Abstract
Exposure to particulate air pollution and socioeconomic risk factors are shown to be independently associated with adverse pregnancy outcomes; however, their confounding relationship is an epidemiological challenge that requires understanding of their shared etiologic pathways affecting fetal-placental development. The purpose of this paper is to explore the etiological mechanisms associated with exposure to particulate air pollution in contributing to adverse pregnancy outcomes and how these mechanisms intersect with those related to socioeconomic status. Here we review the role of oxidative stress, inflammation and endocrine modification in the pathoetiology of deficient deep placentation and detail how the physical and social environments can act alone and collectively to mediate the established pathology linked to a spectrum of adverse pregnancy outcomes. We review the experimental and epidemiological literature showing that diet/nutrition, smoking, and psychosocial stress share similar pathways with that of particulate air pollution exposure to potentially exasperate the negative effects of either insult alone. Therefore, socially patterned risk factors often treated as nuisance parameters should be explored as potential effect modifiers that may operate at multiple levels of social geography. The degree to which deleterious exposures can be ameliorated or exacerbated via community-level social and environmental characteristics needs further exploration.
1. Introduction
Over the last decade, chronic exposure to ambient air pollution has become increasingly recognized as an important risk factor underlying adverse pregnancy outcomes (APOs) [1–9]. In parallel, the associations between socioeconomic status (SES) and APOs are among the most robust findings in perinatal research [10–12], which persist even in settings with universal access to health care [13–16]. While interest in the intersection between health and the social environment is long standing [17–19], renewed attention has been propelled by two independent progressions in quantitative research. The first is the popularization of multilevel statistical models and the ability to separate the individual-level effects from those of their encompassing social and physical environments [20–26]. The second is the emerging research on the biological effects of psychosocial stress on health and its modification by environmental factors. There is now mounting evidence that stress can interact with chemical exposures to exacerbate the toxic effect and the physiological response to a greater extent than either insult (stress or chemical) acting alone [27–31]. Furthermore, the accumulation of low-level exposures to multiple chemicals via multiple sources and pathways shows evidence of dose addition and synergism [32–34]. For example, synergism was observed between aqueous cigarette tar and other respirable particles (e.g., asbestos fibers, particulate matter, and diesel exhaust) [35]. Recognition of these interactions has been incorporated into several conceptual models and study designs of cumulative risk of chemical and nonchemical exposures [36–39] with models recently developed to identify these potentially double-exposed populations [40, 41]. Two complimentary reviews of these models have been recently published [42, 43].
Although the causes of APOs are multifactorial, the placenta plays the main intermediary role between the mother's physical and social environment and the fetus [44–50]. Importantly, a perturbed intrauterine environment inhibiting the fetal growth trajectory may also have long-term adult health implications as suggested by the developmental origins of disease hypothesis [51–53]. Therefore efforts to understand the underlying mechanisms of the physical and social environment that contribute to the disproportionate risk of APOs across the socioeconomic spectrum are required in order to target preventative and restorative interventions. This review will examine how the shared pathoetiological effects of exposure to particulate air pollution and SES act on the fetal-placental unit leading to adverse pregnancy outcomes. This will be accomplished by building on conceptual pathway models of air pollution and SES etiologic mechanisms on APOs [54, 55]. We review the role of the placenta in this context, describing its physiology and obstetrical pathologies followed by a description of particulate air pollution and its toxicokinetics in relation to placentation and how it can lead to APOs. We highlight specific indicators of SES and their biological pathways that intersect with air pollution exposure and how this may contribute to increased susceptibility for APOs. Potential implications and interventions are summarized in the conclusion. Our aim is for this review to be a resource for researchers interested in environmental-perinatal epidemiology. Understanding how correlated social and environmental exposures at times overlap to produce potentially synergistic and modifiable effects will help guide future research and intervention strategies with the aim to improve the overall health of the population [36–40].
2. Person, Place, and Context: The Placental, Physical, and Social Environments
2.1. The Placenta
The mammalian placenta is an intriguing and remarkable organ. Formed from two genetically distinct organisms, it is multifunctional and vital to fetal development yet situated outside the fetal body with a limited life span. Notable characteristics unique to humans and the Great Apes include deep interstitial implantation and a highly invasive hemochorial phenotype thus allowing the direct interaction of maternal blood and fetal chorionic tissues [56]. Interestingly, this particular aspect of placental evolution has less to do with nutrient transfer efficiency than previously thought and more likely implicates the highly regulated maternal-fetal immunological relationship [57–59].
The first trimester is a critical period in pregnancy involving implantation and initial placentation, two events highly susceptible to disturbance. The “Great Obstetrical Syndromes” [60] such as early/recurrent miscarriage, pregnancy induced hypertension and preeclampsia (PIH/PE), fetal growth restriction (FGR), placental abruption, prelabour rupture of the fetal membranes (PROM), and spontaneous preterm labour may share common etiological mechanisms arising from defective deep placentation (DDP) [61, 62]. Together, these conditions may complicate between 17 and 29% of all pregnancies [63] and are for the purpose of this review referred to collectively as APOs. Furthermore, these conditions may lead to epigenetic programming of adult disease susceptibility including obesity, diabetes, cardiovascular and reproductive diseases, all with their own substantial societal costs [52, 64–66]. DDP refers to the shallow invasion of the placental bed into the maternal decidua and myometrium including incomplete remodeling of the uterine spiral arteries [62, 67]. The latter is a vital event during which under normal conditions the endothelial lining of the spiral artery walls is remodeled to accommodate the inundation of maternal blood flow starting in the second trimester [68]. Spiral arteries that fail to undergo this vascular remodeling are not only narrower in diameter but also remain responsive to vasoconstrictive compounds such as stress hormones. The etiological trigger(s) leading to DDP are thought to involve either early placental oxidative stress which triggers an inflammatory response or, vice versa, an atypical inflammatory maternal immune response to the semi-allogenic fetal-placental unit leading to placental oxidative stress and further inflammation [69, 70]. The difference between a normal and an affected pregnancy is a matter of degrees on a continuum with individual biological and behavioural variability nested within the social and physical environment [12, 24–26, 68, 69, 71–73].
2.2. The Physical Environment: Particulate Air Pollution
Air pollution is a general term used to describe the presence of agents (particulates, biologicals, and chemicals) in outdoor or indoor air that negatively impact human health. Several common air pollutants have been associated with APOs, including carbon monoxide (CO), nitrogen dioxide (NO2), sulfur dioxide (SO2), ozone, particulate matter (PM), and polycyclic aromatic hydrocarbons (PAHs) [1]; however, attention has focused on the latter two compounds showing strong molecular evidence of cytotoxicity, mutagenicity, DNA damage, oxidative stress, and inflammation [55, 74–79]. While the observed risks of APOs in relation to air pollution tend to be modest, the population attributable risk can be quite large due to the pervasiveness of exposure to the general population [9]. Significant risks have been observed even in settings with relatively low ambient air pollution exposure [80, 81]. Therefore, a small increase in risk can have a large public health impact. Preterm birth (PTB) and FGR are major risk factors of perinatal mortality and serious infant morbidities contributing to increased health care and societal costs [82–87].
Particulate matter (PM) is a complex mixture of varying chemical and physical properties. It is defined according to particle size into the inhalable coarse fraction (PM10, 2.5–10 μm), the fine respirable fraction (PM2.5, ≤2.5 μm), and the ultrafine fraction (UFP, ≤0.1 μm). Their ubiquity and recognized human health risks have deemed them as toxic [88, 89]. Characterizing PM by particle size is important for several reasons. First, particle size dictates the location of deposition in the respiratory system [88, 90]. Second, particle size can give some indication of its general source and behaviour. For example, PM10 is mainly derived from mechanical processes such as windblown soil, pollen, minerals and dust from roads, farms, and industrial operations. PM10 tends to gravitationally settle in a matter of hours to days. Conversely, PM2.5 is a primary by-product of combustion and atmospheric reactions with precursor gases such as SO2, nitrogen oxides, ammonia, and volatile organic compounds (VOCs). PM2.5 can remain suspended in air for days to weeks and are consequently more prone to long-range transport. Precipitation accounts for 80–90% of PM2.5 removal from the atmosphere [88]. Third, the chemical composition is markedly different between PM10 and PM2.5 mixtures. Derived mainly from the Earth's crust, PM10 typically contains oxides of iron, calcium, silicon, and aluminum, whereas PM2.5 mixtures derived from anthropogenic combustion sources are mainly composed of sulphates, nitrates, ammonium, trace metals, elemental carbon, and organic hydrocarbons (e.g., PAHs) [88]. Chemical differences and relative proportions also differ within the PM10 and PM2.5 mixtures with regional (urban-to-rural) and interurban (urban-to-urban) differences as well as intraurban spatial variation [88, 91–93]. Therefore trimester and demographic differences in residential mobility and intraurban population differences are important study design issues to consider [94, 95]. Finally, PM10, PM2.5, and UFPs differ by their toxicological mechanisms such as their oxidative potential, which may reflect their differences in size, surface area, and/or their chemical constituent compositions, although they tend to be correlated [76, 92, 96, 97]. Transition metals such as copper, nickel, lead, chromium, iron, vanadium, and cobalt among other metals are variably present in ambient air absorbed to PM2.5 [92, 93]. Their direct oxidative action or redox potential to create reactive oxidative species (ROS) is one possible mechanism as to how PM2.5 induces oxidative DNA and protein damage [78, 97].
There is accumulating evidence that suggests UFPs may be the fraction of PM responsible for many of the adverse health effects reported in air pollution studies [78, 79, 97, 98]. UFPs are a small proportion by mass but make up a large proportion in particle number and have gone either unmeasured or misclassified as PM2.5 [88, 98]. Their small size facilitates better tissue penetration deep into lung alveoli and into epithelial cells restricting their clearance via macrophage phagocytosis [98]. Animal studies have shown that UFPs can translocate across the lung epithelium into blood circulation and accumulate in other organs, including the liver, spleen, kidneys, heart, brain, and reproductive organs [98]. The high surface area of UFPs favours the absorption of PAHs and possibly transition metals which has shown to localize in the mitochondria inducing major structural damage. This could be a possible explanation to UFP's exhibited higher oxidative potential compared to larger PM fractions of the same material [79]. Recent attention has been given to proinflammatory and endocrine-disrupting properties of diesel emissions, a major source of UFPs in ambient air [31, 99–101].
Polycyclic aromatic hydrocarbons (PAHs) are organic substances that constitute a class of over 100 individual chemical compounds made up of carbon and hydrogen atoms formed into rings [102]. While toxicological data exist for individual PAHs (benzo[a]pyrene being the most commonly used PAH indicator), they almost always occur as complex mixtures (e.g., soot, tobacco smoke, creosote, and diesel exhaust) [103]. Thus it is difficult and arguably futile to assess the toxicity of individual PAH components only to be compounded by the likelihood of interactions [75, 104, 105]. Combustion of organic matter and fossil fuels is the main source of atmospheric PAHs with their distribution and magnitude concentrated along transportation corridors (road and rail) and land-use areas with heavy industrial activities. However, main stream and environmental tobacco smoke (ETS) remain a leading source of PAH exposure [106]. PAHs are generally nonvolatile (i.e., stable) and have low water solubility. As a consequence, PAHs often bind to PM2.5 and UFP in the atmosphere. Residency times in the atmosphere depend on weather conditions, PAH molecular weight, and the emission source (e.g., stack versus tailpipe) with atmospheric deposition as the main source of PAHs to soil, vegetation, and surface water. Once in aquatic systems, PAHs are often found absorbed to suspended particles or bound to sediments settled on the bottom where they persist or are slowly biodegraded by microorganisms. While PAHs can bioaccumulate in some aquatic and terrestrial organisms, they tend to not biomagnify in food systems due to their metabolism in higher order species [102, 106]. However, it is the inefficient clearance and action of the highly reactive PAH metabolites that are suspected to cause cytotoxicity, mutagenicity, DNA damage, oxidative stress, and tumorgenesis [75, 106].
Much of the work elucidating the mechanisms in which PM and PAHs elicit adverse cellular effects have been conducted using cardiovascular disease (CVD) and lung cancer as models [76–78, 97, 107–109]. Although seemingly different diseases, they share several similarities with DDP and APOs with respect to associated risk factors. First, both APOs and CVD related outcomes are associated with PM exposure levels which vary by SES [40, 110, 111] but are also associated with other socially patterned risk factors such as smoking, poor or inadequate diet, psychosocial stress, obesity, and diabetes [12, 112–114]. CVD and APOs also share many other risk factors such as the presence of systemic inflammation and preexisting hypertension. Interestingly, PIH/PE is a risk factor for maternal CVD later in life and also in the offspring if affected by IUGR [115–117]. CVD and disorders of DDP have similarly affected cellular tissues in their respective target systems (i.e., endothelial cells of the cardiovascular system and in the highly vascularised placenta) which are particularly susceptible to oxidative and inflammatory injury [97, 118]. High plasma homocysteine concentrations are positively associated with vasculopathy and infarction in the placental-uterine and coronary systems increasing the risk of spontaneous PTB and CVD events, respectively [119, 120]. Fittingly, high density lipoprotein cholesterol may be protective against spontaneous PTB and CVD events [120, 121]. Finally, PM and PAH-induced mutagenicity, cytotoxicity, DNA damage, and oxidative stress linked to lung cancer have also been observed in the fetal-placental unit [122, 123], and exposure early in pregnancy may contribute to the risk of congenital anomalies and early (subclinical) pregnancy loss [124–127].
2.3. The Social Environment: Socioeconomic Status, Diet, Smoking, and Allostatic Load
The social environment plays a significant role in maternal and perinatal health with indicators of low socioeconomic status (SES) consistently among the strongest predictors of adverse pregnancy outcomes [10–12]. The causal pathways in which SES contribute to APOs and ill health in general can be conceptualized in terms of “downstream” or mediating exposures, stresses, and behaviours acting on the individual through “upstream” society-level determinants such as poverty, poor education, income inequality, and social discrimination/marginalization over the lifespan [12]. Indicators of low SES associated with PTB and FGR include maternal anthropometry (prepregnancy BMI, height, and gestational weight gain), nutrition and micronutrient status, cigarette use, genital tract infections and inflammation, cocaine and other drug use, physically demanding work, quantity and quality of prenatal care, and psychosocial factors including anxiety, depression, and stress (e.g., lack of social, familial, and marital support, poverty or financial hardship, physical/verbal abuse, and neighbourhood crime) [12, 24, 26, 54]. For the purpose of this review, the focus here will be on three that engage with the oxidative stress and inflammation pathways to potentially interact with exposure to particulate air pollution. They include (1) a diet-micronutrient pathway [55, 128–131], (2) cigarette smoke exposure [35, 132–135], and (3) stress-mediated (allostatic) activation of the HPA-axis and corresponding glucocorticoid production [47, 72, 136–138].
Nutrition and diet can influence perinatal health in opposing directions. Poor/under-nutrition such as high fat/calorie dense food and low micronutrient intake is more prevalent among women from low SES backgrounds which may partly explain higher rates of some APOs [12, 139–142]. Conversely, adequate diet and micronutrient status provides resilience against oxidative stress and inflammation caused by various exposures including air pollution, allostatic stress, infection, or smoking [55, 118, 128, 129, 131, 143]. Maternal exposure to mainstream or environmental cigarette smoke during pregnancy is associated with numerous APOs including congenital anomalies [127, 144–146]. Their exposure prevalence is associated with indicators of low SES as well as other socially patterned risk factors [147–149] and remains one of the most modifiable risk factors with potential for beneficial intervention. Other risk factors associated with low SES such as obesity, pre-existing and gestational diabetes, and hypertension [13, 113, 150] also engage the oxidative stress and inflammatory pathways and could therefore also potentially interact with PM exposure to increase susceptibility to adverse effects as evidenced in studies of cardiovascular health [114, 151, 152]. Recent studies have observed increased risks of preeclampsia and gestational diabetes associated with measures of air pollution [153–156] with one study showing positive effect modification by preexisting and gestational diabetes [154]. Evidence shows that chronic life stressors associated with low SES at multiple levels of organization (individual, household, and community) result in a cumulative biological toll on the body affecting multiple systems and increasing susceptibility to numerous ailments [21, 157–160] including APOs [15, 26, 161, 162].
