Abstract
During metastasis, melanoma cells must be sufficiently deformable to squeeze through extracellular barriers with small pore sizes. We visualize and quantify deformability of single cells using micropipette aspiration and examine the migration potential of a population of melanoma cells using a flow migration apparatus. We artificially stiffen the nucleus with recombinant overexpression of Δ50 lamin A, which is found in patients with Hutchison Gilford progeria syndrome and in aged individuals. Melanoma cells, both WM35 and Lu1205, both show reduced nuclear deformability and reduced cell invasion with the expression of Δ50 lamin A. These studies suggest that cellular aging including expression of Δ50 lamin A and nuclear stiffening may reduce the potential for metastatic cancer migration. Thus, the pathway of cancer metastasis may be kept in check by mechanical factors in addition to known chemical pathway regulation.
Keywords: Laminopathy, metastasis, cell mechanics
Introduction
Metastasis is currently the primary cause of death among cancer patients. The process includes a series of well-orchestrated steps including initial growth from the primary tumor followed by tumor cell intravasation, circulation, adhesion, extravasation and growth of a secondary tumor.(1) The movement and migration of cells from the circulation through the extracellular matrix (ECM) requires modulation of cell mechanics and deformation of subcellular regions.(2) Specifically, we are interested in the deformability of metastatic melanoma cancer cell lines, or the “migration potential” of WM35 and Lu1205.(3; 4) WM35 is a radial growth phase melanoma, wherein the tumor grows along the skin surface rather than in the vertical direction into the blood vessels; these melanomas cannot undergo metastatic events. Lu1205 is a vertical growth phase melanoma cell with cells below the surface and entering into blood vessels; these melanomas are more invasive and are capable of undergoing metastatic events.(5) As such, WM35 is less invasive than Lu1205, but some mutations (eg. B-raf) are similar between the two cell lines.(6) These cell lines have been well characterized for their in vivo metastatic potential with clinical relevance and drug-therapeutic interventions.(7; 8) Generally, invasive metastatic cancer cells are less stiff than cells of the primary tumor,(9) and melanoma motility correlates with low stiffness in vitro.(10) In addition to motility, reduced stiffness may impact adhesion and cell deformability related to extra- and intra-vasation.
Recently, nuclear structure and mechanics has been identified to be important in cancer metastasis.(11) The cell nucleus is the largest and stiffest organelle in the cell, particularly when the cytoskeleton is capable of rapid turnover, as in cancer cells.(12) The increase in the migration potential of cancer cells correlates with a higher ability of cells to squeeze through tight spaces.(13) The length scale of the nucleus can be larger than extracellular barriers(14; 15), and the nucleus has been suggested to be a limiting mechanical factor in allowing cancer extravasation and metastatic tumor formation.(16; 17) The mechanical properties of the nucleus are strongly modulated by the lamina nucleoskeleton, which is primarily located at the inner nuclear envelope and is mainly composed of type V intermediate filaments called lamins. In some cancers lamin concentration and posttranslational modifications vary, and modulation of lamin levels can alter migration of cancer cells.(11; 18)
Mutations in lamin A are also associated with altered nucleoskeletal mechanics and a variety of different diseases.(19; 20) A cryptic splice variant of the LMNA gene causes production of Δ50 lamin A (Δ50LA). Normally, small amounts of this variant are produced, and significant amounts of accumulated Δ50LA are found only with advanced age.(21; 22) A rare, de novo DNA mutation in LMNA causes an enhanced production of Δ50LA, which leads to the premature aging disorder Hutchison Gilford progeria syndrome. In addition to the loss of 50 amino acids (exon 11) from the lamin A tail region and a slightly altered structure,(23) Δ50LA retains a C-terminal farnesyl lipid moiety that enhances membrane association with the inner nuclear membrane.(24) Expression of Δ50LA is associated with increased thickness of the nucleoskeleton as well as increased nucleoskeletal stiffness and reduced nuclear deformation in cultured cells.(25)
In this study, we use melanoma cell lines with varying metastatic capacities to quantify how manipulation of nuclear mechanical properties affects overall cellular deformation and motility through confined spaces. Previous studies have shown that reduction of lamin A increases transmigration of cancer cells.(18) We show the converse: that effective stiffening of the nucleoskeleton by overexpression of Δ50LA prevents deformation of the nucleus through small regions, which also correlates with reduced cell migration.
