 2 (0.38) | 0.1 (-0.1 to 0.3) | 0.51 |
| Physical Function Measures‡ | ||||
| On Medication | ||||
| Modified Physical Performance Test (range, 0-36) | 32 (30, 34)b | 31 (28, 33.5) | 0.0 (-1.0 to 2.0)c | 0.70 |
| Sit to Stand (s) | 9.1 (8, 11.5)b | 11.6 (8.4, 14.9) | -1.5 (-4.1 to-0.2)c | 0.10 |
| Functional Reach (cm) | 29.5 (26.4, 35.6)b | 26.7 (22.5, 30.3) | 3.2 (-0.3 to 6.7)c | 0.87 |
| Timed Up and Go (s) | 6.1 (4.9, 6.9)b | 6.5 (5.2, 8.4) | -0.5 (-1.6 to 0.4)c | 0.28 |
| Berg Balance Scale (range, 0-56) | 55 (53, 56)b | 55 (49, 55.5) | 1.0 (0.0 to 2.0)c | 0.14 |
| Six Minute Walk Test Distance (m) | 507.5 (466.3, 591.6)b | 548.3 (448.7, 607) | -11.3 (-79.9 to 50.8)c | 0.74 |
| Walk Speed (m/s) | 1.9 (1.5, 2.1)b | 1.8 (1.6, 2.1) | -0.01 (-0.3 to -0.02)c | 0.91 |
| Off Medication | ||||
| Modified Physical Performance Test (range, 0-36) | 29 (25.5, 32)b | 27 (22, 30) | 2.0 (-1.0 to 5.0)c | 0.27 |
| Sit to Stand (s) | 12.2 (9.8, 14.6)b | 12.8 (10, 17.9) | 1.1 (-1.4 to 4.3)c | 0.44 |
| Functional Reach (cm) | 28.2 (21.6, 33.7)b | 21.7 (18.9, 27.3) | 4.5 (0.3 to 9.2)c | 0.04 |
| Timed Up and Go (s) | 6.9 (5.5, 8.8)b | 7.9 (5.5, 10.5) | -0.6 (-2.5 to 0.8)c | 0.45 |
| Berg Balance Scale (range, 0-56) | 54 (46, 55)b | 51 (43,55) | 1.0 (-1.0 to 5.0)c | 0.22 |
| Six Minute Walk Test Distance (m) | 475.5 (410.4, 528.8)b | 443.5 (353.1, 546.7) | 21.5 (-66.3 to 89.6)c | 0.71 |
| Walk Speed (m/s) | 1.7 (1.4, 2)b | 1.6 (1.2, 2) | 0.01 (-0.2 to 0.3)c | 0.10 |
mean (1SD)
median with 25th-75 percentiles
Hodges Lehman estimate of location shift with 95% confidence intervals
P-values are for comparison of the treatment groups. All P-values are two-sided
Effects of Exercise on General Functional Performance, Balance, and Functional Mobility across 24 months
Table 2 and the Figure show the main effect of time. When tested on medication, participants demonstrated significant improvements in all measures of physical function with the exception of 6MWT which approached significance (p=.068). Post hoc testing of the on medication data showed that both short term (6 months) and long-term (24 months) improvements in all other physical function measures were significant (Table 2).
Table 2.
The effect of time.
