Abstract
Introduction:
PRONOUNCE compared the efficacy and safety of pemetrexed+carboplatin followed by pemetrexed (Pem+Cb) with paclitaxel+carboplatin+bevacizumab followed by bevacizumab (Pac+Cb+Bev) in patients with advanced nonsquamous non–small-cell lung cancer (NSCLC).
Methods:
Patients ≥18 years of age with stage IV nonsquamous NSCLC (American Joint Committee on Cancer v7.0), and Eastern Cooperative Oncology Group performance status 0/1 were randomized (1:1) to four cycles of induction Pem+Cb (pemetrexed, 500 mg/m2, carboplatin, area under the curve = 6) followed by Pem maintenance or Pac+Cb+Bev (paclitaxel, 200 mg/m2, carboplatin, area under the curve = 6, and bevacizumab, 15 mg/kg) followed by Bev maintenance in the absence of progressive disease or discontinuation. The primary objective was progression-free survival (PFS) without grade 4 toxicity (G4PFS). Secondary end points were PFS, overall survival (OS), overall response rate (ORR), disease control rate (DCR), and safety. Resource utilization was also assessed.
Results:
Baseline characteristics of the patients randomized to Pem+Cb (N = 182) and Pac+Cb+Bev (N = 179) were well balanced between the arms. Median (months) G4PFS was 3.91 for Pem+Cb and 2.86 for Pac+Cb+Bev (hazard ratio = 0.85, 90% confidence interval, 0.7–1.04; p = 0.176); PFS, OS, ORR, or DCR did not differ significantly between the arms. Significantly more drug-related grade 3/4 anemia (18.7% versus 5.4%) and thrombocytopenia (24.0% versus 9.6%) were reported for Pem+Cb. Significantly more grade 3/4 neutropenia (48.8% versus 24.6%), grade 1/2 alopecia (28.3% versus 8.2%), and grade 1/2 sensory neuropathy were reported for Pac+Cb+Bev. Number of hospitalizations and overall length of stay did not differ significantly between the arms.
Conclusions:
Pem+Cb did not produce significantly better G4PFS compared with Pac+Cb+Bev. Pem+Cb was not superior in PFS, OS, ORR, or DCR compared with Pac+Cb+Bev. Both regimens were well tolerated, although, toxicity profiles differed.
Keywords: Advanced nonsquamous non-small-cell lung cancer, Efficacy, Safety, Combination therapy, Pemetrexed, Carboplatin, Paclitaxel, Bevacizumab
Lung cancer is the leading cause of cancer mortality in the United States accounting for more than a quarter of cancer deaths for both men and women.1 Non–small-cell lung cancer (NSCLC) accounts for ~85% of the lung cancer histologies in the United States, and 70% of the patients with NSCLC present with inoperable, locally advanced (stage III) or metastatic (stage IV) disease.2
Patients with stage IV NSCLC and Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 or 1 benefit in survival and quality of life from standard platinum doublet chemotherapy.3–6 No randomized trial has demonstrated superiority of one platinum-based doublet over another for unselected patients.7–10 The three-drug regimen of carboplatin/paclitaxel/bevacizumab induction followed by bevacizumab maintenance was approved for nonsquamous NSCLC based on the well-known ECOG 4599 clinical trial.11 This regimen has been adopted as the new ECOG standard for patients eligible to receive bevacizumab.11 Currently in the United States, less than half of all the new lung cancer diagnoses are made in patients who would have met the eligibility criteria for ECOG 4599 and it is usually estimated that no more than a third of the lung cancer patients in the United States are treated with this regimen.12
Pemetrexed is a multitargeted antifolate approved for first-line, second-line, and maintenance treatment of nonsquamous NSCLC.13–16 A prespecified analysis of a large phase III first-line study showed that pemetrexed/cisplatin produced superior overall survival (OS) to gemcitabine/cisplatin for patients with nonsquamous NSCLC.14,17 In first-line maintenance, pemetrexed was superior to placebo for OS in large phase III studies after initial chemotherapy with pemetrexed or taxane-based regimens.15–17 Superior OS with pemetrexed versus docetaxel also was confirmed in a post hoc analysis of second-line study data for nonsquamous histologies.18 In the US, cisplatin doublets are less often used than carboplatin doublets, and although it has not been directly compared in a phase III trial, pemetrexed/carboplatin is more often used in clinical practice in the US.19,20
PRONOUNCE was designed to assess the efficacy and safety of pemetrexed+carboplatin followed by pemetrexed maintenance (Pem+Cb) versus paclitaxel+carbo platin+bevacizumab followed by bevacizumab maintenance (Pac+Cb+Bev) in patients with advanced nonsquamous NSCLC. PRONOUNCE was designed as a supportive study for the PointBreak study and has similar patient eligibility and monitoring.21
A maximum of four cycles of induction therapy was chosen based on recent reviews and meta-analyses confirming four cycles as the optimal duration of first-line platinum combination therapy.22–25 G4PFS defined by the Common Terminology Criteria for Adverse Events (CTCAE) is a composite end point that includes both efficacy and safety outcomes. The G4PFS primary end point is reasonable for clinical trials comparing regimens where efficacy is likely to be similar, making a less toxic regimen clinically relevant, particularly in the noncurative setting.21,26 The hypothesis for PRONOUNCE trial was that Pem+Cb would be superior to Pac+Cb+Bev for G4PFS.
MATERIALS AND METHODS
Eligibility Criteria
Chemotherapy naïve adults (≥18 years of age) with histologically or cytologically confirmed stage IV (American Joint Committee on Cancer, version 7) nonsquamous NSCLC, ECOG PS 0 or 1, measurable disease by Response Evaluation Criteria in Solid Tumors,27 and adequate organ function were eligible. Stable, treated brain metastases were allowed. Patients were required to be eligible for both pemetrexed and bevacizumab. Women of childbearing potential were required to have a negative pregnancy test, and all the patients for whom pregnancy was a risk were required to use effective contraception. The patients were excluded for any contraindications for pemetrexed or bevacizumab or for general radiotherapy within 2 weeks, stereotactic brain radiotherapy within 7 days, major surgery within 28 days, minor surgery within 7 days, neurosurgical resection or brain biopsy within 3 months before day 1, cycle 1. Additional exclusions included the use of an investigational agent within 30 days of randomization and any serious concomitant disorder that could compromise the ability to adhere to the protocol.
