Abstract
The aim of this study was to examine seasonal and pandemic influenza vaccination coverage in adults from the 2008–2009 season to the 2011–2012 season, including pandemic and post-pandemic seasons in Korea. We collected data of self-reported vaccine use from the Korean Community Health Survey. We also collected information on socioeconomic status and health behaviors in subpopulations. We tested for linear trends among the data to investigate vaccine coverage before and after the pandemic; and multiple logistic regression analyses were performed to identify predictors of obtaining the influenza vaccination. The results revealed a steady increase in vaccination coverage in every subgroup during four consecutive seasons. The highest rate of vaccine coverage (43.6%) occurred two years after the pandemic. Factors associated with vaccine receipt were: older age; lower education level; lower income; and health behaviors such as regular walking and receiving a health check-up. Smoking and drinking alcohol were inversely associated with vaccination. Having a chronic health condition was also a strong predictor of vaccine receipt. Though vaccination coverage rates were high in high-risk groups; disparities in coverage rates were substantial; particularly in young adults. Interventions are needed to minimize the coverage gaps among subgroups and to improve overall vaccination rates.
Keywords: influenza, vaccination coverage, adults, Korea
1. Introduction
Seasonal influenza affects 5% to 10% the global population each year [1] and influenza epidemics cause substantial hospitalizations and complications [2,3]. Young children, the elderly, pregnant women, and people of any age with comorbid conditions such as diabetes, cardiovascular diseases, human immunodeficiency virus, lung disease, kidney disease, and asthma, are more vulnerable to infection and are, therefore, defined as high-risk groups [4,5,6,7,8]. The primary strategy for protection against influenza and prevention of complications associated with the infection is the influenza vaccine [5]. Age and immunocompetence of individuals affect the efficacy and effectiveness of the vaccine, but the World Health Organization (WHO) recommends annual vaccination for all age groups [9,10,11,12,13,14]. Vaccination in high-risk groups, in particular, reduces morbidity and mortality [15,16,17,18,19].
Korea implemented a National Immunization Program (NIP) to increase vaccination against epidemic and pandemic influenza and provide free vaccination to priority groups, which are listed in Table 1 [20]. As part of the NIP, seasonal influenza vaccines are provided each year during the flu season. During the 2009–2010 season, both pandemic and seasonal vaccines were distributed and individuals chose to receive either one or both of the vaccines. Consequently, coverage rates for people aged 65 and older were markedly higher than the average coverage rates in other countries reported by the Organization for Economic Cooperation and Development [21]. During the same season, rates of vaccination for the A(H1N1)pdm09 strain of influenza remained low in young adults in Korea [22].
Table 1.
Priority groups for seasonal and pandemic influenza vaccination.
| Seasonal | Pandemic (2009–2010 Season only) |
|---|---|
| 1. Individuals over 65 years old 2. Financially vulnerable persons 3. Handicapped individuals 4. Soldiers |
1. Healthcare workers |
| 2. Infants and pregnant women | |
| 3. Individuals over 65 years old | |
| 4. Individuals in high-risk groups aged 50 to 64 years | |
| 5. Students (elementary, middle, and high school) | |
| 6. Soldiers | |
| 7. Teachers (preschool and K-12) | |
| 8. Airport, infrastructure, and port workers | |
| 9. Social welfare and child care center residents and workers |
A few studies have reported characteristics associated with vaccine receipt in limited populations within Korea [23,24,25], but no studies have addressed vaccination coverage in all adult age groups before and after the influenza pandemic in Korea. The objectives of this study were to describe the trends in seasonal and pandemic influenza vaccination in adults from the 2008–2009 season to the 2011–2012 season and to define demographic factors associated with vaccine receipt.
2. Methods
2.1. Data Source
For this study, we collected data from the Korean Community Health Survey (KCHS), which was conducted by the Korea Centers for Disease Control and Prevention (KCDC). KCDC has been conducting the annual, nationwide, cross-sectional survey since 2008 and, on the basis of this information, has created health indices of individuals that are comparable among different regions in the country. The sample size for the KCHS is 900 subjects in each of 253 community units, including 16 metropolitan cities and provinces. The KCHS expects a total of 227,700 survey participants each year, but the actual number of respondents is nearly 230,000 [26]. The KCHS uses a two-stage sampling process. The first stage selects a sample area (tong/ban/ri) as a primary sample unit, which is selected according to the number of households in the area using a probability proportional to the sampling method. In the second stage, the number of households in the selected sample tong/ban/ri is identified to create a household directory. Sample households are selected using systematic sampling methods. This process is used to ensure that the sample units are representative of the entire population [27]. For the sample to be statistically representative of the population, the data collected from the survey is weighted based on the sample design [28]. Non-responses and individuals who provide insufficient information on socio-demographic variables and influenza vaccination are excluded and not replaced [27].