| Main Effect of Time
|
6 months vs. Baseline
|
24 months vs. Baseline
|
|||||
|---|---|---|---|---|---|---|---|
| Friedman’s Chi Square Statistic (p-value) Df=4 | Wilcoxon Signed-Rank Test (p-value) | Estimated median change from baseline | 95% CI about the median | Wilcoxon Signed-Rank Test (p-value) | Estimated median change from baseline | 95% CI about the median | |
|
|
|
|
|||||
| ON MEDICATION | |||||||
| mPPT | 47.02 (p<.0001) | 196.5 (p=.0061) | 1.00 | 0.00 to 2.00 | 168.5 (p=.0003) | 2.0 | 1.0 to 3.0 |
| STS | 24.22 (p<.0001) | -247.5 (p=.0055) | -1.08 | -1.37 to -0.38 | -205.5 (p=.0006) | -1.19 | -1.84 to -0.47 |
| FRT | 29.29 (p<.0001) | 304 (p=.0008) | 2.86 | 0.634 to 4.4 | 321.5 (p<.0001) | 3.65 | 2.54 to 6.03 |
| TUG | 55.95 (p<.0001) | -412.5 (p<.0001) | -0.56 | -0.94 to -0.39 | -337.5 (p<.0001) | -0.85 | -1.04 to -0.51 |
| BBS | 17.63 (p=.001) | 103.5 (p=.0029) | 0.00 | 0.00 to 1.00 | 106.5 (p=.0002) | 0.50 | 0.0 to 1.0 |
| 6MWT | 8.73 (p=.068) | 312.5 (p=.0008) | 26.97 | 6.40 to 34.90 | 145 (p=.0266) | 19.96 | -4.88 to 42.52 |
| Walk speed | 25.97 (p<.0001) | 370 (p<.0001) | 0.12 | 0.07 to 0.22 | 274.5 (p<.0001) | 0.22 | 0.05 to 0.38 |
| OFF MEDICATION | |||||||
| mPPT | 65.95 (p<.0001) | 415.5 (p<.0001) | 3.00 | 2.00 to 4.00 | 334.5 (p<.0001) | 3.00 | 2.00 to 5.00 |
| STS | 43.51 (p<.0001) | -329.5 (p=.0001) | -1.93 | -2.73 to -0.83 | -246 (p<.0001) | -2.70 | -4.42 to -1.78 |
| FRT | 20.57 (p=.0004) | 269.5 (p=.0010) | 4.45 | 0.95 to 5.71 | 226 (p<.0001) | 3.81 | 0.95 to 6.67 |
| TUG | 39.63 (p<.0001) | -435.5 (p<.0001) | -0.67 | -1.39 to -0.43 | -260.5 (p<.0001 | -0.99 | -1.60 to -0.57 |
| BBS | 42.15 (p<.0001) | 328.5 (p<.0001) | 2.00 | 1.0 to 4.0 | 202 (p<.0001) | 1.50 | 1.0 to 5.0 |
| 6MWT | 10.50 (p=.033) | 351 (p=.0001) | 32.92 | 10.36 to 49.99 | 123.5 (p=.0614) | 16.76 | -6.25 to 50.90 |
| Walk speed | 24.61 (p<.0001) | -329.5 (p=.0001) | 0.18 | 0.07 to 0.28 | 251.5 (p<.0001) | 0.26 | 0.12 to 0.39 |
CI = confidence interval, mPPT = Modified Physical Performance Test, STS = 5 time sit to stand, FRT = Functional Reach Test, TUG = Timed Up and Go, BBS = Berg Balance Scale, 6MWT = 6 minute walk test, Walk speed = 50ft walking speed.
Figure.
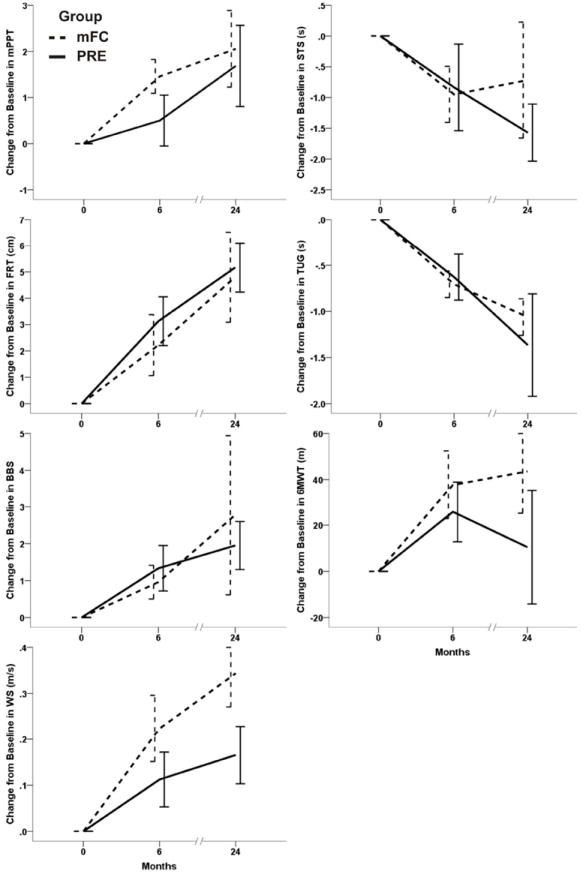
Change from baseline scores for all functional outcome measure in the 2 exercise groups. Data are mean on-medication change (± standard error) at 6 and 24 months for the modified Physical function test (mPPT), five time sit to stand performed as fast as possible (STS), Functional Reach Test (FRT), Timed Up and Go test (TUG), Berg Balance Scale (BBS), 6 minute walk test (6MWT), and walking speed (WS). Note: positive change scores on mPPT, BBS, 6MWT, FRT and walk speed represent improved functional performance while negative change scores on STS and TUG represent improved functional performance.