The study protocol was approved by ethical review boards at each of the participating investigational sites and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All the patients signed written informed consent before the initiation of any study interventions.
Study Design, Treatment, and End points
In this multicenter, randomized, open-label, US-only phase III trial, the eligible patients with nonsquamous NSCLC were randomized in a 1:1 ratio to Pem+Cb or Pac+Cb+Bev by standard protocols including B12 and folate supplementation for pemetrexed and dexamethasone premedication for both the arms. Patients in the Pac+Cb+Bev arm received additional standard prophylaxis against allergic reactions according to the paclitaxel label. Randomization was stratified for disease stage (M1a versus M1b), ECOG PS, and sex. After four cycles of induction therapy every 21 days, maintenance continued until disease progression or intolerance. Planned chemotherapy doses were pemetrexed 500 mg/m2; carboplatin, area under the curve = 6, (as of December 31, 2010, maximum possible dose of 900 mg), paclitaxel 200 mg/m2; bevacizumab 15 mg/kg.
Any required dose reductions, per label indication for each drug or regimen, were maintained for the remainder of the study per CTCAE.28 A maximum of two dose reductions were permitted before the patients were discontinued from the study therapy. Bevacizumab doses were not reduced due to toxicity but bevacizumab was interrupted for any unresolved adverse events (AEs). Upon resolution of select AEs, bevacizumab was resumed at the full dose at the next cycle. The maximum allowable length of any study treatment interruption was 42 days.
The primary objective of this trial was to compare G4PFS between the two treatment arms in the intent-to-treat (ITT) population.29 G4PFS was measured from the date of randomization to the earliest occurrence of the first of grade 4 AEs, disease progression, or death from any cause, regardless of whether or not the event leads to discontinuation. Secondary end points included PFS (gated secondary end point), OS, overall response rate (ORR), disease control rate (DCR = complete response + partial response + stable disease), and safety. Associated resource use data were also collected.
If a patient discontinued study therapy without disease progression, the tumor measurements were performed at 30 days and subsequently every 6 weeks (± 2 weeks) until objective disease progression. Thereafter, the patient was followed every 90 days (± 14 days) for survival.
As a post hoc analysis of the AEs that are important to patients, we have reviewed the symptoms described by Dubey et al.30 in their analysis of data from a questionnaire answered by 464 patients with lung cancer (registered in the Alliance for Lung Cancer Advocacy, Support, and Education database from 2000 to 2002).
Statistical Plan and Analyses
The study was powered for G4PFS; assuming a hazard ratio (HR) of 0.75, approximately 360 randomized patients (180 per arm) were needed for 80% power to detect superiority of the Pem+Cb arm over the Pac+Cb+Bev arm with a two-sided type I error of 0.10. PFS (gated end point) and OS were key secondary end points. Efficacy data were analyzed by ITT using all randomized patients, and safety data were evaluated using CTCAE v3 for patients who received ≥ 1 dose of study treatment (grouped as treated).
All other tests of treatment effects were conducted at a two-sided alpha level of 0.05 and all confidence intervals (CIs) were given at a two-sided 95% level, unless otherwise specified.
For the time-to-event end points, including G4PFS, PFS, and OS, Kaplan-Meier estimates and (nonstratified) log-rank test were employed. Cox regression model (non-stratified) was used to estimate the treatment HR. Fisher’s exact tests were used to compare differences in the ORR and DCR between the two treatment arms. The safety analysis included the maximum grade CTCAEs of laboratory and nonlaboratory events, serious AEs, treatment-emergent AEs, hospitalizations, transfusions, and the use of concomitant medications. Comparisons of the incidences of toxicities and resources use between the two treatment arms were performed using Fisher’s exact test.
RESULTS
Patient Disposition
A CONSORT diagram depicting the patient enrollment and disposition is presented in Figure 1. A total of 496 patients were screened from September 2009 to January 2013 with data cutoff January 31, 2013; 135 patients were excluded for eligibility (n = 105), death (n = 3), or subject decision (n = 27); and 361 patients were randomized (Pem+Cb = 182; Pac+Cb+Bev = 179). Twelve patients in each arm did not receive study treatment after randomization, and the most common reasons included patient decision, entry criteria exclusion, and AEs. At the time of data cutoff (January 31, 2013), 352 patients (n = 177 on Pem+Cb; n = 175 on Pac+Cab+Bev) had discontinued study therapy, and nine patients (n = 5 on Pem+Cb; n = 4 on Pac+Cab+Bev) were continuing study therapy.
FIGURE 1.
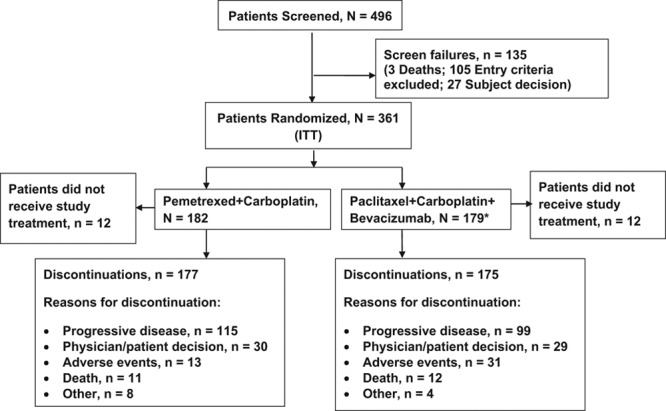
Patient CONSORT flow diagram. ITT, Intent-to-treat. *1 patient randomized to Pac+Cb+Bev but received Pem+Cb, analyzed per ITT population.
