Abstract
Justicia gendarussa methanolic leaf extracts from five different locations in the Southern region of Peninsular Malaysia and two flavonoids, kaempferol and naringenin, were tested for cytotoxic activity. Kaempferol and naringenin were two flavonoids detected in leaf extracts using gas chromatography-flame ionization detection (GC-FID). The results indicated that highest concentrations of kaempferol and naringenin were detected in leaves extracted from Mersing with 1591.80 mg/kg and 444.35 mg/kg, respectively. Positive correlations were observed between kaempferol and naringenin concentrations in all leaf extracts analysed with the Pearson method. The effects of kaempferol and naringenin from leaf extracts were examined on breast cancer cell lines (MDA-MB-231 and MDA-MB-468) using MTT assay. Leaf extract from Mersing showed high cytotoxicity against MDA-MB-468 and MDA-MB-231 with IC50 values of 23 μg/mL and 40 μg/mL, respectively, compared to other leaf extracts. Kaempferol possessed high cytotoxicity against MDA-MB-468 and MDA-MB-231 with IC50 values of 23 μg/mL and 34 μg/mL, respectively. These findings suggest that the presence of kaempferol in Mersing leaf extract contributed to high cytotoxicity of both MDA-MB-231 and MDA-MB-468 cancer cell lines.
1. Introduction
Breast cancer is the second largest cancer after lung cancer in the world and the most common malignancy among women [1]. In Malaysia, the most frequent cancers are breast cancer (18.1%), colorectal cancer (12.3%), and lung cancer (10.2%); these three cancers affect both women and men [2]. Currently, the most common approaches for treating human breast cancer include surgery, radiotherapy, hyperthermia, hormone therapy, and chemotherapy [3].
Breast cancers can be classified by stage, pathology, grade, and expression of oestrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor (Her2/neu) [4]. The two types of breast cancer cells that have gained interest among investigators and medical research laboratories are MDA-MB-231 and MDA-MB-468. MDA-MB-231 cells are characterised as ER-, PR-, and Her2/neu-negative/basal-B mammary carcinoma, while MDA-MB-468 cells are characterised as ER-, PR-, and Her2/neu-negative/basal-A mammary carcinoma [4]. MDA-MB-231 and MDA-MB-468 cells were derived from the pleural effusions of 51-year-old female patients. MDA-MB-231 cells were derived from a Caucasian female, while MDA-MB-468 cells were derived from an African American female [5–7].
There is strong social interest in natural remedies, and more than 80% of the world population considers traditional medicine as their source of primary health care [8]. Indeed, there has been a worldwide effort to discover new anticancer agents from medicinal plants, and various experimental models of natural products have resulted in anticancer agents [9, 10].
One of the potential medicinal plants that is being investigated in our laboratory is J. gendarussa, which is also known by its common name Gendarussa. This plant is a member of the Acanthaceae family that can be found ubiquitously in many countries, including Indonesia, Sri Lanka, India, and Malaysia [11]. The roots and leaf extracts of J. gendarussa have been demonstrated to treat chronic rheumatism, inflammation, bronchitis, headache, arthritis, vaginal discharges, dyspepsia, eye disease, and fever [12].
Previous reports demonstrated that J. gendarussa leaf extracts have been used traditionally as a male contraceptive agent by several ethnic groups in the central part of Papua, Indonesia. This extract is able to inhibit mouse spermatozoa penetration of mice ovum [13]. J. gendarussa methanolic leaves and root extracts showed cytotoxic activity against brine shrimp in the brine shrimp lethality assay with IC50 values of 48.71 μg/mL and 93.25 μg/mL, respectively [14]. In addition, J. gendarussa leaves and stem extracts were reported to have anticancer, antioxidant, antibacterial, antifungal, antiangiogenic, anthelmintic, and hepatoprotective activities [15–23].
