Introduction
In the earliest stages of development in animal systems, the processes necessary for fertilization, maternal and paternal genomic union, mitosis, and cytokinesis are dependent on maternally-supplied molecules that are deposited in the egg during oogenesis. These maternal products -mRNAs, proteins, or other biomolecules- are vital because the fertilized egg is transcriptionally silent for one to many cell cycles prior to zygotic genome activation. If the genes necessary to generate these maternal components, (so-called maternal genes) are disrupted, the result can be disastrous for the development of the zygote. Strict maternal-effect mutations result from the alteration of genes whose zygotic genetic contribution is not necessary for juvenile or adult developmental processes, but that are instead solely required to generate eggs capable of supporting early embryogenesis in the next generation. Females homozygous for these types of mutations are phenotypically normal, but their offspring manifest developmental defects that are present regardless of the genotype of the embryos. In essence, maternal genes affect one of the most evolutionary important adult traits: fertility.
The first lines of evidence suggesting the existence of maternal effect genes surfaced at the turn of the 20th century when breeding studies in snails uncovered an intriguing pattern of inheritance in directionality of shell coiling. By observing offspring from individual snail specimens that were either allowed to self-fertilize or were crossed to snails of the same phenotype, it became apparent that shell coiling direction could best be explained by inferring that a maternal genetic contribution dictated the progeny phenotypes (Boycott and Diver 1923; Boycott et al. 1931; Sturtevant 1923). Later work has illustrated that directionality of shell spiraling is actually related to cytoskeletal dynamics and the tilt angle of mitotic spindles during early cleavage stages (Shibazaki et al. 2004). The phenotypes observed by Boycott and Driver are the result of a recessive maternal-effect mutation in a still-unknown gene that likely regulates the cytoskeleton and mitotic apparatus.
A century later, maternal-effect genes influencing a broad spectrum of processes have been identified in diverse animal systems. While maternal-effect genes have been extensively studied using classical genetics in invertebrate model systems like Drosophila and C. elegans (for reviews, see Kemphues and Strome 1997; St. Johnston and Nüsslein-Volhard 1992), the study of such genes in vertebrates is hampered by the difficulty of genetic analysis in these organisms. Three primary lines of study have had a large impact on our understanding of maternally-driven processes in vertebrates: embryological and reverse genetic approaches in Xenopus laevis (Wylie and Haessman 1997), conditional knockout mutations in the mouse (Roy and Matzuk 2006; Sun et al. 2008), and forward genetics in the zebrafish, which is the subject of this review.
Recent years have seen an explosion of laboratories that, attracted by the embryological and genetic tractability of the zebrafish, have adopted this organism as a model system to study a variety of biological processes (Amatruda et al. 2002; Dooley and Zon 2000; Driever et al. 1994; Kimmel 1989). The study of reproductive biology is no exception to this, as the zebrafish confers a number of advantages to address questions in gamete formation, fertilization, and the initiation of development. This model system allows obtaining large numbers of adults as a source of gonads, as well as progeny for embryological studies. The ability to promote oocyte maturation in vitro (Seki et al. 2008), and to easily obtain mature eggs for in vitro fertilization (Pelegri and Schulte-Merker 1999) adds further temporal control to the experimental system. In addition, the optical clarity of the early egg provides conditions ideal for detailed imaging of developmental processes (Kimmel et al. 1995). Last but not least, the genetic amenability of the zebrafish allows for the identification of maternal- and paternal-effect mutations affecting fertility (Dosch et al. 2004; Pelegri et al. 2004; Pelegri et al. 1999; Wagner et al. 2004). In this regard, the use of ploidy manipulation methods to facilitate genetic screening potentially provides a unique tool for a vertebrate system that may facilitate such screens even in smaller laboratories (Pelegri and Mullins 2004; Pelegri and Schulte-Merker 1999; Streisinger et al. 1981). Together, these characteristics add to a tremendous potential of the zebrafish for the study of vertebrate reproductive biology.
The zebrafish maternal-effect mutations identified in the above screens fall into several classes based on what processes they disrupt. The earliest phenotypes manifest during oogenesis, while mutations during the egg to embryo transition can affect egg activation, fertilization, and cytokinesis. Even later-acting mutations affect cell fate determination, morphogenesis and cell viability. Here, we specifically focus on genes and processes that influence egg activation, fertilization, early cleavage, and germ line determination in this organism, events of crucial importance for reproductive biology, and briefly compare these processes to those as they occur in other studied vertebrate systems. For a more general overview of identified zebrafish maternal-effect genes spanning other types of identified maternal- and paternal-effect genes, including those that affect later processes in development, the reader is directed to other current reviews (Lyman-Gingerich and Pelegri 2007; Putiri and Pelegri 2009).
Fertilization
The development of a functional adult organism from two haploid gametes depends on the initial fusion of maternal and paternal genetic contributions to form the zygotic genome. This composite genome must then be replicated and properly segregated to all of the rapidly multiplying cells of the zygote. Most animal embryos undergo very similar processes during fertilization. Activation of the egg is accomplished by fusion of a sperm with the egg membrane, although in teleosts like the zebrafish, egg activation normally occurs by contact with water and is not dependent on the presence of sperm (Wolenski and Hart 1988). Egg activation triggers the cortical reaction, which involves intracellular calcium release, followed by exocytosis of cortical granules resulting in structural changes in the fertilization membrane that prevent polyspermy (reviewed in Horner and Wolfner 2008; Sardet et al. 2002). Zebrafish eggs are arrested at the second meiotic metaphase and egg activation prompts the completion of meiosis (Streisinger et al. 1981; Selman et al 1993, Dekens et al. 2003), which is shortly followed by the reformation of the female pronuclear membrane (Dekens et al. 2003; our unpublished observations). Through the fusion of the sperm and egg membranes during fertilization, the sperm nucleus enters the egg cytoplasm (Hart et al. 1992). Once inside the egg, the sperm nucleus likely undergoes rapid disassembly and reassembly of the nuclear envelope, as has been shown occurs in many animal species including fish (Iwamatsu and Ohta 1978, Longo 1985). At the same time, sperm-derived centrioles join together with maternally-derived centrosomal components and begin to nucleate the sperm aster by organizing tubulin monomers into a radial array of microtubules (Navara et al. 1994; reviewed in Delattre and Gönczy 2004; Schatten 1994). Growth of this aster and length-dependent pulling forces mediated by anchored cytoplasmic motor proteins are thought to propel the sperm pronucleus towards the center of the embryo (Kimura and Onami 2005; Reinsch and Gonczy 1998). When the lengthening astral microtubules contact the female pronucleus, it begins a relatively rapid migration toward the sperm pronucleus in the direction of the minus ends of the monoaster microtubules (Bestor and Schatten 1981). Once pronuclear apposition is achieved, the two nuclei fuse to form the zygotic nucleus and the embryo proceeds into the first mitotic division. In fish embryos, pronuclear fusion occurs prior to chromosome condensation and nuclear envelope breakdown (NEB) (Iwamatsu and Kobayashi 2002), though in some animals NEB occurs concomitantly with pronuclear apposition and therefore no fusion between the pronuclear membranes is observed (reviewed in Longo 1985).
In zebrafish embryos, sperm entry at fertilization can occur only at a distinct region in the animal pole of the egg known as the micropyle. The micropyle is actually derived from the follicular cell layer that surrounds the maturing oocyte prior to ovulation. The positioning of the single micropyle at the animal pole cortex appears to depend on the establishment of animal-vegetal (AV) polarity both within the maturing oocyte and in the surrounding follicle cells by the asymmetric redistribution of cellular components and molecules. In the zebrafish mutant bucky ball (buc), AV asymmetry fails to be established properly and as a result, multiple micropyles form in the egg, subsequently leading to polyspermy at fertilization (Marlow and Mullins 2008). The buc gene seems to be important for positioning many cellular components in the oocyte, as well as several mRNAs that will eventually reside in the primordial germ cells (Bontems et al. 2009 - see below).
Although most animal oocytes do not contain a localized structure like the fish micropyle where sperm entry must occur, many species do establish an AV axis prior to fertilization that may specify the sub-region wherein sperm entry generally takes place. In frogs, the asymmetric redistribution of specific mRNAs and structures during oogenesis results in a polarized mature oocyte (reviewed in King et al. 2005; Kloc and Etkin 2005) where sperm entry is normally confined to the animal pole (Elinson 1975). The possibility of prepatterning by the establishment of asymmetries in the mammalian oocyte has long been a controversial issue. Several groups have provided evidence that mammalian oocytes are symmetric and non-polar (Hiiragi and Solter 2005; Motosugi et al. 2005). However recent studies in mouse suggest that oocytes are in fact asymmetric and transiently polarized, as illustrated by the invariant asymmetric positioning of organelles in the early stages of oocyte maturation (Kloc et al. 2008). The biological significance of mammalian oocyte polarization remains to be determined.
In the zebrafish, egg activation is associated with a variety of processes such as changes in egg shape, ooplasmic segregation to the blastodisc, and cortical granule release-dependent chorion expansion (reviewed in Yamagani et al. 1992; Hart and Fluck 1995; see also Becker and Hart 1996; Becker and Hart 1999, Leung et al. 2000; Fernández et al. 2006). Several zebrafish maternal-effect mutations (jump start, p11cv) have been identified that affect all of these processes, suggesting a function for the corresponding genes in upstream events in egg activation (Dosch et al. 2004). On the other hand, other mutations affect only a subset of the events associated with egg activation or fertilization, such as chorionic membrane expansion (claustro; Pelegri et al. 2004), ooplasmic segregation (emulsion, dp14nb; Dosch et al. 2004), or pronuclear migration (futile cycle) (Dekens et al. 2003).