The survey is offered only to individuals who provide written informed consent, so their responses might cause volunteer bias to the survey and to the data collected, which would result in positive outcomes for health-related variables. KCHS uses interviewers trained in computer-assisted personal interviewing techniques to collect information. The aim of the survey is to collect information on health behaviors, chronic diseases, vaccination, use of health services, quality of life, and socio-demographic characteristics including gender, age, income, education level, occupation, residency area, and family size. For our study, we used KCHS data from the 2008–2009 season to the 2011–2012 season.
2.2. Study Variables
Socio-demographic variables that we examined in our study included sex (male, female), age group (19–44, 45–64, 65–74, ≥75 years), monthly income (≥2 million Korean won, <2 million Korean Won; 1000 Korean Won = approximately 1 USD), occupation (professional, service/manual worker, others), education level (at least high school, less than high school), residency (metropolitan, urban, rural), and number of chronic illnesses (none, one, two or more). Chronic illnesses included diabetes, asthma, angina, myocardial infarction, stroke, and tuberculosis; all of these are comorbidities for which the KCDC recommends influenza vaccination. The chronic illness variable was defined as a person who had one or more high-risk medical conditions and was receiving medication for treatment at the time of survey completion. Questions included “Have you ever been told by a doctor that you had chronic disease?” and “Are you currently taking any medication for that disease?” Health behavior variables included receiving a health check-up, smoking, drinking alcohol, and regular walking. A health check-up was measured by a single answer (yes/no); this variable was excluded from the survey during the 2010–2011 season. Smoking was divided into three categories: current smoker, former smoker, and never smoker. Regular alcohol consumption was defined as drinking alcohol more than once a month. Regular walking was defined as walking more than 30 min a day at least 5 days a week. Flu seasons last from December to April in Korea and influenza vaccination is recommended from October to December [20]. Therefore, receipt of the influenza vaccination was assessed by asking if the survey participant received a seasonal influenza vaccination (yes/no) in the previous 12 months, which corresponded to September through August and included the winter and early spring peak seasons of epidemics. In the 2009–2010 season, seasonal and pandemic vaccinations were available; in the 2008–2009, 2010–2011, and 2011–2012 seasons, only seasonal influenza vaccination was available.
2.3. Statistical Analysis
We performed the χ2 test to compare vaccination coverage rates among the seasons. We measured annual changes in influenza vaccination coverage using the χ2 test for linear trends. A p-value <0.05 was considered statistically significant. We performed multivariable logistic regression to calculate the adjusted odds ratios; we included age, sex, and other variables that showed a p-value <0.05 in bivariate analysis. Results are expressed with a 95% confidence interval (CI).
All statistical analyses were performed with SAS statistical software (version 9.3, SAS Institute, Cary, NC, USA). The SAS survey procedure was used to account for the complex sampling design of the KCHS. All analyses were weighted to reflect the age and sex of the Korean population.
3. Results
3.1. Influenza Vaccination Coverage in Four Consecutive Seasons
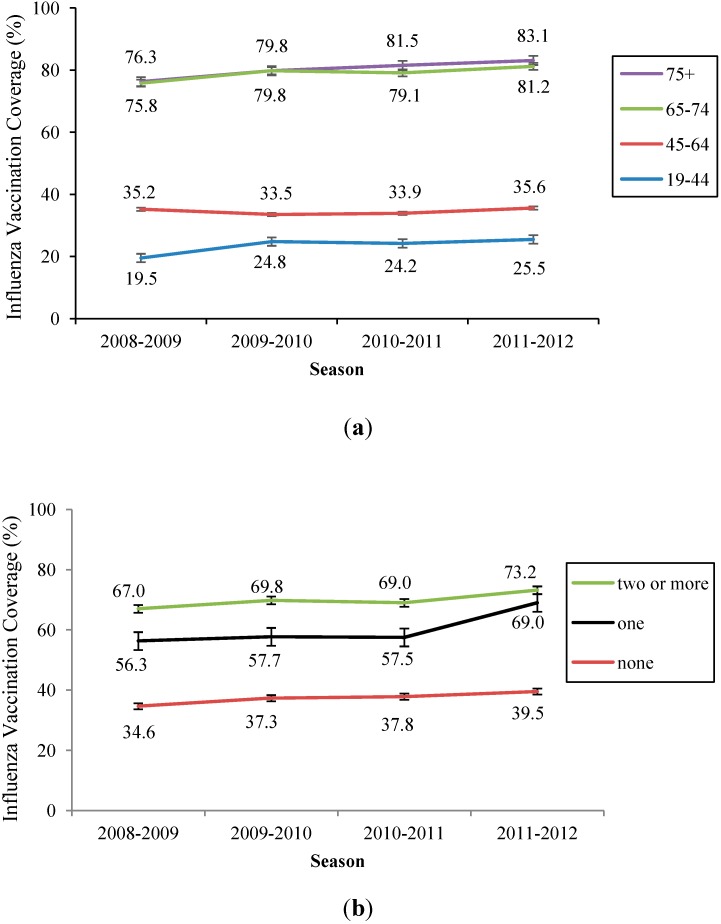
Influenza vaccination coverage (IVC) rates increased during the study period, with overall rates of 37.8% in 2008–2009, 41.0% in 2009–2010, 41.6% in 2010–2011, and 43.6% in 2011–2012 (Table 2). The highest increase in IVC rates occurred between the 2008–2009 and 2009–2010 seasons. IVC rates were higher in vulnerable groups including people with lower income, people with physical jobs, and people with a lower education level. Notably, individuals with lower income showed the highest increase in IVC rates during the four seasons included in our study, increasing steadily from 46.5% in 2008–2009 to 56.1% in 2011–2012. Vaccination rates increased with age and exceeded 80% in the elderly after the pandemic (Figure 1a). IVC among adults with chronic diseases also increased (Figure 1b). Health behaviors such as not smoking, not drinking alcohol, regular walking, and receiving a health check-up were associated with higher IVC rates. Differences in vaccination rates among subgroups were statistically significant (p < 0.05) for each variable.