Phytochemical studies on leaves from J. gendarussa revealed the presence of flavonoids, alkaloids, triterpenoidal saponins, amino acids, aromatic amines, stigmasterol, and lupeol [18, 24–27]. Our previous study on green callus and in vitro leaf extracts of J. gendarussa detected two flavonoids, that is, kaempferol and naringenin using GC-FID [28]. Both flavonoids were also detected in the methanolic leaf extract of J. gendarussa using the same method [29]. Bioactivity studies on both flavonoids found that it exhibited strong antioxidant and inhibitory effects on cholesterol in HepG2 cancer cells [30–32]. Kaempferol also inhibited pancreatic cancer cell (MIAPaCa-2 and Panc-1) proliferation, induced cancer cell apoptosis, and prevented arteriosclerosis [30, 33]. Naringenin demonstrated cytotoxic effects against breast cancer cells (MCF-7) and suppressed apoptosis in mouse leukaemia P388 cells [34–36]. Our previous study on both flavonoids showed strong cytotoxic activity against colonic (HT-29), cervical (HeLa), and pancreatic (BxPC-3) cells [29].
To the best of our knowledge, this is the first study of the effects of J. gendarussa leaf extracts against human breast cancer cell lines (MDA-MB-231 and MDA-MB-468). This study was performed to screen the cytotoxic activities of methanolic leaf extracts from five different locations (Mersing, Muar, Skudai, Batu Pahat, and Pulai) in Johor and two flavonoids (naringenin and kaempferol) against breast cancer cell lines. The quantification of kaempferol and naringenin content in leaf extracts of J. gendarussa using GC-FID was also carried out.
2. Methods and Materials
2.1. Plant Materials
J. gendarussa plants were collected from five different locations in Johor (Mersing, Muar, Skudai, Batu Pahat, and Pulai) and maintained in a greenhouse at the Faculty of Biosciences and Medical Engineering, Universiti Teknologi Malaysia (UTM). The J. gendarussa plant was identified by Dr. Richard Chung Cheng Kong, senior research officer of the Forest Research Institute of Malaysia (FRIM). The voucher specimen (PID-100214-06) was deposited at Herbarium Management Branch, Flora Biodiversity Program, Forest Biodiversity Division, FRIM, Kepong, Selangor, Malaysia.
2.2. General Chemicals
Commercial standards (kaempferol and naringenin) were purchased from Sigma-Aldrich (Subang Jaya, Selangor, Malaysia). Tamoxifen was used as a positive control in the MTT assay. All samples were diluted with 0.1% of dimethylsulfoxide (DMSO), which has no effect on cell viability [37].
2.3. Preparation of Extracts
The J. gendarussa leaves were air-dried for 4 weeks. The dried leaves were ground into small particles and approximately 50 g of small particles was soaked into 1000 mL of methanol at room temperature for 72 hours in a ratio of 1 : 20 (w/v) [10]. The mixtures were filtered through sterile cotton and filtered again using Whatman number 1 filter paper to obtain methanolic supernatants. The filtered methanolic extract was evaporated at 40°C under reduced pressure by using an EYELA N-1000 rotary evaporator (Bohemia, NY, USA). The dried crude extract was kept at 4°C prior to use.
2.4. Quantification of Flavonoids in Leaf Extracts
GC-FID and quantitative analysis were performed according to previously published method [38]. GC-FID (HP-6890N, Agilent, USA) equipped with a HP-5 fused silica capillary column (30.0 m × 0.32 mm ID × 0.25 μm) was used. The temperature programmed was 100°C held for 1 minute and then ramped to 275°C at 10°C/min and held for 17 minutes at 275°C. The injection temperature was 275°C. The flow rate of the carrier gas (helium) was 1 mL/min. A split ratio of 50 : 1 was used. A quantity of 5 μL of leaf extract and standards was injected. The chromatographic data were recorded and processed using Agilent Cerity QA-QC software.
2.5. Cell Culture
MDA-MB-231 (basal-B mammary carcinoma) and MDA-MB-468 (basal-A mammary carcinoma) breast cancer cell lines and CHO (Chinese hamster ovary) normal cell line were obtained from American Type Culture Collection (ATCC) and as a generous gift from Dr. Salehhuddin Hamdan (Animal Cell Culture Laboratory, Faculty of Biosciences and Medical Engineering, UTM). MDA-MB-231 and MDA-MB-468 breast cancer cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM), while CHO normal cells were cultured in Roswell Park Memorial Institute 1640 (RPMI-1640) medium supplemented with 10% v/v foetal bovine serum (FBS), 100 U/mL of penicillin, and 100 μg/mL of streptomycin as a complete growth medium. Cells were maintained in 25 cm2 flasks and incubated in a humidified incubator (CO2 Water-Jacketed Incubator NuAire, Fernbrook Lane, Plymouth, USA) at 37°C with 5% CO2. All materials were obtained from Gibco (Gibco, Bio-Diagnostics, Petaling Jaya, Selangor, Malaysia).
