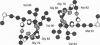Abstract
Dimerization of human glycophorin A in erythrocyte membranes is mediated by specific interactions within the helical transmembrane domain of the protein. Rotational resonance NMR provides a unique approach for obtaining high-resolution structural data in membrane systems and has been used to establish intermolecular contacts in the glycophorin A dimer by using hydrophobic peptides that correspond to the transmembrane sequence. Magnetization exchange rates were measured between [13C]methyl labels in the hydrophobic sequence -G79-V80-M81-A82-G83-V84- located in the middle of the transmembrane domain and specific [13C]carbonyl labels along the peptide backbone across the dimer interface. Significant magnetization exchange was observed only between V80 (13CH3) and G79 (13C = O) and between V84 (13CH3) and G83 (13C = O), indicating that these residues are packed in the dimer interface in a "ridges-ingrooves" arrangement.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the protein subunits. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6162–6166. doi: 10.1073/pnas.84.17.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann B. J., Knowles W. J., Marchesi V. T. Synthetic peptides mimic the assembly of transmembrane glycoproteins. J Biol Chem. 1989 Mar 5;264(7):4033–4037. [PubMed] [Google Scholar]
- Cao H., Bangalore L., Bormann B. J., Stern D. F. A subdomain in the transmembrane domain is necessary for p185neu* activation. EMBO J. 1992 Mar;11(3):923–932. doi: 10.1002/j.1460-2075.1992.tb05131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Levitt M., Richardson D. Helix to helix packing in proteins. J Mol Biol. 1981 Jan 5;145(1):215–250. doi: 10.1016/0022-2836(81)90341-7. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Marchesi V. T. Subunit structure of human erythrocyte glycophorin A. Biochemistry. 1976 Mar 9;15(5):1137–1144. doi: 10.1021/bi00650a028. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N., Fujiyoshi Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature. 1994 Feb 17;367(6464):614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- Landolt-Marticorena C., Williams K. A., Deber C. M., Reithmeier R. A. Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J Mol Biol. 1993 Feb 5;229(3):602–608. doi: 10.1006/jmbi.1993.1066. [DOI] [PubMed] [Google Scholar]
- Lemmon M. A., Flanagan J. M., Hunt J. F., Adair B. D., Bormann B. J., Dempsey C. E., Engelman D. M. Glycophorin A dimerization is driven by specific interactions between transmembrane alpha-helices. J Biol Chem. 1992 Apr 15;267(11):7683–7689. [PubMed] [Google Scholar]
- Lemmon M. A., Flanagan J. M., Treutlein H. R., Zhang J., Engelman D. M. Sequence specificity in the dimerization of transmembrane alpha-helices. Biochemistry. 1992 Dec 29;31(51):12719–12725. doi: 10.1021/bi00166a002. [DOI] [PubMed] [Google Scholar]
- Lemmon M. A., Treutlein H. R., Adams P. D., Brünger A. T., Engelman D. M. A dimerization motif for transmembrane alpha-helices. Nat Struct Biol. 1994 Mar;1(3):157–163. doi: 10.1038/nsb0394-157. [DOI] [PubMed] [Google Scholar]
- Manolios N., Bonifacino J. S., Klausner R. D. Transmembrane helical interactions and the assembly of the T cell receptor complex. Science. 1990 Jul 20;249(4966):274–277. doi: 10.1126/science.2142801. [DOI] [PubMed] [Google Scholar]
- Ponder J. W., Richards F. M. Tertiary templates for proteins. Use of packing criteria in the enumeration of allowed sequences for different structural classes. J Mol Biol. 1987 Feb 20;193(4):775–791. doi: 10.1016/0022-2836(87)90358-5. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Engelman D. M. Membrane protein folding and oligomerization: the two-stage model. Biochemistry. 1990 May 1;29(17):4031–4037. doi: 10.1021/bi00469a001. [DOI] [PubMed] [Google Scholar]
- Silverberg M., Furthmayr H., Marchesi V. T. The effect of carboxymethylating a single methionine residue on the subunit interactions of glycophorin A. Biochemistry. 1976 Apr 6;15(7):1448–1454. doi: 10.1021/bi00652a015. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Hamilton J., Salmon A., Bormann B. J. Rotational resonance NMR determination of intra- and intermolecular distance constraints in dipalmitoylphosphatidylcholine bilayers. Biochemistry. 1994 May 24;33(20):6327–6333. doi: 10.1021/bi00186a036. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Jonas R., Braiman M., Bormann B. J. Structure and orientation of the transmembrane domain of glycophorin A in lipid bilayers. Biochemistry. 1994 May 24;33(20):6334–6341. doi: 10.1021/bi00186a037. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Peersen O. B. Solid-state NMR approaches for studying membrane protein structure. Annu Rev Biophys Biomol Struct. 1992;21:25–47. doi: 10.1146/annurev.bb.21.060192.000325. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Gullick W. J. Neu receptor dimerization. Nature. 1989 Jun 22;339(6226):587–587. doi: 10.1038/339587a0. [DOI] [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- Treutlein H. R., Lemmon M. A., Engelman D. M., Brünger A. T. The glycophorin A transmembrane domain dimer: sequence-specific propensity for a right-handed supercoil of helices. Biochemistry. 1992 Dec 29;31(51):12726–12732. doi: 10.1021/bi00166a003. [DOI] [PubMed] [Google Scholar]