Patient Baseline Characteristics
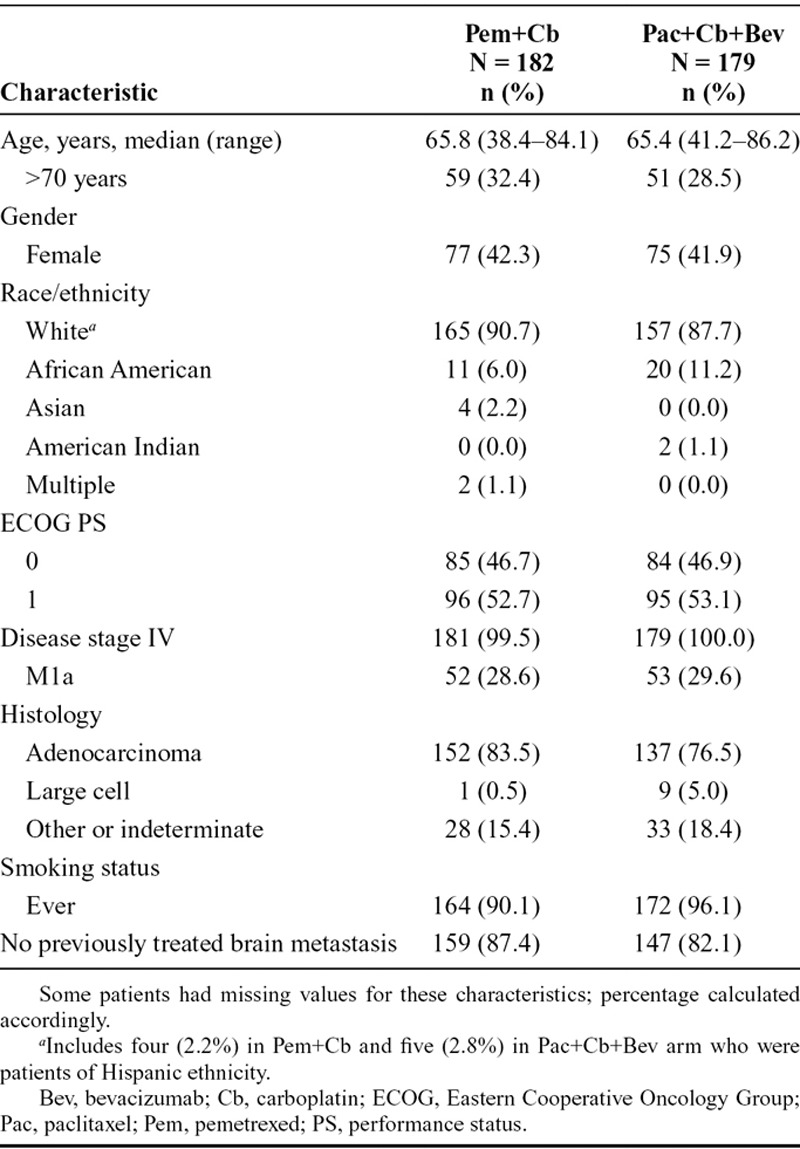
Patient baseline demographic and disease-related characteristics (ITT population) are presented in Table 1. Baseline characteristics were well balanced between the treatment arms. Most patients were white (89.2%) with median age of 65.6 (range, 38.4–86.2) years. No p values were calculated for these characteristics between the arms as the data were descriptive.
TABLE 1.
Patient Baseline Demographic and Disease Characteristics of Intent-to-Treat Population
Treatment
The ITT population included 182 in Pem+Cb and 179 in Pac+Cb+Bev. The median number of cycles (defined as 50% of the patients stay on study treatment) administered was six (range, 1–36+ Pem+Cb and 1–31+ Pac+Cb+Bev). The number of patients completing four cycles of treatment were similar between the two treatment arms: 121 patients (70.8%) in Pem+Cb arm and 113 patients (68.1%) in Pac+Cb+Bev arm. The mean dose intensities in the safety (treated) population were similar for both the arms (%, standard deviation): pemetrexed 92.3 (10.8) and carboplatin 94.1 (11.1) for the Pem+Cb arm; paclitaxel 91.5 (14.9), carboplatin 92.8 (15.5), and bevacizumab 93.3 (15.5) for the Pac+Cb+Bev arm.
Efficacy Measures
In the ITT population, 296 G4PFS events occurred with 152 in Pem+Cb and 144 in Pac+Cb+Bev. Median G4PFS was not statistically significantly different between the two treatment arms as shown by Kaplan-Meier curves (Fig. 2A). For Pem+Cb versus Pac+Cb+Bev, the median G4PFS was 3.91 versus 2.86 months (HR, 0.85, 90% CI, 0.7–1.04, p = 0.176). The median PFS was 4.44 months for Pem+Cb versus 5.49 months for Pac+Cb+Bev (HR, 1.06; 95% CI, 0.84–1.35; p = 0.610) (Fig. 2B). The median OS for Pem+Cb was 10.5 months versus 11.7 months for Pac+Cb+Bev (HR, 1.07; 95% CI, 0.83 to 1.36; p = 0.615) (Fig. 2C). One- and 2-year survival rates were not significantly different between the arms and were 43.7% and 18.0% for Pem+Cb and 48.8% and 17.6% for Pac+Cb+Bev (Fig. 2C). Response rate and DCR were 23.6% and 59.9% for Pem+Cb and 27.4% and 57.0% for Pac+Cb+Bev (p = 0.414 and 0.575, respectively) (Fig. 2B).
FIGURE 2.