Results were similar when tested off medication except that the main effect of time on 6MWT was now significant such that all physical function measures were significantly improved across time. Post hoc testing of the off medication data showed that both short term (6 months) and long-term (24 months) improvements were significant in each of these measures with the exception of 6MWT which was significantly improved from baseline at 6 months only.
Short Term and Long-term Effects of PRE vs. mFC on General Functional Performance, Balance, and Functional Mobility
Comparing PRE and mFC groups, there was no difference between groups in change score from baseline at either 6 months or 24 months on any physical function measure when patients were tested either on or off medication (Table 3).
Discussion
Given the inevitable disease progression in PD, interventions that maintain or promote functional ability are essential in the management of individuals with PD to delay dependence. This study reports functional outcomes from the longest duration clinical PRE exercise trial to date in individuals with PD, the PRET-PD study. The findings showed that 24 months of exercise in individuals with PD significantly improved general functional performance as well as balance and functional mobility. The findings did not support different effects between the type of exercise. These findings were consistent whether patients were tested on or off medication, although the 6MWT was significant off medication and of borderline significance on medication. Taken together the results of this study suggest that long-term, structured exercise can positively impact multiple aspects of physical function in individuals with PD across two years. This positive impact occurred despite increasing dopaminergic medication for each group across two years as detailed in the primary paper21 which showed increasing L-dopa equivalent medication changes in both groups across time, but no between group differences at baseline or 24 months.
In the current study, long-term functional improvement was seen with structured, supervised exercise at both the short term (6 months) and the long-term (24 months) evaluations. The only other previous study to examine the longer term effects of exercise on function in PD was performed by Schenkman and colleagues9 who compared the effects of a flexibility, balance, and function exercise program, a supervised aerobic exercise program, and a home based exercise program utilizing the Fitness Counts exercise program on functional performance and aerobic endurance in individuals with early to mid-stage PD across 16 months. They found that while participants in the flexibility, balance, and function exercise group showed significant improvements in overall function as measured by the Continuous Scale – Physical Functional Performance measure at 4 months compared to the other two groups, this improvement was not maintained in the long-term at either 10 or 16 months. They also showed no between group differences in balance performance as measured by the functional reach test at either 4 or 16 months.
Differences in exercise prescription may in part explain why the current study found long-term functional changes with exercise while the Schenkman et al.9 study found no effect of 16 months of exercise on overall function. The two studies differed substantially in the type of exercises used, the number of weekly exercise sessions performed, the amount of exercise supervision provided, and the mode of long-term exercise supervision (i.e. individual versus group). The results of the current study support the conclusion that exercising with supervision 2 days per week for 6 months and 1 day per week for the remaining 18 months promotes achieving and maintaining functional improvements in patients with PD. Future studies should try to determine the levels and types of exercise supervision to promote cost effective and meaningful functional gains in individuals with PD. Future studies should also strive to investigate the optimal type of exercise to address specific motor and non-motor features of PD. While PRE has been shown to significantly improve strength and the motor signs of PD as measured by UPDRS-III compared to mFC21, the current study showed no difference between these interventions across multiple aspects of function in the same group of patients. Elucidating what aspects of the disease a specific type of exercise impacts and then examining combinations of exercises to target multiple aspects of the motor and non-motor deficits of PD will facilitate targeted exercise prescription at the individual patient level.