2.6. MTT Assay
Cytotoxicity testing was performed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) according to the method reported in previous studies [29, 39]. In this assay, cells were harvested after reaching 80% confluence. Before starting the MTT assay, cells were optimised at different seeding densities ranging from 2.0 × 103 cell/mL to 1.0 × 106 cell/mL in light to determine appropriate seeding number for the experiment. Each well of the microtiter plate (96-well) was filled with 100 μL of cell suspension (MDA-MB-231, MDA-MB-468, and CHO with the seeding number; 5 × 104 cell/mL) in complete growth medium. After 24 hours of incubation, cells were treated with leaf extracts of different concentrations ranging from 7.81 to 1000 μg/mL, with a total well volume of 200 μL with technical replicates. Microtiter plates were further incubated for 72 hours with plant extracts. After 72 hours of incubation, 20 μL of MTT (a stock solution of 5 mg/mL in PBS) was added to each well, and the plates incubated for 4 hours at 37°C. Medium from each well was carefully removed without disturbing the MTT crystals in wells. The MTT formazan crystals were dissolved by the addition of 1 M HCl and 100 mM isopropanol to each well. After solubilising the purple formazan, absorbance was measured using a BioRad microplate reader (Shinagawa-ku, Tokyo, Japan) at a wavelength of 575 nm. Cytotoxic activity was recorded as IC50, which is the concentration necessary to reduce the absorbance of treated cells by 50% compared to the control (untreated cells) [40].
2.7. Statistical Analysis
All samples were run in three replicates. Data obtained were analysed using SPSS software for Windows (SPSS 16.0 for Windows Evaluation Version software, SPSS Inc., USA). The normality of the data was tested using the Shapiro-Wilk test. The data were analysed using the Independence t-test for normal data and Mann-Whitney U test for nonnormal data. The correlations were analysed using the Pearson correlation test [41]. Differences were considered to achieve significance for probability P < 0.05.
3. Results
Phytochemical analysis of J. gendarussa leaf extracts showed that kaempferol and naringenin were quantified from five different locations by GC-FID. Figure 1 shows the distribution of kaempferol and naringenin contents in leaf extracts.
Figure 1.
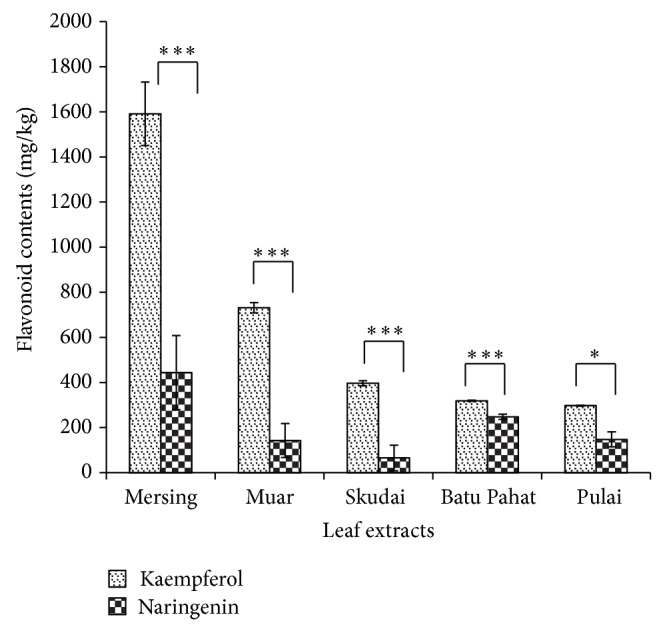
Distribution of kaempferol and naringenin contents in leaf extracts from five different locations. Each result is the mean of 3 replicates. Error bars represent standard deviations (STDEV). Results that are significantly different * P < 0.05, ** P < 0.01, and *** P < 0.001 are marked with an asterisk.
In this study, cytotoxicity of J. gendarussa leaf extracts from five different locations and flavonoids (kaempferol, naringenin, and a mixture of kaempferol and naringenin) were tested against breast cancer cell lines (MDA-MB-231 and MDA-MB-468) and a normal cell line (CHO) using MTT assay. Tamoxifen was used as a positive control. The IC50 values obtained referred to 50% of cells inhibited by plant extracts [42]. In a previous study, cytotoxicity was evaluated based on IC50 values, where IC50 values below 20 μg/mL were considered cytotoxic, from 21 to 40 μg/mL were considered weak cytotoxic, and above 40 μg/mL were not considered cytotoxic [40, 43, 44].