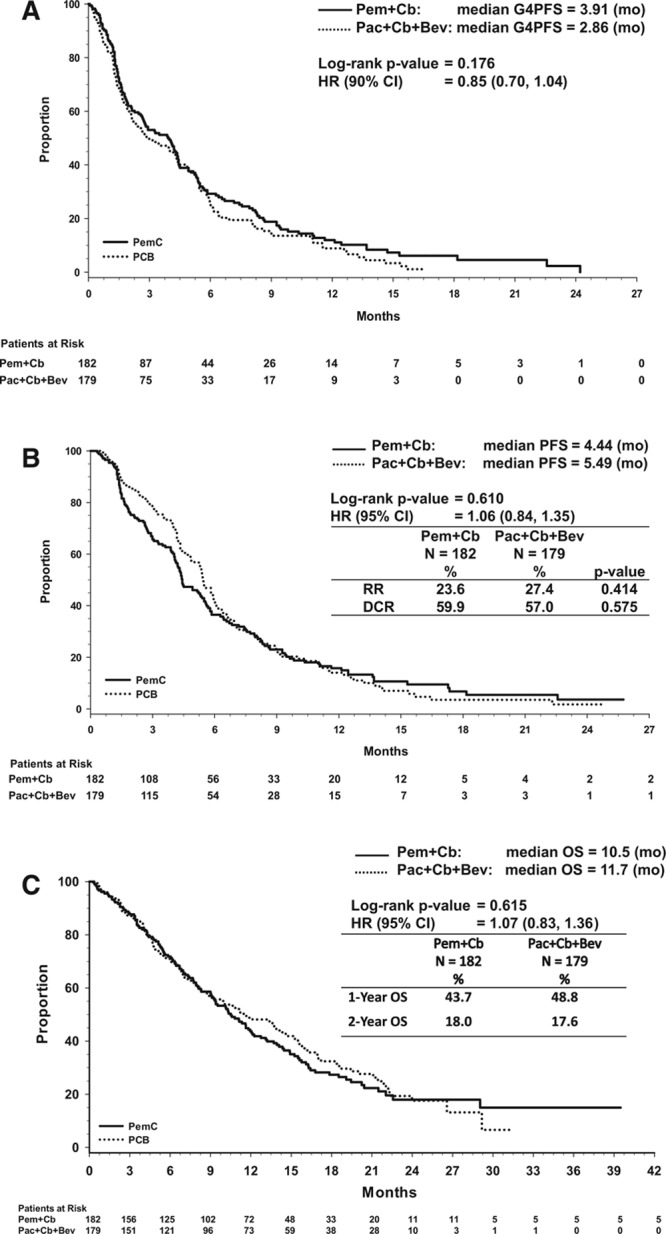
A, Kaplan-Meier plot of primary end point G4 progression-free survival, (B), Kaplan-Meier plot showing the progression-free survival in intent-to-treat population, (C), Kaplan-Meier plot showing the overall survival in intent-to-treat population. Bev, bevacizumab; Cb, carboplatin; G4PFS, grade 4 progression-free survival; HR, hazard ratio; OS, overall survival; Pac, paclitaxel; Pem, pemetrexed; PFS, progression-free survival.
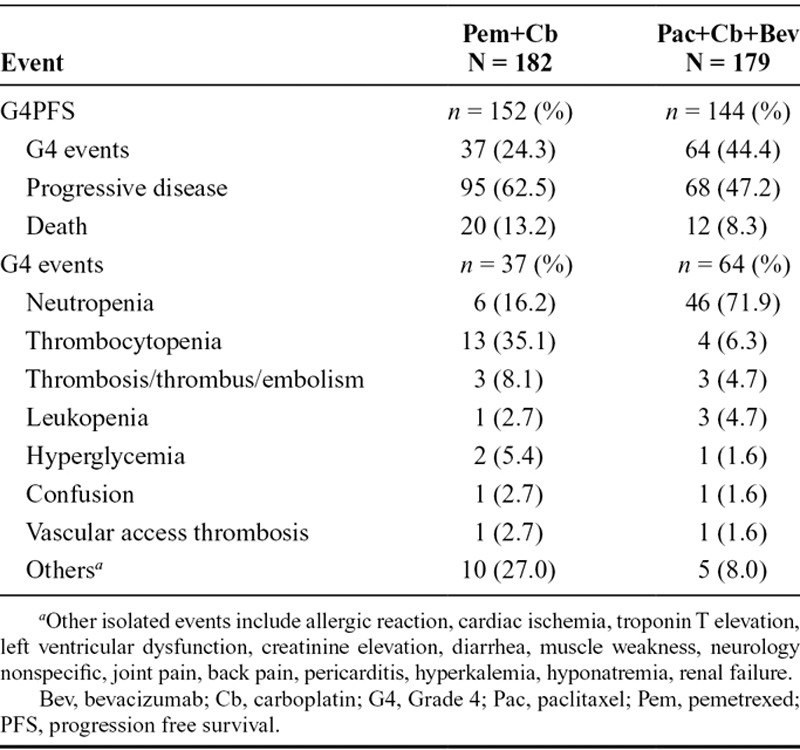
Additional analysis of which G4PFS event (G4AE, progressive disease [PD], or death) occurred first in the ITT population is shown in Table 2. A total of 296 G4PFS events were reported in both the arms. Among these G4PFS events, those occurring first were: 101 (34.1%) G4 AEs; 163 (55.1%) PD; 32 (10.8%) deaths. The most common G4PFS toxicities observed were neutropenia, thrombocytopenia, and thrombosis/embolism (Table 2). The p values for the differences between the two treatment arms were not calculated as the data were descriptive (Table 2).
TABLE 2.
First Occurrences of G4PFS Events in Intent-to-Treat Population
A sensitivity analysis was performed to evaluate the efficacy outcome for patients who received at least one cycle of study treatment (safety population). The efficacy results based on safety population are similar to that based on the ITT population (data not shown).
The preplanned subgroup analyses are shown as Forest plots for G4PFS (Fig. 3A) and for OS (Fig. 3B). The unadjusted HRs (with 95% CIs) shown in these plots do not favor any treatment subgroup.
FIGURE 3.