Whether the dose of PRE or mFC exercise used in the current study was sufficient to produce a clinically relevant improvement in gross motor function across 24 months remains less clear. While PRE has previously been shown to produce a statistically and clinical significant reduction in UPDRS-III scores compared to mFC21, it is not clear that this reduction in the UPDRS-III score will translate into important changes in scaled measures of gross motor function commonly used as outcome measures in individuals with PD. Our understanding of how function is changed by the natural course of PD in the absence of any intervention is a large gap in the current knowledge base which makes understanding of the significance of change scores difficult.46 If we more clearly understood the expected disease related decline in function, understanding long term changes in these measures would be more meaningful. In the meantime, the minimal detectable change (MDC), the smallest amount of difference in score that represents true change beyond random measurement error, can be used. Previous studies report MDCs in patients with PD for the FRT, TUG, BBS, 6MWT and walk speed, but not for the mPPT or STS. The suggested MDC for the FRT in patients with PD has a wide range of means from 4.0-11.5 cm45, 47 and results from the current study achieved that range in one instance (6 month off medication score of 4.45 cm, Table 2). Similarly, for the BBS, only the 6 month off medication change score was within the 2.0 – 5.0 range of MDC in the BBS that has been suggested for patients with PD.45, 47 In contrast, the largest TUG change score of 0.99 seconds at 24 months failed to achieve the recommended MDC of 1.63-11 seconds suggested for patients with mild to moderate PD.45, 47, 48 However, given that the TUG was modified to be performed as fast as possible in this study, this may limit application of MDC in this case. The highest median change in 6MWT in the current study was 34 meters (Table 2, 6 month off medication change) which is considerably lower than the suggested MDC in individuals with PD of 82 meters.45 In contrast, improvements in walking speed at 24 months in the current study of 0.22 m/s when tested on medication and 0.26 m/s when tested off medication are consistent with a suggested MDC for patients with PD of 0.19 m/s47- 0.25m/s45 despite instructions to walk as fast as possible.49 Ceiling effects at the higher levels of functioning were found for some patients in the current study. Since the MDC of functional outcome measures is sensitive to the starting functional ability of the participants such that higher functioning individuals will have a reduced capacity to show change in their functional outcome, relying solely on MDC for interpreting the significance of these findings may not be appropriate. Taken together, the results of the current study suggest that long-term exercise can affect some aspects of functional performance in patients with PD. However, the most conservative interpretation of the current data is that exercise across 24 months can prevent functional decline in patients with PD which, given the progressive nature of the disease, is noteworthy.
One strength of the current study is that participants were tested in both the off and on medication states. Individuals with PD experience significant fluctuations in their medication status throughout the day, and testing functional performance both off and on medication may be necessary to capture all the functional issues that a patient may face. Off medication testing has been shown to be important in some instances of functional testing such as examining predictive validity of the functional gait assessment and the TUG 50 However, testing off medication presents serious challenges for both patient and clinician. Results in functional outcomes in the current study were generally consistent whether patients were tested on or off medication. Therefore, for clinicians and researchers interested in documenting changes in functional performance in patients with PD with exercise, the results of the current study suggest that testing on medication, controlling for when the last dose of medication was taken as was done in this study, might be adequate.
The current study has some limitations. First, the study was powered on the primary outcome measure, and not on the secondary functional measures examined here. In addition, the lack of a no exercise group prevents us from examining how functional performance would have changed across time without intervention. Also, although subjects were expected to have completed a total of 208 exercise sessions at the end of 2 years, we did not have data to quantify the exact number of exercise sessions performed. Future studies may find that using an activity monitor or electronic verification of adherence will help to enable quantitative data collection on adherence. We also did not measure patient satisfaction to examine whether the functional performance changes seen here were meaningful to the patients themselves. Similarly, while assessments were performed in the laboratory setting to promote consistency and standardization of testing in a controlled environment, it may not reflect real life performance of the individuals in their home and community. Future studies should address measures of self-perceived and community functional improvements. Given the age range and the moderate stage of disease severity of the patients studied, application of these results to older individuals in more advanced stages of the disease is limited. The choice of functional performance measures used was guided by previous literature at the time of study development. However, the span of disease severity demonstrated by the patients in the study resulted in ceiling effects at the higher levels of functioning and several outliers for lower functioning patients, which prevented a parametric statistical analysis. These outliers were mainly due to instances of gait freezing during the dynamic assessments. More recent understanding of appropriate measures to assess functional outcomes in individuals with PD51 should be considered for future studies. These include measures such as the Mini-BESTest52 to assess balance and fall risk and the Functional Gait Assessment53 to test balance and mobility. The ceiling effects observed in some of the measures used in the current study such as the BBS and mPPT, and the fact that both groups of patients enrolled in this study were high functioning at baseline might have precluded detection of a differential effect between groups. It is possible that if more sensitive measures of function had been employed, or statistical power increased then a differential effect may have been exhibited between the groups on functional outcomes. However, after careful examination of the means and variance of the functional measures it seems unlikely that this is the explanation for the lack of between group effects for all of the measures. The results of this study suggest that both mFC and PRE exercise can benefit function in PD. Given that functional ability particularly in older adults requires not just muscle strength, but the ability to utilize muscle force in a timely and effective way54, 55, it is perhaps not surprising that PRE didn’t have a more pronounced effect on function in patients with PD than mFC. Future studies should strive to identify the best combination of exercises to address multiple aspects of functional performance in individuals with PD.