In futile cycle (fue) mutant embryos, pronuclear congression fails and the maternal and paternal nuclei do not fuse (Fig. 1A) (Dekens et al. 2003). Despite the disruption of the nuclei, these zygotes undergo multiple rounds of cytokinesis, resulting in large numbers of anucleate blastomeres. fue embryos complete meiosis normally, construct robust asters, and undergo normal centrosomal duplication and segregation, but mitotic spindles never form (Fig. 1B) (Dekens et al. 2003). In addition, recent evidence suggests that the sperm monoaster forms normally in fue embryos (our unpublished data), though distribution of molecular motors and membrane proteins known to be involved in migration has yet to be assessed. The fue mutation is unique in its array of phenotypic attributes and is one of only a few examples of genetic lesions that uncouple cytokinesis from karyokinesis.
Figure 1.

Nuclear congression and chromosomal segregation defects in futile cycle embryos shortly after fertilization. (A) At 10 minutes post-fertilization (mpf), DAPI staining of nuclei indicates that the second meiotic division has completed with extrusion of the second polar body (asterisks), and maternal (arrow heads) and paternal pronuclei are not closely apposed in either wild-type (WT) or fue embryos. By approximately15 mpf, WT pronuclei have completed migration and are beginning to fuse while fue pronuclei are still separate entities, having failed to congress. At 25 mpf, WT embryos show a characteristic metaphase chromosome arrangement. In fue embryos, DNA appears to have condensed but there are still three distinct chromosome clusters showing that maternal and paternal genomes have not intermixed. (B) At anaphase in the 2-cell embryo, separating chromosomes (in blue), and asters with spindles (indicated by red α-tubulin antibody staining) are apparent in WT embryos. In contrast, fue embryos show abnormally localized chromosome clusters (near the furrow in this case) and completely lack spindles, although asters are present.
In many mammalian and amphibian embryos, as well as in echinoderms, ctenophores and nematodes, the processes that bring pronuclei together are very similar to those at work in fish (Chambers 1939; Navara et al. 1994; Payne et al. 2003a; Reinsch and Karsenti 1997; Rouviere et al. 1994 - reviewed in Schatten 1982; Strome and Hill 1988). An exception is found in rodent species, where pronuclear migration occurs by the action of acentriolar microtubule arrays positioned throughout the egg (reviewed in Schatten 1994). The analysis of pronuclear congression in sea urchin has added another interesting variation to this theme, as these large eggs depend on the contraction of a nuclear actin network to deliver the female pronucleus to within reach of the sperm-derived monoaster (Lénárt et al. 2005). In primate and bovine species, several components, including the molecular motor dynein, as well as proteins of the nuclear envelope are important for pronuclear migration (Payne et al. 2003a; Payne et al. 2003b). Although maternal-effect mutations in these types of genes have not been identified in mammalian species, the gene products involved are very likely maternally contributed.
Centrosome inheritance and the cell cycle
Centrosomes, the microtubule organizing centers composed of a pair centrioles surrounded by pericentriolar material, are instrumental in spindle formation and ciliogenesis (reviewed in Bornens 2002; Strnad and Gönczy 2008). Because of their role in spindle pole organization, maintaining two and only two centrosomes is essential. Variations in this number cause defects in cell division and can result in aneuploidy and unregulated cell growth (reviewed in Acilan and Saunders 2008; Delattre and Gönczy 2004). In the same way that organisms have achieved a method to avoid ploidy duplication during the fusion of male and female gametes (through reductional division during meiosis to generate haploid gametes), mechanisms have also developed during gametogenesis to avoid a duplication of centrosomes during animal fertilization (reviewed in Delattre and Gönczy 2004; Schatten 1994). One common strategy, which is exemplified in the zebrafish, is for the sperm to provide centrioles to an acentriolar egg (Fig. 2, left column). Ultrastructural analysis has shown that the zebrafish sperm contains a pair of centrioles (Kessel et al. 1983). The centriolar contribution from the sperm is essential for embryogenesis. Even if embryos can be forced to develop in the absence of other sperm components such as paternal chromosomes, as when generating haploid embryos using sperm whose DNA has been inactivated with UV light (Walker 1999), sperm-derived centrioles must be present to facilitate chromosome segregation and cytokinesis.
Figure 2.
Centrosome biogenesis and cell division in wild-type embryos (left column), and embryos from mothers (middle column) or fathers (right column) mutant for cellular atoll/sas-6. Sperm-provided centrioles are indicated in green, centrioles duplicated in the zygote are indicated in white, light green circles indicate reconstituted centrosomes. Centriole duplication in the zygote depends on the presence of functional Cellular atoll/Sas-6 maternal product in the cytoplasm. Maternally mutant embryos arrest cell divisions after the first cell cycle due to the lack of centriolar biogenesis in the zygote. Paternally mutant embryos exhibit a delay in the first cell division while the single sperm-derived centriole replicates, but a normal cleavage pattern ensues thereafter. In paternal mutants, ongoing DNA replication during the delayed first cell division cycle results in whole genome duplication to generate tetraploid embryos.
Maintenance of centrioles during cell division involves the duplication of the centrioles themselves in a process where preexisting mother centrioles facilitate the assembly of adjacent daughter centrioles (reviewed in Song et al. 2008). Once centrioles have duplicated, the separation of pericentriolar material surrounding the two pairs of duplicated centrioles generates two centrosomes in preparation for cell division. In recent years, a flurry of elegant studies have delineated a pathway for centriole and centrosomal duplication, largely driven by findings in the nematode C. elegans and in cultured human cells (reviewed in Leidel and Gönczy 2005; Song et al. 2008). One gene required for centriolar duplication is sas-6, which corresponds to the maternal-effect zebrafish mutation cellular atoll (Yabe et al. 2007). In embryos from cellular atoll/sas-6 mutant mothers, cell division fails in a fraction of the blastomeres (Fig. 2, middle column). These embryos show defects in centrosome duplication and consequently in the formation of bipolar spindles, chromosome segregation and (because of the role of the bipolar spindle in furrow induction as described below) cytokinesis.
Remarkably, the cellular atoll mutation results not only in a maternal-effect, but also a paternal-effect phenotype (Yabe et al. 2007). Embryos derived from the fertilization of wild-type eggs with sperm derived from males homozygous for cellular atoll/sas-6 exhibit a delay in cell division during the first cell cycle, followed by an initiation of a normal cleavage pattern at a time coincident with the second cell cycle (Fig. 2, right column). Such embryos likely inherit a single centriole from the sperm cell, as opposed to the normal centriolar pair. The delay in cell division during the first cell cycle presumably allows duplication (using maternal centriolar factors present in the wild-type egg) of the single centriole into a centriolar pair capable of driving cell division in subsequent cell cycles. Conversely, embryos derived from homozygous cellular atoll/sas-6 females crossed to wild-type males exhibit defects in cell division starting at the second cell cycle. In such maternally mutant cellular atoll/sas-6 embryos, daughter centrioles are absent due to the lack of centriole duplication during the first cell cycle, but the first mitotic division occurs normally because the spindle can be organized by the unduplicated sperm-derived centrioles alone. These two sets of defects show distinct and complementary roles for maternal and paternal cellular atoll/sas-6 function during early development, a situation similar to that previously described for centriolar biogenesis genes in C. elegans (O’Connell et al. 2001).
Of significant interest, cells arrested by defects in centriolar duplication continue DNA replication, resulting in the doubling of the genome (Yabe et al. 2007). This not only provides a potential new tool for ploidy manipulation in the zebrafish but also suggests new scenarios in which mutations present in an animal lineage may facilitate the appearance and fixation of genome duplication events that occur during evolution.
Many organisms including Xenopus (Kato et al. 2002) and C. elegans (Wolf et al. 1978) have sperm that, as in zebrafish, contribute a pair of centrioles to an acentriolar egg. However, there is significant variability in this pattern (reviewed in Delattre and Gönczy 2004; Schatten 1994). Species such as humans (Manandhar et al. 2000) and Drosophila (Callaini et al. 1999) generate sperm with only one centriole, which necessitates an additional round of centriole duplication prior to the first mitosis. Even more strikingly, mice (Manandhar et al. 2000) and some parthenogenetic insect species (Callaini et al. 1999) do not provide centrioles through the sperm, and the early embryonic divisions are driven by spindles nucleated via a centrosome-independent pathway (reviewed in Wadsworth and Khodjakov 2004). The variation in centriolar inheritance in diverse animal species undoubtedly contributes to reproductive barriers both in natural settings and in experimental manipulations.
Cell division
The fertilized zebrafish embryo begins its first cleavage at minute 40 post-fertilization and continues to undergo a cell division cycle approximately every 15 minutes for about 10 cell cycles, when the cell cycle slows down at the midblastula transition (Kane and Kimmel 1993). The first several cell divisions appear to be specialized, possibly due to the large size of the embryo and the need to redistribute cell fate determinants present in the egg. Such specializations include large-scale rearrangements of the cytoskeleton, and the association of furrow formation with slow calcium waves and the localization of cell fate determinants.