Results
To quantify the migration potential of WM35 and Lu1205, we adapted an in vitro flow-pore assay to measure the cell's ability to (i) escape from flow, (ii) translocate through the endothelial layer and (iii) crawl into tight interstitial spaces (schematic in Figure 1A). Previously, studies using this flow migration chamber have shown the importance of adhesion (by αv adhesion molecules) and subsequent transendothelial migration in cancer metastasis.(26) Theoretical in vitro flow migration results have been validated using in vivo models.(8; 27)
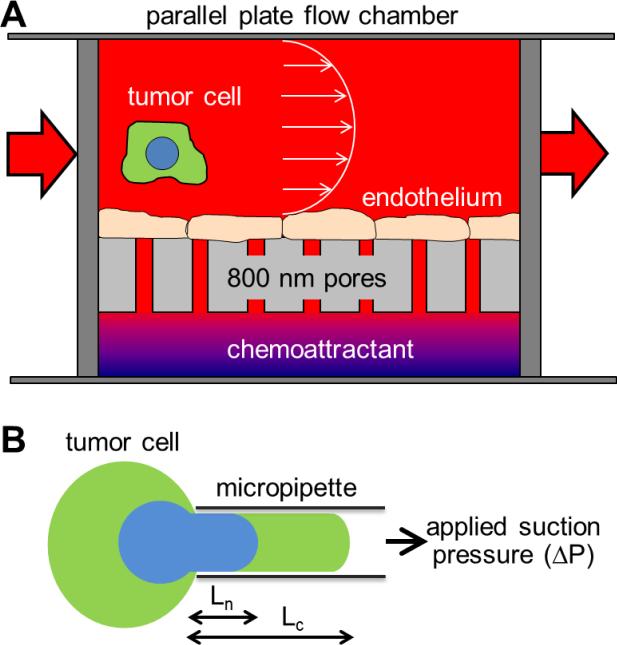
Figure 1. Schematic of experimental apparati used for this study.
(A) Migration potential was measured with a parallel plate flow chamber above a Boyden chamber with layer of endothelial cells cultured in between. Tumor cells circulate through the flow chamber, and metastatic cells are able to adhere to the endothelial cells, pass through endothelial cells, and migrate through the 8 μm pores toward the chemoattractant. Cells able to reach the bottom of the appartus are then counted by microscopy. (B) Deformability of individual cells and subcellular structures are measured by micropipette aspiration. Suspended cells are aspirated into a glass micropipette by applied suction pressure. By imaging the cell and nucleus independently, relative deformability can be measured.
We mimicked flow through post capillary venules by culturing a layer of endothelial cells under a parallel plate flow chamber and on top of the polycarbonate surface of a modified 48-well Boyden chamber with 8 μm pores. Below the pores, soluble collagen IV was added as a chemoattractant for cells. We measured the number of cells able to migrate to the bottom surface over 4 hours under low shear stress (0.625 dyn/cm2). Similar to previous reports of migration potential,(8; 28) we found 44 ± 2 and 105 ± 15 cells per field of view for WM35 and Lu1205, respectively (compared with experimental data later in Figure 4C). As expected, the more metastatic Lu1205 cells showed a statistically higher degree of cellular migration.
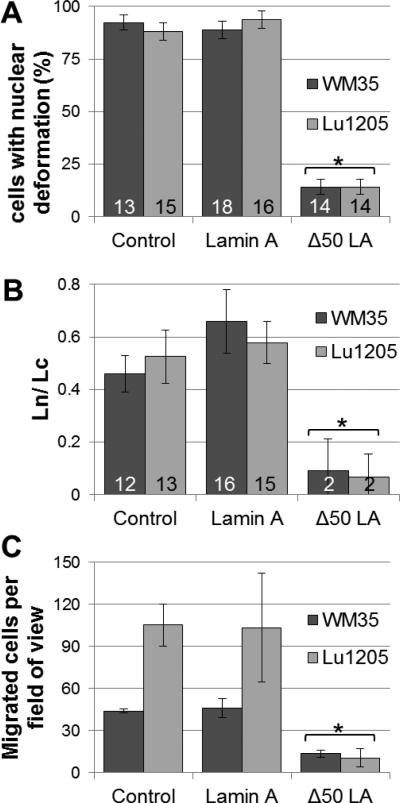
Figure 4. Stiffening nuclei with Δ50LA causes altered cellular deformation.
(A) The ability of the nucleus to deform into the micropipette while cells are being aspirated is given a binary value, and percent of cells with nuclear deformability is quantified. Few cells expressing Δ50LA show nuclear deformation into the micropipette compared with nearly 100% for untransfected control cells or cells exogenously expressing lamin A. (n is the number of cells measured per sample) (B) For cells that show a nuclear deformation into the pipette, the ratio of Ln/Lc is much lower for Δ50LA expressing cells. (n is the number of cell with multiple measurements per cell) (C) The expression of Δ50LA severely reduced the migration potential, calculated as the number of cells which can escape from the circulation domain through the endothelial later and through the pores of a polycarbonate filter (Figure 1A). Statistical differences from control (* is p < 0.05 from relevant control).