The concept of allostasis and allostatic load/overload has been proposed to describe the individual stress response to an event as a necessary and adaptive process thereby removing the implicit negative connotation attached to the term “stress” [163]. Stress can be positive or tolerable when it improves function and performance and may have long-term adaptive benefits. However, this may depend on available coping resources such as one's psychological resistance, resilience, and ability to recover. Negative or toxic stress occurs when real or perceived environmental/social demands, or the anticipation of such, become too extreme or unpredictable thereby exceeding one's (perceived) ability to cope (e.g., no sense of control, adverse childhood experiences, and other forms of trauma) [164, 165]. Therefore, allostasis is the multisystem biological response that promotes adaptation using system mediators such as cortisol, (nor)epinephrine, vasopressin, renin, and glucagon, whereas allostatic load and overload is the cumulative toll (wear and tear) on biological systems after prolonged or poorly regulated (hyper/hypoactivated) allostatic responses [165, 166]. For example, the cardiovascular system is extremely sensitive to stress in terms of increased blood pressure; however, metabolic disorders such as diabetes and obesity as well as immune function impairment are also linked to chronic stress. Furthermore, lifestyle coping mechanisms as a response to chronic stress have the ability to either buffer or exasperate the effect (e.g., exercise, diet, sleep, and social interactions or lack thereof) [163]. Therefore in light of the above, it is our belief that the fetal-placental unit is the site where the physical and social environments converge and interact to influence reproductive health which we describe further below.
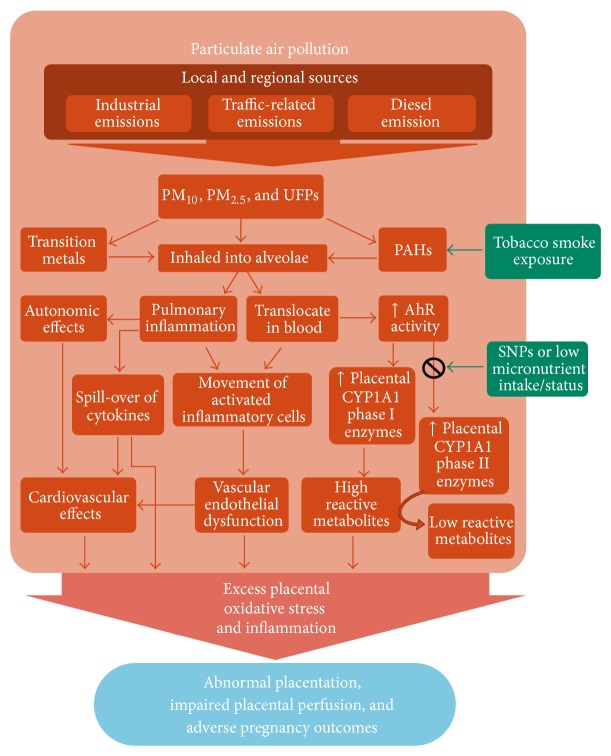
Figure 1 illustrates the interconnectedness between particulate air pollution (PM/PAH) and SES on how they may act discretely or in a combined manner to yield APOs. Using Figure 1 as a guide, the following text will review the two major mechanisms (oxidative stress and inflammation) through which the physical and social environments are believed to negatively affect the fetal-placental unit and how they may combine/interact to lead to the multifactorial nature of APOs.
Figure 1.
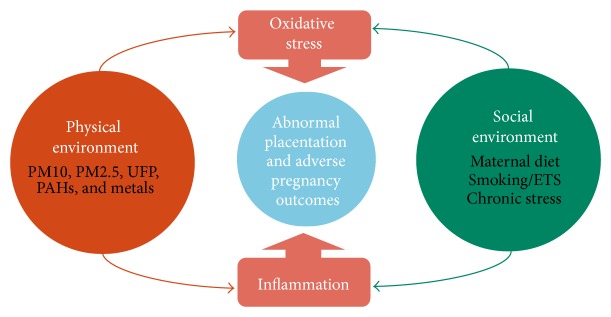
A conceptual framework of the shared mechanisms of socioeconomic determinants and particulate air pollution exposure contributing to adverse pregnancy outcomes. The physical environment (orange) consisting of particulate air pollution and the social environment (green) consisting of community and individual-level social factors/stressors converge to affect the fetal-placental environment (blue) via oxidative stress and inflammatory mechanisms potentially leading to adverse pregnancy outcomes.
3. Biological Mechanisms Leading to Adverse Pregnancy Outcomes
3.1. Oxidative Stress
Aptly known as “The Oxygen Paradox,” oxygen is both essential and toxic to the multicellular aerobic organisms whose very evolution was dependent on leveraging this anaerobic waste by-product into a higher energy producing advantage [167]. Observed in all mammals, a steep oxygen tension gradient from 20% in our atmosphere to 3-4% oxygen concentration in most internal tissues is the primary defense against oxidative damage. Secondary and tertiary layers of protection include antioxidant defenses as well as damage removal, repair, and apoptotic response systems [168, 169]. These genetically adaptive responses are upregulated in the presence of reactive oxygen species (ROS) generated as natural by-products of cellular aerobic metabolism and exposure to various toxins. Oxidative stress occurs when there is an imbalance between pro- and antioxidant capacity.
For example, superoxide is the most common intracellular ROS in mammals. It is produced by the mitochondria as a metabolic by-product but also from the metabolism of various growth factors, drugs, and toxins by oxidizing enzymes such as NADPH-oxidase and cytochrome P450 (CYP450). Superoxide is reduced by superoxide dismutase (SOD) into hydrogen peroxide (H2O2) which is then further reduced into water by glutathione peroxidase (GPx) and catalase. Under normal physiological conditions H2O2 acts as intracellular secondary messengers; however, it's accumulation along with superoxide can react with free iron ions or nitric oxide to form highly toxic hydroxyl (OH∙) or peroxynitrite (ONOO−) ions, respectively [70, 168]. Free iron is a common metal found absorbed to PM, and the antioxidant heme oxygenase-1 (HO-1) facilitates its conjugation and removal through the increased availability of ferritin thereby preventing the formation of reactive hydroxyl molecules [92, 170–172]. Deficiencies in HO-1 have been associated with several APOs such as recurrent miscarriage, FGR, and preeclampsia [171, 172].
Common antioxidants include enzymatic (e.g., SOD, GPx, catalase, and HO-1) and nonenzymatic compounds (e.g., vitamin C and E, glutathione, β-carotene, and ubiquinone) [118]. Genetic polymorphisms and/or micronutrient deficiencies in antioxidant enzymes precursors can impair antioxidant capacity, while chronic exposure to toxicants, psychosocial stress, bacteria, viruses, and other inducers of inflammation can foster prooxidant burden [70, 77, 118, 172]. Oxidative stress is unavoidable; however, under optimal conditions the presence of ROS leads to homeostatic adaptation and are safely removed. Failure to effectively manage oxidative stress can result in altered cellular function as excess ROS degrade lipids, proteins, and DNA potentially initiating pathological processes. Refer to [168] for an extensive review on the role of cellular ROS in pregnancy outcomes.
3.2. Inflammation and Immunologic Alterations
It is well recognized that the maternal immune system plays a central role throughout the entire pregnancy, from preimplantation to parturition, and is influenced by the inflammatory response of the mother to her environment as well as to her partner. Alternative to previously hypothesized [173], the maternal immune system is not passive or suppressed during implantation and development of the semiallogeneic placenta and fetus. Rather, it exerts executive influence on the establishment and progression of the pregnancy as an immune-mediated quality control mechanism to maximize maternal and offspring health [44, 173]. This is achieved by favouring pro- or anti-inflammatory environments at different times during pregnancy for different purposes. For instance, implantation, initial placentation, and parturition are characterized by a proinflammatory environment whereas an anti-inflammatory state prevails for most of midgestation [174]. The favoured localized immunological response however is highly modified by the infectious, inflammatory, stress, nutritional, and metabolic status of the individual and thus can be influenced by environmental agents such as PM [175–177] and/or available coping, social, and nutritional resources [44, 128, 164, 178]. Therefore, inflammation is believed to be one pathway involved in both PM and SES-mediated APOs.
Chronic and acute inflammation is a complex response process mediated by a real or perceived attack from foreign substances. The innate immune response is the rapid automatic response to externally originating (exogenous) substances such as pathogens, but also from internal (endogenous) danger signals including products of trauma, ischemia, necrosis, or oxidative stress [179]. The response includes the release of proinflammatory signaling cytokine proteins such as interleukins IL-1β, IL-6, and tumour necrosis factor (TNF-α) which serve to recruit neutrophils to affected tissues. However, the recruited neutrophils release ROS and hydrolytic proinflammatory enzymes (inducible nitric oxide synthase (iNOS), cyclooxygenase (COX-2), and prostaglandins (PG-E2)) which disturb normal cells in addition to affected tissues. This in turn leads to increased ROS and oxidative stress [180, 181]. The placenta plays a role at the maternal-fetal interface as the main producer of endocrine steroid and protein hormones as well as the immunologic barrier between mother and fetus which positively interact for the success of the pregnancy [44, 173]. This is achieved through a nonlinear series of positive and negative feedback pathways with the stimulation or suppression of molecules with pro- and anti-immunosuppressant properties (interleukins, galectins, placental growth factor, and human chorionic gonadotropin (hCG)) [182–184]. The production of these cytokines, chemokines, and other immune-regulatory agents mediates the coordination, migration, and function of several maternal immune cells (e.g., uterine natural killer cells (uNK)) that participate in early pregnancy events such as endometrial receptivity of embryo implantation, tissue remodeling, immune tolerance, and vascular adaptation to invading placental trophoblast cells [44, 182–184]. Interference or aberrant production/secretion of these substances by various stressors including infection, toxins, and those acting through the HPA-axis may result in the impaired maternal immune response leading to the hallmark DDP syndrome complications described above (early pregnancy loss, PIH/PE, PROM, FGR, and premature labour, Figure 2) [44, 61, 69, 134, 175, 185–187].
Figure 2.
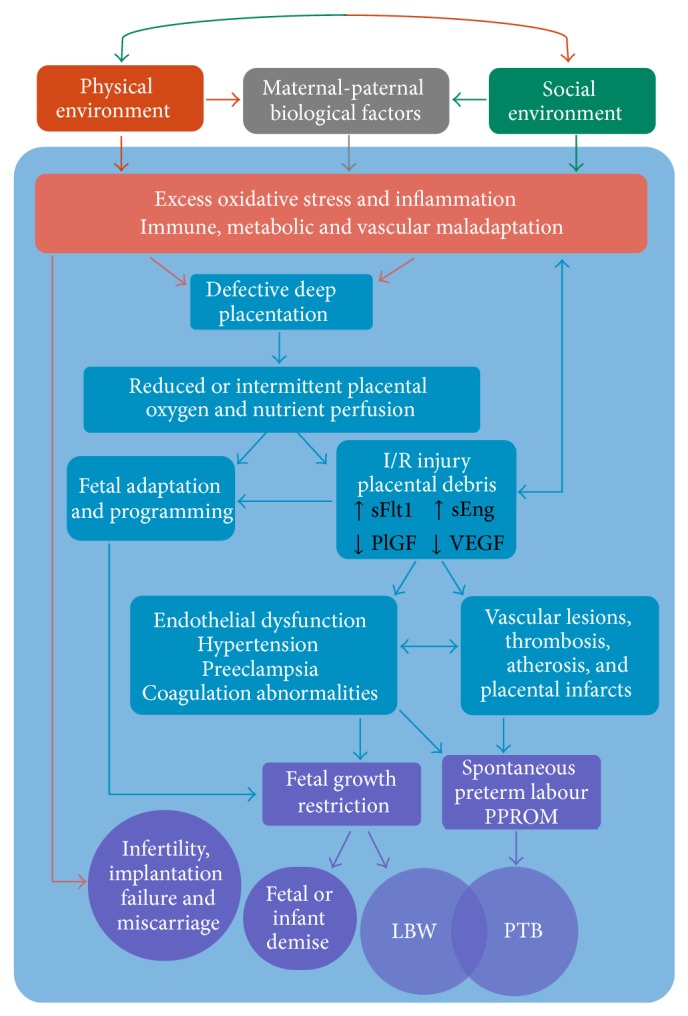
Proposed pathways contributing to adverse pregnancy outcomes. The co-presence of maternal and paternal biological factors can result in protection or increased susceptibility to the interaction with the physical and social environments. Cumulative negative exposures early in pregnancy resulting in excess oxidative stress and inflammation may cause a cascade of events leading to defective deep placentation. Depending on the degree of severity, the reduced transplacental perfusion can result in various pathologies associated with a range of obstetric complications and outcomes [60, 61, 69, 70].
3.3. Mechanisms of Oxidative Stress and Inflammation Involved in Adverse Perinatal Outcomes
3.3.1. Impaired Fertility and (Recurrent) Miscarriage
Due to immortal time bias, miscarriage is not easily measured in population or cohort studies without careful design methodologies [188, 189]; however, associations between infertility and air pollution have been made [190, 191]. Oxidative stress has shown to have a direct effect on fertility and embryo development. For example, obese mice showed increased ROS synthesis and oxidation in oocytes with a reduced ability of zygotes to develop to the blastocyst stage providing evidence that impaired cellular antioxidant capacity can limit successful ovulation and fertilization [118]. Dividing mitotic cells are particularly sensitive to oxidative damage and are shown to enter a transient growth-arrested state as a protective mechanism until the stress has passed. Thus, severe or chronic oxidative stress may hamper cell division or cause cellular necrosis reducing or terminating embryo viability [72, 169]. Alternatively, an exaggerated inflammatory state via a viral, toxic, and/or allostatic load could lead to a maternal immune maladaptation to conception restricting trophoblast stem cell accumulation in the early embryo responsible for the production of hormones that enables successful implantation (Figure 2) [44, 72].
Oxidative stress is implicated in first trimester miscarriage from premature placental perfusion of maternal oxygenated blood and accompanying ROS into the early embryonic environment [192]. Early embryo development occurs in a low oxygen state, and it is not until the tenth to twelfth week of gestation that maternal blood begins to gradually infiltrate the intervillous space of the yet fully developed placenta. The limited oxygen environment is thought to act as a protective mechanism against the deleterious and teratogenic effects of ROS on early stem cells at a time of extensive cell division [64, 138]. This early hypoxic environment also plays a vital physiological role in placental cell type differentiation switching from proliferative villous cytotrophoblasts into invasive extravillous trophoblasts (EVT) important in spiral artery remodeling [193]. At the end of the first trimester, oxygen tension rises sharply which coincides with the infusion of oxygenated maternal blood into the placenta and triggers an apoptotic cascade that serves to establish the definitive discoid placenta. However, in 70% of early miscarriage cases EVT invasion is insufficient allowing for the premature onset of maternal intraplacental circulation and its consequential burst of ROS on the conceptus [70, 192]. Severe cases may result in pregnancy failure while more modest cases may initiate fetal-maternal adaption to impaired spiral artery remodeling leading to the DDP pathology and further complications later in pregnancy such as FGR and PIH/PE (Figure 2) [69, 70, 193].