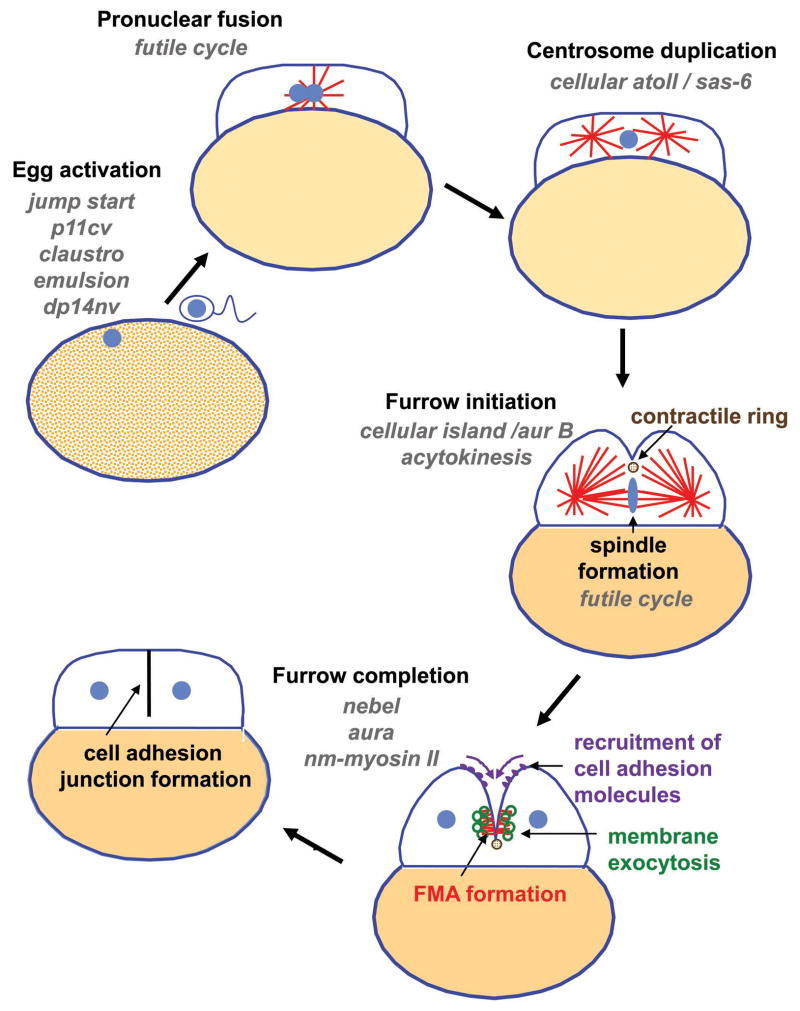
As in other systems, furrows are induced in cortical regions overlying the spindle apparatus, halfway between the spindle poles (reviewed in Glotzer 2004; a summary of the main events in cell division is presented in Fig. 3). Previous studies have indicated the ability of multiple signals to induce furrow formation in a variety of animal cells (Bringmann et al. 2007; Bringmann and Hyman 2005; Chen et al. 2008). These studies have indicated the presence of redundant signals for furrow initiation, derived both from the spindle midzone and astral microtubules. However, the fact that futile cycle mutants, which lack spindle midzones, exhibit largely normal cell divisions (Dekens et al. 2003), suggests that asters contribute a dominant signal for furrow initiation in zebrafish embryos. The dominance of an astral signal is possibly an adaptation for carrying out cell divisions in large blastomeres, as one might expect distal regions of the blastomeres would be too distant to be influenced by the centrally located spindle. A mutation in the maternal-effect gene cellular island appears to affect this astral-derived signal, and allows formation of the furrow only in the middle-most region of the blastomeres, likely induced by the underlying spindle (Yabe et al. 2009). cellular island has been shown to encode the zebrafish Aurora B kinase (Yabe et al. 2009), a component of the chromosomal passenger complex thought to be involved in furrow initiation (reviewed in Ruchaud et al. 2007).
Figure 3.
Diagram representing the main events associated with fertilization and cell division in the early zebrafish embryo. Maternal-effect mutants that affect various processes during this period are listed in grey type. See text for details.
The initiation of furrow formation is associated with enrichment of actin at the furrow, including the formation of an actomyosin contractile ring at the furrow proper (Urven et al. 2006). While the actomyosin ring is thought to provide a primary force for membrane invagination associated with the initiation of furrow formation, it is intriguing that this process still occurs in the presence of drugs expected to interfere with myosin activity (Urven et al. 2006). This suggests the possibility that other processes may also facilitate furrow contraction in these large cells, such as membrane bending factors like BAR-domain proteins (Cory and Cullen 2007).
In addition to the contractile ring apparatus, other major cytoskeletal structures appear at the initiating furrow. Flanking the contractile ring at either side of the furrow, enrichments of punctate f-actin appear and soon develop into lamellipodia extending across the extracellular space towards the center of the furrow (Urven et al. 2006). In another identified maternal-effect mutation, in the gene acytokinesis, f-actin dynamics during furrow formation are abnormal, although it is unclear what precise elements are affected in the mutant (Kishimoto et al. 2004). Coincident with furrow actin recruitment, an array of parallel microtubules forms oriented perpendicular to the furrow (Jesuthasan 1998). This array may be analogous to midzone microtubules observed during the completion of cytokinesis in cultured cells and in smaller cells in embryos at later stages of development. Similar to the case of the midzone, the FMA has been proposed to mediate the addition of intraembryonically derived membrane vesicles to the furrow site (Danilchik et al. 1998; Jesuthasan 1998; Pelegri et al. 1999), which has been postulated occurs in many early animal embryos in order to achieve the increase in surface area required for cell division (reviewed in Otegui et al. 2005).
The furrow-associated cytoskeletal networks present in the early zebrafish embryo become rearranged as the furrow matures. The lamellipodia derived from pericleavage f-actin enrichments fuse at the center of the furrow and eventually replace the contractile ring with intense accumulations of f-actin cables (Urven et al. 2006). Concomitant with this process, newly exocytosed membrane is added to the furrow. At the same time, cell adhesion junction components, such as β-catenin and integrins accumulate at the furrow, likely via movements along the cortex and by their presence in exocytosing intraembryonic vesicles (Jesuthasan 1998; Urven et al. 2006). Together, new intraembryonically-derived membrane and cell adhesion junction components assemble a wall-like domain of adhesive membrane at the inter-blastomeric boundaries.
Another major cytoskeletal rearrangement that occurs during furrow maturation is the reorganization of FMA tubules. While initially the FMA forms at the furrow as an array where tubules are parallel to each other and perpendicular to the furrow, during furrow maturation the outer ends of the microtubules (those closest to the furrow, presumably their plus ends) appear to translocate towards the distal ends of the furrow (Jesuthasan 1998; Pelegri et al. 1999; Urven et al. 2006). There they aggregate as compact structures prior to their disassembly after furrow completion. Inhibition of myosin activity results in the stabilization of the FMA and the absence of these movements, indicating that myosin motors play a role in these processes (Urven et al. 2006). This striking process of tubule reorganization may be a necessary step in the disassembly of the FMA, which, as mentioned below, has potentially been co-opted to facilitate germ plasm compaction. However, the precise molecular mechanisms involved in this process remain to be determined.
An interesting feature of furrow formation in the early zebrafish embryo is its association with waves of intracellular calcium release, or slow calcium waves (Chang and Meng 1995; Créton et al. 1998; Webb et al. 1997). For the first three cell cycles, furrow ingression is immediately preceded by a cortical calcium wave possibly involved in signaling furrow initiation. Another, more prominent wave that occurs during maximal membrane contraction reaches deeper into the cytoplasm, and may be involved in the events associated with this process such as membrane addition and cytoskeletal reorganization. In particular, inhibition of calcium waves using a calcium chelator results in defects in both furrow deepening and the recruitment of peri-cleavage f-actin at the furrow (Lee et al. 2003; Li et al. 2008). Interestingly, these slow calcium waves originate from the center of the blastodisc and move toward the distal end of the furrow (Chang and Meng 1995; Créton et al. 1998; Webb et al. 1997), mirroring the described progression of cytoskeletal rearrangements in the FMA. This raises the additional possibility, which remains to be tested, that these calcium waves may be providing a medial-to-distal directionality to these rearrangements. This idea is also consistent with the fact that these slow calcium waves are detectable in the first three cell cycles, which are precisely those in which the FMA is most apparent. It is therefore possible that calcium waves have evolved to facilitate the organization of the cytoskeleton in the very large blastomeres of the early embryo, possibly also in association with the segregation of the germ plasm (see below). An interesting line of research will involve the precise role of calcium waves in furrow organization, as well as the mechanism of medial-to-distal orientation during furrow maturation.
In addition to a role for myosin activity in furrow maturation, several maternal-effect mutations have been found that affect the completion of cytokinesis, such as those in the genes nebel and aura. In the case of nebel, the mutation has been shown to interfere with proper FMA formation and to result in the mislocalization of FMA remnants in non-distal regions of the furrow (Pelegri et al. 1999), suggesting that nebel may be important for the directionality of furrow reorganization.
The events described above may be conserved in furrow formation in lower vertebrates, as Xenopus embryos exhibit actin-dependent extensions across the extracellular space (Danilchik and Brown 2008), as well as FMA-dependent membrane addition (Danilchik et al. 1998). However, large-scale cytoskeletal rearrangements such as those of the FMA have not been reported in the mouse embryo. These differences may reflect a lesser requirement for large-scale membrane addition in the slower cycling mouse blastomeres, as well as the lack of a requirement for maternally-inherited germ plasm segregation in this organism (see below). A role for calcium waves associated with egg activation and originating at the sperm entry site is widely conserved in animal species, including zebrafish, Xenopus, and mice (reviewed in Ducibella and Fissore 2008; Webb and Miller 2000). However, calcium waves associated with furrow formation have only been reported in teleost fish such as zebrafish (Chang and Meng 1995; Créton et al. 1998; Webb et al. 1997) and medaka (Fluck et al. 1991) and a more general role for these waves in cytokinesis in other species remains to be established.
Segregation of germ plasm components
One of the earliest cell fate decisions in the animal embryo is the differentiation of germ line precursors from the soma. In many animal species, this decision involves the segregation of the germ plasm, a specialized cytoplasm containing specific mRNA and protein products, which confers the germ cell fate to the cells inheriting it (reviewed in Cinalli et al. 2008; Wylie 2000). In the zebrafish, germ plasm components are stored in the oocyte in at least two separate domains (summarized in Fig. 4). Some germ plasm mRNA components, such as those encoded by the genes dead end (Weidinger et al. 2003), nanos (Köprunner et al. 2001) and vasa (Yoon et al. 1997), are present in aggregates in the animal pole of the oocyte (Theusch et al. 2006). mRNAs for other germ plasm components, such as daz-like and bruno-like, are initially stored at the vegetal pole of the oocyte (Maegawa et al. 1999; Suzuki et al. 2000).