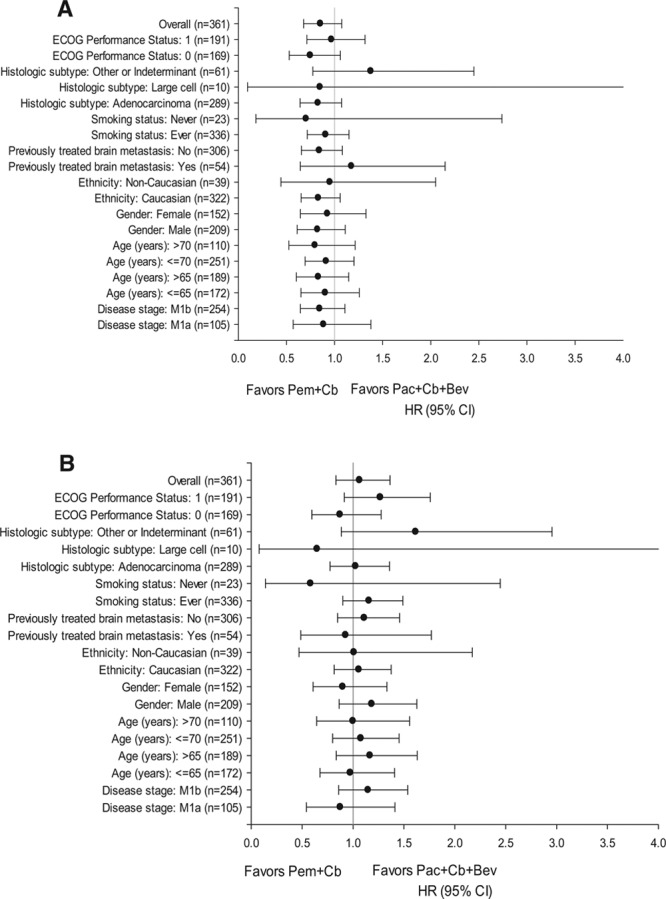
A, Subgroup analysis of G4 progression-free survival, and (B), overall survival, hazard ratio (95% CI). Bev, bevacizumab; Cb, carboplatin; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; Pac, paclitaxel; Pem, pemetrexed; CI, confidence interval.
Safety Measures
Grade 3/4 drug-related toxicities that were significantly different between the treatment arms in the safety population were (Pem+Cb versus Pac+Cb+Bev): anemia (18.7% versus 5.4%, p < 0.001), neutropenia (24.6% versus 48.8%, p < 0.001), and thrombocytopenia (24.0% versus 9.6%, p < 0.001). Hemorrhage was numerically higher in the Pem+Cb arm (1.2% versus 0.00%), and thrombosis/embolism was numerically higher in the Pac+Cb+Bev arm (0.0% versus 2.4%); these differences did not reach statistical significance. Grade 3 and grade 4 drug-related AEs in each treatment arm are presented in Table 3.
TABLE 3.
Drug-Related Grade 3 and 4 Common Terminology Criteria for Adverse Events in Patients (Safety Population)
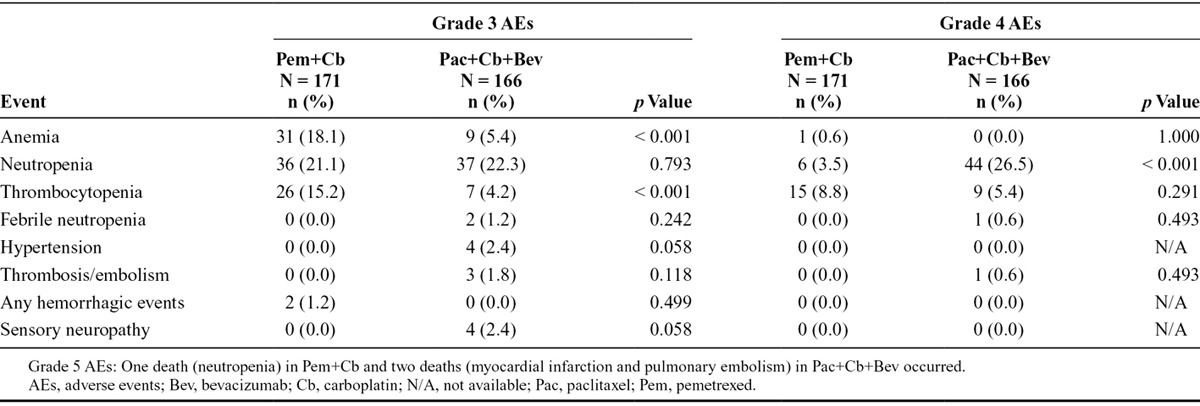
Drug-related events considered most important to patients such as nausea, vomiting, infections, alopecia, and sensory neuropathy30 were analyzed post hoc in this study. Grade 1 (21.7% versus 7.6%), and 2 (8.4% versus 0.6%), sensory neuropathy were significantly more common with Pac+Cb+Bev compared with Pem+Cb (P < 0.001). Grade 1 nausea was significantly (p = 0.003) higher in Pem+Cb (29.8%) compared with Pac+Cb+Bev (15.7%). However, grade 2 nausea was not significantly different between the two arms (17.0% versus 13.3%, p = 0.365). Grade 1 (16.3% versus 5.8%) and 2 (12.0% versus 2.3%) alopecia were significantly more common with Pac+Cb+Bev compared with Pem+Cb (p < 0.001). The other nonhematological grade 3/4 events including fatigue, febrile neutropenia, nausea, and vomiting were similar between the treatment arms. For patients with G4PFS other than PD as the initial event, the incidence of subsequent PD was 18 of 37 for arm A and 22 of 64 for arm B.
The patients who developed G4AEs during the induction phase (one to four cycles) were 33 of 37 (89.2%) in Pem+Cb and 60 of 64 (93.6%) in Pac+Cb+Bev and in the maintenance phase (> 4 cycles), 4 of 37 (10.8%) in Pem+Cb and 3 of 64 (4.8%) in Pac+Cb+Bev.
Forty-nine patients died during the study or within 30 days of discontinuation (Pem+Cb, n = 24 [14.0%]; Pac+Cb+Bev, n = 25 [15.1%]). Possible drug-related deaths were one patient (0.6%) in Pem+Cb due to neutropenia and two patients (1.2%) in Pac+Cb+Bev due to myocardial infarction and pulmonary embolism. Deaths not deemed to be related to study drug by the investigator were attributed to progression of lung cancer (8.2% versus 12.0%) and AEs of other causes (5.3% versus 1.8%) including pneumonia, respiratory failure, myocardial infarction, stroke, and renal failure in the Pem+Cb arm; and respiratory failure, intracranial bleeding, and nonspecific AEs in the Pac+Cb+Bev arm.