Table 1 represents the IC50 values of J. gendarussa leaf extracts, flavonoids, and tamoxifen. Overall, tamoxifen showed cytotoxic activity against CHO and MDA-MB-231 cells with IC50 values of 8 μg/mL and 12 μg/mL, respectively, compared to MDA-MB-468 cell with IC50 values of 27 μg/mL.
Table 1.
Comparison of IC50 values between J. gendarussa leaf extracts, flavonoids, and tamoxifen in breast cancer cell lines.
| IC50 values (μg/mL) | |||
|---|---|---|---|
| MDA-MB-231 | MDA-MB-468 | CHO | |
| Leaf extract | |||
| Mersing | 40 | 23 | 28 |
| Muar | 275 | 160 | 108 |
| Skudai | 61 | 259 | 88 |
| Batu Pahat | 538 | 398 | 190 |
| Pulai | 250 | 299 | 305 |
| Compounds | |||
| Kaempferol | 34 | 23 | 14 |
| Naringenin | 238 | 70 | 21 |
| Mixture of kaempferol and naringenin | 43 | 44 | NT |
| Tamoxifen | 12 | 27 | 8 |
NT: not tested.
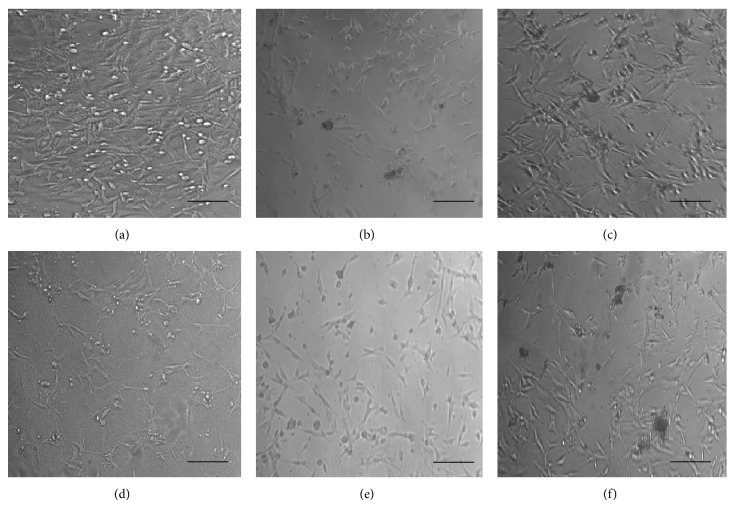
Morphological changes of cells were observed under an inverted fluorescence microscope (Nikon ECLIPSE Ti-S, Shinagawa-ku, Tokyo, Japan) (100x magnification) after 72 hours of treatment. The methanolic leaf extracts from various locations were used to treat MDA-MB-231 cancer cell lines and revealed morphology changes (Figures 2(b), 2(c), 2(d), 2(e), and 2(f)) compared to nontreated cells (Figure 2(a)).
Figure 2.
Morphology changes of MDA-MB-231 cells when treated with leaf extracts. (a) MDA-MB-231 cells without any treatment; (b) leaf extract from Mersing (IC50: 40 μg/mL); (c) leaf extract from Muar (IC50: 275 μg/mL); (d) leaf extract from Skudai (IC50: 61 μg/mL); (e) leaf extract from Batu Pahat (IC50: 538 μg/mL); and (f) leaf extract from Pulai (IC50: 250 μg/mL). Scale bars: 100 μM.