Figure 4.
Segregation of germ plasm components during cell division in the early zebrafish embryo. Top row of images: side views of embryos showing initially distinct localization of animal and vegetal germ plasm aggregates, and the animally-directed movement of the latter during the first cell cycles to the cells forming at the animal pole. Middle row of images: animal views of embryos. Animal germ plasm components are initially bound to a cortical filamentous network likely composed of f-actin (leftmost diagram). Peripheral movement of this network by the action of astral microtubules facilitates germ plasm aggregation and leads to the recruitment of germ plasm as a rod-like structure at the forming furrow (second and third diagram from left). Cytoskeletal rearrangements of the FMA are associated with further translocation and aggregation of the germ plasm at the distal ends of the mature furrow (fourth diagram from left). This process is repeated for the second furrow, resulting in four stable germ plasm aggregates (rightmost diagram). After their animally-directed movement, vegetal germ plasm components become anchored to the distal end of the compacted animal germ plasm, generating two associated subcompartments (rightmost diagrams in all rows). Bottom row: close up, in an animal view, of the cytoskeleton and associated germ plasm aggregates during the stages shown in the middle row diagrams. See text for additional details.
These distinct localization patterns become established during oogenesis. As the oocyte matures, a number of germ plasm mRNAs transiently localize to the Balbiani body, a mitochondria-rich structure present in the forming oocyte (Guraya 1969), and are transported to the vegetal pole of the oocyte (Kosaka et al. 2007). However, at later stages of oocyte formation, daz-like RNA remains anchored at the vegetal pole while nanos and vasa mRNAs become more ubiquitously distributed (Kosaka et al. 2007). Recent studies have shown that bucky ball function, discussed above because of its requirement for proper oocyte polarity and placement of the micropyle, is essential for Balbiani body formation (Marlow and Mullins 2008). The Balbiani body appears to be homologous to the mitochondrial cloud present in developing Xenopus oocytes, which is also rich in mitochondria and has a role in the segregation of the germ plasm (Minakhina and Stewards 2005). Cloning of the bucky ball gene shows that it encodes a vertebrate-specific protein whose mRNA and protein are localized to the Balbiani body (Bontems et al. 2009). bucky ball is the first gene shown to be essential for the formation of this important conserved structure.
The process of fertilization initiates separate mechanisms that bring the animal pole and vegetal pole germ plasm mRNAs together as four distinct aggregates at the distal ends of the furrows for the first and second cleavage cycles. The animal pole germ plasm mRNA aggregates appear to be bound to an f-actin cytoskeletal network randomly arranged in the forming blastodisc (Knaut et al. 2000; Theusch et al. 2006). During the first and second cleavage cycles, this cytoskeletal network, and with it the animal germ plasm mRNA aggregates, becomes aligned circumferentially, possibly as a result of the binding of f-actin filaments to astral microtubules, and the subsequent centrifugal translocation caused by the outwardly growing asters. These asters are arranged as a monoaster during the first cell cycle, nucleated by the sperm-derived reconstituted centrosome, and diasters during the following cycles, nucleated by centrosomes at the spindle poles (Theusch et al. 2006). The peripheral translocation and alignment of the actin network appears to be completed by the second or third cell cycle, delineating the time period in which this process mediates germ plasm segregation (Theusch et al. 2006; Yabe et al. 2007). The circumferential alignment of the germ plasm-containing network during the first and second cell cycle appears to facilitate the fusion of neighboring germ plasm aggregates into larger, multimodular structures in a process of pre-aggregation that precedes furrow formation (Theusch et al. 2006). As furrows begin to form, these germ plasm pre-aggregates are recruited to the furrow in a rod-like structure (Yoon et al. 1997), which is associated with the distal ends of the FMA tubules (Knaut et al. 2000; Pelegri et al. 1999; Theusch et al. 2006). The precise mechanism by which this process occurs is not fully understood. However, one possible model is that germ plasm aggregates become recruited to the furrow by hitchhiking on the f-actin network that is being driven towards the furrow by elongating astral microtubules from opposite spindle poles. Recruitment of a rod-like germ plasm aggregate at the initiating furrow is thereafter dependent on the induction of putative anchors to stabilize these larger aggregates in the furrow region. Thus, astral microtubules may serve a dual function, both in the induction of the furrow itself (see above) and in bringing together germ plasm aggregates at the forming furrow.
As the furrow matures, the tilting and enrichment of FMA tubules to the distal ends of the furrow (Jesuthasan 1998) are associated with the distal translocation and compaction of the recruited germ plasm (Urven et al. 2006). FMA tubule tilting and enrichment is also spatially linked to germ plasm recruitment in nebel mutants, where these enrichments occur in non-distal regions of the furrow (Pelegri et al. 1999). In wild-type embryos, it is clear that non-muscle myosin II function is required in this coordinated process, as functional inhibition of this motor by small molecule inhibitors results in a stabilized FMA accompanied by defects in the transition of germ plasm from rod-like to the more compact, distally-enriched aggregate (Urven et al. 2006). One potential way in which myosin activity may be involved in this process is if it acts to mediate the translocation of microtubule ends along the actin cytoskeleton aligned at the furrow, a process that in turn may drive germ plasm distal recruitment. However, this possibility remains to be explored.
As the animal germ plasm aggregates become recruited to the furrow, other aggregates, containing vegetally localized germ plasm RNAs such as daz-like and bruno-like, initiate a process of translocation towards the animal pole. In the case of daz-like, rapid translocation towards the recruited germ plasm has been shown to occur along the egg cortex (Theusch et al. 2006), although bulk translocation of mRNAs also occurs during the streaming of the ooplasm towards the forming blastodisc throughout the early cleavage stages (Maegawa et al. 2002). The cytoskeletal requirements for the cortical, animally-directed translocation of daz-like mRNA have not been precisely determined, but may involved arrays of microtubules present at the egg cortex (Jesuthasan and Strähle 1997). The aggregates originally found at the vegetal pole become anchored to the four clusters of animally localized germ plasm mRNAs present at the distal end of the mature furrows (Hashimoto et al. 2004). Remarkably, within these structures, the originally animal and vegetal germ plasm mRNAs occupy linked, yet largely non-overlapping regions of the aggregate: animal mRNAs are present in the part of the aggregate closest to the internal end of the blastodisc, while the vegetal germ plasm RNA daz-like is present in a more distal region (Theusch et al. 2006). Even more remarkably, this subcompartmentalized structure is maintained during the early cellular cleavages. The significance of these two separate pathways for germ plasm recruitment and the subsequent compartmentalization of the germ plasm remains unknown, but may be related to the complex regulation of germ plasm segregation and/or its function during these early developmental stages.
Other products have been also found to localize to the zebrafish germ plasm, such as the RNAs for the gene askopos (Blaser et al. 2005) and tudor-domain-containing protein 7 (TDRD7; Mishima et al. 2006), as well as proteins for bruno-like (Hashimoto et al. 2006), bucky ball (Bontems et al. 2009) and the Argonaute family member zebrafish Piwi protein (Ziwi; Houwing et al, 2007). Interestingly, overexpression of bucky ball by mRNA injection in the early embryo results in ectopic germ plasm aggregates, suggesting that this protein is important for the stabilization of germ plasm anchors (Bontems et al. 2009). It is possible that Bucky Ball acts as a limiting component of such germ plasm anchors at the furrow, and is normally depleted each cell cycle thus resulting in the characteristic pattern of four and only four germ plasm aggregates. Although beyond the scope of this review, 3′UTR regions of various genes, such as bucky ball, vasa, nanos and daz-like have been shown mediate RNA localization during oogenesis and early embryogenesis (Bontems et al. 2009; Knaut et al. 2002; Kosaka et al. 2007), as well as mRNA stability and translation in the embryo (Köprunner et al. 2001; Knaut et al. 2002; Wolke et al. 2002). Recent studies have also begun to uncover a regulatory role for micro RNAs and Dead end protein in the posttranscriptional regulation of germ plasm mRNAs in primordial germ cells (Mishima et al. 2006; Kedde et al. 2007) and suggest a role for Ziwi-mediated RNA silencing in germ cell survival (Houwing et al. 2007).
Thus, the zebrafish germ plasm is segregated by a complex series of events, involving the astral microtubule-dependent preaggregation and recruitment of animal germ plasm aggregates to the furrow as well as a process of distal compaction associated with FMA tubule rearrangements. These events generate a structure to which a second germ plasm sub-domain containing vegetally-derived germ plasm products is added. Together, these processes result in the generation of four large germ plasm aggregates in diametrically opposite regions of the embryo. This complex yet elegant strategy solves an intrinsic problem associated with the oocyte-to-embryo transition: how to segregate the components of a large macromolecular structure to a small subset of cells in the cleaving embryo. In the case of the zebrafish, this problem appears to be solved by a local gathering of subcomponents to generate larger aggregates. The process of cell division, which mediates the partitioning of the embryo in a symmetrical manner, may be an ideal system to co-opt towards this goal.
During further divisions in the early blastula embryo, the four germ plasm aggregates become incorporated into four primordial germ cells (Braat et al. 2000; Theusch et al. 2006; Yoon et al. 1997). Interestingly, these primordial germ cells continue to divide, although during these stages only one daughter cell inherits the germ plasm aggregate such that only four cells contain germ plasm (Braat et al. 1999; Knaut et al. 2000; Yoon et al. 1997). Little is known about the cellular processes involved in the segregation of germ plasm during this asymmetric cell division, which likely relies on maternal products as it occurs prior to the activation of zygotic expression at the midblastula transition. During asymmetric cell division, the germ plasm is associated with one of the two spindle poles (Braat et al. 1999; Knaut et al. 2000). This is reminiscent of the role of centrosomes in asymmetric division of stem cells (reviewed in Gonzalez 2008; Yamashita and Fuller 2008), highlighting once more the importance of this cellular structure in the early division and patterning of the embryo. The asymmetric segregation of germ plasm in these early stages may serve to regulate the number of primordial germ cells available to the embryo. Maternally-derived components of the germ plasm are also likely involved in the initiation of zygotically-driven programs of germ cell specification in the 1000-cell blastula embryo (Knaut et al. 2000). Thus, another reason for the early asymmetric segregation of the germ plasm may be to achieve the inheritance of this structure without diminishing its inductive potential during cell division.