3.3.2. Pregnancy Induced Hypertension, Preeclampsia, and Prelabour Rupture of Membranes
While oxidative stress and inflammation are conditions of normal pregnancy, they are consistently elevated in cases of PIH/PE and central in its pathology. PIH/PE stems from a defect in early trophoblast invasion insufficient to fully convert the spiral arteries into low-resistance channels [68, 194]. The retention of smooth muscle cells remains active to circulating vasoconstricting agents such as stress hormones (e.g., glucocorticoids) and other stimulants. The diminished and intermittent perfusion of maternal blood into the intravillous space produces transient hypoxia resulting in a chronic ischaemia-reperfusion (I/R) type injury. This further provokes ROS synthesis and excess shedding of placental microvesicles which have proinflammatory, antiangiogenic, and procoagulant activity initiating endothelial dysfunction [68–70]. Elevated circulating levels of placental debris and ROS biomarkers in the placental tissues of preeclamptic women are well documented [68, 179, 194]. Similarly, PROM can be considered part of the DDP syndrome but may represent a phenotype resulting from a less severe DDP pathophysiology compared to preeclampsia [61, 62]. Excess oxidative stress arising from multiple causes (infection, inflammation, smoking, and cocaine use) has been implicated in PROM in addition to its role in DDP [70]. Both PIH/PE and PROM are leading causes of preterm birth, while PIH/PE is a major risk factor for FGR (Figure 2) [69]. Deficiencies in HO-1 have been associated with preeclampsia as well as morphological changes in the placenta and elevations in maternal blood pressure. The bioactive HO-1 metabolites CO and bilirubin may protect against preeclampsia through their vasodilatory properties and the suppression of the antiangiogenic factor sFlt, respectively [171, 172].
3.3.3. Fetal Growth Restriction
FGR has many causes however often arises from placental insufficiency due to compromised supply of oxygen and nutrients to the fetus which may have both short- and long-term health consequences on the offspring [51, 82, 195]. FGR is strongly associated with early onset or more severe cases of preeclampsia, and there is a clear etiological link between FGR and DDP as it involves abnormal placentation and reduced uteroplacental blood flow (Figure 2) [62, 70]. Alternatively, perturbed calcium homeostasis can induce chronic low-level stress within the endoplasmic reticulum leading to suppressed protein synthesis and a reduced growth trajectory of the placenta [70]. Cadmium, an environmental toxin and highly present in cigarette smoke, is a major antagonist of cellular calcium activities (transport, uptake, and binding) as well as in the transfer of other nutrients and zinc homeostasis within the placenta [134, 185, 196]. Furthermore, cadmium is a known endocrine disruptor shown to impair hormone synthesis in the placenta including progesterone and leptin, important hormones in early pregnancy [49, 175, 186]. Both smoking and air pollution exposure were associated with lower birth weights along with low blood progesterone levels and high placental cadmium concentrations compared to a non-exposed control group [135].
3.3.4. Spontaneous Preterm Labour and Birth
Inflammation is proposed as one potential mechanism leading to spontaneous preterm labour, both with intact membranes or PROM [177]. The classification of patients who deliver preterm can be categorized into two non-mutually exclusive clusters: those who present with inflammatory lesions (e.g., acute chorioamnionitis and funisitis) and those with vascular lesions who tend to have longer gestational periods [61]. The consequence of uteroplacental ischemia as a result of such lesions will depend on the severity, the timing, and duration of the insult. While a complete blockage of uterine arteries will lead to fetal death, less severe ischemia will result in different clinical phenotypes as a result of adaptive mechanisms for fetal survival. This may include fetal growth restriction if chronic underperfusion of oxygen and nutrients persists, the onset of maternal hypertension to sustain or increase uterine blood flow, and/or the initiation of preterm labour as a maternal/fetal adaptation to continued growth restriction in utero (Figure 2) [61, 197]. Cardiovascular lesions indicating thrombosis and atherosis are shown to be indirectly caused by exposure to PM2.5 and UFPs via inflammatory and/or oxidative injury [97].
4. The Physical and Social Environments and Their Relation to Adverse Perinatal Outcomes
4.1. PM-Induced Oxidative Stress and Inflammatory Mechanisms
Exposure to PM2.5 and its constituents, including PAHs and metals, induce oxidative stress and inflammation in many biological systems through various means (Figure 3) [48, 77–79, 97, 176, 177, 198]. One method is the direct generation of ROS from free radicals and oxidants on particle surfaces including soluble transition metals such as iron, copper, chromium, and vanadium. As mentioned above, free iron can react with available superoxide or hydrogen peroxide to form highly reactive hydroxyl radicals [70, 77]. PAHs and other organic molecules absorbed to PM2.5 and UFPs may account for a large proportion of their oxidative potential due to their ability to enter the cell and disrupt the mitochondria [79]. Altered function of mitochondria may produce excess quantities of NADPH-oxidase which in turn generates large amounts of cellular superoxide, a process already in overdrive throughout pregnancy but particularly in the first trimester [70, 77]. Interpolated ambient PM10 exposure was shown to be negatively associated with the number of placental mitochondrial DNA, a molecular marker of mitochondrial disruption and inflammation. This association was reversed with increasing distance from major roads, a proxy for traffic-related air pollution [48].
Figure 3.
Proposed pathways of particulate air pollution contributing to oxidative stress and inflammation leading to adverse pregnancy outcomes. Exposure to PM and its associated constituents of transition metals, PAHs, and other organic molecules affect the cardiovascular and metabolic systems which are highly active throughout pregnancy. For example, detoxification of PAHs and other organic toxins activate AhR signalling resulting in additional oxidative stress if antioxidant defenses are limited or impaired [55, 79, 98, 108, 109, 199].
Alternatively, PM/PAH mediated oxidative stress can be induced by the activation of the inflammation system. Immunotoxic compounds can promote the release of proinflammatory cytokines, TNF-α, and COX-2, which in turn act in a positive feedback loop to generate more ROS and oxidative stress [77]. For example, modelled PM10 and PM2.5 exposure has been positively associated with elevated C-reactive protein (CRP) levels, a biomarker of systemic inflammation, in both maternal first trimester blood and fetal cord blood in a dose-dependent manner [176, 200]. CRP is produced in the liver and part of the acute-phase response released during inflammatory reactions from cytokines produced in the lungs. Raised CRP is a risk factor for cardiovascular disease as a marker of unstable atheromatous plagues leading to thrombosis and ischemic events [97]. Exposure to diesel exhaust in healthy human volunteers resulted in pulmonary inflammation in addition to systemic inflammation, prothrombotic changes, and other cardiovascular effects consequent of proinflammatory events [99, 201]. This hyper proinflammatory state, along with oxidative stress, is hypothesized to contribute to several APOs [69, 70, 174, 181, 202].
Indirectly, the cellular detoxification of PAHs can induce oxidative stress and cytotoxicity by forming potent ROS metabolite by-products. Specifically, PAHs and other organic xenobiotics (notably PCBs and dioxins) are detoxified by the cytochrome P-450 (CYP) superfamily of Phase I and Phase II metabolizing enzymes. The expression of these enzymes is highly modulated by genetic polymorphisms, steroid/sex hormones such as glucocorticoids, insulin, estrogens, and progesterone, and micronutrient/dietary deficiencies [74, 75, 128, 203, 204]. Furthermore, hypoxia, infection, and inflammation are shown, in general, to downregulate CYP enzymes which may affect the clearance and bioavailability of growth factors, hormones, drugs, and toxins [203, 205]. CYP has numerous isoforms which are expressed in many tissues especially the liver. CYP1A1 is the only isoform also significantly expressed in the placenta throughout pregnancy responsible for metabolizing steroid/sex hormones, growth factors, and fatty acids in addition to toxins [75]. These exogenous and endogenous substances act as ligands to activate the aryl hydrocarbon receptor (AhR), a transcription factor that mediates the biotransformation of such ligands (PAHs, estradiol, etc.) into more polar and bioavailable metabolites by upregulating CYP enzymes. However, certain metabolites of PAHs (e.g., o-quinones, arene oxide, and diol epoxide) bind to DNA, RNA, and protein macromolecules to form toxic adducts that disrupt DNA replication and are considered mutagenic [72, 75]. Such DNA adducts have been found in newborn cord-blood positively correlated with maternal exposure to PAHs [50]. PAHs have also shown to significantly decrease the accumulation of trophoblast stem cells in the early placenta thereby limiting their differentiation into other cell types vital for hormone synthesis and ongoing placental development, a process that could contribute to DDP [72]. Direct prenatal exposure to airborne PAHs has been associated with FGR with an increased exposure-related risk in the first trimester [206, 207]. Secondary (Phase II) metabolizing enzymes are required to further detoxify reactive PAH-metabolites in which their inefficient clearance results in prolonged exposure leading to sustained cytotoxicity and mutagenicity. Phase II enzymes include glutathione s-transferases (GSTs), UDP-glucuronosyltransferases (UGTs), NAD(P)H-dependent quinone oxidoreductase-1 (NQO1), and aldehyde dehydrogenase-3 (ALDH3) [75, 205].
4.2. Maternal Diet and Micronutrient Intake
Adequate diet and micronutrient status provides resilience against oxidative stress and inflammation caused by various exposures including air pollution, allostatic stress, infection, and smoking (Figure 4) [55, 118, 128, 129, 131, 143]. Many micronutrients such as essential trace metals are vital cofactors in several antioxidant enzyme systems. For example, copper and zinc are necessary in the production of SOD. Similarly, selenium and its incorporation into the amino acid selenocysteine are required for the functionality of all selenoenzymes, including GPx and GST. Thus, selenium is essential in several aspects of human health, particularly conditions involving oxidative stress and inflammation such as CVD, immune function, cancer, and reproduction, but also thyroid regulation and brain diseases [208, 209].
Figure 4.
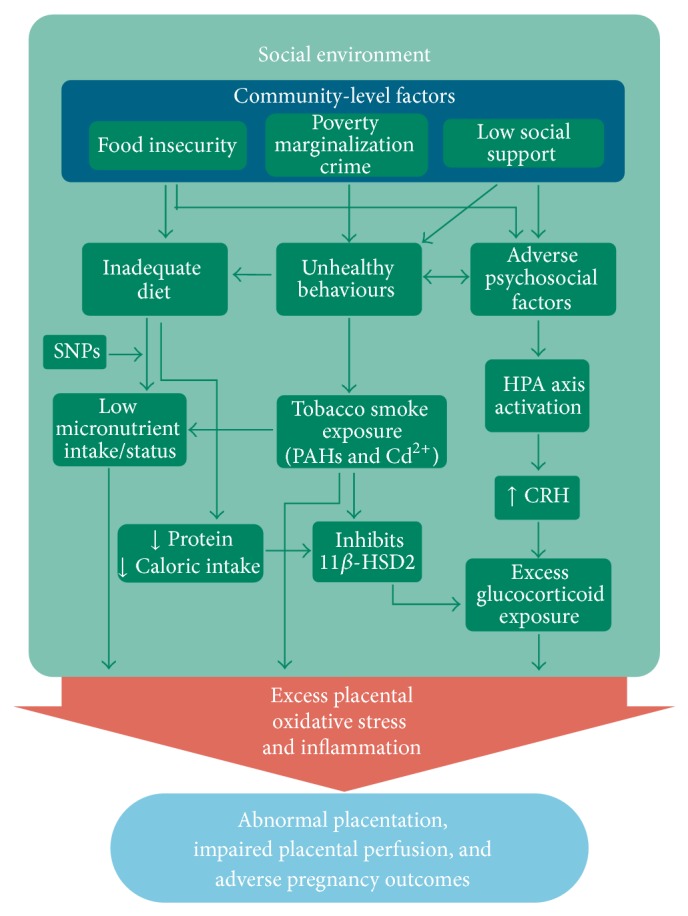
Proposed pathways of how the social environment interacts to produce excess systemic and placental oxidative stress and inflammation leading to adverse pregnancy outcomes. The pregnant woman is nested within and influenced by neighbourhood/community-level factors which can exasperate or buffer the individual-level biological and behavioural factors [24, 26, 54, 128, 224, 225].
ROS may have direct effects on oocyte quality and appears to be modulated by dietary antioxidant supplements [118]. Women who are obese tend to have higher rates of infertility that correlate with increased levels of oxidative stress biomarkers in their blood as excess glucose availability leads to higher mitochondrial ROS synthesis [70, 118]. Selenium deficiency and corresponding reduced GPx activity has been documented in cases of recurrent miscarriage and spontaneous abortions [210–212] and has also been associated with preeclampsia and preterm birth [213, 214]. However, given the supposed role of oxidative stress in preeclampsia, treatment with certain antioxidants (notably vitamins C and E) has not produced reliable preventative results in experimental trials [69]. One hypothesis is that inappropriate antioxidant regiment and/or administration too late in gestation are responsible and new therapeutic candidates include melatonin and selenium [118]. Interestingly, national programs in Finland and New Zealand fortifying food with selenium have been associated with a significant reduction in the rate of preeclampsia [215].
Oxidative stress negatively affects the placental transport of amino acids and glucose [45]. Furthermore, fatty acids and low density lipid (LDL) cholesterols necessary for the placental synthesis of oestrogens and progesterone are particularly vulnerable to oxidative injury [216]. Regulation of placental nutrient transport is controlled by several different mechanism, including imprinted genes, placental signaling pathways, various cytokines, and hormones such as insulin, leptin, glucocorticoids, and oestrogens (for review see [45]). The major placental transfer mechanisms include simple diffusion of lipophilic substances (e.g., oxygen, CO2, fatty acids, steroids, fat soluble vitamins, and anesthetic gases), restricted diffusion of hydrophilic substances, facilitated diffusion via a membrane bound carrier (e.g., glucose and other carbohydrates), and active transport which requires energy (e.g., amino acids, iron, calcium, and other divalent cations) [45, 217]. Placental physiology, including spiral artery remodeling and placental villous surface area are major determinants dictating placental transport capacity, and the degree of placental developmental disruption correlates with the severity of obstetrical complications associated with DDP [51, 62].
Nutrition and diet can influence perinatal health in opposing directions (i.e., it can be an antagonist or agonist). Poor/undernutrition such as high fat/calorie dense food and low micronutrient intake is more prevalent among women from low SES backgrounds which may partly explain higher rates of some APOs [12, 139–142]. On the other hand, good nutrition and supplemental vitamin intake is capable of reducing the toxicity of everyday environmental stressors as well as preventing certain APOs and congenital anomalies as shown with the successful reduction of neural tube defects with folic acid [128, 143, 218]. Nutritional and/or genetically induced deficiencies in folate and vitamins B6 and B12 can disrupt the homocysteine-to-methionine pathway resulting in hyperhomocysteinemia (HHC), a known risk factor of cardiovascular morbidities (thrombosis, lesions, and infarcts) and markers of oxidative stress [54, 119, 219, 220]. HHC may similarly affect the highly vascularized placenta and has been associated with decidual vasculopathy and preterm birth [54, 120]. Omega-3 fatty acids abundant from eating salmon were shown to improve markers of oxidative stress [221], which may impart neurodevelopmental resilience against stressors [222, 223]. Dietary phytophenols from fruits, vegetables, herbs, and spices have shown to have antioxidant and anti-inflammatory properties capable of reducing infection-induced inflammatory and contractile pathways in human gestational tissues [129]. Significant differences in pregnancy outcomes between Dominicans and African Americans both exposed to similar levels of PAHs in New York city neighbourhoods were thought to be due to healthful dietary/cultural practices in the Dominican immigrant population [206].