To remove contributions from cellular adhesion and force generation, we measured the deformability of individual live cells using micropipette aspiration. Micropipette aspiration simulates the high strain deformation experienced by cancer cells invading extracellular matrix environments with micrometer size scales. There are numerous methods to mechanically characterize cells including microparticle tracking, magnetic twisting cytometry and atomic force microscopy.(29) However, micropipettes allow for simultaneous visualization of different subcellular features during cell deformation.(30; 31) Nuclei can easily be visualized in live cells using the membrane permeable DNA dye Hoeschst 33342. From visualization of deformation of the cell membrane (Lc) and nucleus (Ln), we are able to measure cell deformation and the contribution of the nucleus (Figures 1B and 2).
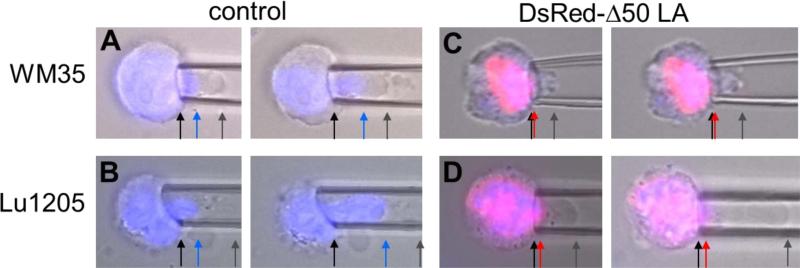
Figure 2. Imaging during micropipette aspiration of cells shows nuclear and cellular deformation.
Representative overlayed images of cells (brightfield, blue nucleus and DsRed-Δ50LA) with increasing strain into a micropipette. Cells were aspirated at constant pressure and images from the left panel to the right were taken with 60-120 sec of additional creep. Arrows have been added to highlight boundaries. (A) Aspiration of WM35 cells shows that the cell and nucleus are able to deform into the micropipette. Cell deformation (gray arrow) is longer than nuclear deformation (blue arrow). (B) The nucleus of Lu1205 cells appears more deformable with the cell. (C) Expression of DsRed-Δ50LA prevents the nucleus from entering the pipette (red arrow). In WM35 cells, the cell also showed reduced deformability. (D) Lu1205 cells were able to deform, but the DsRed-Δ50LA-stiffened nucleus was unable to deform into the pipette. For length scale, micropipettes of 5-8 μm inner diameter were used.
With increasing time after fixed aspiration pressure through the micropipette, we observe the cell deforming into the pipette (Figure 2, 3). In both the WM35 and Lu1205 cases, we observed that the cell membrane and other cellular structures deform 14 ± 2 μm (p = 0.08 between WM35 and Lu1205) into the pipette before the portion of the cell containing the nucleus enters the pipette (Figure 3, x-axis). The initial deformation of the nucleus into the pipette is higher for the WM35 than for Lu1205 (Figure 3, WM35 data above Lu1205 data). This slightly higher minimum strain may be a function of nucleoskeletal mechanics or intracellular connections. Following this minimal strain, the nucleus of Lu1205 flows much more easily than WM35, as shown by the slope of nuclear deformation normalized to cellular deformation, Ln/Lc (Figure 3, inset). This suggests that after the initiation of deformation the nuclei in the Lu1205 are more easily able to deform through the small spaces. This higher contribution of the nucleus to cell deformability can also be visualized in Figure 2B versus 2A.
Figure 3. Cell and nuclear deformation during micropipette aspiration.
For four cells of each condition (each shown in a different color), more than 10 measurements per cell show increasing deformation of the cell (Lc) matched with increasing deformation of the nucleus (Ln). The linear slopes, Ln versus LC, are determined for each cell and all have an r2 > 0.9. The averages of these slopes (Ln/Lc) are shown in the inset with standard deviation error. Nuclei in Lu1205 cells deform more readily than in WM35 suggesting different contributions of subcellular structures to cellular deformability.
We stiffened the nucleoskeleton with the exogenous overexpression of DsRed Δ50LA (see Methods and Supplemental Figure 1). Previously, we have shown in cells expressing Δ50LA, both exogenous and from patients with Hutchison Gilford progeria syndrome, that they have stiffer nuclei and less adaptable to deformation than control cells.(25) As expected, the nuclei of both WM35 and Lu1205 cells expressing Δ50LA were significantly less deformable than untransfected control cells (Figure 2C,D). With the Δ50LA-stiffened nuclei, fewer nuclei were able to enter the pipette (Figure 2, 4A). That is, in most cells the nucleus did not deform into the pipette at all despite the cell deforming with increased aspiration pressure. Of the nuclei that were able to enter the pipette, the nuclei only deformed slightly, represented by the ratio Ln/Lc, compared with the control (Figure 4B). The data for Ln/Lc appear slightly different between figure 3 and figure 4B. Figure 3 represents the stepwise data of 4 cells for each condition with >10 measurements per cell with increasing aspiration. For figure 4, a large number of cells (>13 cells per condition) were examined with 1-2 aspiration lengths per cell for comparison of control cells with Δ50LA expressing cells.