In conclusion, 24-months of supervised and structured exercise was effective at improving functional outcomes that span general functional performance, balance and functional ambulation in individuals with moderate PD. While PRE was previously shown to be significantly better than a non-progressive mFC program at improving the UPDRS-III scores, upper extremity muscle strength and movement speed21, the current study showed that both PRE and mFC performed under weekly supervision across 2 years can benefit functional performance of individuals with PD. Clinicians should strive to include structured and supervised exercise in the long-term plan of care for individuals with PD which should continue beyond any short term intervention
Table 3.
Between group treatment effects at 6 and 24 months.
| mFC Group vs. PRE Group at 6 months
|
mFC Group vs. PRE Group at 24 months
|
|||||
|---|---|---|---|---|---|---|
| Between group difference in median change from baseline(SE) | 95% CI for the median change | Pa | Between group difference in median change from baseline(SE) | 95% CI for the median change | P | |
|
|
|
|||||
| ON MEDICATION | ||||||
| mPPT | 1.0 (0.5) | -2.0 to 0.0 | .18 | 0.0 (0.8) | -2.0 to 1.0 | .74 |
| STS | 0.1 (0.7) | -1.2 to 1.5 | .89 | 0.6 (0.7) | -0.9 to 1.9 | .40 |
| FRT | 1.3 (1.7) | -4.4 to 2.2 | .46 | -1.6 (1.7) | -4.8 to 1.9 | .31 |
| TUG | 0.1 (0.3) | -0.6 to 0.4 | .80 | -0.1 (0.3) | -0.7 to 0.4 | .55 |
| BBS | 0.0 (0.3) | -1.0 to 0.0 | .66 | 0.0 (0.5) | -2.0 to 0.0 | .28 |
| 6MWT | 15.1 (17.9) | -21.6 to 48.5 | .31 | 24.5 (26.4) | -23.8 to 79.5 | .29 |
| Walk speed | 0.0 (0.1) | -0.1 to 0.3 | .49 | 0.2 (0.1) | -0.0 to 0.3 | .08 |
| OFF MEDICATION | ||||||
| mPPT | 1.0 (1.0) | -1.0 to 3.0 | .21 | -2.0 (1.0) | -4.0 to 0.0 | .10 |
| STS | -0.8 (0.8) | -2.4 to 0.9 | .33 | 1.3 (1.3) | -1.1 to 4.1 | .38 |
| FRT | -2.9 (1.8) | -6.0 to 0.9 | .10 | -2.2 (2.3) | -6.7 to 2.5 | .27 |
| TUG | 0.1 (0.4) | -0.7 to 0.8 | .96 | 0.5 (0.6) | -0.4 to 2.0 | .24 |
| BBS | 0.0 (0.8) | -2.0 to 1.0 | .59 | -1.0 (1.5) | -5.0 to 1.0 | .21 |
| 6MWT | 0.2 (19.2) | -40.1 to 35.2 | 1.0 | -48.9 (27.1) | -101.6 to 4.6 | .06 |
| Walk speed | -0.1 (0.1) | -0.3 to 0.1 | .29 | 0.2 (0.1) | -0.1 to 0.4 | 0.33 |
P-value from the Wilcoxon ranked sums test.
Median change = estimated median change score from baseline, SE = standard error, CI = confidence interval, PRE = progressive resistance exercise group, mFC – modified fitness counts group, mPPT = Modified Physical Performance Test, STS = 5 time sit to stand, FRT = Functional Reach Test, TUG = Timed Up and Go, BBS = Berg Balance Scale, 6MWT = 6 minute walk test, Walk speed = 50ft walking speed.
Acknowledgments
This study was supported by a grant from the National Institute of Neurological Disorders and Stroke (R01-NS28127). Additional work on the manuscript was supported by the Foundation for Physical Therapy (MR PODS-II) and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000050 (MR).