4.3. Maternal Smoking and Environmental Tobacco Smoke (ETS) Exposure
Maternal smoking during pregnancy and exposure to ETS remain to be two modifiable risk factors with the greatest potential for beneficial interventions (Figure 4). Their association with numerous APOs including congenital anomalies is well documented [127, 144–146], as have their associated prevalence with indicators of low SES and other socially patterned risk factors [147–149]. The mechanisms involved leading to APOs have been well reviewed [132, 134]; however, it is notable that the two main toxins present in tobacco smoke can also be absorbed to PM2.5 (PAHs more so than cadmium). Cadmium (Cd) exposure readily interferes with the active transport of essential minerals to the fetus, particularly zinc and calcium [46, 135, 196, 226–228]. Cadmium and lead (Pb) exposure has also been shown to reduce glycogen concentrations thereby potentially limiting available glucose to the fetus [229]. Cadmium has shown to disrupt placental leptin synthesis, a hormone with several vital functions including placental angiogenesis, immunomodulation, amino acid and fatty acid transport, as well as fetal pancreatic development important in the regulation of insulin-like growth factors and fetal body fat accumulation [49, 51]. Finally, synergistic effects in the generation of oxidative hydroxyl radicals have been observed between tobacco smoke and both ambient PM2.5 and diesel exhaust particles specifically [35]. Interestingly, the counterintuitive association between smoking and lower risk of preeclampsia was recently shown to vary according to the timing and intensity of smoking [230]. It is possible that the increased exposure to CO from smoking in late gestation acts as a vasodilator and at the same time inhibits the release of sFlt-1, a hallmark antiangiogenic factor implicated in the endothelial dysfunction present in preeclampsia [115, 230].
4.4. Allostatic Stress and Glucocorticoid Exposure
Reviewed elsewhere [163], the brain is the primary target and mediating organ through which SES-related stress pathways are translated to other body systems via the hypothalamic-pituitary-adrenal (HPA) axis. The HPA-axis is actively involved in several biological systems, including the cardiovascular, metabolic, immunological, and endocrinal effects in both mother and fetus to promote allostatic adaptation [165, 231]. Here, the neuroendocrine hormones of the HPA axis, corticotrophin releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and glucocorticoids (GC), respectively, coordinate the biological response via feedback loops. The human placenta is also capable of releasing CRH and other neuropeptides which interact with the HPA axis to regulate the maternal stress response as well as other normal pregnancy functions [47]. Proper levels of in utero glucocorticoids are essential for successful embryo implantation, fetal organ maturation, and the initiation of labour with glucocorticoid levels gradually increasing over the course of gestation. Normally, levels of maternal cortisol rise sharply in the third trimester causing the release of placental CRH in a positive adrenal-placental feedback loop. Placental CRH stimulates fetal cortisol secretion which in turn suppresses placental progesterone and activates the release of prostaglandins and oxytocin to promote uterine contractions [47, 232]. However, early and increased levels of fetal glucocorticoids can impair growth and predispose to adult-onset diseases [136, 233, 234]. The placental enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) protects the fetus from excess endogenous glucocorticoids by converting active cortisol into inactive cortisone. 11β-HSD2 is hormonally regulated making it susceptible to endocrine disruption from chemical and nonchemical stressors such as maternal anxiety, inflammation, infection, cadmium exposure, and low caloric intake [136, 138, 224, 235, 236]. Placental hypoxia associated with PIH/PE has been shown to suppress 11β-HSD2 activity which may be an adaptive response to counteract compromised fetal growth by allowing more cortisol to reach the fetus for organ development. Low concentrations/activities of 11β-HSD2 and high levels of cortisol have been associated with PTB and FGR [136, 237, 238], two outcomes also associated with poor maternal psychosocial/mental health [233, 234, 239].
Factors affecting 11β-HSD2 activity that are associated with low SES include allostatic overload leading to the excess production of glucocorticoids that can overwhelm the fetal protective mechanism (Figure 4) [136, 231, 240]. Indirectly, allostatic load is capable of disrupting the metabolic system leading to impaired glucose tolerance, insulin resistance, diabetes, and/or obesity, all of which are risk factors for various APOs [138, 165, 231]. General maternal undernutrition and/or a low dietary protein intake has been shown to impair placental glucose transport and inhibit 11β-HSD2 activity in pregnant rats leading to FGR, indicating a possible mechanism through poor diet [224, 241]. Additionally, cadmium has also shown to inhibit 11β-HSD2 activity in both human and rodent placentas [225], and prenatal cadmium exposure has been shown to increase fetal corticosterone concentrations in rats which resulted in reduced birth weights [236]. This suggests a possible mechanism from active or passive tobacco smoke exposure or ambient PM2.5 exposure [135, 242, 243]. Collectively, it is possible for the cumulative exposures of PM2.5, smoking, ETS, poor dietary intake, and other SES-related factors to interact through the same 11β-HSD2 mechanism to increase the risk of impaired fetal growth (Figure 4).
5. Discussion
The ubiquitous exposure to particulate air pollution and its constituents (e.g., PAHs and metals) is but one class of environmental contaminants that can act through oxidative stress, inflammation, and/or endocrine disruption to promote developmental toxicity and adverse perinatal health [177, 244, 245]. Summarized in Figure 2, a perturbed early in utero environment can lead to defective deep placentation resulting in a cascade of fetal-placental adaptive mechanisms contributing to a range of pregnancy complications and adverse outcomes [60]. Here the underlying biological, social, and physical risk factors likely intersect to produce excessive or atypical oxidative stress, inflammatory response, and biological antagonism to either initiate the defective deep placentation pathology and/or contribute to the severity of its phenotype. Socioeconomic disparities are known to confound the environmental exposure effects; however, they may also act as potential effect modifiers given their overlapping etiological mechanisms with PM2.5 exposure. While the traditional biomedical paradigm that views populations as a collection of independent individuals has yielded useful information regarding risk factors, elucidating the intersecting pathways involved in APOs will require placing individual biologic and behavioural determinants within the social and spatial context [22, 246]. It is now well recognized that SES operates at multiple levels of organization, and neighbourhood or community-level factors can work to either ameliorate or exacerbate certain risk factors [15, 24–26]. The healthy migrant paradox exemplifies these effects in which home country, education, and neighbourhood qualities combine to modify the expected perinatal outcomes often observed with low income households [161, 247].
The SES risk factors that overlap or interact with the PM-mediated mechanisms include smoking, nutrition, and psychosocial stress acting through the HPA-axis and allostatic load. Given this knowledge, interventions aimed at ameliorating these factors may be the best way to counteract the negative influences of low SES and air pollution exposure on fetal development. Maternal smoking continues to be one of the most modifiable risk factors to lower the risk of APOs [134, 147]. Furthermore, maternal smoking also tends to interact negatively with nutrient intake and status [133, 248]. Smokers in general have poorer nutritional profiles than nonsmokers with both behavioural and biological factors independently accounting for the differences in micronutrients such as folate and essential vitamins and minerals [133, 248–250]. While smokers tend to have lower dietary nutrient intakes, they also have an accelerated requirement for micronutrients due to increased inflammatory cell turnover caused by the oxidative stress of smoking, an effect more pronounced among heavy and long-time smokers [248]. These interacting effects of smoking and nutrition are further compounded by their association with other indicators of low SES such as low education and income contributing to allostatic load [139, 251]. Nutrient intake may be ameliorative after an insult has occurred as shown in rat models of fetal alcohol syndrome where an omega-3 fatty acid enriched diet reversed the cellular effects of prenatal ethanol exposure on the fetal brain [222]. Therefore with respect to policy interventions, nutrition in the form of improved food security and micronutrient intake may serve to counteract the negative influences of both low SES and air pollution exposure [252–257].
The complex mixture of particulate air pollution, especially PM2.5 and UFPs which includes absorbed PAHs and various metals, also emerges as an important target for risk reduction and management. The deserved scrutiny stems from their ubiquity in the environment, the myriad of emission sources, and their established association with APOs [88, 98, 258]. The pervasiveness of PM2.5 and UFPs in the environment means that a high proportion of people are exposed resulting in a high etiological fraction. Therefore, even a modest reduction in exposure will have a large population effect with reduction of the societal costs of APOs [259]. Notably, their sources are primarily local, such as vehicle emissions and industrial land-use. This makes them modifiable risk factors that can be addressed at the municipal and provincial/state level with better urban planning to reduce vehicle traffic, increasing access to green-space and enforcing air quality regulations [260–263].
Not unlike the accumulation of evidence on smoking and health outcomes or that of air pollution on cardiovascular and pulmonary health [264], the epidemiological and toxicological research over the past two decades has established a consistent dose-response association with high biological specificity, temporality, and plausibility [3, 55, 177]. Taken in concert, these characteristics and further corroborating research should lend strength for evidence-based policy for intervention strategies targeting high risk areas in order to reduce the environmental burden of disease attributed to particulate air pollution [98, 265, 266].
6. Conclusion
Adverse pregnancy outcomes such as fetal growth restriction and preterm birth are a public health priority of global importance. We have brought together the multidisciplinary literature on the current state of evidence linking the physical and social environment to specific adverse pregnancy outcomes. The evidence suggests that various exposures, whether socially or environmentally determined, may be interpreted by the fetoplacental unit in similar ways resulting in a common pathological foundation for adverse outcomes, namely, deficient deep placentation. Given this background, well planned future epidemiology studies using multilevel models exploring various biological effects of the social and physical environment will have the potential to provide the evidence to establish crucial windows of fetal vulnerability with an aim to identify and mitigate modifiable risk factors.
Acknowledgments
This research was funded by a Canadian Institute of Health Research Grant (IPH-98849). Dr. Arbour is also funded through a Michael Smith Foundation for Health Research Salary Award. Funders provided no role in the direction of this review paper.
Acronyms
- AhR:
Aryl hydrocarbon receptor
- ACTH:
Adrenocorticotropic hormone
- APO:
Adverse pregnancy/perinatal outcome
- Cd:
Cadmium
- CRH:
Corticotropin releasing hormone
- COX-2:
Cyclooxygenase-2
- CO:
Carbon monoxide
- CRP:
C-reactive protein
- CVD:
Cardiovascular disease
- CYP:
Cytochrome P450
- DPP:
Defective deep placentation
- ETS:
Environmental tobacco smoke
- EVT:
Extravillous trophoblast
- IUGR or FGR:
Intrauterine or fetal growth restriction
- HHC:
Hyperhomocysteinemia
- HPA axis:
Hypothalamus-pituitary-adrenal axis
- I/R injury:
Ischemic-reperfusion injury
- GPx:
Glutathione peroxidase
- GST:
Glutathione-S-transferase
- LBW:
Low birth weight
- LDL:
Low Density lipoproteins
- NO, NO2:
Nitrogen oxide/dioxide
- PAH:
Polycyclic aromatic hydrocarbons
- PAP:
Particulate air pollution
- PIH/PE:
Pregnancy induced hypertension/preeclampsia
- PM:
Particulate matter
- pPROM:
(Premature) Prelabour rupture of membranes
- PTB:
Preterm birth
- ROS:
Reactive oxygen species
- SES:
Socioeconomic status
- SNP:
Single nucleotide polymorphism
- SOD:
Superoxide dismutase
- sEng:
Soluble endoglin
- sFlt:
Soluble fms-like tyrosine kinase
- UFP:
Ultrafine particles
- uNK (cells):
Uterine natural killer
- 11β-HSD2:
11β-Hydroxysteroid dehydrogenase type 2.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Šrám R. J., Binková B., Dejmek J., Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environmental Health Perspectives. 2005;113(4):375–382. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah P. S., Balkhair T. Air pollution and birth outcomes: a systematic review. Environment International. 2011;37(2):498–516. doi: 10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Backes C. H., Nelin T., Gorr M. W., Wold L. E. Early life exposure to air pollution: how bad is it? Toxicology Letters. 2013;216(1):47–53. doi: 10.1016/j.toxlet.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maisonet M., Correa A., Misra D., Jaakkola J. J. K. A review of the literature on the effects of ambient air pollution on fetal growth. Environmental Research. 2004;95(1):106–115. doi: 10.1016/j.envres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Protano C., Scalise T., Orsi G. B., Vitali M. A systematic review of benzene exposure during pregnancy and adverse outcomes on intrauterine development and birth: still far from scientific evidence. Annali di Igiene. 2012;24(6):451–463. [PubMed] [Google Scholar]
- 6.Bosetti C., Nieuwenhuijsen M. J., Gallus S., Cipriani S., La Vecchia C., Parazzini F. Ambient particulate matter and preterm birth or birth weight: a review of the literature. Archives of Toxicology. 2010;84(6):447–460. doi: 10.1007/s00204-010-0514-z. [DOI] [PubMed] [Google Scholar]
- 7.Lacasaña M., Esplugues A., Ballester F. Exposure to ambient air pollution and prenatal and early childhood health effects. European Journal of Epidemiology. 2005;20(2):183–199. doi: 10.1007/s10654-004-3005-9. [DOI] [PubMed] [Google Scholar]
- 8.Glinianaia S. V., Rankin J., Bell R., Pless-Mulloli T., Howel D. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology. 2004;15(1):36–45. doi: 10.1097/01.ede.0000101023.41844.ac. [DOI] [PubMed] [Google Scholar]
- 9.Dadvand P., Parker J., Bell M. L., et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environmental Health Perspectives. 2013;121(3):267–373. doi: 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker J. D., Schoendorf K. C., Kiely J. L. Associations between measures of socioeconomic status and low birth weight, small for gestational age, and premature delivery in the United States. Annals of Epidemiology. 1994;4(4):271–278. doi: 10.1016/1047-2797(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox M. A., Smith S. J., Johnson I. R., Maynard P. V., Chilvers C. E. The effect of social deprivation on birthweight, excluding physiological and pathological effects. British Journal of Obstetrics and Gynaecology. 1995;102(11):918–924. doi: 10.1111/j.1471-0528.1995.tb10882.x. [DOI] [PubMed] [Google Scholar]