There appear to be remarkable similarities between the processes of germ plasm recruitment in zebrafish and those described in Xenopus (reviewed in King et al. 2005; Kloc and Etkin 2005). As in zebrafish, Xenopus germ plasm components including mRNAs for xDaz-like (Houston et al. 1998) and the nanos homologue Xcat2 (Mosquera et al. 1993), initiate their segregation during oogenesis within a mitochondrial-rich structure, the mitochondrial cloud. Similarly, in both cases germ plasm components become localized to the vegetal pole of the egg. Moreover, as in zebrafish, Xenopus germ plasm forms four large aggregates associated with a network of microtubules (Robb et al. 1996), which are later inherited by four blastomeres (MacArthur et al. 2000). However, one striking difference between these two systems is that in the zebrafish a subset of germ plasm components becomes distributed to non-vegetal regions of the growing oocyte, so that these components are present at the animal pole of the egg, not its vegetal pole, soon after fertilization (see above). The precise reason for this intriguing difference is unclear but is potentially related to embryonic anatomy, since furrows only cleave the animal region of the embryo in teleosts, as opposed to the entirety in the amphibian egg. Maternal germ plasm containing Vasa protein has also been observed to accumulate at the base of the membrane furrows of the early cleavage stage chick embryo (Tsunekawas et al. 2000), in a manner very reminiscent to that in which germ plasm is localized in the zebrafish. Thus, the inheritance of a maternal germ plasm appears to be a widely conserved feature in animal development.
Even so, a number of animal species including Urodeles (newts, salamanders) and mammals do not contain a recognizable maternally-inherited germ plasm. Instead, in these species germ cells are induced during gastrulation from within the embryonic ectoderm by signals originating in extraembryonic tissues (reviewed in McLaren 2003). In spite of these differences, many of the genes present in the zebrafish germ plasm, such as nanos and dead end, have been found to have roles in germ cell development in the mouse (reviewed in Saga 2008)). Similarly, the gene for the zebrafish germ plasm component vasa is specifically expressed in germ cells in a variety of species, from planaria (Shibata et al. 1999) to humans (Anderson et al. 2007). Thus, individual components of germ cell determination pathways appear to be widely conserved through evolution, even if the developmental pathways in which these components act exhibit species-specific differences.
Conclusions and future directions
Studies in the zebrafish are gaining momentum in providing novel genetic entry points into processes relevant to reproductive biology, such as the mechanisms of gametogenesis, fertilization, cell division, and cell fate specification, including the determination of the germ line. Knowledge gained in these ongoing studies will complement those in other vertebrate model systems. Even in mammals, where zygotic gene activation occurs at an earlier stage of development, an increasing number of studies highlight an essential role for maternal genes in early development (reviewed in Roy and Matzuk 2006; see also Lykke-Andersen et al. 2008; Tang et al. 2007; Wan et al. 2008). Thus, the detailed analysis of maternal genes in the zebrafish promises to be widely applicable to other systems, including humans.
Further characterization of maternal-effect mutations and more generally, the maternal contribution necessary for normal development, will likely yield important insights into problems of reproduction and infertility. Several promising oocyte-based methodologies in reproductive biology and other biomedical applications are dependent on maternal factors, including nuclear transfer during animal cloning and the generation of specific embryonic stem cell lines. In addition, as we gain more knowledge about maternally-driven processes in a variety of model systems, interspecies comparison will reveal not only the similarities in these systems, but also their differences. This will in turn be instrumental for our understanding of zygotic barriers important in animal speciation during the process of evolution, as well serve to illustrate the variables important for successful interspecies nuclear transfer technologies applied to the conservation of endangered species. In the future, it will be of high interest to observe how advances in our understanding of the basic biology of the maternal to zygotic transition in the animal embryo may result in the development of corresponding applications in reproductive biology.
Table 1.
Zebrafish maternal-effect mutations discussed in this manuscript.
| General Category | Zebrafish Genes | Process Affected | References | Homologues |
|---|---|---|---|---|
| Oogenesis | bucky ball | Balbiani body & germ plasm assembly, oocyte polarity | Dosch et al. 2004, Marlow et al. 2008, Bontems et al. 2009 | Homologues of bucky ball are found in many vertebrates, including chick, human, & frog (Bontems et al. 2009) |
| Egg activation/fertilization | jump start p11cv | Cortical granule release, egg morphological changes, cytoplasmic segregation to blastodisc | Dosch et al. 2004 | Molecular identity unknown |
| emulsion dp14nb | Cytoplasmic segregation to blastodisc | Dosch et al. 2004 | Molecular identity unknown | |
| claustro | Expansion of chorionic membrane | Pelegri et al. 2004 | Molecular identity unknown | |
| futile cycle | Pronuclear congression, spindle formation | Dekens et al. 2003, Pelegri et al. 2004 | Molecular identity unknown | |
| Cytokinesis | cellular atoll | Centriole duplication | Dosch et al. 2004, Yabe et al. 2007 | Homologues of cellular atol/sas6 have been studied in human cell lines (Dammermann et al. 2004, Leidel et al. 2005, Strnad et al. 2007), mouse epithethial cells (Vladar and Stearns 2007), as well as in several invertebrate systems (Dammermann et al. 2004, Peel et al. 2007) |
| acytokinesis | Cleavage furrow initiation | Kishimoto et al. 2004 | Molecular identity unknown | |
| cellular island | Cleavage furrow completion | Dosch et al. 2004, Yabe et al. 2009 | Homologues of cellular island/aurora kinase B are found in vertebrates, flies, worms, & yeast (reviewed in Carmena and Earnshaw 2003; see also Terada et al. 1998, Murata-Hori et al. 2002) | |
| nebel | Cleavage furrow maturation and organization | Pelegri et al. 1999, 2004 | Molecular identity unknown | |
| aura | Cleavage furrow maturation | Pelegri et al. 2004 | Molecular identity unknown |
Acknowledgments
We thank members of our laboratory for discussions and apologize to scientists whose work we have not cited due to restricted space. R.L. was partially supported by a Predoctoral Fellowship from the National Science Foundation, and work in the Pelegri laboratory is funded by grant RO1GM65303 from the National Institutes of Health.
References
- Acilan C, Saunders WS. A tale of too many centrosomes. Cell. 2008;134:572–575. doi: 10.1016/j.cell.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Amatruda JF, Shepard JL, Stern HM, Zon LI. Zebrafish as a cancer model system. Cancer Cell. 2002;1:229–231. doi: 10.1016/s1535-6108(02)00052-1. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PTK. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol. 2007;7:136. doi: 10.1186/1471-213X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KA, Hart NH. The cortical actin cytoskeleton of unactivated zebrafish eggs: spatial organization and distribution of filamentous actin, non-filamentous actin, and myosin-II. Mol Reprod Dev. 1996;43:536–547. doi: 10.1002/(SICI)1098-2795(199604)43:4<536::AID-MRD17>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Becker KA, Hart NH. Reorganization of filamentous actin and myosin-II in zebrafish eggs correlates temporally and spatially with cortical granule exocytosis. J Cell Sci. 1999;112:97–110. doi: 10.1242/jcs.112.1.97. [DOI] [PubMed] [Google Scholar]
- Bestor TH, Schatten G. Anti-tubulin immunofluorescence microscopy of microtubules present during the pronuclear movement of sea urchin fertilization. Dev Biol. 1981;1:80–91. doi: 10.1016/0012-1606(81)90220-7. [DOI] [PubMed] [Google Scholar]
- Blaser H, Eisenbeiss S, Neumann M, Reichman-Fried M, Thisse B, Thisse C, Raz E. Transition from non-motile behaviour to directed migration during early PGC development in zebrafish. J Cell Sci. 2005;118:4027–4038. doi: 10.1242/jcs.02522. [DOI] [PubMed] [Google Scholar]
- Bontems F, Stein A, Marlow F, Lyautey J, Gupta T, Mullins MC, Dosch R. Bucky ball organizes germ plasm assembly in zebrafish. Curr Biol. 2009;19:414–422. doi: 10.1016/j.cub.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14:25–34. doi: 10.1016/s0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- Boycott AE, Diver C. On the inheritance of sinistrality in Limnaea peregra. Proc R Soc Lond B. 1923:207–213. [Google Scholar]
- Boycott AE, Diver C, Garstang SL, Turner FM. The inheritance of sinistrality in Limnaea peregra (Mollusca, Pulmonata) Phil Trans R Sox Lond B. 1931:51–131. [Google Scholar]