Table 2.
Demographic characteristics and influenza vaccination rates from the 2008–2009 season to the 2011–2012 season.
| Variables | 2008–2009 Seasonal | 2009–2010 Seasonal and Pandemic | 2010–2011 Seasonal | 2011–2012 Seasonal | Test for linear trend * |
|---|---|---|---|---|---|
| Number of Individuals (vaccination Coverage, %) | p-value | ||||
| Socioeconomic variables | |||||
| Sex | |||||
| Male | 107,080 (32.4) | 104,575 (36.6) | 103,017 (36.5) | 102,898 (38.4) | <0.001 |
| Female | 123,635 (42.6) | 124,654 (44.7) | 126,209 (45.8) | 126,023 (47.8) | <0.001 |
| Age (years) | |||||
| 19–44 | 95,611 (19.5) | 90,970 (24.8) | 87,472 (24.2) | 85,060 (25.5) | <0.001 |
| 45–64 | 83,419 (35.2) | 84,005 (33.5) | 85,130 (33.9) | 85,757 (35.6) | <0.001 |
| 65–74 | 33,958 (75.8) | 34,823 (79.8) | 35,050 (79.1) | 35,483 (81.2) | <0.001 |
| ≥75 | 17,727 (76.3) | 19,431 (79.8) | 21,574 (81.5) | 22,621 (83.1) | <0.001 |
| Income † | |||||
| ≥2000 per month | 109,699 (29.2) | 104,669 (35.5) | 109,798 (31.7) | 119,165 (33.6) | <0.001 |
| <2000 per month | 112,716 (46.5) | 103,916 (49.8) | 101,784 (52.3) | 96,476 (56.1) | <0.001 |
| Occupation | |||||
| Professional | 72,118 (24.7) | 68,231 (28.0) | 70,694 (27.5) | 71,868 (29.3) | <0.001 |
| Service/physical | 66,141 (39.7) | 60,208 (41.5) | 70,925 (42.9) | 73,042 (45.5) | <0.001 |
| Others ‡ | 92,294 (46.8) | 86,883 (50.3) | 87,267 (52.0) | 83,800 (54.2) | <0.001 |
| Education level | |||||
| At least high school | 123,800 (25.9) | 124,649 (29.3) | 124,178 (29.3) | 125,419 (31.2) | <0.001 |
| Less than high school | 106,646 (51.7) | 103,956 (55.1) | 104,557 (56.2) | 103,069 (58.7) | <0.001 |
| Residency | |||||
| Metropolitan | 68,647 (30.7) | 67,747 (34.6) | 67,921 (35.6) | 67,852 (37.4) | <0.001 |
| Urban | 61,499 (31.5) | 60,947 (34.5) | 60,837 (35.4) | 61,903 (36.9) | <0.001 |
| Rural | 97,311 (47.1) | 97,318 (49.6) | 97,234 (49.9) | 94,991 (52.5) | <0.001 |
| Chronic illness § | |||||
| None | 198,682 (34.6) | 191,414 (37.3) | 189,346 (37.8) | 188,180 (39.5) | <0.001 |
| One | 27,566 (56.3) | 31,777 (57.7) | 33,473 (57.5) | 34,120 (60.2) | <0.001 |
| Two or more | 4467 (67.0) | 6,038 (69.8) | 6407 (69.0) | 6621 (73.2) | <0.001 |
| Health behaviors | |||||
| Health check-up †† | |||||
| No | 88,572 (24.3) | 86,883 (29.8) | - | 76,567 (30.7) | <0.001 |
| Yes | 142,143 (46.3) | 142,346 (47.9) | - | 152,354 (50.1) | <0.001 |
| Smoking | |||||
| Never smoker | 144,588 (40.7) | 144,859 (43.6) | 143,886 (44.3) | 144,249 (46.2) | <0.001 |
| Former smoker | 31,168 (44.5) | 33,551 (47.8) | 36,612 (47.9) | 36,831 (50.9) | <0.001 |
| Current smoker | 54,779 (26.3) | 50,741 (29.2) | 48,679 (28.9) | 47,812 (29.9) | <0.001 |
| Drink alcohol | |||||
| No | 114,699 (46.6) | 113,314 (49.6) | 111,358 (50.8) | 112,986 (53.0) | <0.001 |
| Yes | 115,931 (29.1) | 115,726 (32.6) | 117,743 (32.9) | 115,880 (34.4) | <0.001 |
| Regular walking | |||||
| <30 min/5days/week | 113,656 (36.6) | 129,781 (40.2) | 133,215 (40.8) | 136,324 (43.2) | <0.001 |
| ≥30 min/5days/week | 116,896 (39.0) | 98,988 (42.1) | 95,576 (42.7) | 92,343 (44.2) | <0.001 |
| Total | 230,715 (37.8) | 229,229 (41.0) | 229,226 (41.6) | 229,921 (43.6) | <0.001 |
Notes: * χ2 for linear trend between the 2008–2009 and 2011–2012 seasons; † Units: Korean Chun-Won (₩1000); ‡ Others include soldiers, college students, and housewives; § Patients were diagnosed with one or more of the following diseases by a doctor and were receiving a medication for the disease: diabetes, stroke, heart disease (angina or myocardial infarction), hepatitis, or tuberculosis; †† Variable was excluded from the questionnaire in the 2010–2011 season.