- 12.Kramer M. S., Séguin L., Lydon J., Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatric and Perinatal Epidemiology. 2000;14(3):194–210. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- 13.Joseph K. S., Liston R. M., Dodds L., Dahlgren L., Allen A. C. Socioeconomic status and perinatal outcomes in a setting with universal access to essential health care services. CMAJ. 2007;177(6):583–590. doi: 10.1503/cmaj.061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Z.-C., Wilkins R., Kramer M. S. Effect of neighbourhood income and maternal education on birth outcomes: a population-based study. Canadian Medical Association Journal. 2006;174(10):1415–1420. doi: 10.1503/cmaj.051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumenshine P., Egerter S., Barclay C. J., Cubbin C., Braveman P. Socioeconomic disparities in adverse birth outcomes: a systematic review. The American Journal of Preventive Medicine. 2010;39(3):263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Petersen C. B., Mortensen L. H., Morgen C. S., Madsen M., Schnor O., Arntzen A., Gissler M., Cnattingius S., Andersen A.-M. N. Socio-economic inequality in preterm birth: a comparative study of the Nordic countries from 1981 to 2000. Paediatric and Perinatal Epidemiology. 2009;23(1):66–75. doi: 10.1111/j.1365-3016.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- 17.MacMahon B., Kovar M. G., Feldman J. J. Infant mortality rates: socioeconomic factors. United States. Vital and Health Statistics Series 1: Programs and Collection Procedures. 1972;22(14):1–68. [PubMed] [Google Scholar]
- 18.Antonovsky A. Social class and the major cardiovascular diseases. Journal of Chronic Diseases. 1968;21(2):65–106. doi: 10.1016/0021-9681(68)90098-2. [DOI] [PubMed] [Google Scholar]
- 19.Cassel J. The contribution of the social environment to host resistance: the Fourth Wade Hampton Frost Lecture. The American Journal of Epidemiology. 1976;104:107–123. doi: 10.1093/oxfordjournals.aje.a112281. [DOI] [PubMed] [Google Scholar]
- 20.Diez-Roux A. V. Bringing context back into epidemiology: variables and fallacies in multilevel analysis. American Journal of Public Health. 1998;88(2):216–222. doi: 10.2105/AJPH.88.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickett K. E., Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. Journal of Epidemiology and Community Health. 2001;55(2):111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMichael A. J. Prisoners of the proximate: loosening the constraints on epidemiology in an age of change. American Journal of Epidemiology. 1999;149(10):887–897. doi: 10.1093/oxfordjournals.aje.a009732. [DOI] [PubMed] [Google Scholar]
- 23.Sampson R. J., Morenoff J. D., Gannon-Rowley T. Assessing “neighborhood effects”: social processes and new directions in research. Annual Review of Sociology. 2002;28:443–478. doi: 10.1146/annurev.soc.28.110601.141114. [DOI] [Google Scholar]
- 24.Meng G., Thompson M. E., Hall G. B. Pathways of neighbourhood-level socio-economic determinants of adverse birth outcomes. International Journal of Health Geographics. 2013;12, article 32 doi: 10.1186/1476-072X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metcalfe A., Lail P., Ghali W. A., Sauve R. S. The association between neighbourhoods and adverse birth outcomes: a systematic review and meta-analysis of multi-level studies. Paediatric and Perinatal Epidemiology. 2011;25(3):236–245. doi: 10.1111/j.1365-3016.2011.01192.x. [DOI] [PubMed] [Google Scholar]
- 26.Morenoff J. D. D. Neighborhood mechanisms and the spatial dynamics of birth weight. American Journal of Sociology. 2003;108(5):976–1017. doi: 10.1086/374405. [DOI] [PubMed] [Google Scholar]
- 27.Clougherty J. E., Rossi C. A., Lawrence J., Long M. S., Diaz E. A., Lim R. H., McEwen B., Koutrakis P., Godleski J. J. Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environmental Health Perspectives. 2010;118(6):769–775. doi: 10.1289/ehp.0901631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortin M. C., Cory-Slechta D. A., Ohman-Strickland P., Nwankwo C., Yanger T. S., Todd A. C., Moynihan J., Walton J., Brooks A., Fiedler N. Increased lead biomarker levels are associated with changes in hormonal response to stress in occupationally exposed male participants. Environmental Health Perspectives. 2012;120(2):278–283. doi: 10.1289/ehp.1103873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cory-Slechta D. A., Virgolini M. B., Rossi-George A., Thiruchelvam M., Lisek R., Weston D. Lifetime consequences of combined maternal lead and stress. Basic and Clinical Pharmacology and Toxicology. 2008;102(2):218–227. doi: 10.1111/j.1742-7843.2007.00189.x. [DOI] [PubMed] [Google Scholar]
- 30.Gump B. B., Reihman J., Stewart P., Lonky E., Granger D. A., Matthews K. A. Blood lead (Pb) levels: further evidence for an environmental mechanism explaining the association between socioeconomic status and psychophysiological dysregulation in children. Health Psychology. 2009;28(5):614–620. doi: 10.1037/a0015611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolton J. L., Huff N. C., Smith S. H., Mason S. N., Foster W. M., Auten R. L., Bilbo S. D. Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environmental Health Perspectives. 2013;121(9):1075–1082. doi: 10.1289/ehp.1306560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kortenkamp A., Faust M., Scholze M., Backhaus T. Low-level exposure to multiple chemicals: reason for human health concerns? Environmental Health Perspectives. 2007;115(supplement):106–114. doi: 10.1289/ehp.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpenter D. O., Arcaro K., Spink D. C. Understanding the human health effects of chemical mixtures. Environmental Health Perspectives. 2002;110(1):25–42. doi: 10.1289/ehp.02110s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rider C. V., Furr J. R., Wilson V. S., Gray L. E., Jr. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. International Journal of Andrology. 2010;33(2):443–462. doi: 10.1111/j.1365-2605.2009.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valavanidis A., Vlachogianni T., Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. International Journal of Environmental Research and Public Health. 2009;6(2):445–462. doi: 10.3390/ijerph6020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Fur P. L., Evans G. W., Hubal E. A. C., Kyle A. D., Morello-Frosch R. A., Williams D. R. Vulnerability as a function of individual and group resources in cumulative risk assessment. Environmental Health Perspectives. 2007;115(5):817–824. doi: 10.1289/ehp.9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morello-Frosch R., Shenassa E. D. The environmental “Riskscape” and social inequality: implications for explaining maternal and child health disparities. Environmental Health Perspectives. 2006;114(8):1150–1153. doi: 10.1289/ehp.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su J. G., Morello-Frosch R., Jesdale B. M., Kyle A. D., Shamasunder B., Jerrett M. An index for assessing demographic inequalities in cumulative environmental hazards with application to Los Angeles, California. Environmental Science and Technology. 2009;43(20):7626–7634. doi: 10.1021/es901041p. [DOI] [PubMed] [Google Scholar]
- 39.Gee G. C., Payne-Sturges D. C. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environmental Health Perspectives. 2004;112(17):1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molitor J., Su J. G., Molitor N.-T., et al. Identifying vulnerable populations through an examination of the association between multipollutant profiles and poverty. Environmental Science & Technology. 2011;45(18):7754–7760. doi: 10.1021/es104017x. [DOI] [PubMed] [Google Scholar]
- 41.National Environmental Justice Advisory Council . Nationally Consistent Environmental Justice Screening Approaches. Washington, DC, USA: National Environmental Justice Advisory Council; 2010. [Google Scholar]
- 42.Linder S. H., Sexton K. Conceptual models for cumulative risk assessment. The American Journal of Public Health. 2011;101(1):S74–S81. doi: 10.2105/AJPH.2011.300318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sexton K., Linder S. H. Cumulative risk assessment for combined health effects from chemical and nonchemical stressors. American Journal of Public Health. 2011;101(supplement 1):S81–S88. doi: 10.2105/AJPH.2011.300118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robertson S. A. Immune regulation of conception and embryo implantation-all about quality control? Journal of Reproductive Immunology. 2010;85(1):51–57. doi: 10.1016/j.jri.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Lager S., Powell T. L. Regulation of nutrient transport across the placenta. Journal of Pregnancy. 2012;2012 doi: 10.1155/2012/179827.179827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAleer M. F., Tuan R. S. Cytotoxicant-induced trophoblast dysfunction and abnormal pregnancy outcomes: role of zinc and metallothionein. Birth Defects Research Part C: Embryo Today: Reviews. 2004;72(4):361–370. doi: 10.1002/bdrc.20024. [DOI] [PubMed] [Google Scholar]
- 47.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain, Behavior, and Immunity. 2005;19(4):296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Janssen B. G., Munters E., Pieters N., Smeets K., Cox B., Cuypers A., Fierens F., Penders J., Vangronsveld J., Gyselaers W., Nawrot T. S. Placental mitochondrial DNA content and particulate air pollution during in Utero life. Environmental Health Perspectives. 2012;120(9):1346–1352. doi: 10.1289/ehp.1104458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stasenko S., Bradford E. M., Piasek M., Henson M. C., Varnai V. M., Jurasović J., Kušec V. Metals in human placenta: focus on the effects of cadmium on steroid hormones and leptin. Journal of Applied Toxicology. 2010;30(3):242–253. doi: 10.1002/jat.1490. [DOI] [PubMed] [Google Scholar]
- 50.Jedrychowski W. A., Perera F. P., Tang D., et al. The relationship between prenatal exposure to airborne polycyclic aromatic hydrocarbons (PAHs) and PAH-DNA adducts in cord blood. Journal of Exposure Science and Environmental Epidemiology. 2013;23(4):371–377. doi: 10.1038/jes.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sankaran S., Kyle P. M. Aetiology and Pathogenesis of IUGR. Best Practice and Research: Clinical Obstetrics and Gynaecology. 2009;23(6):765–777. doi: 10.1016/j.bpobgyn.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Gluckman P. D., Hanson M. A., Pinal C. The developmental origins of adult disease. Maternal and Child Nutrition. 2005;1(3):130–141. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swanson J. M., Entringer S., Buss C., Wadhwa P. D. Developmental origins of health and disease: environmental exposures. Seminars in Reproductive Medicine. 2009;27(5):391–402. doi: 10.1055/s-0029-1237427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kramer M. S., Goulet L., Lydon J., Séguin L., McNamara H., Dassa C., Platt R. W., Chen M. F., Gauthier H., Genest J., Jr., Kahn S., Libman M., Rozen R., Masse A., Miner L., Asselin G., Benjamin A., Klein J., Koren G. Socio-economic disparities in preterm birth: causal pathways and mechanisms. Paediatric and Perinatal Epidemiology. 2001;15(supplement 2):104–123. doi: 10.1046/j.1365-3016.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 55.Kannan S., Misra D. P., Dvonch J. T., Krishnakumar A. Exposures to airbone particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environmental Health Perspectives. 2006;114(11):1636–1642. doi: 10.1289/ehp.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carter A. M., Pijnenborg R. Evolution of invasive placentation with special reference to non-human primates. Best Practice & Research: Clinical Obstetrics & Gynaecology. 2011;25(3):249–257. doi: 10.1016/j.bpobgyn.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Martin R. D. The evolution of human reproduction: a primatological perspective. The American Journal of Physical Anthropology. 2007;45:59–84. doi: 10.1002/ajpa.20734. [DOI] [PubMed] [Google Scholar]
- 58.Capellini I., Venditti C., Barton R. A. Placentation and maternal investment in mammals. American Naturalist. 2011;177(1):86–98. doi: 10.1086/657435. [DOI] [PubMed] [Google Scholar]
- 59.Chucri T. M., Monteiro J. M., Lima A. R., Salvadori M. L. B., Junior J. R. K., Miglino M. A. A review of immune transfer by the placenta. Journal of Reproductive Immunology. 2010;87(1-2):14–20. doi: 10.1016/j.jri.2010.08.062. [DOI] [PubMed] [Google Scholar]
- 60.Brosens I., Pijnenborg R., Vercruysse L., Romero R. The “great Obstetrical Syndromes” are associated with disorders of deep placentation. American Journal of Obstetrics & Gynecology. 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romero R., Kusanovic J. P., Chaiworapongsa T., Hassan S. S. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Practice and Research: Clinical Obstetrics and Gynaecology. 2011;25(3):313–327. doi: 10.1016/j.bpobgyn.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khong Y., Brosens I. Defective deep placentation. Best Practice and Research: Clinical Obstetrics and Gynaecology. 2011;25(3):301–311. doi: 10.1016/j.bpobgyn.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 63.Andraweera P. H., Dekker G. A., Roberts C. T. The vascular endothelial growth factor family in adverse pregnancy outcomes. Human Reproduction Update. 2012;18(4):436–457. doi: 10.1093/humupd/dms011.dms011 [DOI] [PubMed] [Google Scholar]
- 64.Barker D. J. P. The origins of the developmental origins theory. Journal of Internal Medicine. 2007;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 65.McMillen I. C., Robinson J. S. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiological Reviews. 2005;85(2):571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 66.Heindel J. J. Role of exposure to environmental chemicals in the developmental basis of reproductive disease and dysfunction. Seminars in Reproductive Medicine. 2006;24(3):168–177. doi: 10.1055/s-2006-944423. [DOI] [PubMed] [Google Scholar]
- 67.Pijnenborg R., Vercruysse L., Brosens I. Deep placentation. Best Practice & Research: Clinical Obstetrics & Gynaecology. 2011;25(3):273–285. doi: 10.1016/j.bpobgyn.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Jauniaux E., Poston L., Burton G. J. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Human Reproduction Update. 2006;12(6):747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sibai B., Dekker G., Kupferminc M. Pre-eclampsia. The Lancet. 2005;365(9461):785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 70.Burton G. J., Jauniaux E. Oxidative stress. Best Practice & Research Clinical Obstetrics & Gynaecology. 2011;25:287–299. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wlodarczyk B. J., Palacios A. M., Chapa C. J., Zhu H., George T. M., Finnell R. H. Genetic basis of susceptibility to teratogen induced birth defects. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics. 2011;157(3):215–226. doi: 10.1002/ajmg.c.30314. [DOI] [PubMed] [Google Scholar]
- 72.Rappolee D. A., Awonuga A. O., Puscheck E. E., Zhou S., Xie Y. Benzopyrene and experimental stressors cause compensatory differentiation in placental trophoblast stem cells. Systems Biology in Reproductive Medicine. 2010;56(2):168–183. doi: 10.3109/19396360903431638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perera F., Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reproductive Toxicology. 2011;31(3):363–373. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lewtas J. Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutation Research: Reviews in Mutation Research. 2007;636(1–3):95–133. doi: 10.1016/j.mrrev.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Stejskalova L., Pavek P. The function of cytochrome P450 1A1 enzyme (CYP1A1) and aryl hydrocarbon receptor (AhR) in the placenta. Current Pharmaceutical Biotechnology. 2011;12(5):715–730. doi: 10.2174/138920111795470994. [DOI] [PubMed] [Google Scholar]