Once we determined changes in single cell deformability, we re-examined metastasis using the flow chamber setup from Figure 1. Similar to the single cell experiments, we observed a statistical reduction in migration potential of both WM35 and Lu1205 cells with the overexpression of Δ50LA (Figure 4C). However, in every experimental condition the overexpression of lamin A had no statistical effect compared with untransfected control cells (Figure 4A, B, C and see Discussion).
Discussion
Cancer arises from genetic instability and expression of oncogenes, which include genes that regulate cell motility, migration, proliferation, and survival.(32) Measuring changes in the expression of these oncogenes can be used for cancer detection and mapping of intracellular protein pathways involved with cancer progression.(1; 33; 34) Simultaneously, nuclear structure and morphology are usually altered in cancer cells.(35) Nuclear morphology serves as a key diagnostic tool to identify the presence and type of cancer,(36; 37) and lamins are differentially expressed and localized in some cancers.(38; 39) Further, the altered nuclear architecture may allow for the increased deformability of the cancer cell as indicated by our results. Studies of cancer cell mechanics suggest that most cancer cells are more deformable than healthy cells and stiffness is related with their metastatic potential.(36) Obviously, a deformable nucleus allows for cellular deformation and migration through tight interstitial spaces.(40) Also, our previous work has shown that melanoma deformability enhances adhesion to endothelial cells under shear flow by increasing contact area.(41; 42)
While expression of exogenous wild type lamin A had little effect on migration of melanoma cells, expression of Δ50LA decreased migration potential of both WM35 and Lu1205 cells through a layer of endothelial cells by nearly 80% under low shear stresses. We were surprised that the exogenous overexpression of lamin A had no effect, but we suggest that the added lamins were insufficient to determine differences using these methodologies; perhaps more sensitive biophysical characterization would yield a statistical difference. Expression of Δ50LA pathologically increases the stiffness of the nucleoskeleton(25) resulting in stiffening and reduced responsiveness of the nucleus.(43) Overexpression of Δ50LA generally results in over-accumulation of lamins within the nucleoskeleton as well as other structural nuclear, cytoplasmic and cytoskeletal defects.(25; 44; 45) Alteration of nuclear structure and stiffness with the expression of Δ50LA may impact other cellular processes including intracellular force generation and transduction, cell adhesion and gene expression. Our own work in fibroblasts has shown that expression of Δ50LA reduces motility of cells through tight spaces from an increased nuclear resistance and from decreased actin-myosin force generation.(40) Independent of nuclear structure, altered cytoskeletal organization mediated by substrate stiffness has been shown to affect the movement of tumor cells into tight interstitial spaces.(15) Thus, the coupled intracellular mechanisms of invasiveness and motility as they relate to resistance and driving force of cell movement will need to be further elucidated. However, we find that nuclear deformability is drastically impacted in single suspended cells with the expression of Δ50LA measured by micropipette aspiration where internal cytoskeletal stress fibers are lost.(46) Thus, micropipette aspiration measures cell deformation independent of adhesion and force generation. This agrees with previous studies of nuclear stiffening with the expression of Δ50LA found in isolated nuclei.(25)
In measuring bulk migration potential we observe large differences in invasion between control Lu1205 and WM35 cells that are less obvious in single cell deformability assays. The increased invasion of the Lu1205 over WM35 is consistent with the increased metastatic pathogenesis described in the introduction. There is a small increase in the ability of Lu1205 nuclei to deform within cells after a minimum strain compared with WM35 (quantified as Ln/Lc in Figure 3), but the differences from applied extracellular forces are substantially less than the bulk migration assay. As such, we suggest that the cell-type differences in bulk invasion and transmigration between Lu1205 and WM35 cells may reflect altered adhesion, force generation, etc. Flow cytometry studies by Zhang et al. have shown lower expression of adhesion molecules such as ICAM-1 and αvβ3 on WM35 cells compared to Lu1205 melanoma cells.(3) Collectively, these results show that there is a change in cell migration between these WM35 and Lu1205 cell lines dependent on the expression of adhesion molecules. However, modulating nuclear stiffness with Δ50LA reduces invasiveness of both cell types in our migration assay. Interestingly, we see that differences in invasion and migration observed between Lu1205 and WM35 are lost with the expression of Δ50LA. We suggest that the motility is so greatly attenuated with the expression of Δ50LA that differences between cell types and the contributions of adhesion are lost.
Expression of Δ50LA, which causes the premature aging disease Hutchison Gilford progeria syndrome, is not a viable treatment option unless it can be activated in only cancer cells. We suggest that other mechanisms of nuclear stiffening may inhibit cancer cell migration: many DNA intercalating drugs are currently available to treat cancer by preventing or reducing DNA replication. These drugs may also be screened for the possibility of stiffening nuclei and reducing invasion and transmigration.