- Braat AK, van de Water S, Goos H, Bogerd J, Zivkovic D. Vasa protein expression and localization in the zebrafish. Mech Dev. 2000;95:271–274. doi: 10.1016/s0925-4773(00)00344-0. [DOI] [PubMed] [Google Scholar]
- Braat AK, Zandbergen T, van de Water S, Goos HJT, Zivkovic D. Characterization of zebrafish primordial germ cells: morphology and early distribution of vasa RNA. Dev Dyn. 1999;216:153–167. doi: 10.1002/(SICI)1097-0177(199910)216:2<153::AID-DVDY6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Bringmann H, Cowan CR, Kong J, Hyman AA. LET-99, GOA-1/GPA-16, and GPR-1/2 are required for aster-positioned cytokinesis. Curr Biol. 2007;17:185–191. doi: 10.1016/j.cub.2006.11.070. [DOI] [PubMed] [Google Scholar]
- Bringmann H, Hyman AA. A cytokinesis furrow is positioned by two consecutive signals. Nature. 2005;436:731–734. doi: 10.1038/nature03823. [DOI] [PubMed] [Google Scholar]
- Callaini G, Riparbelli MG, Dallai R. Centrosome inheritance in insects: fertilization and parthenogenesis. Biol Cell. 1999;91:355–366. [PubMed] [Google Scholar]
- Carmena M, Earnshaw WC. The cellular geography or aurora kinases. Nature Rev Mol Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- Chambers EL. The movement of the egg nucleus in relation to the sperm aster in the echinoderm egg. J Exp Biol. 1939:409–424. [Google Scholar]
- Chang DC, Meng C. A localized elevation of cytosolic free calcium is associated with cytokinesis in the zebrafish embryo. J Cell Biol. 1995;131:1539–1545. doi: 10.1083/jcb.131.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Foss M, Zhang D. Redundant mechanisms recruit actin into the contractile ring in silkworm spermatocytes. PLoS Biol. 2008;6:e209. doi: 10.1371/journal.pbio.0060209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinalli RM, Rangan P, Lehmann R. Germ cells are forever. Cell. 2008;132:559–562. doi: 10.1016/j.cell.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Cory GOC, Cullen PJ. Membrane curvature: the power of bananas, zeppelins and boomerangs. Curr Biol. 2007;17:R455–R457. doi: 10.1016/j.cub.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Créton R, Speksnijder JE, Jaffe LF. Patterns of free calcium in zebrafish embryos. J Cell Sci. 1998;111:1613–1622. doi: 10.1242/jcs.111.12.1613. [DOI] [PubMed] [Google Scholar]
- Dammermann A, Müller-Reichert T, Pelletier L, Habermann B, Desai A, Oegema K. Centriole assembly requires both centriolar and pericentriolar material proteins. Dev Cell. 2004;7:815–829. doi: 10.1016/j.devcel.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Danilchik MV, Brown EE. Membrane dynamics of cleavage furrow closure in Xenopus laevis. Dev Dyn. 2008;237:565–579. doi: 10.1002/dvdy.21442. [DOI] [PubMed] [Google Scholar]
- Danilchik MV, Funk WC, Brown EE, Larkin K. Requirement for microtubules in new membrane formation during cytokinesis of Xenopus embryos. Dev Biol. 1998;194:47–60. doi: 10.1006/dbio.1997.8815. [DOI] [PubMed] [Google Scholar]
- Dekens MPS, Pelegri FJ, Maischein H-M, Nüsslein-Volhard C. The maternal-effect gene futile cycle is essential for pronuclear congression and mitotic spindle assembly in the zebrafish zygote. Development. 2003;130:3907–3916. doi: 10.1242/dev.00606. [DOI] [PubMed] [Google Scholar]
- Delattre M, Gönczy P. The arithmetic of centrosome biogenesis. J Cell Sci. 2004;117:1619–1629. doi: 10.1242/jcs.01128. [DOI] [PubMed] [Google Scholar]
- Dooley K, Zon LI. Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev. 2000;10:252–256. doi: 10.1016/s0959-437x(00)00074-5. [DOI] [PubMed] [Google Scholar]
- Dosch R, Wagner DS, Mintzer KA, Runke G, Wiemelt AP, Mullins MC. Maternal control of vertebrate development before the midblastula transition: mutants from the zebrafish I. Dev Cell. 2004;6:771–780. doi: 10.1016/j.devcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Driever W, Stemple D, Schier A, Solnica-Krezel L. Zebrafish: genetic tools for studying vertebrate development. Trends Genet. 1994;10:152–159. doi: 10.1016/0168-9525(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol. 2008;315:257–279. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinson RP. Site of sperm entry and a cortical contraction associated with egg activation in the frog Rana pipiens. Dev Biol. 1975;47:257–268. doi: 10.1016/0012-1606(75)90281-x. [DOI] [PubMed] [Google Scholar]
- Fernández J, Valladares M, Fuentes R, Ubilla A. Reorganization of cytoplasm in the zebrafish oocyte and egg during early steps of ooplasmic segregation. Dev Dyn. 2006;235:656–671. doi: 10.1002/dvdy.20682. [DOI] [PubMed] [Google Scholar]
- Fluck RA, Miller AL, Jaffe LF. Slow Calcium Waves Accompany Cytokinesis in Medaka Fish eggs. The Journal of Cell Biology. 1991;115:1259–1265. doi: 10.1083/jcb.115.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. Cleavage furrow positioning. J Cell Biol. 2004;164:347–351. doi: 10.1083/jcb.200310112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. Centrosome function during stem cell division: the devil is in the details. Curr Opin Cell Biol. 2008;20:694–698. doi: 10.1016/j.ceb.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Guraya SS. In: The cell and molecular biology of fish oogenesis. Sauer HW, editor. New York: Karger; 1969. [PubMed] [Google Scholar]
- Hart NH, Becker KA, Wolenski JS. The sperm entry site during fertilization of the zebrafish egg: localization of actin. Mol Rep Dev. 1992;32:217–228. doi: 10.1002/mrd.1080320306. [DOI] [PubMed] [Google Scholar]
- Hart NH, Fluck RA. Cytoskeleton in teleost eggs and early embryos: contributions to cytoarchitecture and motile events. Curr Top Dev Biol. 1995;31:343–381. doi: 10.1016/s0070-2153(08)60233-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Maegawa S, Nagai T, Yamaha E, Suzuki H, Yasuda K, Inoue K. Localized maternal factors are required for zebrafish germ cell formation. Dev Biol. 2004;268:152–161. doi: 10.1016/j.ydbio.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Suzuki H, Kageyama H, Yasuda K, Inoue K. Bruno-like protein is localized to zebrafish germ plasm during the early cleavage stages. Gene Exp Patterns. 2006;6:201–205. doi: 10.1016/j.modgep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hiiragi T, Solter D. Mechanism of first cleavage specification in the mouse egg: is our body plan set at day 0? Cell Cycle. 2005;5:661–664. doi: 10.4161/cc.4.5.1680. [DOI] [PubMed] [Google Scholar]
- Horner VL, Wolfner MF. Transitioning from egg to embryo: triggers and mechanisms of egg activation. Dev Dyn. 2008;237:527–544. doi: 10.1002/dvdy.21454. [DOI] [PubMed] [Google Scholar]
- Houston DW, Zhang J, Maines JZ, Wasserman SA, King ML. A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule. Development. 1998;125:171–180. doi: 10.1242/dev.125.2.171. [DOI] [PubMed] [Google Scholar]
- Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, van den Elst H, Filippov DV, Blaser H, Raz E, Moens CB, Plasterk RHA, Hannon GJ, Draper BW, Ketting RF. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell. 2007;129:69–82. doi: 10.1016/j.cell.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T, Kobayashi H. Electron microscopic observations of karyogamy in the fish egg. Dev Growth Differ. 2002;5:357–363. doi: 10.1046/j.1440-169x.2002.00649.x. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T, Ohta T. Electron microscopic observation on sperm penetration and pronuclear formation in the fish egg. J Exp Zool. 1978;205:157–180. doi: 10.1002/jez.1402050202. [DOI] [PubMed] [Google Scholar]
- Jesuthasan S. Furrow-associated microtubule arrays are required for the cohesion of zebrafish blastomeres following cytokinesis. J Cell Sci. 1998;111:3695–3703. doi: 10.1242/jcs.111.24.3695. [DOI] [PubMed] [Google Scholar]
- Jesuthasan S, Strähle U. Dynamic microtubules and specification of the zebrafish embryonic axis. Curr Biol. 1997;7:31–42. doi: 10.1016/s0960-9822(06)00025-x. [DOI] [PubMed] [Google Scholar]
- Kane DA, Kimmel CB. The zebrafish midblastula transition. Development. 1993;119:447–456. doi: 10.1242/dev.119.2.447. [DOI] [PubMed] [Google Scholar]
- Kato Y, Habas R, Katsuyama Y, Näär AM, He X. A component of the ARC/Mediator complex required for TGFβ/Nodal signalling. Nature. 2002;418:641–646. doi: 10.1038/nature00969. [DOI] [PubMed] [Google Scholar]
- Kedde M, Strasser MJ, Boldajipour B, Oude Vrielink JAF, Slanchev K, le Sage C, Nagel R, Voorhoeve M, van Duijse J, Andersson Ørom U, Lund AH, Perrakis A, Raz E, Agami R. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell. 2007;131:1273–1286. doi: 10.1016/j.cell.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Strome S. Fertilization and establishment of polarity in the embryo. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Cold Spring Harbor Laboratory Press; 1997. pp. 335–359. [PubMed] [Google Scholar]
- Kessel RG, Beams HW, Tung HN, Roberts R. Unusual particle arrays in the plasma membrane of zebrafish spermatozoa. J Ultrastruct Res. 1983;84:268–274. [Google Scholar]
- Kimmel C, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development in the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimmel CB. Genetics and early development of zebrafish. Trends Genet. 1989;5:283–288. doi: 10.1016/0168-9525(89)90103-0. [DOI] [PubMed] [Google Scholar]