Figure 1.
(a) Influenza vaccination coverage by age group; (b) Influenza vaccination coverage according to number of chronic illnesses.
3.2. Determinants of Influenza Vaccination Receipt
For seasonal and pandemic IVC, the following factors predicted a higher likelihood of vaccine receipt according to multiple logistic regression analysis (Table 3): female gender, elderly age group (65–74 years old and ≥75 years old), low income, engaging in service/physical work or other jobs, receiving a health check-up, and having a chronic illness. In the 2011–2012 season, participants in the ≥75 years old age group were the most likely to receive a vaccine (OR 8.12, 95% CI 7.75–8.50), followed by the 65–74 years old age group (OR 6.23, 95% CI 6.00–6.48). Receiving a health check-up was also a strong predictor for IVC (OR 2.28, 95% CI 2.23–2.33). Those who self-reported as a current smoker or regular alcohol drinker were less likely to receive a vaccination than never and former smokers and non-drinkers, respectively.
Table 3.
Adjusted odds ratios of demographic characteristics associated with influenza vaccine receipt from the 2008–2009 season to the 2011–2012 season.
| Variables | 2008–2009 | 2009–2010 | 2010–2011 | 2011–2012 | ||||
|---|---|---|---|---|---|---|---|---|
| Adjusted OR | 95% CI | Adjusted OR | 95% CI | Adjusted OR | 95% CI | Adjusted OR | 95% CI | |
| Socioeconomic variables | ||||||||
| Sex | ||||||||
| Male | 1 | 1 | 1 | 1 | ||||
| Female | 1.32 | (1.28–1.36) | 1.12 | (1.07–1.14) | 1.27 | (1.23–1.31) | 1.29 | (1.25–1.33) |
| Age (years) | ||||||||
| 19–44 | 1 | 1 | 1 | 1 | ||||
| 45–64 | 1.42 | (1.38–1.45) | 1.00 | (0.97–1.02) | 1.21 | (1.18–1.24) | 0.99 | (0.97–1.02) |
| 65–74 | 6.73 | (6.49–6.98) | 6.57 | (6.31–6.83) | 7.54 | (7.26–7.82) | 6.23 | (6.00–6.48) |
| ≥75 | 7.93 | (7.57–8.30) | 7.22 | (6.87–7.58) | 9.16 | (8.75–9.60) | 8.12 | (7.75–8.50) |
| Income † | ||||||||
| ≥2000 per month | 1 | 1 | 1 | 1 | ||||
| <2000 per month | 1.14 | (1.11–1.16) | 1.02 | (1.00–1.05) | 1.07 | (1.05–1.10) | 1.14 | (1.11–1.16) |
| Occupation | ||||||||
| Professional | 1 | 1 | 1 | 1 | ||||
| Service/physical | 1.08 | (1.05–1.11) | 1.00 | (0.97–1.03) | 1.05 | (1.02–1.08) | 1.07 | (1.04–1.10) |
| Others ‡ | 1.25 | (1.22–1.29) | 1.24 | (1.20–1.27) | 1.11 | (1.08–1.14) | 1.24 | (1.20–1.27) |
| Education level | ||||||||
| At least high school | 1 | 1 | 1 | 1 | ||||
| Less than high school | 1.23 | (1.20–1.26) | 1.02 | (1.00–1.05) | 1.27 | (1.24–1.30) | 1.31 | (1.28–1.34) |
| Residency | ||||||||
| Metropolitan | 1 | 1 | 1 | 1 | ||||
| Urban | 1.07 | (1.04–1.10) | 1.07 | (1.04–1.10) | 1.04 | (1.01–1.07) | 1.04 | (1.02–1.07) |
| Rural | 1.35 | (1.32–1.38) | 1.34 | (1.31–1.38) | 1.18 | (1.15–1.21) | 1.21 | (1.18–1.24) |
| Chronic illness § | ||||||||
| None | 1 | 1 | 1 | 1 | ||||
| One | 1.29 | (1.26–1.33) | 1.39 | (1.34–1.43) | 1.34 | (1.30–1.38) | 1.38 | (1.34–1.42) |
| Two or more | 1.59 | (1.49–1.70) | 1.62 | (1.51–1.73) | 1.59 | (1.49–1.70) | 1.80 | (1.68–1.92) |
| Health behaviors | ||||||||
| Health check–up †† | ||||||||
| No | 1 | 1 | - | 1 | ||||
| Yes | 2.48 | (2.43–2.54) | 2.15 | (2.10–2.20) | - | 2.28 | (2.23–2.33) | |
| Smoking | ||||||||
| Never smoker | 1 | 1 | 1 | 1 | ||||
| Former smoker | 1.04 | (1.01–1.08) | 0.97 | (0.93–1.01) | 1.03 | (1.00–1.07) | 1.08 | (1.05–1.12) |