Beyond possible treatment options, we speculate that these results may suggest some rational for the low expression of Δ50LA that occurs in individuals without HGPS during natural aging, albeit at a lower rate than in patients with HGPS.(22) Decreased metastasis can be statistically associated with old age,(47) but the impact of age in cancer progression is still a subject of high controversy and may depend on type of cancer.(47) Previous reviews have reported that expression of the Δ50LA variant in nature is a cryptic error and serves no function, and lamin A appears to be expendable to some degree.(48) However, we suggest that the nuclear stiffening that is associated with aging may serve some purpose. DNA is constantly being mutated by replication errors and mutagens, and although there are molecular and cellular checkpoints to correct for these mutations, the probability of a cancer-causing error occurring increases with time. Thus, like other aspects of cellular aging (including telomere length decrease, senescence pathways, etc.(49)), Δ50LA accumulation may be part of cellular programming. We are hypothesizing that the accumulation of the Δ50LA splice variant with age and subsequent nuclear stiffening may be playing a role to reduce risks or severity of metastatic cancers. However, this hypothesis is extraordinarily difficult to test since accumulation of Δ50LA in normal individuals takes decades to accumulate,(22) which is beyond the scope of any experiment or reasonable animal model.
Materials and methods
Cell culture
Low passage WM35 cells were kindly provided by Dr. Meenhard Herlyn (Wistar Institute)(6) and were maintained in Roswell Park Memorial Institute 1640 medium (RPMI 1640; Biosource, Inc.). Lu1205 metastatic melanoma cell lines were kindly provided by Dr. Gavin Robertson (Penn State Hershey Medical Center)(50) and maintained in Dulbecco's modified Eagle's medium (Life Technologies). HUVEC (Human umbilical vein endothelial cells) were obtained from American Type Culture Collection (ATCC) and maintained in F12-K medium supplemented with 30 μg/ml of endothelial cell growth factors and 50 μg/ml heparin (Mallinckrodt Baker). All media was supplemented with 10 % fetal bovine serum (FBS; Biosource) and 100 units/ml penicillin-streptomycin (Biosource).
Transfection with cDNAs
WM35 and Lu1205 were transfected with DsRed tagged lamin cDNA: lamin A or Δ50LA. GFP-plasmids,(25) a kind gift of Tom Misteli (NCI NIH) were subcloned into the DsRed plasmid (Clontech) and confirmed via sequencing. Cells were transfected with Trans-It 2020 transfection reagent (Mirus) as per the manufacturer's instructions. At 48 hours post transfection, images of 20-40 transfected nuclei at 63x were obtained for adherent cells. Projected area of nuclei was compared between DsRed lamin A and DsRed Δ50LA to see if nuclear size was altered with expression (Supplemental Figure 1A). Nuclear area was statistically similar between DsRed lamin A and DsRed Δ50LA for each cell type (p>0.1 by Students t-test). Mean fluorescence intensity of the DsRed from DsRed lamin A or DsRed Δ50LA expressed in the Lu1205 or WM35 was normalized to the background (in ImageJ). Expression levels of DsRed were always at statistically similar levels between experiments (p>0.1 for normalized intensity by Students t-test), and expression did not vary greatly between samples (Supplemental Figure 1B). To test proper lamin localization, we imaged nuclei segments across nuclei (Supplemental Figure 1C, inset) and examined fluorescence intensity along the line for greater than 10 nuclei (Supplemental Figure 1C). All lamins localized to the nucleus, at the nuclear lamina, and there was no dramatic change in lamin localization between the samples examined (Supplemental Figure 1D).
Flow migration assay
We have previously described and characterized the flow migration assay in detail.(4; 8; 26; 27; 51) Briefly, HUVECs were grown to confluence on fibronectin-coated 6-10 μm deep polycarbonate filters (NeuroProbe). See Supplemental Figure 2 for characterization of the monolayer. The wells on the bottom plate of the chamber were filled with HUVEC media with 2% bovine serum albumin (BSA) except for the middle 12 wells, which were coated with soluble collagen type IV (BD Bioscience antibody 354233, 100 μg/ml, optimized previously(52)). Collagen IV was found to be a specific chemoattractant to melanoma cells and, unlike other chemoattractants such as serum, does not affect the migration of endothelial cells layered on the top part of polycarbonate filters.(52) The apparatus was assembled as in Figure 1 with cells on the filter in between a flow chamber and collagen-filled wells. The assembled migration chamber was placed in a flow loop and cells in media with 2.5 × 106 melanoma cells were injected into the flow migration chamber at 0.625 dyn/cm2. The flow rate was calculated to produce the desired fluid shear stress using parallel plate flow equation.(53) Cells and media were immediately perfused into the chamber, initially at a flow rate 2 mL/min, which was then increased to a desired experimental rate (0-20 mL/min). The entire chamber was placed in a 37°C, 5% CO2 incubator.