- 76.de Kok T. M. C. M., Driece H. A. L., Hogervorst J. G. F., Briedé J. J. Toxicological assessment of ambient and traffic-related particulate matter: a review of recent studies. Mutation Research: Reviews in Mutation Research. 2006;613(2-3):103–122. doi: 10.1016/j.mrrev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Risom L., Møller P., Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutation Research. 2005;592(1-2):119–137. doi: 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 78.Valavanidis A., Fiotakis K., Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. Journal of Environmental Science and Health, Part C: Environmental Carcinogenesis and Ecotoxicology Reviews. 2008;26(4):339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- 79.Li N., Sioutas C., Cho A., Schmitz D., Misra C., Sempf J., Wang M., Oberley T., Froines J., Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environmental Health Perspectives. 2003;111(4):455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brauer M., Lencar C., Tamburic L., Koehoorn M., Demers P., Karr C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environmental Health Perspectives. 2008;116(5):680–686. doi: 10.1289/ehp.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu S., Krewski D., Shi Y., Chen Y., Burnett R. T. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environmental Health Perspectives. 2003;111(14):1773–1778. doi: 10.1289/ehp.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Resnik R. Intrauterine growth restriction. Obstetrics and Gynecology. 2002;99(3):490–496. doi: 10.1016/S0029-7844(01)01780-X. [DOI] [PubMed] [Google Scholar]
- 83.Behrman R. E., Butler A. S., editors. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC, USA: National Acadamies Press; 2007. [PubMed] [Google Scholar]
- 84.Petrou S. Economic consequences of preterm birth and low birthweight. BJOG. 2003;110(supplement 20):117–123. [PubMed] [Google Scholar]
- 85.Saigal S., Doyle L. W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet. 2008;371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 86.Williams R. L., Creasy R. K., Cunningham G. C., Hawes W. E., Norris F. D., Tashiro M. Fetal growth and perinatal viability in California. Obstetrics and Gynecology. 1982;59(5):624–632. [PubMed] [Google Scholar]
- 87.Boyd D. R., Genuis S. J. The environmental burden of disease in Canada: respiratory disease, cardiovascular disease, cancer, and congenital affliction. Environmental Research. 2008;106(2):240–249. doi: 10.1016/j.envres.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 88.Priority Substances List Assessment Report for Respirable Particulate Matter Less Than 10 microns. Ottawa, Canada: Environment Canada, Health Canada; 2000. [Google Scholar]
- 89.US Environmental Protection Agency . Integrated Science Assessment for Particulate Matter (Final Report) Washington, DC, USA: US Environmental Protection Agency; 2009. [PubMed] [Google Scholar]
- 90.Dockery D. W., Pope C. A. Acute respiratory effects of particulate air pollution. Annual Review of Public Health. 1994;15:107–132. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- 91.Harrison R. M., Jones A. M., Lawrence R. G. Major component composition of PM10 and PM2.5 from roadside and urban background sites. Atmospheric Environment. 2004;38(27):4531–4538. doi: 10.1016/j.atmosenv.2004.05.022. [DOI] [Google Scholar]
- 92.Schroeder W. H., Dobson M., Kane D. M., Johnson N. D. Toxic trace elements associated with airborne particulate matter: a review. Journal of the Air Pollution Control Association. 1987;37(11):1267–1285. doi: 10.1080/08940630.1987.10466321. [DOI] [PubMed] [Google Scholar]
- 93.Boogaard H., Kos G. P. A., Weijers E. P., Janssen N. A. H., Fischer P. H., van der Zee S. C., de Hartog J. J., Hoek G. Contrast in air pollution components between major streets and background locations: Particulate matter mass, black carbon, elemental composition, nitrogen oxide and ultrafine particle number. Atmospheric Environment. 2011;45(3):650–658. doi: 10.1016/j.atmosenv.2010.10.033. [DOI] [Google Scholar]
- 94.Bell M. L., Belanger K. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. Journal of Exposure Science and Environmental Epidemiology. 2012;22(5):429–438. doi: 10.1038/jes.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bell M. L., Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environmental Health Perspectives. 2012;120(12):1699–1704. doi: 10.1289/ehp.1205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boogaard H., Janssen N. A. H., Fischer P. H., Kos G. P. A., Weijers E. P., Cassee F. R., van der Zee S. C., de Hartog J. J., Brunekreef B., Hoek G. Contrasts in oxidative potential and other particulate matter characteristics collected near major streets and background locations. Environmental Health Perspectives. 2012;120(2):185–191. doi: 10.1289/ehp.1103667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Donaldson K., Stone V., Seaton A., MacNee W. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environmental Health Perspectives. 2001;109(supplement):523–527. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.HEI Review Panel on Ultrafine Particles . Understanding the Health Effects of Ambient Ultrafine Particles. Boston, Mass, USA: HEI Perspectives; 2013. [Google Scholar]
- 99.Ghio A. J., Sobus J. R., Pleil J. D., Madden M. C. Controlled human exposures to diesel exhaust. Swiss Medical Weekly. 2012;142 doi: 10.4414/smw.2012.13597.w13597 [DOI] [PubMed] [Google Scholar]
- 100.Weldy C. S., Liu Y., Liggitt H. D., Chin M. T. In utero exposure to diesel exhaust air pollution promotes adverse intrauterine conditions, resulting in weight gain, altered blood pressure, and increased susceptibility to heart failure in adult mice. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0088582.e88582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takeda K., Tsukue N., Yoshida S. Endocrine-disrupting activity of chemicals in diesel exhaust and diesel exhaust particles. Environmental Sciences. 2004;11(1):33–45. [PubMed] [Google Scholar]
- 102.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Polycyclic Aromatic Hydrocarbons. Atlanta, Ga, USA: Agency for Toxic Substances and Disease Registry (ATSDR); 1995. [PubMed] [Google Scholar]
- 103.Gladen B. C., Zadorozhnaja T. D., Chislovska N., Hryhorczuk D. O., Kennicutt M. C., II, Little R. E. Polycyclic aromatic hydrocarbons in placenta. Human & Experimental Toxicology. 2000;19(11):597–603. doi: 10.1191/096032700671433928. [DOI] [PubMed] [Google Scholar]
- 104.Environmental Protection Agency . Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. Washington, DC, USA: U.S. Environmental Protection Agency; 2000. [Google Scholar]
- 105.Líbalová H., Krčková S., Uhlířová K., Milcová A., Schmuczerová J., Ciganek M., Kléma J., Machala M., Šrám R. J., Topinka J. Genotoxicity but not the AhR-mediated activity of PAHs is inhibited by other components of complex mixtures of ambient air pollutants. Toxicology Letters. 2014;225(3):350–357. doi: 10.1016/j.toxlet.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 106.Environment Canada, Health Canada . Priority Substances List Assessment Report for Polycyclic Aromatic Hydrocarbons. Ottawa, Canada: Environment Canada, Health Canada; 1994. [Google Scholar]
- 107.Li X. Y., Gilmour P. S., Donaldson K., MacNee W. Free radical activity and pro-inflammatory effect of particulate air pollution (PM10) in vivo and in vitro. Thorax. 1996;51(12):1216–1222. doi: 10.1136/thx.51.12.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miller M. R., Shaw C. A., Langrish J. P. From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiology. 2012;8(4):577–602. doi: 10.2217/fca.12.43. [DOI] [PubMed] [Google Scholar]
- 109.Simkhovich B. Z., Kleinman M. T., Kloner R. A. Air pollution and cardiovascular injury: epidemiology, toxicology, and mechanisms. Journal of the American College of Cardiology. 2008;52(9):719–726. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 110.Morello-Frosch R., Zuk M., Jerrett M., Shamasunder B., Kyle A. D. Understanding the cumulative impacts of inequalities in environmental health: implications for policy. Health Affairs. 2011;30(5):879–887. doi: 10.1377/hlthaff.2011.0153. [DOI] [PubMed] [Google Scholar]
- 111.Krewski D., Jerrett M., Burnett R. T., et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Research Report (Health Effects Institute) 2009;140:5–114. [PubMed] [Google Scholar]
- 112.Tanuseputro P., Manuel D. G., Leung M., Nguyen K., Johansen H. Risk factors for cardiovascular disease in Canada. Canadian Journal of Cardiology. 2003;19(11):1249–1259. [PubMed] [Google Scholar]
- 113.Lee D. S., Chiu M., Manuel D. G., Tu K., Wang X., Austin P. C., Mattern M. Y., Mitiku T. F., Svenson L. W., Putnam W., Flanagan W. M., Tu J. V. Trends in risk factors for cardiovascular disease in Canada: temporal, socio-demographic and geographic factors. CMAJ. 2009;181(3-4):E55–E66. doi: 10.1503/cmaj.081629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kannan S., Dvonch J. T., Schulz A. J., Israel B. A., Mentz G., House J., Max P., Reyes A. G. Exposure to fine particulate matter and acute effects on blood pressure: Effect modification by measures of obesity and location. Journal of Epidemiology and Community Health. 2010;64(1):68–74. doi: 10.1136/jech.2008.081836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Powe C. E., Levine R. J., Karumanchi S. A. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123(24):2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Poston L. Endothelial dysfunction in pre-eclampsia. Pharmacological Reports. 2006;58:69–74. [PubMed] [Google Scholar]
- 117.Louey S., Thornburg K. L. The prenatal environment and later cardiovascular disease. Early Human Development. 2005;81(9):745–751. doi: 10.1016/j.earlhumdev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 118.Poston L., Igosheva N., Mistry H. D., Seed P. T., Shennan A. H., Rana S., Karumanchi S. A., Chappell L. C. Role of oxidative stress and antioxidant supplementation in pregnancy disorders. The American Journal of Clinical Nutrition. 2011;94(6):1980S–1985S. doi: 10.3945/ajcn.110.001156. [DOI] [PubMed] [Google Scholar]
- 119.Mangoni A. A., Jackson S. H. D. Homocysteine and cardiovascular disease: current evidence and future prospects. The American Journal of Medicine. 2002;112(7):556–565. doi: 10.1016/S0002-9343(02)01021-5. [DOI] [PubMed] [Google Scholar]
- 120.Kramer M. S., Kahn S. R., Rozen R., et al. Vasculopathic and thrombophilic risk factors for spontaneous preterm birth. International Journal of Epidemiology. 2009;38(3):715–723. doi: 10.1093/ije/dyp167. [DOI] [PubMed] [Google Scholar]
- 121.Assmann G., Nofer J.-R. Atheroprotective effects of high-density lipoproteins. Annual Review of Medicine. 2003;54:321–341. doi: 10.1146/annurev.med.54.101601.152409. [DOI] [PubMed] [Google Scholar]
- 122.Bocskay K. A., Tang D., Orjuela M. A., Liu X., Warburton D. P., Perera F. P. Chromosomal aberrations in cord blood are associated with prenatal exposure to carcinogenic polycyclic aromatic hydrocarbons. Cancer Epidemiology Biomarkers& Prevention. 2005;14(2):506–511. doi: 10.1158/1055-9965.EPI-04-0566. [DOI] [PubMed] [Google Scholar]
- 123.Perera F., Tang D., Whyatt R., Lederman S. A., Jedrychowski W. DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiology Biomarkers and Prevention. 2005;14(3):709–714. doi: 10.1158/1055-9965.EPI-04-0457. [DOI] [PubMed] [Google Scholar]
- 124.Lupo P. J., Langlois P. H., Reefhuis J., Lawson C. C., Symanski E., Desrosiers T. A., Khodr Z. G., Agopian A. J., Waters M. A., Duwe K. N., Finnell R. H., Mitchell L. E., Moore C. A., Romitti P. A., Shaw G. M. Maternal occupational exposure to polycyclic aromatic hydrocarbons: effects on gastroschisis among offspring in the national birth defects prevention study. Environmental Health Perspectives. 2012;120(6):910–915. doi: 10.1289/ehp.1104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mattison D. R. Environmental exposures and development. Current Opinion in Pediatrics. 2010;22(2):208–218. doi: 10.1097/MOP.0b013e32833779bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brent R. L. Environmental causes of human congenital malformations: the pediatrician’s role in dealing with these complex clinical problems caused by a multiplicity of environmental and genetic factors. Pediatrics. 2004;113(4):957–968. [PubMed] [Google Scholar]
- 127.Hackshaw A., Rodeck C., Boniface S. Maternal smoking in pregnancy and birth defects: A systematic review based on 173 687 malformed cases and 11.7 million controls. Human Reproduction Update. 2011;17(5):589–604. doi: 10.1093/humupd/dmr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cetin I., Berti C., Calabrese S. Role of micronutrients in the periconceptional period. Human Reproduction Update. 2009;16(1):80–95. doi: 10.1093/humupd/dmp025. [DOI] [PubMed] [Google Scholar]
- 129.Lim R., Barker G., Wall C. A., Lappas M. Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium. Molecular Human Reproduction. 2013;19(7):451–462. doi: 10.1093/molehr/gat015. [DOI] [PubMed] [Google Scholar]
- 130.Guxens M., Aguilera I., Ballester F., et al. Prenatal exposure to residential air pollution and infant mental development: modulation by antioxidants and detoxification factors. Environmental Health Perspectives. 2012;120(1):144–149. doi: 10.1289/ehp.1103469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pedersen M., Schoket B., Godschalk R. W., Wright J., von Stedingk H., Törnqvist M., Sunyer J., Nielsen J. K., Merlo D. F., Mendez M. A., Meltzer H. M., Lukács V., Landström A., Kyrtopoulos S. A., Kovács K., Knudsen L. E., Haugen M., Hardie L. J., Gützkow K. B., Fleming S., Fthenou E., Farmer P. B., Espinosa A., Chatzi L., Brunborg G., Brady N. J., Botsivali M., Arab K., Anna L., Alexander J., Agramunt S., Kleinjans J. C., Segerbäck D., Kogevinas M. Bulky DNA adducts in cord blood, maternal fruit-and-vegetable consumption, and birth weight in a European mother-child study (NewGeneris) Environmental Health Perspectives. 2013;121(10):1200–1206. doi: 10.1289/ehp.1206333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zdravkovic T., Genbacev O., McMaster M. T., Fisher S. J. The adverse effects of maternal smoking on the human placenta: a review. Placenta. 2005;26:S81–S86. doi: 10.1016/j.placenta.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 133.Northrop-Clewes C. A., Thurnham D. I. Monitoring micronutrients in cigarette smokers. Clinica Chimica Acta. 2007;377(1-2):14–38. doi: 10.1016/j.cca.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 134.Jauniaux E., Burton G. J. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Human Development. 2007;83(11):699–706. doi: 10.1016/j.earlhumdev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 135.Sorkun H. C., Bir F., Akbulut M., Divrikli U., Erken G., Demirhan H., Duzcan E., Elci L., Celik I., Yozgatli U. The effects of air pollution and smoking on placental cadmium, zinc concentration and metallothionein expression. Toxicology. 2007;238(1):15–22. doi: 10.1016/j.tox.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 136.Marciniak B., Patro-Malysza J., Poniedziałek-Czajkowska E., Kimber-Trojnar Z., Leszczyńska-Gorzelak B., Oleszczuk J. Glucocorticoids in pregnancy. Current Pharmaceutical Biotechnology. 2011;12(5):750–757. doi: 10.2174/138920111795470868. [DOI] [PubMed] [Google Scholar]
- 137.Clougherty J. E., Kubzansky L. D. A framework for examining social stress and susceptibility to air pollution in respiratory health. Environmental Health Perspectives. 2009;117(9):1351–1358. doi: 10.1289/ehp.0900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mairesse J., Lesage J., Breton C., Bréant B., Hahn T., Darnaudéry M., Dickson S. L., Seckl J., Blondeau B., Vieau D., Maccari S., Viltart O. Maternal stress alters endocrine function of the feto-placental unit in rats. The American Journal of Physiology—Endocrinology and Metabolism. 2007;292(6):E1526–E1533. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- 139.Haste F. M., Brooke O. G., Anderson H. R., Bland J. M., Peacock J. L. Social determinants of nutrient intake in smokers and non-smokers during pregnancy. Journal of Epidemiology and Community Health. 1990;44(3):205–209. doi: 10.1136/jech.44.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Haste F. M., Brooke O. G., Anderson H. R., Bland J. M. The effect of nutritional intake on outcome of pregnancy in smokers and non-smokers. British Journal of Nutrition. 1991;65(3):347–354. doi: 10.1079/BJN19910095. [DOI] [PubMed] [Google Scholar]
- 141.Rifas-Shiman S. L., Rich-Edwards J. W., Kleinman K. P., Oken E., Gillman M. W. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. Journal of the American Dietetic Association. 2009;109(6):1004–1011. doi: 10.1016/j.jada.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baker P. N., Wheeler S. J., Sanders T. A., Thomas J. E., Hutchinson C. J., Clarke K., Berry J. L., Jones R. L., Seed P. T., Poston L. A prospective study of micronutrient status in adolescent pregnancy. The American Journal of Clinical Nutrition. 2009;89(4):1114–1124. doi: 10.3945/ajcn.2008.27097. [DOI] [PubMed] [Google Scholar]