| Current smoker | 0.79 | (0.77–0.82) | 0.74 | (0.72–0.77) | 0.75 | (0.72–0.77) | 0.78 | (0.75–0.80) |
| Drinking alcohol | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 0.86 | (0.85–0.88) | 0.87 | (0.85–0.89) | 0.88 | (0.86–0.90) | 0.85 | (0.83–0.87) |
| Regular walking | ||||||||
| <30 min/5days/week | 1 | 1 | 1 | 1 | ||||
| ≥30 min/5days/week | 1.12 | (1.10–1.15) | 1.14 | (1.12–1.16) | 1.14 | (1.11–1.16) | 1.12 | (1.10–1.14) |
Notes: † Units: Korean Chun-Won (₩1,000); ‡ Others include soldiers, college students, and housewives; § Patients were diagnosed with one or more of the following diseases by a doctor and were receiving a medication for the disease: diabetes, stroke, heart disease (angina or myocardial infarction), hepatitis, or tuberculosis; †† Variable was excluded from the questionnaire in the 2010–2011 season.
4. Discussion
We summarized IVC rates in four consecutive flu seasons in Korea. The large sample of KCHS data revealed a steady increase in self-reported influenza vaccine receipt. The highest increase in vaccination rate was observed between the 2008–2009 and 2009–2010 seasons. This increased vaccine uptake might be explained by the fear of pandemic influenza. Also, in the 2009–2010 season, two vaccines (seasonal and pandemic) were available, which can also explain the increase in IVC rates in the 2009–2010 season.
Overall, IVC increased with age, with the elderly reporting the highest rates of vaccination. People aged 65 years or older had markedly higher vaccination rates than individuals in other countries [29,30,31,32,33,34,35,36], and this group exceeded the WHO vaccination target of 75% [1]. Many other countries have coverage gaps that vary from 4% to 70% between young adults and the elderly [37,38,39,40] and our study revealed great disparities in IVC between these age groups in Korea. Vaccination rates in young adults were 20% to 30%, which is similar to rates in the United States [38]. IVC coverage in people aged 45–64 years was 35.2%, but the rates decreased during the pandemic and then slowly recovered, as shown in Table 1. Coverage gaps between subgroups according to socioeconomic variables differed from other countries. In this study, vulnerable groups such as people of older age, people with a lower education level, people with lower income, people with physical jobs, people residing in rural areas, and females, showed higher rates of IVC. These results are similar to those previously reported from studies in Korea and China [24,29,30]. The highest increase in vaccine coverage was observed in people with lower income. Usually, education levels are low in the elderly, which led to employment in physical jobs with low income, such as those in agriculture and fisheries. However, each of these factors is defined as a vulnerable group in Korea and 253 regional healthcare centers provide free vaccines to vulnerable groups each year. According to studies from other countries, higher education level, higher income, and professional jobs are associated with higher rates of vaccine coverage [31,32,33].
Smoking and drinking alcohol were inversely associated with vaccine receipt, and receiving health check-ups and regular walking were positively associated with vaccination. These results indicate that engaging in health behaviors is a predictor of influenza vaccination; this finding has been previously reported by other studies [30,31,41,42].