After running flow migration experiments at 37°C for 4 hr, the number of melanoma cells that migrated through the endothelial layer onto the bottom of the filter was counted on an inverted fluorescent microscope (Nikon microscope and EZ Coolsnap camera). One field of view from each of the 12 wells was imaged using a 10X objective (Supplemental Figure 3). Results were averaged from the 12 wells and reported as cells per field of view; roughly cells per 0.5 mm2.
Micropipette experiments and imaging
Live cells were trypsinized and resuspended in fresh media for more than 30 minutes and less than 2 hours for aspiration. Cell suspensions in coverslip-bottom dishes (Mattek) were maintained in a large heated chamber with 5% CO2 and humidity control (PeCon and Leica) on an inverted microscope (Leica DMI6000) using a 63x (1.4 NA) oil immersion objective. 1 μg/mL each of Hoeschst 33342 and propidium iodide (Life Technologies) was added to the media to label the nucleus and test for membrane integrity, respectively. Images were analyzed using the Leica AF6000 software and ImageJ.
Micropipette aspiration technique
Micropipettes were pulled from capillary tubes (ID of 1 mm from World Precision Instruments) using a micropipette puller (PMP-102 MicroData Instruments). Pipettes of inner diameter 5 – 8 μm were used with aspiration pressures of 0.2 – 2 kPa to induce different levels of strain. In every case, data was only considered in which the initial diameter of the cell was 1.5 - 2.5 times the diameter of the pipette and the length of the cell, Lc, was greater than the radius of the pipette. We were focused primarily on the relative deformability of subcellular components with respect to one another rather than the overall mechanical properties of the cell. As such, we did not record the absolute pressure. For suspended cells, there were not obvious differences in nuclear or cellular size, similar to adhered cells (Supplemental Figure 1). As we have shown previously, cell diameters above 2.5x the size of the pipette only impacts the local region; below 1.5, cells are aspirated into the pipette and do not adequately deform.(31) Variations in mechanics of nuclei,(25) cells(31) and cell aggregates(54) measured by MPA do not correlate significantly with variations of pipette size and aspiration pressures within the range used in this study.
The inside and the outside of the pipette were passivated with 1% filtered BSA. The back-filled pipette was connected via a Narishige micromanipulation system in parallel with a water-filled manometer connected to a pressure transducer (World Precision Instruments). Initially, the individual cell and pipette were aligned in the absence of pressure. Aspiration pressure was applied and held constant. Imaging was initiated immediately after setting the constant pressure. The images were acquired first every 5 s for 20-30 s and for every 10 s after that for 90-120 s. Images were acquired in brightfield and fluorescent modes for propidium iodide and Hoechst 33342. DsRed was also captured with the propodium iodide channel in the case of transfected cells. For analysis, only cells that did not contain red fluorescent DNA (propidium iodide) throughout the experiment were used; propidium iodide positive cells were rarely observed. Micropipette aspiration experiments were run by multiple investigators on different days with different passage cells.
Statistical analysis
Statistical comparisons were performed using Student's t-test for 2 comparisons or via one-way ANOVA for multiple samples. Results represent at least 3 independent experiments and are shown as averages ± SEM. Results were considered significant at p < 0.05.
Supplementary Material
Acknowledgements
We kindly acknowledge funding from the NIH (CA-125707 to CD) and NSF (CBET 0954421 CAREER to KND).
Footnotes
A.J.S.R., P.K., A.S., C.D. and K.N.D. declare they have no conflicts of interest. No human or animal studies were carried out by the authors for this article.