Contributor Information
Janey Prodoehl, Physical Therapy Program, Midwestern University, 555 31st Street, Downers Grove, IL 60515 (USA).
Miriam Rafferty, Department of Kinesiology and Nutrition and Graduate Program in Neuroscience, University of Illinois, Chicago, Illinois.
Fabian J. David, Department of Kinesiology and Nutrition, University of Illinois, Chicago, Illinois.
Cynthia Poon, Department of Kinesiology and Nutrition, University of Illinois, Chicago, Illinois.
David E. Vaillancourt, Department of Applied Physiology and Kinesiology, University of Florida, Gainesville, Florida.
Cynthia L. Comella, Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois.
Sue Leurgans, Rush Alzheimer’s Disease Center and Departments of Neurological Sciences & Preventive Medicine, Rush University Medical Center, Chicago, Illinois.
Wendy M. Kohrt, Division of Geriatric Medicine, University of Colorado School of Medicine, Aurora, Colorado.
Daniel M. Corcos, Departments of Kinesiology and Nutrition, Bioengineering, and Psychology, University of Illinois, Chicago, Illinois; Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois.
Julie A. Robichaud, Department of Kinesiology and Nutrition, University of Illinois, Chicago, Illinois.
References
- 1.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet neurology. 2006 Jun;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaki JM, Martin W, Suchowersky O, Weiner WJ, Lang AE. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002 Jan 8;58(1):11–17. doi: 10.1212/wnl.58.1.11. [DOI] [PubMed] [Google Scholar]
- 3.Pahwa R, Factor SA, Lyons KE, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006 Apr 11;66(7):983–995. doi: 10.1212/01.wnl.0000215250.82576.87. [DOI] [PubMed] [Google Scholar]
- 4.Weaver FM, Follett K, Stern M, et al. Bilateral Deep Brain Stimulation vs Best Medical Therapy for Patients With Advanced Parkinson Disease A Randomized Controlled Trial. Jama-J Am Med Assoc. 2009 Jan 7;301(1):63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suchowersky O, Gronseth G, Perlmutter J, et al. Practice Parameter: neuroprotective strategies and alternative therapies for Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006 Apr 1;66(7):976–982. doi: 10.1212/01.wnl.0000206363.57955.1b. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL. An exercise intervention to prevent falls in people with Parkinson’s disease: a pragmatic randomised controlled trial. Journal of neurology, neurosurgery, and psychiatry. 2011 Nov;82(11):1232–1238. doi: 10.1136/jnnp-2011-300919. [DOI] [PubMed] [Google Scholar]
- 7.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. The New England journal of medicine. 2012 Feb 9;366(6):511–519. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petzinger GM, Fisher BE, Van Leeuwen JE, et al. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25(Suppl 1):S141–145. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schenkman M, Hall DA, Baron AE, Schwartz RS, Mettler P, Kohrt WM. Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial. Physical therapy. 2012 Nov;92(11):1395–1410. doi: 10.2522/ptj.20110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keus SH, Bloem BR, Hendriks EJ, Bredero-Cohen AB, Munneke M Practice Recommendations Development G. Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Movement disorders : official journal of the Movement Disorder Society. 2007 Mar 15;22(4):451–460. doi: 10.1002/mds.21244. quiz 600. [DOI] [PubMed] [Google Scholar]
- 11.Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson’s disease: a systematic review across the disability spectrum. Journal of neurologic physical therapy : JNPT. 2009 Mar;33(1):14–26. doi: 10.1097/NPT.0b013e3181990fcc. [DOI] [PubMed] [Google Scholar]
- 12.Burini D, Farabollini B, Iacucci S, et al. A randomised controlled cross-over trial of aerobic training versus Qigong in advanced Parkinson’s disease. Europa medicophysica. 2006 Sep;42(3):231–238. [PubMed] [Google Scholar]
- 13.Shulman LM, Katzel LI, Ivey FM, et al. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA neurology. 2013 Feb;70(2):183–190. doi: 10.1001/jamaneurol.2013.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skidmore FM, Patterson SL, Shulman LM, Sorkin JD, Macko RF. Pilot safety and feasibility study of treadmill aerobic exercise in Parkinson disease with gait impairment. Journal of rehabilitation research and development. 2008;45(1):117–124. doi: 10.1682/jrrd.2006.10.0130. [DOI] [PubMed] [Google Scholar]