When IVC was evaluated in occupation subgroups, higher rates of vaccination were observed in ‘others (soldiers, students, and housewives)’ than in professional and service/physical workers. This may be because the soldiers in the ‘others’ group are obligated to receive the vaccine soon after enlisting in the army. Having a chronic health condition was also positively associated with vaccination, as other studies have reported [24,25,31,34]. Younger people, people with higher income, people with a higher education level, people who do not receive health check-ups, current smokers, and regular alcohol consumers showed a relatively low IVC of approximately 30%. Coverage gaps between subgroups did not narrow and were similar from the 2008–2009 season through the 2011–2012 season. During the influenza pandemic in 2009, many countries offered free vaccination to high-risk groups with campaigns designed to build herd immunity and promote public awareness of the infection. The Korean government prepared vaccines to cover 39% of the total population and offered free vaccination to high-risk groups first [22]. The IVC was lower than expected during the pandemic, but, according to our study, overall IVC rates increased in the year and two years following the pandemic season. This trend is different from other studies that reported that vaccination rates decreased or remained unchanged after the pandemic in China [22,30,37], the United States [38], and Europe [33,39,40,43,44]. One of the reasons that Korea showed comparatively higher IVC might be the NIP, which fully funds the influenza vaccine and administration fee for vulnerable groups (Table 1). The NIP covers more beneficiaries of free vaccination than other countries [22,45]. Additionally, domestically manufactured influenza vaccines might have contributed to high IVC in Korea, since it was easier to distribute a sufficient number of vaccines [46]. The WHO has emphasized ‘sufficient influenza vaccine provision’ as a major factor in increasing IVC [5].
To augment overall IVC, policies targeting young adults will offer the most benefit. In Korea, children and young adults are more commonly infected with influenza and the resulting socioeconomic burden from infection is considerable [47,48,49]. To implement effective interventions that promote public awareness of influenza and reduce the disparities of IVC among subgroups, strategies that focus on these subpopulations must be combined with the current immunization program [50,51].
For this study, we used a large sample of data that comprised approximately 230,000 survey respondents each year. This data is sufficient to provide information on general vaccination coverage rates and trends, but several limitations exist. First, children are a high-risk group for influenza, but IVC in children could not be evaluated in our study because the KCHS only includes adults aged ≥19 years. Therefore, this study does not represent IVC rates of all populations in Korea. Second, self-reported influenza vaccination might be subject to recall bias. Finally, other chronic conditions such as malignancy, renal diseases, and acquired immune deficiency syndrome, which confer a high risk for influenza complications, were not included in the survey [5]. Thus, data on risk groups in this study do not fully represent all of the risk groups in Korea.
5. Conclusions
In summary, our results show a steady increase in IVC among adults in Korea from before the pandemic in 2008–2009 to after the pandemic in 2011–2012. IVC rates in Korea are higher than in other countries. However, disparities between subgroups were substantial in people with higher income, people with a higher education level, people who do not receive a health check-up, current smokers, regular alcohol consumers, and young people. Vaccination promotion targeting these specific subgroups should be included in the NIP to increase vaccine coverage. Also, future research should be conducted to define the reasons that young adults abstain from influenza vaccination.
Acknowledgments
This study was based on the Korean Community Health Surveys (KCHS) from 2008–2009 to 2011–2012, which was supported by the Korea Centers for Diseases Control and Prevention (KCDC), Division of Chronic Disease Control.
Author Contributions
Hye Jung Yang wrote the manuscript and analyzed the data. Sung-il Cho interpreted the data and reviewed the manuscript. Both of the authors read and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.World Health Organization (WHO) WHO position paper. Wkly. Epidemiol. Rec. 2005;33:279–287. [Google Scholar]
- 2.CDC Prevention and control of seasonal influenza with vaccines. MMWR. 2013;62:1–43. [PubMed] [Google Scholar]
- 3.Simonsen L., Fukuda K. The impact of influenza epidemics on hospitalizations. J. Infect. Dis. 2000;181:831–837. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 4.Terebu P., Uyeki T. Impact of influenza on young children and the shaping of United Sates influenza vaccine policy. Pediatr. Infect. Dis. 2003;22:S231–S235. doi: 10.1097/01.inf.0000092194.33331.66. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Vaccines against influenza WHO position paper—Novermber 2012. Wkly. Epidemiol. Rec. 2012;47:461–476. [PubMed] [Google Scholar]
- 6.Jain S., Kamimoto L. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N. Engl. J. Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 7.Mallia P., Johnston S.L. Influenza infection and COPD. Int. J. COPD. 2007;2:55–64. doi: 10.2147/copd.2007.2.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuzil K.M., Reed G.W. Influenza-associated morbidity and mortality in young and middle-aged women. JAMA. 1999;281:901–907. doi: 10.1001/jama.281.10.901. [DOI] [PubMed] [Google Scholar]