In the safety population, the number of patients with at least one hospitalization was not significantly different between the treatment arms (Pem+Cb, n = 59 [34.5%]; Pac+Cb+Bev, n = 53 [31.9%], p = 0.645). Similarly, the mean (SD) number of hospitalized days did not differ between the arms (Pem+Cb, 8.2 [6.79]; Pac+Cb+Bev, 8.8 [7.33], p = 0.682). Significantly, more patients treated with Pem+Cb received at least one red blood cell transfusion compared with Pac+Cb+Bev (35.7% versus 12.7%, p < 0.001). No differences were observed for platelet transfusions between the treatment arms (Pem+Cb, 5.8% versus Pac+Cb+Bev, 4.2%, p = 0.621).
Use of rescue antiemetics (64.3% in Pem+Cb versus 60.8% in Pac+Cb+Bev, p = 0.574), analgesics (88.9% in Pem+Cb versus 92.2% in Pac+Cb+Bev, p = 0.355), and antibiotics (53.2% in Pem+Cb versus 59.0% in Pac+Cb+Bev, p = 0.323) did not differ significantly between the treatment arms. Use of erythropoetic stimulating agents was significantly higher in Pem+Cb (19.9%) compared with Pac+Cb+Bev (7.2%) (p < 0.001), whereas the use of granulocyte colony stimulating factors was significantly lower in Pem+Cb (17.0%) compared with Pac+Cb+Bev (30.1%) (p = 0.005).
Postdiscontinuation Therapies
Second-line therapy and beyond was determined by the treating physician. Among the ITT population, the use of second-line treatment was similar between the two treatment arms (47.3% in Pem+Cb versus 52.5% in Pac+Cb+Bev, p = 0.344). Treatments were similar between the two treatment arms except for docetaxel (26.4% in Pem+Cb versus 6.1% in Pac+Cb+Bev, p < 0.001) and pemetrexed (8.8% in Pem+Cb versus 34.1% in Pac+Cb+Bev, p < 0.001). Other therapies administered to ≥5% of the patients but not significantly different in Pem+Cb versus Pac+Cb+Bev included: bevacizumab (7.7% versus 12.8%), carboplatin (9.9% versus 16.2%), erlotinib (9.3% versus 7.8%), gemcitabine (5.5% versus 5.0%), and paclitaxel (6.0% versus 7.3%).
DISCUSSION
The randomized phase III PRONOUNCE study demonstrated that Pem+Cb was not superior to Pac+Cb+Bev for either the primary end point of G4PFS (p = 0.176) or for the secondary efficacy end points of PFS, OS, ORR, and DCR. This trial attempted for the first time to prospectively measure the novel primary end point of G4PFS. Although G4PFS has not been validated, a previous clinical trial that showed similar survival with pemetrexed versus docetaxel13 was retrospectively analyzed for OS without grade 3/4 toxicity and suggested a benefit-to-risk favoring pemetrexed compared with docetaxel in the second-line treatment of patients with NSCLC.26 A similar primary end point (survival without grade 3/4 toxicity) was also used in a phase III clinical trial comparing pemetrexed/carboplatin with docetaxel/carboplatin as first-line treatment for advanced, nonsquamous NSCLC.31 This end point (survival without grade 3/4 toxicity) also has been analyzed post hoc in a large phase III trial.32 The current study prospectively evaluated PFS without grade 4 toxicity and did not show superior benefit-to-risk for Pem+Cb compared with Pac+Cb+Bev. Both the treatments showed similar severity and temporal onset of toxicity, although the specific toxicities differed between the arms. The G4PFS end point as applied here did not discriminate between different, similarly graded AEs that may have different significance for patient quality of life.30
The G4PFS end point was hoped to provide an indication of toxicity and efficacy. A substantial number of patients met this end point from a toxicity standpoint although still responding to their assigned regimen and continued on study. As might be expected, the majority of non-PD G4PFS end points occurred in the first four cycles of therapy during platinum doublet/triplet treatment. This provides some reassurance about the tolerability of maintenance with either pemetrexed or bevacizumab.
Regular weekly complete blood cell counts in the experimental setting compared with clinical practice may have contributed to the observed higher incidences of laboratory toxicities, sometimes referred to as paper toxicities. If the G4PFS end point is used in future studies, consideration should be given to excluding nonclinically significant laboratory toxicities and to including some lower grade toxicities such as grade 2 neuropathy or alopecia, that are considered clinically relevant to the patients. The practice of weekly laboratory testing in asymptomatic participants in clinical trials where this is not standard in a nonstudy population treated with similar regimens should be carefully considered for future studies.
The PFS and OS values reported here are comparable with other recently reported phase III trials with pemetrexed and platinum combinations and with other chemotherapy and bevacizumab combinations.11,14,21,33 Because of the small number of patients, we did not evaluate the differences in safety and efficacy in the >70 years age subgroup. Earlier studies have shown conflicting outcomes with or without differences by age groups.34,35 These data when analyzed will be reported elsewhere.
The efficacy of the control arm (Pac+Cb+Bev) in this study showed lower median survivals compared with previous trials11,21 possibly due to differences in the study populations and smaller sample size. The estimation of median OS in the control arm was 11.66 months with 95% CI (9.17–14.32), which is similar to the outcome in the ECOG 4599 study (12.3 months).11
Patients experienced acceptable and expected toxicities on both the arms of this study based upon comparable rates of total AEs reported in previous pemetrexed plus platinum-based regimens21,33 and bevacizumab combinations.11
Several AEs considered most important to patients30 including grade 1 and 2 alopecia and sensory neuropathy were significantly more common with Pac+Cb+Bev than with Pem+Cb (p < 0.001), whereas grade 1 nausea was significantly more common in patients receiving Pem+Cb (p = 0.003). Other grades of sensory neuropathy, febrile neutropenia, fatigue, nausea, and vomiting were similar between the treatment arms. The specific toxicity profiles and resource use differed by regimen.