References
- 1.Liotta LA. Cancer cell invasion and metastasis. Sci Am. 1992;266:54–9. 62–3. doi: 10.1038/scientificamerican0292-54. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–27. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang P, Ozdemir T, Chung CY, Robertson GP, Dong C. Sequential binding of alphaVbeta3 and ICAM-1 determines fibrin-mediated melanoma capture and stable adhesion to CD11b/CD18 on neutrophils. J Immunol. 2011;186:242–54. doi: 10.4049/jimmunol.1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng HH, Liang S, Henderson AJ, Dong C. Regulation of interleukin-8 expression in melanoma-stimulated neutrophil inflammatory response. Exp Cell Res. 2007;313:551–9. doi: 10.1016/j.yexcr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowson AN, Magro CM, Mihm MC. Prognosticators of melanoma, the melanoma report, and the sentinel lymph node. Mod Pathol 19 Suppl. 2006;2:S71–87. doi: 10.1038/modpathol.3800517. [DOI] [PubMed] [Google Scholar]
- 6.Karasic TB, Hei TK, Ivanov VN. Disruption of IGF-1R signaling increases TRAIL-induced apoptosis: a new potential therapy for the treatment of melanoma. Exp Cell Res. 2010;316:1994–2007. doi: 10.1016/j.yexcr.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70:6071–82. doi: 10.1158/0008-5472.CAN-09-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang S, Sharma A, Peng HH, Robertson G, Dong C. Targeting mutant (V600E) B-Raf in melanoma interrupts immunoediting of leukocyte functions and melanoma extravasation. Cancer Res. 2007;67:5814–20. doi: 10.1158/0008-5472.CAN-06-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swaminathan V, Mythreye K, O'Brien ET, Berchuck A, Blobe GC, Superfine R. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011;71:5075–80. doi: 10.1158/0008-5472.CAN-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe T, Kuramochi H, Takahashi A, Imai K, Katsuta N, et al. Higher cell stiffness indicating lower metastatic potential in B16 melanoma cell variants and in (-)-epigallocatechin gallate-treated cells. J Cancer Res Clin Oncol. 2012 doi: 10.1007/s00432-012-1159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denais C, Lammerding J. Nuclear mechanics in cancer. Advances in experimental medicine and biology. 2014;773:435–70. doi: 10.1007/978-1-4899-8032-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11:512–22. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou HW, Li QS, Lee GY, Kumar AP, Ong CN, Lim CT. Deformability study of breast cancer cells using microfluidics. Biomed Microdevices. 2009;11:557–64. doi: 10.1007/s10544-008-9262-8. [DOI] [PubMed] [Google Scholar]
- 14.Polacheck WJ, Zervantonakis IK, Kamm RD. Tumor cell migration in complex microenvironments. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci U S A. 2012;109:10334–9. doi: 10.1073/pnas.1118073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 2011;23:55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds NH, Ronan W, Dowling EP, Owens P, McMeeking RM, McGarry JP. On the role of the actin cytoskeleton and nucleus in the biomechanical response of spread cells. Biomaterials. 2014;35:4015–25. doi: 10.1016/j.biomaterials.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 18.Harada T, Swift J, Irianto J, Shin JW, Spinler KR, et al. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. The Journal of cell biology. 2014;204:669–82. doi: 10.1083/jcb.201308029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:a000760. doi: 10.1101/cshperspect.a000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchison CJ, Alvarez-Reyes M, Vaughan OA. Lamins in disease: why do ubiquitously expressed nuclear envelope proteins give rise to tissue-specific disease phenotypes? J Cell Sci. 2001;114:9–19. doi: 10.1242/jcs.114.1.9. [DOI] [PubMed] [Google Scholar]
- 21.Scaffidi P, Gordon L, Misteli T. The cell nucleus and aging: tantalizing clues and hopeful promises. PLoS Biol. 2005;3:e395. doi: 10.1371/journal.pbio.0030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–63. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin Z, Kalinowski A, Dahl KN, Buehler MJ. Structure and stability of the lamin A tail domain and HGPS mutant. Journal of structural biology. 2011;175:425–33. doi: 10.1016/j.jsb.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalinowski A, Qin Z, Coffey K, Kodali R, Buehler MJ, et al. Calcium causes a conformational change in lamin A tail domain that promotes farnesyl-mediated membrane association. Biophysical journal. 2013;104:2246–53. doi: 10.1016/j.bpj.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:10271–6. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voura EB, Ramjeesingh RA, Montgomery AM, Siu CH. Involvement of integrin alpha(v)beta(3) and cell adhesion molecule L1 in transendothelial migration of melanoma cells. Mol Biol Cell. 2001;12:2699–710. doi: 10.1091/mbc.12.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanna P, Chung CY, Neves RI, Robertson GP, Dong C. CD82/KAI expression prevents IL-8-mediated endothelial gap formation in late-stage melanomas. Oncogene. 2013 doi: 10.1038/onc.2013.249. [DOI] [PubMed] [Google Scholar]
- 28.Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Physiol Cell Physiol. 2005;288:C831–9. doi: 10.1152/ajpcell.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman BD, Crocker JC. Cell mechanics: dissecting the physical responses of cells to force. Annu Rev Biomed Eng. 2009;11:259–88. doi: 10.1146/annurev.bioeng.10.061807.160511. [DOI] [PubMed] [Google Scholar]