- 15.Kurtais Y, Kutlay S, Tur BS, Gok H, Akbostanci C. Does treadmill training improve lower-extremity tasks in Parkinson disease? A randomized controlled trial. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2008 May;18(3):289–291. doi: 10.1097/JSM.0b013e318170626d. [DOI] [PubMed] [Google Scholar]
- 16.Hackney ME, Earhart GM. Tai Chi improves balance and mobility in people with Parkinson disease. Gait & posture. 2008 Oct;28(3):456–460. doi: 10.1016/j.gaitpost.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Archives of physical medicine and rehabilitation. 2003 Aug;84(8):1109–1117. doi: 10.1016/s0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 18.Schenkman M, Cutson TM, Kuchibhatla M, et al. Exercise to improve spinal flexibility and function for people with Parkinson’s disease: a randomized, controlled trial. Journal of the American Geriatrics Society. 1998 Oct;46(10):1207–1216. doi: 10.1111/j.1532-5415.1998.tb04535.x. [DOI] [PubMed] [Google Scholar]
- 19.Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson’s disease: a comparison of Argentine tango and American ballroom. Journal of rehabilitation medicine : official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2009 May;41(6):475–481. doi: 10.2340/16501977-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabilitation and neural repair. 2012 Feb;26(2):132–143. doi: 10.1177/1545968311421614. [DOI] [PubMed] [Google Scholar]
- 21.Corcos DM, Robichaud JA, David FJ, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2013 Mar 27; doi: 10.1002/mds.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane database of systematic reviews. 2013;9 doi: 10.1002/14651858.CD002817.pub4. CD002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen NE, Canning CG, Sherrington C, et al. The effects of an exercise program on fall risk factors in people with Parkinson’s disease: a randomized controlled trial. Movement disorders : official journal of the Movement Disorder Society. 2010 Jul 15;25(9):1217–1225. doi: 10.1002/mds.23082. [DOI] [PubMed] [Google Scholar]
- 24.Ebersbach G, Ebersbach A, Edler D, et al. Comparing exercise in Parkinson’s disease--the Berlin LSVT(R)BIG study. Movement disorders : official journal of the Movement Disorder Society. 2010 Sep 15;25(12):1902–1908. doi: 10.1002/mds.23212. [DOI] [PubMed] [Google Scholar]
- 25.Wichmann R, Walde-Douglas M. Parkinson’s Disease: Fitness Counts. United States: National Parkinson Foundation, Inc.; 1998. [Google Scholar]
- 26.Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. The journals of gerontology Series A, Biological sciences and medical sciences. 2000 Jun;55(6):M350–355. doi: 10.1093/gerona/55.6.m350. [DOI] [PubMed] [Google Scholar]
- 27.Canadian Society for Exercise Physiology. Physical Activity Readiness Questionnaire-PAR-Q (revised 2002) [August 20, 2012]; http://uwfitness.uwaterloo.ca/PDF/par-q.pdf.
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.David FJ, Rafferty MR, Robichaud JA, et al. Progressive resistance exercise and Parkinson’s disease: a review of potential mechanisms. Parkinson’s disease. 2012;2012:124527. doi: 10.1155/2012/124527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cianci H. Parkinson’s Disease: Fitness Counts. 3. National Parkinson Foundation; 2006. [Google Scholar]
- 31.Feigenbaum MS, Pollock ML. Prescription of resistance training for health and disease. Medicine and science in sports and exercise. 1999 Jan;31(1):38–45. doi: 10.1097/00005768-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Pollock ML, Franklin BA, Balady GJ, et al. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation. 2000 Feb 22;101(7):828–833. doi: 10.1161/01.cir.101.7.828. [DOI] [PubMed] [Google Scholar]
- 33.Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7(1):2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 34.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994 Mar;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 35.Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. Journal of gerontology. 1990 Nov;45(6):M192–197. doi: 10.1093/geronj/45.6.m192. [DOI] [PubMed] [Google Scholar]