- 143.Hennig B., Ormsbee L., McClain C. J., Watkins B. A., Blumberg B., Bachas L. G., Sanderson W., Thompson C., Suk W. A. Nutrition can modulate the toxicity of environmental pollutants: Implications in risk assessment and human health. Environmental Health Perspectives. 2012;120(6):771–774. doi: 10.1289/ehp.1104712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salmasi G., Grady R., Jones J., McDonald S. D. Environmental tobacco smoke exposure and perinatal outcomes: a systematic review and meta-analyses. Acta Obstetricia et Gynecologica Scandinavica. 2010;89(4):423–441. doi: 10.3109/00016340903505748. [DOI] [PubMed] [Google Scholar]
- 145.Dejmek J., Solansky I., Podrazilová K., Šrám R. J. The exposure of nonsmoking and smoking mothers to environmental tobacco smoke during different gestational phases and fetal growth. Environmental Health Perspectives. 2002;110(6):601–606. doi: 10.1289/ehp.02110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Castles A., Adams E. K., Melvin C. L., Kelsch C., Boulton M. L. Effects of smoking during pregnancy: five meta-analyses. American Journal of Preventive Medicine. 1999;16(3):208–215. doi: 10.1016/S0749-3797(98)00089-0. [DOI] [PubMed] [Google Scholar]
- 147.Erickson A. C., Arbour L. T. Heavy smoking during pregnancy as a marker for other risk factors of adverse birth outcomes: a population-based study in British Columbia, Canada. BMC Public Health. 2012;12(1, article 102) doi: 10.1186/1471-2458-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mohsin M., Bauman A. E. Socio-demographic factors associated with smoking and smoking cessation among 426,344 pregnant women in New South Wales, Australia. BMC Public Health. 2005;5, article 138 doi: 10.1186/1471-2458-5-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schneider S., Maul H., Freerksen N., Pötschke-Langer M. Who smokes during pregnancy? An analysis of the German Perinatal Quality Survey 2005. Public Health. 2008;122(11):1210–1216. doi: 10.1016/j.puhe.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 150.Krishnan S., Cozier Y. C., Rosenberg L., Palmer J. R. Socioeconomic status and incidence of type 2 diabetes: results from the black women's health study. American Journal of Epidemiology. 2010;171(5):564–570. doi: 10.1093/aje/kwp443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dubowsky S. D., Suh H., Schwartz J., Coull B. A., Gold D. R. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environmental Health Perspectives. 2006;114(7):992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen J. C., Cavallari J. M., Stone P. H., Christiani D. C. Obesity is a modifier of automatic cardiac responses to fine metal particulates. Environmental Health Perspectives. 2007;115(7):1002–1006. doi: 10.1289/ehp.9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Malmqvist E., Jakobsson K., Tinnerberg H., Rignell-Hydbom A., Rylander L. Gestational diabetes and preeclampsia in association with air pollution at levels below current air quality guidelines. Environmental Health Perspectives. 2013;121(4):488–493. doi: 10.1289/ehp.1205736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pereira G., Haggar F., Shand A. W., Bower C., Cook A., Nassar N. Association between pre-eclampsia and locally derived traffic-related air pollution: a retrospective cohort study. Journal of Epidemiology and Community Health. 2013;67(2):147–152. doi: 10.1136/jech-2011-200805. [DOI] [PubMed] [Google Scholar]
- 155.Dadvand P., Figueras F., Basagaña X., Beelen R., Martinez D., Cirach M., Schembari A., Hoek G., Brunekreef B., Nieuwenhuijsen M. J. Ambient air pollution and preeclampsia: a spatiotemporal analysis. Environmental Health Perspectives. 2013;121(11-12):1365–1371. doi: 10.1289/ehp.1206430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu J., Ren C., Delfino R. J., Chung J., Wilhelm M., Ritz B. Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the South Coast Air Basin of california. Environmental Health Perspectives. 2009;117(11):1773–1779. doi: 10.1289/ehp.0800334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gruenewald T. L., Karlamangla A. S., Hu P., Stein-Merkin S., Crandall C., Koretz B., Seeman T. E. History of socioeconomic disadvantage and allostatic load in later life. Social Science and Medicine. 2012;74(1):75–83. doi: 10.1016/j.socscimed.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Seeman T. E., McEwen B. S., Rowe J. W., Singer B. H. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(8):4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bird C. E., Seeman T., Escarce J. J., Basurto-Dávila R., Finch B. K., Dubowitz T., Heron M., Hale L., Merkin S. S., Weden M., Lurie N. Neighbourhood socioeconomic status and biological “wear and tear” in a nationally representative sample of US adults. Journal of Epidemiology and Community Health. 2010;64(10):860–865. doi: 10.1136/jech.2008.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Roux A. V. D. Investigating neighborhood and area effects on health. American Journal of Public Health. 2001;91(11):1783–1789. doi: 10.2105/AJPH.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Auger N., Giraud J., Daniel M. The joint influence of area income, income inequality, and immigrant density on adverse birth outcomes: a population-based study. BMC Public Health. 2009;9, article 237 doi: 10.1186/1471-2458-9-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tegethoff M., Greene N., Olsen J., Schaffner E., Meinlschmidt G. Stress during pregnancy and offspring pediatric disease: a national cohort study. Environmental Health Perspectives. 2011;119(11):1647–1652. doi: 10.1289/ehp.1003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mcewen B. S., Gianaros P. J. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McEwen B. S. Brain on stress: how the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Karatsoreos I. N., McEwen B. S. Psychobiological allostasis: Resistance, resilience and vulnerability. Trends in Cognitive Sciences. 2011;15(12):576–584. doi: 10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 166.Sapolsky R. M., Romero L. M., Munck A. U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/er.21.1.55. [DOI] [PubMed] [Google Scholar]
- 167.Davies K. J. Oxidative stress: the paradox of aerobic life. Biochemical Society Symposium. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- 168.Al-Gubory K. H., Fowler P. A., Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. The International Journal of Biochemistry & Cell Biology. 2010;42(10):1634–1650. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 169.Davies K. J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50(4-5):279–289. doi: 10.1080/15216540051081010. [DOI] [PubMed] [Google Scholar]
- 170.Ferenbach D. A., Kluth D. C., Hughes J. Hemeoxygenase-1 and renal ischaemia-reperfusion injury. Nephron—Experimental Nephrology. 2010;115(3):e33–e37. doi: 10.1159/000313828. [DOI] [PubMed] [Google Scholar]
- 171.Levytska K., Kingdom J., Baczyk D., Drewlo S. Heme oxygenase-1 in placental development and pathology. Placenta. 2013;34(4):291–298. doi: 10.1016/j.placenta.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 172.Zhao H., Wong R. J., Kalish F. S., Nayak N. R., Stevenson D. K. Effect of heme oxygenase-1 deficiency on placental development. Placenta. 2009;30(10):861–868. doi: 10.1016/j.placenta.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Munoz-Suano A., Hamilton A. B., Betz A. G. Gimme shelter: the immune system during pregnancy. Immunological Reviews. 2011;241(1):20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 174.Prins J. R., Gomez-Lopez N., Robertson S. A. Interleukin-6 in pregnancy and gestational disorders. Journal of Reproductive Immunology. 2012;95(1-2):1–14. doi: 10.1016/j.jri.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 175.Gerhard I., Waibel S., Daniel V., Runnebaum B. Impact of heavy metals on hormonal and immunological factors in women with repeated miscarriages. Human Reproduction Update. 1998;4(3):301–309. doi: 10.1093/humupd/4.3.301. [DOI] [PubMed] [Google Scholar]
- 176.Lee P.-C., Talbott E. O., Roberts J. M., Catov J. M., Sharma R. K., Ritz B. Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology. 2011;22(4):524–531. doi: 10.1097/EDE.0b013e31821c6c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vadillo-Ortega F., Osornio-Vargas A., Buxton M. A., Sánchez B. N., Rojas-Bracho L., Viveros-Alcaráz M., Castillo-Castrejón M., Beltrán-Montoya J., Brown D. G., O'Neill M. S. Air pollution, inflammation and preterm birth: a potential mechanistic link. Medical Hypotheses. 2014;82(2):219–224. doi: 10.1016/j.mehy.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McEwen B. S., Tucker P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. American Journal of Public Health. 2011;101(supplement 1):S131–S139. doi: 10.2105/AJPH.2011.300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Redman C. W. G., Tannetta D. S., Dragovic R. A., et al. Review: does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33:S48–S54. doi: 10.1016/j.placenta.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 180.Pleil J. D., Sheldon L. S. Adapting concepts from systems biology to develop systems exposure event networks for exposure science research. Biomarkers. 2011;16(2):99–105. doi: 10.3109/1354750X.2010.541565. [DOI] [PubMed] [Google Scholar]
- 181.Than N. G., Romero R., Tarca A. L., Draghici S., Erez O., Chaiworapongsa T., Mee Kim Y., Kim S. K., Vaisbuch E., Tromp G. Mitochondrial manganese superoxide dismutase mRNA expression in human chorioamniotic membranes and its association with labor, inflammation, and infection. Journal of Maternal-Fetal and Neonatal Medicine. 2009;22(11):1000–1013. doi: 10.3109/14767050903019676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bowen J. M., Chamley L., Keelan J. A., Mitchell M. D. Cytokines of the placenta and extra-placental membranes: Roles and regulation during human pregnancy and parturition. Placenta. 2002;23(4):257–273. doi: 10.1053/plac.2001.0782. [DOI] [PubMed] [Google Scholar]
- 183.Bowen J. M., Chamley L., Mitchell M. D., Keelan J. A. Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta. 2002;23(4):239–256. doi: 10.1053/plac.2001.0781. [DOI] [PubMed] [Google Scholar]
- 184.Than N. G., Romero R., Kim C. J., McGowen M. R., Papp Z., Wildman D. E. Galectins: Guardians of eutherian pregnancy at the maternal-fetal interface. Trends in Endocrinology and Metabolism. 2012;23(1):23–31. doi: 10.1016/j.tem.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Thompson J., Bannigan J. Cadmium: toxic effects on the reproductive system and the embryo. Reproductive Toxicology. 2008;25(3):304–315. doi: 10.1016/j.reprotox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 186.Henson M. C., Chedrese P. J. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Experimental Biology and Medicine. 2004;229(5):383–392. doi: 10.1177/153537020422900506. [DOI] [PubMed] [Google Scholar]
- 187.Dunn A. J. The HPA axis and the immune system: a perspective. In: del Rey A., Chrousos G. P., Besedovsky H. O., editors. NeuroImmune Biology. Vol. 7. NeuroImmune Biology, Elsevier; 2007. pp. 3–15. [Google Scholar]
- 188.Venners S. A., Wang X., Chen C., Wang L., Chen D., Guang W., Huang A., Ryan L., O'Connor J., Lasley B., Overstreet J., Wilcox A., Xu X. Paternal smoking and pregnancy loss: a prospective study using a biomarker of pregnancy. The American Journal of Epidemiology. 2004;159(10):993–1001. doi: 10.1093/aje/kwh128. [DOI] [PubMed] [Google Scholar]
- 189.Ronnenberg A. G., Venners S. A., Xu X., Chen C., Wang L., Guang W., Huang A., Wang X. Preconception B-vitamin and homocysteine status, conception, and early pregnancy loss. American Journal of Epidemiology. 2007;166(3):304–312. doi: 10.1093/aje/kwm078. [DOI] [PubMed] [Google Scholar]
- 190.Slama R., Bottagisi S., Solansky I., Lepeule J., Giorgis-Allemand L., Sram R. Short-term impact of atmospheric pollution on fecundability. Epidemiology. 2013;24(6):871–879. doi: 10.1097/EDE.0b013e3182a702c5. [DOI] [PubMed] [Google Scholar]
- 191.Nieuwenhuijsen M. J., Basagaña X., Dadvand P., Martinez D., Cirach M., Beelen R., Jacquemin B. Air pollution and human fertility rates. Environment International. 2014;70:9–14. doi: 10.1016/j.envint.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 192.Jauniaux E., Watson A. L., Hempstock J., Bao Y.-P., Skepper J. N., Burton G. J. Onset of maternal arterial blood flow and placental oxidative stress: a possible factor in human early pregnancy failure. The American Journal of Pathology. 2000;157(6):2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wallace A. E., Fraser R., Cartwright J. E. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Human Reproduction Update. 2012;18(4):458–471. doi: 10.1093/humupd/dms015.dms015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Redman C. W. G., Sargent I. L. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30:38–42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 195.Gagnon R. Placental insufficiency and its consequences. European Journal of Obstetrics Gynecology and Reproductive Biology. 2003;110:S99–S107. doi: 10.1016/S0301-2115(03)00179-9. [DOI] [PubMed] [Google Scholar]
- 196.Lin. F.-J., Fitzpatrick J. W., Iannotti C. A., Martin D. S., Mariani B. D., Tuan R. S. Effects of cadmium on trophoblast calcium transport. Placenta. 1997;18(4):341–356. doi: 10.1016/S0143-4004(97)80069-0. [DOI] [PubMed] [Google Scholar]
- 197.Morgan T. K., Tolosa J. E., Mele L., Wapner R. J., Spong C. Y., Sorokin Y., Dudley D. J., Peaceman A. M., Mercer B. M., Thorp J. M., O'sullivan M. J., Ramin S. M., Rouse D. J., Sibai B. Placental villous hypermaturation is associated with idiopathic preterm birth. Journal of Maternal-Fetal and Neonatal Medicine. 2013;26(7):647–653. doi: 10.3109/14767058.2012.746297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bollati V., Marinelli B., Apostoli P., Bonzini M., Nordio F., Hoxha M., Pegoraro V., Motta V., Tarantini L., Cantone L., Schwartz J., Bertazzi P. A., Baccarelli A. Exposure to metal-rich particulate matter modifies the expression of candidate MicroRNAs in peripheral blood leukocytes. Environmental Health Perspectives. 2010;118(6):763–768. doi: 10.1289/ehp.0901300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Slama R., Darrow L., Parker J., Woodruff T. J., Strickland M., Nieuwenhuijsen M., Glinianaia S., Hoggatt K. J., Kannan S., Hurley F., Kalinka J., Šrám R., Brauer M., Wilhelm M., Henrich J., Ritz B. Meeting report: atmospheric pollution and human reproduction. Environmental Health Perspectives. 2008;116(6):791–798. doi: 10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.van den Hooven E. H., de Kluizenaar Y., Pierik F. H., et al. Chronic air pollution exposure during pregnancy and maternal and fetal c-reactive protein levels: the generation R study. Environmental Health Perspectives. 2012;120(5):746–751. doi: 10.1289/ehp.1104345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Salvi S., Blomberg A., Rudell B., Kelly F., Sandström T., Holgate S. T., Frew A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. American Journal of Respiratory and Critical Care Medicine. 1999;159(3):702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- 202.Wei S.-Q., Fraser W., Luo Z.-C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstetrics and Gynecology. 2010;116(2):393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- 203.Monostory K., Pascussi J.-M., Kóbori L., Dvorak Z. Hormonal regulation of CYP1A expression. Drug Metabolism Reviews. 2009;41(4):547–572. doi: 10.1080/03602530903112284. [DOI] [PubMed] [Google Scholar]
- 204.Collier A. C., Tingle M. D., Paxton J. W., Mitchell M. D., Keelan J. A. Metabolizing enzyme localization and activities in the first trimester human placenta: the effect of maternal and gestational age, smoking and alcohol consumption. Human Reproduction. 2002;17(10):2564–2572. doi: 10.1093/humrep/17.10.2564. [DOI] [PubMed] [Google Scholar]
- 205.Vondráček J., Umannová L., Machala M. Interactions of the aryl hydrocarbon receptor with inflammatory mediators: beyond CYP1A regulation. Current Drug Metabolism. 2011;12(2):89–103. doi: 10.2174/138920011795016827. [DOI] [PubMed] [Google Scholar]