- Kimura A, Onami S. Computer simulations and image processing reveal length-dependent pulling force as the primary mechanism for C. elegans male pronuclear migration. Dev Cell. 2005;8:765–775. doi: 10.1016/j.devcel.2005.03.007. [DOI] [PubMed] [Google Scholar]
- King ML, Messitt TJ, Mowry KL. Putting RNAs in the right place at the right time: RNA localization in the frog oocyte. Biol Cell. 2005;1:19–33. doi: 10.1042/BC20040067. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Koshida S, Furutani-Seiki M, Kondoh H. Zebrafish maternal-effect mutations causing cytokinesis defects without affecting mitosis or equatorial vasa deposition. Mech Dev. 2004;121:79–89. doi: 10.1016/j.mod.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kloc M, Etkin LD. RNA localization mechanisms in oocytes. J Cell Sci. 2005;118:269–282. doi: 10.1242/jcs.01637. [DOI] [PubMed] [Google Scholar]
- Kloc M, Jaglarz M, Dougherty M, Steward MD, Nel-Themaat L, Bilinski S. Mouse early oocytes are transiently polar: three dimensional and ultrastructural analysis. Exp Cell Res. 2008;17:3245–3254. doi: 10.1016/j.yexcr.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaut H, Pelegri F, Bohmann K, Schwarz H, Nüsslein-Volhard C. Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically prior to germ line specification. J Cell Biol. 2000;149:875–888. doi: 10.1083/jcb.149.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaut H, Steinbeisser H, Schwarz H, Nüsslein-Volhard C. An evolutionary conserved region in the vasa 3′UTR targets RNA translation to the germ cells in the zebrafish. Curr Biol. 2002;12:454–466. doi: 10.1016/s0960-9822(02)00723-6. [DOI] [PubMed] [Google Scholar]
- Köprunner M, Thisse C, Thisse B, Raz E. A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes Dev. 2001;15:2877–2885. doi: 10.1101/gad.212401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K, Kawakami K, Sakamoto H, Inoue K. Spatiotemporal localization of germ plasm RNAs during zebrafish oogenesis. Mech Dev. 2007;124:279–289. doi: 10.1016/j.mod.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Lee KW, Webb SE, Miller AL. Ca2+ released via IP3 receptors is required for furrow deepening during cytokinesis in zebrafish embryos. Int J Dev Biol. 2003;47:411–421. [PubMed] [Google Scholar]
- Leidel S, Delattre M, Cerutti L, Baumer K, Gönczy P. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nature Cell Biol. 2005;7:115–124. doi: 10.1038/ncb1220. [DOI] [PubMed] [Google Scholar]
- Leidel S, Gönczy P. Centrosome duplication and nematodes: recent insights from an old relationship. Dev Cell. 2005;9:317–325. doi: 10.1016/j.devcel.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Lénárt P, Bacher CP, Daigle N, Hand AR, Eils R, Terasaki M, Ellenberg J. A contractile nuclear actin network drives chromosome congression in oocytes. Nature. 2005;436:812–818. doi: 10.1038/nature03810. [DOI] [PubMed] [Google Scholar]
- Leung CF, Webb SE, Miller AL. On the mechanism of ooplasmic segregation in single-cell zebrafish embryos. Dev Growth Differ. 2000;42:29–40. doi: 10.1046/j.1440-169x.2000.00484.x. [DOI] [PubMed] [Google Scholar]
- Li WM, Webb SE, Chan CM, Miller AL. Multiple roles of the furrow deepening Ca2+ transient during cytokinesis in zebrafish embryos. Dev Biol. 2008;316:228–248. doi: 10.1016/j.ydbio.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Longo FJ. Pronuclear events during fertilization. In: Metz CB, Monroy A, editors. Biology of Fertilization. Orlando: Academic Press; 1985. pp. 251–298. [Google Scholar]
- Lykke-Andersen K, Gilchrist MJ, Grabarek JB, Das P, Miska E, Zernicka-Goetz M. Maternal argonaute 2 is essential for early mouse development at the maternal-zygotic transition. Mol Biol Cell. 2008;19:4383–4392. doi: 10.1091/mbc.E08-02-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman-Gingerich J, Pelegri F. Maternal factors in fish oogenesis and embryonic development. In: Babin PJ, Cerdá J, Lubzens E, editors. The fish oocyte: from basic studies to biotechnological applications. Dordrecht: Springer; 2007. pp. 141–174. [Google Scholar]
- MacArthur H, Houston DW, Bubunenko M, Mosquera L, King ML. DEADSouth is a germ plasm specific DEAD-box RNA helicase in Xenopus related to eIF4A. Mech Dev. 2000;95:291–295. doi: 10.1016/s0925-4773(00)00357-9. [DOI] [PubMed] [Google Scholar]
- Maegawa S, Yamashita M, Yasuda K, Inoue K. Zebrafish DAZ-like protein controls translation via the sequence ‘CUUC’. Genes Cells. 2002;7:971–984. doi: 10.1046/j.1365-2443.2002.00576.x. [DOI] [PubMed] [Google Scholar]
- Maegawa S, Yasuda K, Inoue K. Maternal mRNA localization of zebrafish DAZ-like gene. Mech Dev. 1999;81:223–226. doi: 10.1016/s0925-4773(98)00242-1. [DOI] [PubMed] [Google Scholar]
- Manandhar G, Simerly C, Schatten G. Centrosome reduction during mammalian spermiogenesis. Curr Top Dev Biol. 2000;49:343–363. doi: 10.1016/s0070-2153(99)49017-9. [DOI] [PubMed] [Google Scholar]
- Marlow FL, Mullins MC. Bucky ball functions in Balbiani body assembly and animal-vegetal polarity in the oocyte and follicle cell layer in zebrafish. Dev Biol. 2008;321:40–50. doi: 10.1016/j.ydbio.2008.05.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- Minakhina S, Stewards R. Axes formation and RNA localization. Curr Opin Genet Dev. 2005;15:416–421. doi: 10.1016/j.gde.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Mishima Y, Giraldez AJ, Takeda Y, Fujiwara T, Sakamoto H, Schier AF, Inoue K. Differential regulation of germline mRNAs in soma and germ cells by zebrafish miR-430. Curr Biol. 2006;16:2135–2142. doi: 10.1016/j.cub.2006.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquera L, Forristall C, Zhou Y, King ML. A mRNA localized to the vegetal cortex of Xenopus oocytes encodes a protein with a nanos-like zinc finger domain. Development. 1993;117:377–386. doi: 10.1242/dev.117.1.377. [DOI] [PubMed] [Google Scholar]
- Motosugi N, Bauer T, Polanski Z, Solter D, Hiiragi T. Polarity of the mouse embryo is established at blastocyst and is not prepatterned. Genes Dev. 2005;19:1081–1092. doi: 10.1101/gad.1304805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata-Hori M, Wang Y-I. The kinase activity of Aurora B is required for kinetochore-microtubule interactions during mitosis. Curr Biol. 2002;12:894–899. doi: 10.1016/s0960-9822(02)00848-5. [DOI] [PubMed] [Google Scholar]
- Navara CS, First NL, Schatten G. Microtubule organization in the cow during fertilization, polyspermy, parthenogenesis, and nuclear transfer: the role of the sperm aster. Dev Biol. 1994;1:29–40. doi: 10.1006/dbio.1994.1064. [DOI] [PubMed] [Google Scholar]
- O’Connell KF, Caron C, Kopish KR, Hurd DD, Kemphues KJ, Li Y, White JG. The C. elegans zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell. 2001;105:547–558. doi: 10.1016/s0092-8674(01)00338-5. [DOI] [PubMed] [Google Scholar]
- Otegui MS, Verbrugghe KJ, Skop AR. Midbodies and phragmoblasts: analogous structures involved in cytokinesis. Trends Cell Biol. 2005;15:404–413. doi: 10.1016/j.tcb.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C, Rawe V, Ramalho-Santos V, Simerly C, Schatten G. Preferentially localized dynein and perinuclear dynactin associate with nuclear pore complex proteins to mediate genomic union during mammalian fertilization. J Cell Sci. 2003a;23:4727–4738. doi: 10.1242/jcs.00784. [DOI] [PubMed] [Google Scholar]
- Payne C, StJohn JC, Ramalho-Santos J, Schatten G. LIS1 association with dynactin is required for nuclear motility and genomic union in the fertilized mammalian oocyte. Cell Motil Cytoskeleton. 2003b;4:245–251. doi: 10.1002/cm.10151. [DOI] [PubMed] [Google Scholar]
- Peel N, Stevens NR, Basto R, Raff JW. Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr Biol. 2007;17:834–843. doi: 10.1016/j.cub.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegri F, Dekens MPS, Schulte-Merker S, Maischein H-M, Weiler C, Nüsslein-Volhard C. Identification of recessive maternal-effect mutations in the zebrafish using a gynogenesis-based method. Dev Dyn. 2004;231:325–336. doi: 10.1002/dvdy.20145. [DOI] [PubMed] [Google Scholar]
- Pelegri F, Knaut H, Maischein H-M, Schulte-Merker S, Nüsslein-Volhard C. A mutation in the zebrafish maternal-effect gene nebel affects furrow formation and vasa RNA localization. Curr Biol. 1999;9:1431–1440. doi: 10.1016/s0960-9822(00)80112-8. [DOI] [PubMed] [Google Scholar]
- Pelegri F, Mullins M. Genetic screens for maternal-effect mutations. In: Detrich HW, Westerfield M, Zon LI, editors. The Zebrafish: 2nd Edition Genetics, Genomics and informatics. San Diego: Elsevier Academic Press; 2004. pp. 21–51. [Google Scholar]
- Pelegri F, Schulte-Merker S. A gynogenesis-based screen for maternal-effect genes in the zebrafish, Danio rerio. In: Detrich W, Zon LI, Westerfield M, editors. The Zebrafish: Genetics and Genomics. San Diego: Academic Press; 1999. pp. 1–20. [DOI] [PubMed] [Google Scholar]
- Putiri E, Pelegri F. Passing the torch at the oocyte to embryo transition. Trends Dev Biol. 2009 In press. [Google Scholar]
- Reinsch S, Gonczy P. Mechanisms of nuclear positioning. J Cell Sci. 1998;16:2283–2295. doi: 10.1242/jcs.111.16.2283. [DOI] [PubMed] [Google Scholar]
- Reinsch S, Karsenti E. Movement of nuclei along microtubules in Xenopus egg extracts. Curr Biol. 1997;3:211–214. doi: 10.1016/s0960-9822(97)70092-7. [DOI] [PubMed] [Google Scholar]