- 36.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. 1991 Feb;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 37.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Canadian Medical Association journal. 1985 Apr 15;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- 38.Schenkman M, Cutson TM, Kuchibhatla M, Chandler J, Pieper C. Reliability of impairment and physical performance measures for persons with Parkinson’s disease. Physical therapy. 1997 Jan;77(1):19–27. doi: 10.1093/ptj/77.1.19. [DOI] [PubMed] [Google Scholar]
- 39.Schenkman M, Ellis T, Christiansen C, et al. Profile of functional limitations and task performance among people with early- and middle-stage Parkinson disease. Physical therapy. 2011 Sep;91(9):1339–1354. doi: 10.2522/ptj.20100236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brusse KJ, Zimdars S, Zalewski KR, Steffen TM. Testing functional performance in people with Parkinson disease. Physical therapy. 2005 Feb;85(2):134–141. [PubMed] [Google Scholar]
- 41.Dibble LE, Hale TF, Marcus RL, Droge J, Gerber JP, LaStayo PC. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2006 Sep;21(9):1444–1452. doi: 10.1002/mds.20997. [DOI] [PubMed] [Google Scholar]
- 42.Schilling BK, Pfeiffer RF, Ledoux MS, Karlage RE, Bloomer RJ, Falvo MJ. Effects of moderate-volume, high-load lower-body resistance training on strength and function in persons with Parkinson’s disease: a pilot study. Parkinson’s disease. 2010;2010:824734. doi: 10.4061/2010/824734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Physical therapy. 2001 Feb;81(2):810–818. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- 44.Qutubuddin AA, Pegg PO, Cifu DX, Brown R, McNamee S, Carne W. Validating the Berg Balance Scale for patients with Parkinson’s disease: a key to rehabilitation evaluation. Archives of physical medicine and rehabilitation. 2005 Apr;86(4):789–792. doi: 10.1016/j.apmr.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Physical therapy. 2008 Jun;88(6):733–746. doi: 10.2522/ptj.20070214. [DOI] [PubMed] [Google Scholar]
- 46.Dibble LE, Cavanaugh JT, Earhart GM, Ellis TD, Ford MP, Foreman KB. Charting the progression of disability in Parkinson disease: study protocol for a prospective longitudinal cohort study. BMC neurology. 2010;10:110. doi: 10.1186/1471-2377-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim LI, van Wegen EE, de Goede CJ, et al. Measuring gait and gait-related activities in Parkinson’s patients own home environment: a reliability, responsiveness and feasibility study. Parkinsonism & related disorders. 2005 Jan;11(1):19–24. doi: 10.1016/j.parkreldis.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Huang SL, Hsieh CL, Wu RM, Tai CH, Lin CH, Lu WS. Minimal detectable change of the timed “up & go“ test and the dynamic gait index in people with Parkinson disease. Physical therapy. 2011 Jan;91(1):114–121. doi: 10.2522/ptj.20090126. [DOI] [PubMed] [Google Scholar]
- 49.Dibble LE, Hale TF, Marcus RL, Gerber JP, LaStayo PC. High intensity eccentric resistance training decreases bradykinesia and improves Quality Of Life in persons with Parkinson’s disease: a preliminary study. Parkinsonism & related disorders. 2009 Dec;15(10):752–757. doi: 10.1016/j.parkreldis.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Foreman KB, Addison O, Kim HS, Dibble LE. Testing balance and fall risk in persons with Parkinson disease, an argument for ecologically valid testing. Parkinsonism & related disorders. 2011 Mar;17(3):166–171. doi: 10.1016/j.parkreldis.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kegelmeyer D, Terry Ellis T, Esposito A, et al. Taking The Step Over The Edge: How To Apply The Recommendations For Use Of Outcome Measures In PD. Paper presented at: Combined Sections Meeting, American Physical Therapy Association; Las Vegas, NV. Feb 02, 2014. [Google Scholar]
- 52.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. Journal of rehabilitation medicine : official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2010 Apr;42(4):323–331. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Physical therapy. 2004 Oct;84(10):906–918. [PubMed] [Google Scholar]
- 54.Butler AA, Lord SR, Rogers MW, Fitzpatrick RC. Muscle weakness impairs the proprioceptive control of human standing. Brain research. 2008 Nov 25;1242:244–251. doi: 10.1016/j.brainres.2008.03.094. [DOI] [PubMed] [Google Scholar]
- 55.Muehlbauer T, Besemer C, Wehrle A, Gollhofer A, Granacher U. Relationship between strength, power and balance performance in seniors. Gerontology. 2012;58(6):504–512. doi: 10.1159/000341614. [DOI] [PubMed] [Google Scholar]