- 206.Choi H., Rauh V., Garfinkel R., Tu Y., Perera F. P. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environmental Health Perspectives. 2008;116(5):658–665. doi: 10.1289/ehp.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Choi H., Wang L., Lin X., Spengler J. D., Perera F. P. Fetal window of vulnerability to airborne polycyclic aromatic hydrocarbons on proportional intrauterine growth restriction. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035464.e35464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rayman M. P. The importance of selenium to human health. The Lancet. 2000;356(9225):233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 209.Chen J., Berry M. J. Selenium and selenoproteins in the brain and brain diseases. Journal of Neurochemistry. 2003;86(1):1–12. doi: 10.1046/j.1471-4159.2003.01854.x. [DOI] [PubMed] [Google Scholar]
- 210.Al-Kunani A. S., Knight R., Haswell S. J., Thompson J. W., Lindow S. W. The selenium status of women with a history of recurrent miscarriage. British Journal of Obstetrics and Gynaecology. 2001;108(10):1094–1097. doi: 10.1016/S0306-5456(01)00253-4. [DOI] [PubMed] [Google Scholar]
- 211.Zachara B. A., Dobrzyński W., Trafikowska U., Szymański W. Blood selenium and glutathione peroxidases in miscarriage. British Journal of Obstetrics and Gynaecology. 2001;108(3):244–247. doi: 10.1016/S0306-5456(00)00030-9. [DOI] [PubMed] [Google Scholar]
- 212.Howard J. M., Davies S., Hunnisett A. Red cell magnesium and glutathione peroxidase in infertile women—effects of oral supplementation with magnesium and selenium. Magnesium Research. 1994;7(1):49–57. [PubMed] [Google Scholar]
- 213.Dobrzynski W., Trafikowska U., Trafikowska A., Pilecki A., Szymanski W., Zachara B. A. Decreased selenium concentration in maternal and cord blood in preterm compared with term delivery. Analyst. 1998;123(1):93–97. doi: 10.1039/a704884j. [DOI] [PubMed] [Google Scholar]
- 214.Mistry H. D., Wilson V., Ramsay M. M., Symonds M. E., Pipkin F. B. Reduced selenium concentrations and glutathione peroxidase activity in preeclamptic pregnancies. Hypertension. 2008;52(5):881–888. doi: 10.1161/HYPERTENSIONAHA.108.116103. [DOI] [PubMed] [Google Scholar]
- 215.Vanderlelie J., Perkins A. V. A. Selenium and preeclampsia: a global perspective. Pregnancy Hypertension. 2011;1(3-4):213–224. doi: 10.1016/j.preghy.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 216.Piasek M., Blanuša M., Kostial K., Laskey J. W. Placental cadmium and progesterone concentrations in cigarette smokers. Reproductive Toxicology. 2001;15(6):673–681. doi: 10.1016/S0890-6238(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 217.Rurak D. Placental development and function. In: Harding R., Bocking A. D., editors. Fetal Growth and Development. Cambridge, Mass, USA: Cambridge University Press; 2001. [Google Scholar]
- 218.Kramer M. S., Kahn S. R., Platt R. W., et al. Antioxidant vitamins, long-chain fatty acids, and spontaneous preterm birth. Epidemiology. 2009;20(5):707–713. doi: 10.1097/EDE.0b013e3181a818c5. [DOI] [PubMed] [Google Scholar]
- 219.Dragani A., Falco A., Santilli F., Basili S., Rolandi G., Cerasa L., Lattanzio S., Ciabattoni G., Patrono C., Dav̀ G. Oxidative stress and platelet activation in subjects with moderate hyperhomocysteinaemia due to MTHFR 677 C→T polymorphism. Thrombosis and Haemostasis. 2012;108(3):533–542. doi: 10.1160/TH11-12-0899. [DOI] [PubMed] [Google Scholar]
- 220.De Chiara B., Sedda V., Parolini M., Campolo J., De Maria R., Caruso R., Pizzi G., Disoteo O., Dellanoce C., Corno A. R., Cighetti G., Parodi O. Plasma total cysteine and cardiovascular risk burden: action and interaction. The Scientific World Journal. 2012;2012 doi: 10.1100/2012/303654.303654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.García-Rodríguez C. E., Mesa M. D., Olza J., Vlachava M., Kremmyda L.-S., Diaper N. D., Noakes P. S., Miles E. A., Ramírez-Tortosa M. C., Liaset B., Frøyland L., Rossary A., Farges M.-C., Vasson M.-P., Aguilera C. M., Helmersson-Karlqvist J., Godfrey K. M., Calder P. C., Basu S., Gil Á. Does consumption of two portions of salmon per week enhance the antioxidant defense system in pregnant women? Antioxidants& Redox Signaling. 2012;16(12):1401–1406. doi: 10.1089/ars.2012.4508. [DOI] [PubMed] [Google Scholar]
- 222.Patten A. R., Brocardo P. S., Christie B. R. Omega-3 supplementation can restore glutathione levels and prevent oxidative damage caused by prenatal ethanol exposure. Journal of Nutritional Biochemistry. 2013;24(5):760–769. doi: 10.1016/j.jnutbio.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 223.Roy S., Kale A., Dangat K., Sable P., Kulkarni A., Joshi S. Maternal micronutrients (folic acid and vitamin B12) and omega 3 fatty acids: implications for neurodevelopmental risk in the rat offspring. Brain and Development. 2012;34(1):64–71. doi: 10.1016/j.braindev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 224.Bertram C., Trowern A. R., Copin N., Jackson A. A., Whorwood C. B. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11β-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142(7):2841–2853. doi: 10.1210/en.142.7.2841. [DOI] [PubMed] [Google Scholar]
- 225.Yang K., Julan L., Rubio F., Sharma A., Guan H. Cadmium reduces 11β-hydroxysteroid dehydrogenase type 2 activity and expression in human placental trophoblast cells. American Journal of Physiology: Endocrinology and Metabolism. 2006;290(1):E135–E142. doi: 10.1152/ajpendo.00356.2005. [DOI] [PubMed] [Google Scholar]
- 226.Ronco A. M., Garrido F., Llanos M. N. Smoking specifically induces metallothionein-2 isoform in human placenta at term. Toxicology. 2006;223(1-2):46–53. doi: 10.1016/j.tox.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 227.Kippler M., Hoque A. M. W., Raqib R., Öhrvik H., Ekström E.-C., Vahter M. Accumulation of cadmium in human placenta interacts with the transport of micronutrients to the fetus. Toxicology Letters. 2010;192(2):162–168. doi: 10.1016/j.toxlet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 228.Agency for Toxic Substances and Disease Registry . Toxicological Profile for Cadmium. Atlanta, Ga, USA: Agency for Toxic Substances and Disease Registry; 2012. [PubMed] [Google Scholar]
- 229.Nampoothiri L. P., Gupta S. Biochemical effects of gestational coexposure to lead and cadmium on reproductive performance, placenta, and ovary. Journal of Biochemical and Molecular Toxicology. 2008;22(5):337–344. doi: 10.1002/jbt.20246. [DOI] [PubMed] [Google Scholar]
- 230.Engel S. M., Scher E., Wallenstein S., Savitz D. A., Alsaker E. R., Trogstad L., Magnus P. Maternal active and passive smoking and hypertensive disorders of pregnancy: Risk with trimester-specific exposures. Epidemiology. 2013;24(3):379–386. doi: 10.1097/EDE.0b013e3182873a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Reynolds R. M., Labad J., Buss C., Ghaemmaghami P., Räikkönen K. Transmitting biological effects of stress in utero: implications for mother and offspring. Psychoneuroendocrinology. 2013;38(9):1843–1849. doi: 10.1016/j.psyneuen.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 232.Goldenberg R. L., Culhane J. F., Iams J. D., Romero R. Epidemiology and causes of preterm birth. The Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sandman C. A., Wadhwa P. D., Chicz-DeMet A., Dunkel-Schetter C., Porto M. Maternal stress, HPA activity, and fetal/infant outcome. Annals of the New York Academy of Sciences. 1997;814:266–275. doi: 10.1111/j.1749-6632.1997.tb46162.x. [DOI] [PubMed] [Google Scholar]
- 234.Sandman C. A., Glynn L., Schetter C. D., Wadhwa P., Garite T., Chicz-DeMet A., Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27(6):1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 235.Duthie L., Reynolds R. M. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuroendocrinology. 2013;98(2):106–115. doi: 10.1159/000354702. [DOI] [PubMed] [Google Scholar]
- 236.Ronco A. M., Urrutia M., Montenegro M., Llanos M. N. Cadmium exposure during pregnancy reduces birth weight and increases maternal and foetal glucocorticoids. Toxicology Letters. 2009;188(3):186–191. doi: 10.1016/j.toxlet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 237.Kajantie E., Dunkel L., Turpeinen U., Stenman U. H., Wood P. J., Nuutila M., Andersson S. Placental 11β-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. Journal of Clinical Endocrinology and Metabolism. 2003;88(1):493–500. doi: 10.1210/jc.2002-021378. [DOI] [PubMed] [Google Scholar]
- 238.Aufdenblatten M., Baumann M., Raio L., Dick B., Frey B. M., Schneider H., Surbek D., Hocher B., Mohaupt M. G. Prematurity is related to high placental cortisol in preeclampsia. Pediatric Research. 2009;65(2):198–202. doi: 10.1203/PDR.0b013e31818d6c24. [DOI] [PubMed] [Google Scholar]
- 239.Federenko B. I. S., Wadhwa P. D. Women's mental health during pregnancy influences fetal and infant. CNS Spectrums. 2004;9:198–206. doi: 10.1017/s1092852900008993. [DOI] [PubMed] [Google Scholar]
- 240.Szanton S. L., Gill J. M., Allen J. K. Allostatic load: a mechanism of socioeconomic health disparities? Biological Research for Nursing. 2005;7(1):7–15. doi: 10.1177/1099800405278216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lesage J., Hahn D., Léonhardt M., Blondeau B., Bréant B., Dupouy J. P. Maternal undernutrition during late gestation-induced intrauterine growth restriction in the rat is associated with impaired placental GLUT3 expression, but does not correlate with endogenous corticosterone levels. Journal of Endocrinology. 2002;174(1):37–43. doi: 10.1677/joe.0.1740037. [DOI] [PubMed] [Google Scholar]
- 242.Salpietro C. D., Gangemi S., Minciullo P. L., Briuglia S., Merlino M. V., Stelitano A., Cristani M., Trombetta D., Saija A. Cadmium concentration in maternal and cord blood and infant birth weight: a study on healthy non-smoking women. Journal of Perinatal Medicine. 2002;30(5):395–399. doi: 10.1515/JPM.2002.061. [DOI] [PubMed] [Google Scholar]
- 243.Llanos M. N., Ronco A. M. Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities. Reproductive Toxicology. 2009;27(1):88–92. doi: 10.1016/j.reprotox.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 244.Al-Gubory K. H. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reproductive BioMedicine Online. 2014;29(1):17–31. doi: 10.1016/j.rbmo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 245.Younglai E. V., Wu Y. J., Foster W. G. Reproductive toxicology of environmental toxicants: emerging issues and concerns. Current Pharmaceutical Design. 2007;13(29):3005–3019. doi: 10.2174/138161207782110499. [DOI] [PubMed] [Google Scholar]
- 246.Diez-Roux A. V. On genes, individuals, society, and epidemiology. American Journal of Epidemiology. 1998;148(11):1027–1032. doi: 10.1093/oxfordjournals.aje.a009578. [DOI] [PubMed] [Google Scholar]
- 247.Auger N., Luo Z.-C., Platt R. W., Daniel M. Do mother's education and foreign born status interact to influence birth outcomes? Clarifying the epidemiological paradox and the healthy migrant effect. Journal of Epidemiology and Community Health. 2008;62(5):402–409. doi: 10.1136/jech.2007.064535. [DOI] [PubMed] [Google Scholar]
- 248.Alberg A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology. 2002;180(2):121–137. doi: 10.1016/S0300-483X(02)00386-4. [DOI] [PubMed] [Google Scholar]
- 249.Okumura K., Tsukamoto H. Folate in smokers. Clinica Chimica Acta. 2011;412(7-8):521–526. doi: 10.1016/j.cca.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 250.Kim D. B., Oh Y. S., Yoo K. D., Lee J. M., Park C. S., Ihm S. H., Jang S. W., Shim B. J., Kim H. Y., Seung K. B., Rho T. H., Kim J. H. Passive smoking in never-smokers is associated with increased: plasma homocysteine levels. International Heart Journal. 2010;51(3):183–187. doi: 10.1536/ihj.51.183. [DOI] [PubMed] [Google Scholar]
- 251.Haste F. M., Brooke O. G., Anderson H. R., Bland J. M., Shaw A., Griffin J., Peacock J. L. Nutrient intakes during pregnancy: observations on the influence of smoking and social class. The American Journal of Clinical Nutrition. 1990;51(1):29–36. doi: 10.1093/ajcn/51.1.29. [DOI] [PubMed] [Google Scholar]
- 252.Astley S. B., Lindsay D. G. Conclusions. Molecular Aspects of Medicine. 2002;23(1–3):287–291. doi: 10.1016/S0098-2997(02)00019-5. [DOI] [PubMed] [Google Scholar]
- 253.Wang M. C., Kim S., Gonzalez A. A., MacLeod K. E., Winkleby M. A. Socioeconomic and food-related physical characteristics of the neighbourhood environment are associated with body mass index. Journal of Epidemiology and Community Health. 2007;61(6):491–498. doi: 10.1136/jech.2006.051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Seligman H. K., Bindman A. B., Vittinghoff E., Kanaya A. M., Kushel M. B. Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (NHANES) 1999–2002. Journal of General Internal Medicine. 2007;22(7):1018–1023. doi: 10.1007/s11606-007-0192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Adams E. J., Grummer-Strawn L., Chavez G. Food insecurity is associated with increased risk of obesity in California women. Journal of Nutrition. 2003;133(4):1070–1074. doi: 10.1093/jn/133.4.1070. [DOI] [PubMed] [Google Scholar]
- 256.Von Braun J., Bouis H., Kumar S., Pandya-Lorch R. Improving Food Security of the Poor: Concept, Policy, and Programs. Washington, DC, USA: International Food Policy Research Institute; 1992. [Google Scholar]
- 257.Laraia B., Epel E., Siega-Riz A. M. Food insecurity with past experience of restrained eating is a recipe for increased gestational weight gain. Appetite. 2013;65:178–184. doi: 10.1016/j.appet.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Parker J. D., Rich D. Q., Glinianaia S. V., Leem J. H., Wartenberg D., Bell M. L., Bonzini M., Brauer M., Darrow L., Gehring U., Gouveia N., Grillo P., Ha E., van den Hooven Hooven E. H., Jalaludin B., Jesdale B. M., Lepeule J., Morello-Frosch R., Morgan G. G., Slama R., Pierik F. H., Pesatori A. C., Sathyanarayana S., Seo J., Strickland M., Tamburic L., Woodruff T. J. The international collaboration on air pollution and pregnancy outcomes: initial results. Environmental Health Perspectives. 2011;119(7):1023–1028. doi: 10.1289/ehp.1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rojas-Rueda D., de Nazelle A., Teixidó O., Nieuwenhuijsen M. Health impact assessment of increasing public transport and cycling use in Barcelona: a morbidity and burden of disease approach. Preventive Medicine. 2013;57(5):573–579. doi: 10.1016/j.ypmed.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 260.Dadvand P., de Nazelle A., Triguero-Mas M., Schembari A., Cirach M., Amoly E., Figueras F., Basagaña X., Ostro B., Nieuwenhuijsen M. Surrounding greenness and exposure to air pollution during pregnancy: an analysis of personal monitoring data. Environmental Health Perspectives. 2012;120(9):1286–1290. doi: 10.1289/ehp.1104609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yao Z., Zhang Y., Shen X., Wang X., Wu Y., He K. Impacts of temporary traffic control measures on vehicular emissions during the Asian Games in Guangzhou, China. Journal of the Air & Waste Management Association. 2013;63(1):11–19. doi: 10.1080/10962247.2012.724041. [DOI] [PubMed] [Google Scholar]
- 262.Boogaard H., Janssen N. A. H., Fischer P. H., Kos G. P. A., Weijers E. P., Cassee F. R., van der Zee S. C., de Hartog J. J., Meliefste K., Wang M., Brunekreef B., Hoek G. Impact of low emission zones and local traffic policies on ambient air pollution concentrations. Science of the Total Environment. 2012;435-436:132–140. doi: 10.1016/j.scitotenv.2012.06.089. [DOI] [PubMed] [Google Scholar]
- 263.Jackson L. E. The relationship of urban design to human health and condition. Landscape and Urban Planning. 2003;64(4):191–200. doi: 10.1016/S0169-2046(02)00230-X. [DOI] [Google Scholar]
- 264.Pope C. A., III Review: epidemiological basis for particulate air pollution health standards. Aerosol Science and Technology. 2000;32(1):4–14. doi: 10.1080/027868200303885. [DOI] [Google Scholar]
- 265.World Health Organization . Health Effects of Particulate Matter: Policy Implications for Countries in Eastern Europe, Caucasus and Central Asia. Vol. 50. Copenhagen, Denmark: World Health Organization; 2013. [Google Scholar]
- 266.Hänninen O., Knol A. B., Jantunen M., Lim T.-A., Conrad A., Rappolder M., Carrer P., Fanetti A.-C., Kim R., Buekers J., Torfs R., Iavarone I., Classen T., Hornberg C., Mekel O. C. L. Environmental burden of disease in Europe: assessing nine risk factors in six countries. Environmental Health Perspectives. 2014;122(5):439–446. doi: 10.1289/ehp.1206154. [DOI] [PMC free article] [PubMed] [Google Scholar]