- 9.Osterholm M.T., Kelley N.S. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 10.Puig-Barbera J., Diez-Domingo J. Effectiveness of the 2011–2011 seasonal influenza vaccine in preventing confirmed influenza hospitalizations in adults: A case-case comparison, case-control study. Vaccine. 2012;30:5714–5720. doi: 10.1016/j.vaccine.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard M.P., Tam J.S. The 2009 A (H1N1) influenza virus pandemic: A review. Vaccine. 2010;28:4895–4902. doi: 10.1016/j.vaccine.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Song J.Y., Cheong H.J. Effectiveness of the pandemic influenza A/H1N1 2009 monovalent vaccine in Korea. Vaccine. 2011;29:1395–1398. doi: 10.1016/j.vaccine.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 13.Blank P.R., Szucs T.D. Increasing influenza vaccination coverage in recommended population groups in Europe. Expert Rev. Vaccines. 2009;8:425–433. doi: 10.1586/erv.09.7. [DOI] [PubMed] [Google Scholar]
- 14.Simonsen L., Taylor R.J. Mortality benefits of influenza vaccination in elderly people: An ongoing controversy. Lancet Infect. Dis. 2007;7:658–666. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- 15.Nicho K.L., Nording J. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N. Engl. J. Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 16.Kausz A., Pahari D. The value of vaccination in chronic kidney disease. Semin. Dial. 2004;17:9–11. doi: 10.1111/j.1525-139X.2004.17104.x. [DOI] [PubMed] [Google Scholar]
- 17.Lau D., Eurich D.T. Effectiveness of influenza vaccination in working-age adults with diabetes: A population-based cohort study. Thorax. 2013;68:658–663. doi: 10.1136/thoraxjnl-2012-203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramarz P., Ciancio B. Sesonal and pandemic influenza vaccines for the elderly and other risk groups: A review of available data. Pol. Arch. Med. Wewn. 2009;119:654–659. [PubMed] [Google Scholar]
- 19.Lin H.C., Chiu H.F. Association of influenza vaccination and reduced risk of stroke hospitalization among the elderly. Int. J. Environ. Res. Public Health. 2014;11:3639–3649. doi: 10.3390/ijerph110403639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korea Centers for Disease Control and Prevention . 2013–2014 Instruction Guidelines for Influenza. KCDC; Seoul, Korea: 2013. [Google Scholar]
- 21.OECD Health at a Glance 2011: OECD Indicators, OECD Publishing, 2011. [(accessed on 3 December 2013)]. Available online: http://dx.doi.org/10.1787/health_glance-2011-en.
- 22.Lee Y.K., Kwon Y. 2009–2010 novel influenza A (H1N1) vaccination coverage in the Republic of Korea. Am. J. Infect. Control. 2012;40:481–483. doi: 10.1016/j.ajic.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Heo J.Y., Chang S.H. Risk perception, preventive behaviors, and vaccination coverage in the Korean population during the 2009–2010 pandemic influenza A (H1N1); Comparison between high-risk group and non-high-risk group. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0064230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu S.Y., Kim S.H. Influenza vaccination among adults 65 years or older: A 2009–2010 community health survey in the Honam region of Korea. Int. J. Environ. Res. Public Health. 2011;8:4197–4296. doi: 10.3390/ijerph8114197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kee S.Y., Lee J.S. Influenza vaccine coverage rates and perceptions on vaccination in South Korea. J. Infect. 2007;55:273–281. doi: 10.1016/j.jinf.2007.04.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y.T., Choi B.Y. Overview of Korean community health survey. J. Korean Med. Assoc. 2012;55:74–83. doi: 10.5124/jkma.2012.55.1.74. [DOI] [Google Scholar]
- 27.Rim H., Kim H. Validity of self-reported healthcare utilization data in the community health survey in Korea. J. Korean Med. Sci. 2011;26:1409–1414. doi: 10.3346/jkms.2011.26.11.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh D.H., Kim S.A. Prevalence and correlates of depressive symptoms in Korean adults: Results of a 2009 Korean community health survey. J. Korean Med. Sci. 2013;28:128–135. doi: 10.3346/jkms.2013.28.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kee S.Y., Cheong H.J. Influenza vaccination coverage rate and factors associated with vaccination in people with chronic disease. Infect. Chemother. 2011;43:406–411. doi: 10.3947/ic.2011.43.5.406. [DOI] [Google Scholar]
- 30.Wu S., Yang P. Influenza vaccination coverage rates among adults before and after the 2009 influenza pandemic and the reasons for non-vaccination in Beijing, China: A cross-sectional study. BMC Public Health. 2013;13:1–8. doi: 10.1186/1471-2458-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takayama M., Wetmore C.M. Characteristics associated with the uptake of influenza vaccination among adults in the United States. Prev. Med. 2012;54:358–362. doi: 10.1016/j.ypmed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Wada K., Smith D.R. Influenza vaccination uptake among the working age population of Japan: Results from a national cross-sectional survey. PLoS One. 2013;8:1–6. doi: 10.1371/journal.pone.0059272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaux S., van Cauteren D. Influenza vaccination coverage against seasonal and pandemic influenza and their determinants in France: A cross-sectional survey. BMC Public Health. 2011;11:1–9. doi: 10.1186/1471-2458-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIntyre A.F., Gonzalez-Feliciano A.G. Flu Vaccination Coverage, United States, 2011–12 Influenza Season. [(accessed on 29 April 2014)]; Available online: http://www.cdc.gov/flu/pdf/fluvaxview/vax-coverage-1112estimates.pdf.