- 30.Dong C, Skalak R, Sung KL, Schmid-Schonbein GW, Chien S. Passive deformation analysis of human leukocytes. J Biomech Eng. 1988;110:27–36. doi: 10.1115/1.3108402. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro AJ, Tottey S, Taylor RW, Bise R, Kanade T, et al. Mechanical characterization of adult stem cells from bone marrow and perivascular niches. Journal of biomechanics. 2012;45:1280–7. doi: 10.1016/j.jbiomech.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCaffrey LM, Macara IG. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 2011;21:727–35. doi: 10.1016/j.tcb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Vasko V, Saji M, Hardy E, Kruhlak M, Larin A, et al. Akt activation and localisation correlate with tumour invasion and oncogene expression in thyroid cancer. J Med Genet. 2004;41:161–70. doi: 10.1136/jmg.2003.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakraborty AK, Kolesnikova N, Sousa Jde F, Espreafico EM, Peronni KC, Pawelek J. Expression of c-Met proto-oncogene in metastatic macrophage x melanoma fusion hybrids: implication of its possible role in MSH-induced motility. Oncol Res. 2003;14:163–74. doi: 10.3727/000000003771013062. [DOI] [PubMed] [Google Scholar]
- 35.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004;4:677–87. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 36.Gal N, Weihs D. Intracellular mechanics and activity of breast cancer cells correlate with metastatic potential. Cell Biochem Biophys. 2012;63:199–209. doi: 10.1007/s12013-012-9356-z. [DOI] [PubMed] [Google Scholar]
- 37.Bista RK, Uttam S, Hartman DJ, Qiu W, Yu J, et al. Investigation of nuclear nano-morphology marker as a biomarker for cancer risk assessment using a mouse model. J Biomed Opt. 2012;17:066014. doi: 10.1117/1.JBO.17.6.066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z, Wu L, Weng D, Xu D, Geng J, Zhao F. Reduced expression of lamin A/C correlates with poor histological differentiation and prognosis in primary gastric carcinoma. J Exp Clin Cancer Res. 2009;28:8. doi: 10.1186/1756-9966-28-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butin-Israeli V, Adam SA, Goldman AE, Goldman RD. Nuclear lamin functions and disease. Trends Genet. 2012;28:464–71. doi: 10.1016/j.tig.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Booth-Gauthier EA, Du V, Ghibaudo M, Rape AD, Dahl KN, Ladoux B. Hutchinson-Gilford progeria syndrome alters nuclear shape and reduces cell motility in three dimensional model substrates. Integr Biol (Camb) 2013;5:569–77. doi: 10.1039/c3ib20231c. [DOI] [PubMed] [Google Scholar]
- 41.Leyton-Mange J, Yang S, Hoskins MH, Kunz RF, Zahn JD, Dong C. Design of a side-view particle imaging velocimetry flow system for cell-substrate adhesion studies. J Biomech Eng. 2006;128:271–8. doi: 10.1115/1.2165689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Slattery MJ, Hoskins MH, Liang S, Dong C, Du Q. Monte carlo simulation of heterotypic cell aggregation in nonlinear shear flow. Math Biosci Eng. 2006;3:683–96. doi: 10.3934/mbe.2006.3.683. [DOI] [PubMed] [Google Scholar]
- 43.Philip JT, Dahl KN. Nuclear mechanotransduction: response of the lamina to extracellular stress with implications in aging. J Biomech. 2008;41:3164–70. doi: 10.1016/j.jbiomech.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2004;101:8963–8. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kubben N, Voncken JW, Demmers J, Calis C, van Almen G, et al. Identification of differential protein interactors of lamin A and progerin. Nucleus. 2010;1:513–25. doi: 10.4161/nucl.1.6.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maloney JM, Nikova D, Lautenschlager F, Clarke E, Langer R, et al. Mesenchymal stem cell mechanics from the attached to the suspended state. Biophys J. 2010;99:2479–87. doi: 10.1016/j.bpj.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamstra DA, Bae K, Pilepich MV, Hanks GE, Grignon DJ, et al. Older age predicts decreased metastasis and prostate cancer-specific death for men treated with radiation therapy: meta-analysis of radiation therapy oncology group trials. International journal of radiation oncology, biology, physics. 2011;81:1293–301. doi: 10.1016/j.ijrobp.2010.07.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fong LG, Ng JK, Lammerding J, Vickers TA, Meta M, et al. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J Clin Invest. 2006;116:743–52. doi: 10.1172/JCI27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol. 2012;13:579–90. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]
- 50.Sharma A, Tran MA, Liang S, Sharma AK, Amin S, et al. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-Raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res. 2006;66:8200–9. doi: 10.1158/0008-5472.CAN-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong C, Slattery M, Liang S. Micromechanics of tumor cell adhesion and migration under dynamic flow conditions. Front Biosci. 2005;10:379–84. doi: 10.2741/1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hodgson L, Henderson AJ, Dong C. Melanoma cell migration to type IV collagen requires activation of NF-kappaB. Oncogene. 2003;22:98–108. doi: 10.1038/sj.onc.1206059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang S, Slattery MJ, Wagner D, Simon SI, Dong C. Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation. Annals of biomedical engineering. 2008;36:661–71. doi: 10.1007/s10439-008-9445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ribeiro AS, Dahl KN. The nucleus as a central structure in defining the mechanical properties of stem cells. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:831–4. doi: 10.1109/IEMBS.2010.5626785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.