In addition to the traditionally measured toxicities, financial burden is becoming recognized as an additional toxicity that may affect a patient’s potential course of treatment or quality of life.36–39 Using data from the PRONOUNCE trial, a post hoc cost analysis estimated differences in costs of treatment and related patient care between the two study regimens. Clinical data, resource use, and postdiscontinuation therapy data were used to estimate preprogression and postprogression inputs to which the respective 2013 unit costs were applied. The costs were obtained from three sources: Truven Health Analytics (drug wholesale acquisition costs), Centers for Medicare and Medicaid Services (drug administration and transfusion costs), and HCUPnet (hospital charges for treating toxicities). Results suggest that the average total cost of treatment with Pem+Cb was $4,690 less than Pac+Cb+Bev ($30,334 versus $35,024), and Pem+Cb was cost-saving in 99% of 10,000 iterations of a probabilistic sensitivity analysis that used Monte Carlo methods.40
The recently published results for the PointBreak study comparing Pem+C+Bev followed by Pem+Bev to Pac+C+Bev followed by Bev showed no statistical difference in OS, though PFS was statistically longer in the pemetrexed arm; different toxicities were noted between the two study arms. Similarly, this PRONOUNCE trial that compared Pem+Cb followed by Pem to Pac+Cb+Bev followed by Bev demonstrated no significant difference of efficacy for OS and PFS between the treatment arms, and again different toxicities were noted. Comparison of the results of these two studies are informative when choosing a chemotherapy regimen for a given patient who may not tolerate certain regimens and reassures the clinicians that the standard of care therapy is being offered to each patient based upon individual preferences and comorbid conditions.
In conclusion, the PRONOUNCE study objective of superiority of G4PFS was not met; no superiority was observed for the standard end points such as PFS, OS, ORR, or DCR in Pem+Cb compared with Pac+Cb+Bev. The safety profiles of both the treatment regimens are reasonable, though different. Differing toxicity profiles are often a deciding factor considered by treating oncologists when choosing an effective regimen for nonsquamous NSCLC.
ACKNOWLEDGMENTS
This study was funded by Eli Lilly and Company, Indianapolis, IN. The authors wish to thank all the patients and the investigators who participated in this clinical trial.
Footnotes
Disclosure: Borys Hrinczenko, J. Thaddeus Beck, Manuel R. Modiano, Robert W. Weaver, David R. Spigel, and Helen J. Ross have no conflict of interest to declare. Ralph G. Zinner received funding for research from Eli Lilly and Company. Petros G. Nikolinakos participated in the advisory boards meetings and received honoraria from Eli Lilly and Company. David M. Waterhouse was on the speaker’s bureau for Eli Lilly and Company and received honoraria in the past. Ramaswamy Govindan is a consultant for Boehringer Ingelheim, GlaxoSmithKline, Pfizer, Merck, Bayer, Covidien, Bristol-Myers Squibb, Genentech, and Mallinckrodt. Coleman K. Obasaju, Jingyi Liu, Andrew G. Koustenis, Katherine B. Winfree, Symantha A. Melemed, Susan C. Guba, Waldo I. Ortuzar, Durisala Desaiah, and Joseph A. Treat are employees of Eli Lilly and Company and may hold company stock.
Data from this study were presented at the 2013 annual meeting of American Society for Clinical Oncology, Chicago, IL, on June 3, 2013 (Abstract# LBA 8003) and at the World Conference on Lung Cancer, Sydney, Australia, October 28, 2013 (Abstract# P1.10–033).
ClinicalTrials.gov Identifier: NCT00948675.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obasaju CK, Raju RN, Stinchcombe T, et al. Final results of a randomized phase II trial of pemetrexed (P) + carboplatin (Cb) ± enzastaurin (E) versus docetaxel (D) + Cb as first-line treatment of patients (pts) with stage IIIB/IV non–small-cell lung cancer (NSCLC). J Clin Oncol. 2009;27(suppl abstract 8037):15s. [Google Scholar]
- 4.Grilli R, Oxman AD, Julian JA. Chemotherapy for advanced non-small-cell lung cancer: how much benefit is enough? J Clin Oncol. 1993;11:1866–1872. doi: 10.1200/JCO.1993.11.10.1866. [DOI] [PubMed] [Google Scholar]
- 5.Marino P, Pampallona S, Preatoni A, Cantoni A, Invernizzi F. Chemotherapy vs supportive care in advanced non-small-cell lung cancer. Results of a meta-analysis of the literature. Chest. 1994;106:861–865. doi: 10.1378/chest.106.3.861. [DOI] [PubMed] [Google Scholar]
- 6.Souquet PJ, Chauvin F, Boissel JP, et al. Polychemotherapy in advanced non small cell lung cancer: a meta-analysis. Lancet. 1993;342:19–21. doi: 10.1016/0140-6736(93)91882-m. [DOI] [PubMed] [Google Scholar]
- 7.Chang AY, Kim K, Glick J, Anderson T, Karp D, Johnson D. Phase II study of taxol, merbarone, and piroxantrone in stage IV non-small-cell lung cancer: the Eastern Cooperative Oncology Group results. J Natl Cancer Inst. 1993;85:388–392. doi: 10.1093/jnci/85.5.388. [DOI] [PubMed] [Google Scholar]
- 8.Murphy WK, Fossella FV, Winn RJ, et al. Phase II study of taxol in patients with untreated advanced non-small-cell lung cancer. J Natl Cancer Inst. 1993;85:384–388. doi: 10.1093/jnci/85.5.384. [DOI] [PubMed] [Google Scholar]
- 9.Scagliotti GV, De Marinis F, Rinaldi M, et al. Italian Lung Cancer Project. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–4291. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- 10.Schiller JH, Harrington D, Belani CP, et al. Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 11.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 12.Somer RA, Sherman E, Langer CJ. Restrictive eligibility limits access to newer therapies in non-small-cell lung cancer: the implications of Eastern Cooperative Oncology Group 4599. Clin Lung Cancer. 2008;9:102–105. doi: 10.3816/CLC.2008.n.015. [DOI] [PubMed] [Google Scholar]