- 35.Bentele H., Bergsaker M.R. Vaccination coverage for seasonal influenza among residents and health care workers in Norwegian nursing homes during the 2012/13 season, a cross-sectional study. BMC Public Health. 2014;14:1–6. doi: 10.1186/1471-2458-14-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroneman M., van Essen G.A. Influenza vaccination coverage and reasons to refrain among high-risk persons in four European countries. Vaccine. 2006;24:622–628. doi: 10.1016/j.vaccine.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L., Su Q. Seasonal influenza vaccination coverage rate of target groups in selected cities and provinces in China by season (2009/10 to 2011/12) PLoS One. 2013;8:1–7. doi: 10.1371/annotation/9e189146-9c58-44d0-8bff-347bde7018e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CDC Surveillance of influenza vaccination coverage—United States, 2007–08 through 2011–12 influenza seasons. MMWR. 2013;62:1–28. [PubMed] [Google Scholar]
- 39.Mereckiene J., Cotter S. Seasonal influenza immunization in Europe. Overview of recommendations and vaccination coverage for three seasons: pre-pandemic (2008/09), pandemic (2009.10) and post-pandemic (2010/11) Euro. Sruveill. 2014;19:1–11. doi: 10.2807/1560-7917.es2014.19.16.20780. [DOI] [PubMed] [Google Scholar]
- 40.Caille-Brillet A.L., Raude J. Trends in influenza vaccination behaviors—Results from the CoPanFlu cohort, France, 2006 to 2011. Euro. Surveill. 2013;18:1–10. doi: 10.2807/1560-7917.es2013.18.45.20628. [DOI] [PubMed] [Google Scholar]
- 41.Nelson D.E., Bland S. State Trend in health risk factors and receipt of clinical preventive service among US adults during the 1990s. JAMA. 2002;287:2659–2667. doi: 10.1001/jama.287.20.2659. [DOI] [PubMed] [Google Scholar]
- 42.CDC Surveillance for certain health behaviors among states and selected local areas—United States, 2010. MMWR. 2013;62:1–75. [PubMed] [Google Scholar]
- 43.Pinto C.S., Nunes B. Trends in influenza vaccination coverage in Portugal from 1998 to 2010: Effect of major pandemic threats. BMC Public Health. 2013;13:1–10. doi: 10.1186/1471-2458-13-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castilla J., Martinez-Baz I. Trends in influenza vaccine coverage among primary healthcare workers in Spain, 2008–2011. Prev. Med. 2013;57:206–211. doi: 10.1016/j.ypmed.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 45.de Lataillade C., Auvergne S. 2005 and 2006 seasonal influenza vaccination coverage rates in 10 countries in Africa, Asia Pacific, Europe, Latin America and the Middle East. J. Public Health Policy. 2009;1:83–101. doi: 10.1057/jphp.2008.40. [DOI] [PubMed] [Google Scholar]
- 46.Palache A. Seasonal influenza vaccine provision in 157 countries (2004–2009) and the potential influence of national public health policies. Vaccine. 2011;29:9459–9466. doi: 10.1016/j.vaccine.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 47.Kim W.J. Novel influenza A/H1N1 pandemic: Current status and prospects. J. Korean Med. Assoc. 2009;52:787–794. doi: 10.5124/jkma.2009.52.8.787. [DOI] [Google Scholar]
- 48.Kim H.S., Kim J.H. Fatal cases of 2009 pandemic influenza A(H1N1) in Korea. J. Korean Med. Sci. 2011;26:22–27. doi: 10.3346/jkms.2011.26.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suh M., Kang D.R. Socioeconomic burden of influenza in the Republic of Korea, 2007–2010. PLoS One. 2013;8:1–7. doi: 10.1371/journal.pone.0084121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoo B-.K. How to improve influenza vaccination rates in the U.S. J. Prev. Med. Public Health. 2011;44:141–148. doi: 10.3961/jpmph.2011.44.4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stinchfield P.K. Practice-proven interventions to increase vaccination rates and broaden the immunization season. Amer. J. Med. 2008;121:S11–S21. doi: 10.1016/j.amjmed.2008.05.003. [DOI] [PubMed] [Google Scholar]