- Robb DL, Heasman J, Raats J, Wylie C. A kinesin-like protein is required for germ plasm aggregation in Xenopus. Cell. 1996;87:823–831. doi: 10.1016/s0092-8674(00)81990-x. [DOI] [PubMed] [Google Scholar]
- Rouviere C, Houliston E, Carre D, Chang P, Sardet C. Characteristics of pronuclear migration in Beroe ovata. Cell Motil Cytoskeleton. 1994;4:301–311. doi: 10.1002/cm.970290403. [DOI] [PubMed] [Google Scholar]
- Roy A, Matzuk MM. Deconstructing mammalian reproduction: using knockouts to define fertility pathways. Reproduction. 2006;131:207–219. doi: 10.1530/rep.1.00530. [DOI] [PubMed] [Google Scholar]
- Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- Saga Y. Mouse germ cell development during embryogenesis. Curr Opin Genet Dev. 2008;18:337–341. doi: 10.1016/j.gde.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Sardet C, Prodon F, Dumollard R, Chang P, Chenevert J. Structure and function of the egg cortex from oogenesis through fertilization. Dev Biol. 2002;241:1–23. doi: 10.1006/dbio.2001.0474. [DOI] [PubMed] [Google Scholar]
- Schatten G. Motility during fertilization. Int Rev Cytol. 1982;79:59–163. doi: 10.1016/s0074-7696(08)61673-3. [DOI] [PubMed] [Google Scholar]
- Schatten G. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol. 1994;165:299–335. doi: 10.1006/dbio.1994.1256. [DOI] [PubMed] [Google Scholar]
- Seki S, Kouya T, Tsuchiya R, Valdez DM, Jin B, Hara T, Saida N, Kasai M, Edashige K. Development of a reliable in vitro maturation system for zebrafish oocytes. Reproduction. 2008;135:285–292. doi: 10.1530/REP-07-0416. [DOI] [PubMed] [Google Scholar]
- Selman K, Walace RA, Sarka A, Qi X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J Morphol. 1993;218:203–224. doi: 10.1002/jmor.1052180209. [DOI] [PubMed] [Google Scholar]
- Shibata N, Umesono Y, Orii H, Sajurai T, Watanabe K, Agata K. Expression of vasa(vas)-related genes in germline cells and totipotent somatic stem cells of planarians. Developmental Biology. 1999;206:73–87. doi: 10.1006/dbio.1998.9130. [DOI] [PubMed] [Google Scholar]
- Shibazaki Y, Shimizu M, Kuroda R. Body handedness is directed by genetically determined cytoskeletal dynamics in the early embryo. Curr Biol. 2004;16:1462–1467. doi: 10.1016/j.cub.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Song MH, Miliaras NB, Peel N, O’Connell KF. Centrioles: some self-assembly required. Curr Opin Cell Biol. 2008;20:688–693. doi: 10.1016/j.ceb.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D, Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992;68:201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio) Nature. 1981;291:293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- Strnad P, Gönczy P. Mechanisms of procentriole formation. Trends Cell Biol. 2008;18:389–396. doi: 10.1016/j.tcb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Strnad P, Leidel S, Vinogradova T, Euteneuer U, Khodjakov A, Gönczy P. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev Cell. 2007;13:203–213. doi: 10.1016/j.devcel.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S, Hill DP. Early embryogenesis in Caenorhabditis elegans: the cytoskeleton and spatial organization of the zygote. BioEssays. 1988;5:145–149. doi: 10.1002/bies.950080504. [DOI] [PubMed] [Google Scholar]
- Sturtevant AH. Inheritance of direction of coiling in Limnaea. Science. 1923;1501:269–270. doi: 10.1126/science.58.1501.269. [DOI] [PubMed] [Google Scholar]
- Sun Q-Y, Liu K, Kikuchi K. Oocyte-specific knockout: a novel in vivo approach for studying gene functions during folliculogenesis, oocyte maturation, fertilization, and embryogenesis. Biol Reprod. 2008;79:1014–1020. doi: 10.1095/biolreprod.108.070409. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Maegawa S, Nishibu T, Sugiyama T, Yasuda K, Inoue K. Vegetal localization of the maternal mRNA encoding an EDEN-BP/Bruno-like protein in zebrafish. Mech Dev. 2000;93:205–209. doi: 10.1016/s0925-4773(00)00270-7. [DOI] [PubMed] [Google Scholar]
- Tang F, Kaneda M, O’Carroll D, Hajkova P, Barton SC, Sun YA, Lee C, Tarkhovsky A, Lao K, Surani MA. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007;21:644–648. doi: 10.1101/gad.418707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y, Tatsuka M, Suzuki F, Yasuda Y, Fujita W, Otsu M. AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO J. 1998;17:667–676. doi: 10.1093/emboj/17.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theusch EV, Brown KJ, Pelegri F. Separate pathways of RNA recruitment lead to the compartmentalization of the zebrafish germ plasm. Dev Biol. 2006;292:129–141. doi: 10.1016/j.ydbio.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Tsunekawas N, Noito M, Sakai Y, Nishida T, Noce T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development. 2000;127:2741–2750. doi: 10.1242/dev.127.12.2741. [DOI] [PubMed] [Google Scholar]
- Urven LE, Yabe T, Pelegri F. A role for non-muscle myosin II function in furrow maturation in the early zebrafish embryo. J Cell Sci. 2006;119:4342–4352. doi: 10.1242/jcs.03197. [DOI] [PubMed] [Google Scholar]
- Vladar EK, Stearns T. Molecular characterization of centriole assembly in ciliated epithelial cells. J Cell Biol. 2007;178:31–42. doi: 10.1083/jcb.200703064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth P, Khodjakov A. E pluribus unum: towards a universal mechanism for spindle assembly. Trends Cell Biol. 2004;14:413–419. doi: 10.1016/j.tcb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wagner DS, Dosch R, Mintzer KA, Wiemelt AP, Mullins MC. Maternal control of development at the midblastula transition and beyond: mutants from the zebrafish II. Dev Cell. 2004;6:781–790. doi: 10.1016/j.devcel.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Walker C. Haploid screens and gamma-ray mutagenesis. In: Detrich WH, Westerfield M, Zon LI, editors. The zebrafish: Genetics and genomics. San Diego: Academic Press; 1999. pp. 43–70. [Google Scholar]
- Wan L-B, Pan H, Hannenhalli S, Cheng Y, Ma J, Fedoriw A, Lobanenkov V, Latham KE, Schultz RM, Bartolomei MS. Maternal depletion of CTCF reveals multiple functions during oocyte and preimplantation embryo development. Development. 2008;135:2729–2738. doi: 10.1242/dev.024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SE, Lee KW, Karplus E, Miller AL. Localized calcium transients accompany furrow positioning, propagation, and deepening during the early cleavage period of zebrafish embryos. Dev Biol. 1997;192:78–92. doi: 10.1006/dbio.1997.8724. [DOI] [PubMed] [Google Scholar]
- Webb SE, Miller AL. Calcium signalling during zebrafish embryonic development. Bioessays. 2000;22:113–123. doi: 10.1002/(SICI)1521-1878(200002)22:2<113::AID-BIES3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Weidinger G, Stebler J, Slanchev K, Dumstrei K, Wise C, Lovell-Badge R, Thisse C, Thisse B, Raz E. dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol. 2003;13:1429–1434. doi: 10.1016/s0960-9822(03)00537-2. [DOI] [PubMed] [Google Scholar]
- Wolf N, Hirsh D, McIntosh JR. Spermatogenesis in males of the free-living nematode, Caenorhabditis elegans. J Ultrastruct Res. 1978;63:155–169. doi: 10.1016/s0022-5320(78)80071-9. [DOI] [PubMed] [Google Scholar]
- Wolenski JS, Hart NH. Sperm incorporation independent of fertilization cone formation in the danio egg. Dev Growth Diff. 1988;30:619–628. doi: 10.1111/j.1440-169X.1988.00619.x. [DOI] [PubMed] [Google Scholar]
- Wolke U, Widinger G, Köprunner M, Raz E. Multiple levels of postranscriptional control lead to germ line-specific gene expression in the zebrafish. Curr Biol. 2002;12:289–294. doi: 10.1016/s0960-9822(02)00679-6. [DOI] [PubMed] [Google Scholar]
- Wylie C. Germ cells. Curr Opin Genet Dev. 2000;10:410–413. doi: 10.1016/s0959-437x(00)00105-2. [DOI] [PubMed] [Google Scholar]
- Wylie CC, Haessman J. What my mother told me: examining the roles of maternal gene products in a vertebrate. TCB. 1997;7:459–461. doi: 10.1016/S0962-8924(97)01151-3. [DOI] [PubMed] [Google Scholar]
- Yabe T, Ge X, Lindeman R, Nair S, Runke G, Mullins M, Pelegri F. The maternal-effect gene cellular island encodes Aurora B kinase and is involved in furrow formation and microtubule reorganization in the early zebrafish embryo. PLoS Genet. 5:e100518. doi: 10.1371/journal.pgen.1000518. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe T, Ge X, Pelegri F. The zebrafish maternal-effect gene cellular atoll encodes the centriolar component Sas-6 and defects in its paternal function promote whole genome duplication. Dev Biol. 2007;312:44–60. doi: 10.1016/j.ydbio.2007.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagani K, Hamazaki TS, Yasumasu S, Masuda K, Iuchi I. Molecular and cellular basis of formation, hardening, and breakdown of the egg envelope in fish. Int Rev Cytol. 1992;136:51–92. doi: 10.1016/s0074-7696(08)62050-1. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Fuller MT. Asymmetric centrosome behavior and the mechanisms of stem cell division. J Cell Biol. 2008;180:261–266. doi: 10.1083/jcb.200707083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Kawakami K, Hopkins N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development. 1997;124:3157–3165. doi: 10.1242/dev.124.16.3157. [DOI] [PubMed] [Google Scholar]