- 13.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 14.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 15.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 16.Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 17.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist. 2009;14:253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 18.Peterson P, Park K, Fossella F, et al. Is pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a phase III trial of pemetrexed vs. docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC). J Thorac Oncol. 2007;2:s851. [Google Scholar]
- 19.Zinner RG, Fossella FV, Gladish GW, et al. Phase II study of pemetrexed in combination with carboplatin in the first-line treatment of advanced nonsmall cell lung cancer. Cancer. 2005;104:2449–2456. doi: 10.1002/cncr.21480. [DOI] [PubMed] [Google Scholar]
- 20.Scagliotti GV, Kortsik C, Dark GG, et al. Pemetrexed combined with oxaliplatin or carboplatin as first-line treatment in advanced non-small cell lung cancer: a multicenter, randomized, phase II trial. Clin Cancer Res. 2005;11(2 Pt 1):690–696. [PubMed] [Google Scholar]
- 21.Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:4349–4357. doi: 10.1200/JCO.2012.47.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith IE, O’Brien ME, Talbot DC, et al. Duration of chemotherapy in advanced non-small-cell lung cancer: a randomized trial of three versus six courses of mitomycin, vinblastine, and cisplatin. J Clin Oncol. 2001;19:1336–1343. doi: 10.1200/JCO.2001.19.5.1336. [DOI] [PubMed] [Google Scholar]
- 23.Socinski MA, Schell MJ, Peterman A, et al. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J Clin Oncol. 2002;20:1335–1343. doi: 10.1200/JCO.2002.20.5.1335. [DOI] [PubMed] [Google Scholar]
- 24.Socinski MA, Stinchcombe TE. Duration of first-line chemotherapy in advanced non small-cell lung cancer: less is more in the era of effective subsequent therapies. J Clin Oncol. 2007;25:5155–5157. doi: 10.1200/JCO.2007.13.4015. [DOI] [PubMed] [Google Scholar]
- 25.Lustberg MB, Edelman MJ. Optimal duration of chemotherapy in advanced non-small cell lung cancer. Curr Treat Options Oncol. 2007;8:38–46. doi: 10.1007/s11864-007-0020-6. [DOI] [PubMed] [Google Scholar]
- 26.Pujol JL, Paul S, Chouaki N, et al. Survival without common toxicity criteria grade 3/4 toxicity for pemetrexed compared with docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC): a risk-benefit analysis. J Thorac Oncol. 2007;2:397–401. doi: 10.1097/01.JTO.0000268672.57002.69. [DOI] [PubMed] [Google Scholar]
- 27.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 28.[NCI] National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) 2006. available from http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf;Internet; accessed 22 Apr. 2014. [Google Scholar]
- 29.Zinner RG, Saxman SB, Peng G, Monberg MJ, Ortuzar WI. Treatment rationale and study design for a randomized trial of pemetrexed/carboplatin followed by maintenance pemetrexed versus paclitaxel/carboplatin/bevacizumab followed by maintenance bevacizumab in patients with advanced non-small-cell lung cancer of nonsquamous histology. Clin Lung Cancer. 2010;11:352–357. doi: 10.3816/CLC.2010.n.045. [DOI] [PubMed] [Google Scholar]
- 30.Dubey S, Brown RL, Esmond SL, Bowers BJ, Healy JM, Schiller JH. Patient preferences in choosing chemotherapy regimens for advanced non-small cell lung cancer. J Support Oncol. 2005;3:149–154. [PubMed] [Google Scholar]
- 31.Rodrigues-Pereira J, Kim JH, Magallanes M, et al. A randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011;6:1907–1914. doi: 10.1097/JTO.0b013e318226b5fa. [DOI] [PubMed] [Google Scholar]
- 32.Scagliotti GV, Park K, Patil S, et al. Survival without toxicity for cisplatin plus pemetrexed versus cisplatin plus gemcitabine in chemonaïve patients with advanced non-small cell lung cancer: a risk-benefit analysis of a large phase III study. Eur J Cancer. 2009;45:2298–2303. doi: 10.1016/j.ejca.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 34.Ramalingam SS, Dahlberg SE, Langer CJ, et al. Eastern Cooperative Oncology Group. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol. 2008;26:60–65. doi: 10.1200/JCO.2007.13.1144. [DOI] [PubMed] [Google Scholar]
- 35.Langer CJ, Socinski MA, Patel JD, et al. Efficacy and safety of paclitaxel and carboplatin with bevacizumab for the first-line treatment of patients with nonsquamous non-small cell lung cancer (NSCLC): analyses based on age in the phase 3 PointBreak and E4599 trials [IASLC abstract MO06.12]. J Thorac Oncol. 2013;8(suppl 2):S291. [PubMed] [Google Scholar]
- 36.Wong YN, Egleston BL, Sachdeva K, et al. Cancer patients’ trade-offs among efficacy, toxicity, and out-of-pocket cost in the curative and noncurative setting. Med Care. 2013;51:838–845. doi: 10.1097/MLR.0b013e31829faffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18:381–390. doi: 10.1634/theoncologist.2012-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zafar SY, Abernethy AP. Financial toxicity, part I: a new name for a growing problem. Available at: http://www.cancernetwork.com/practice-policy/financial-toxicity-part-i-new-name-growing-problem. Accessed April 22, 2014. [PMC free article] [PubMed] [Google Scholar]
- 39.Zafar SY, Abernethy AP. Financial toxicity, part II: how can we help with the burden of treatment-related costs? Available at: http://www.cancernetwork.com/practice-policy/financial-toxicity-part-ii-how-can-we-helpburden-treatment-related-costs. Accessed April 22, 2014. [PubMed] [Google Scholar]
- 40.Graham CN, Knox H, Winfree KB, et al. Cost-minimization analysis of pemetrexed/carboplatin with maintenance pemetrexed (PemC) versus paclitaxel/carboplatin/bevacizumab with maintenance bevacizumab (PCB) in nonsquamous non-small cell lung cancer (NS-NSCLC). ASCO Quality Care Symposium 2013 [Google Scholar]