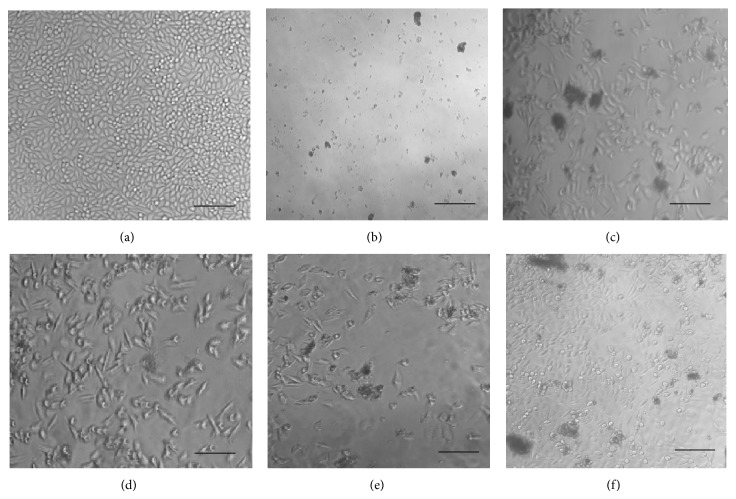
Morphological changes were revealed after methanolic leaf extract treatment of MDA-MB-468 cancer cell lines (Figures 3(b), 3(c), 3(d), 3(e), and 3(f)) compared to nontreated cells (Figure 3(a)).
Figure 3.
Morphological changes of MDA-MB-468 cells when treated with leaf extracts. (a) MDA-MB-468 cells without any treatment; (b) leaf extract from Mersing (IC50: 23 μg/mL); (c) leaf extract from Muar (IC50: 160 μg/mL); (d) leaf extract from Skudai (IC50: 259 μg/mL); (e) leaf extract from Batu Pahat (IC50: 398 μg/mL); and (f) leaf extract from Pulai (IC50: 299 μg/mL). Scale bars: 100 μM.
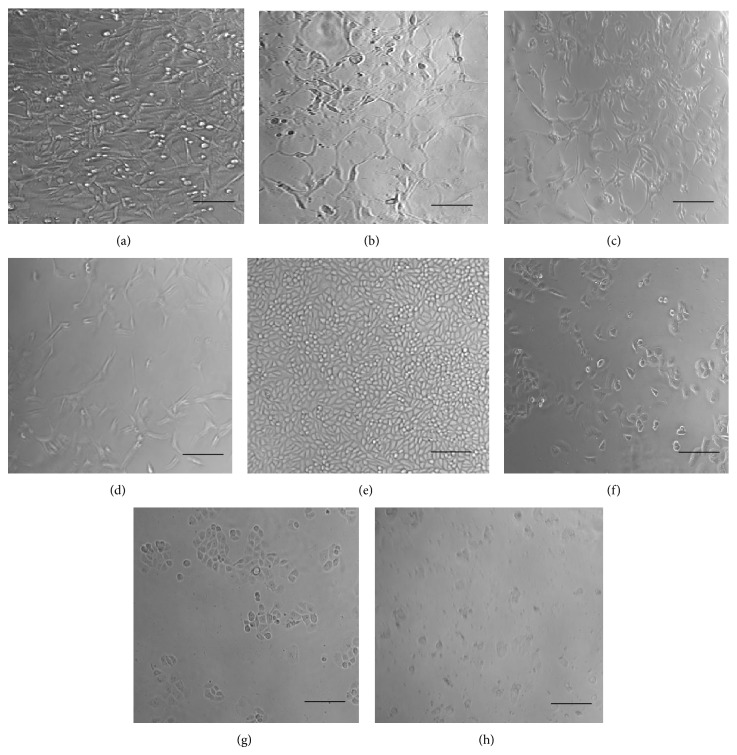
The morphology changes of MDA-MB-231 (Figures 4(b), 4(c), and 4(d)) and MDA-MB-468 (Figures 4(f), 4(g), and 4(h)) cancer cell lines when treated with kaempferol, naringenin and a mixture of kaempferol and naringenin compared to nontreated MDA-MB-231 and MDA-MB-468 cancer cell lines (Figures 4(a) and 4(e)), respectively.
Figure 4.
Morphology of MDA-MB231 and MDA-MB-468 cells when treated with kaempferol, naringenin and a mixture of kaempferol and naringenin. (a) MDA-MB-231 cells without any treatment, control; (b) MDA-MB-231 cells treated with kaempferol (IC50: 34 μg/mL); (c) MDA-MB-231 cells treated with naringenin (IC50: 238 μg/mL); (d) MDA-MB-231 cells treated with a mixture of kaempferol and naringenin (IC50: 43 μg/mL); (e) MDA-MB-468 cells without any treatment, control; (f) MDA-MB-468 cells treated with kaempferol (IC50: 23 μg/mL); (g) MDA-MB-468 cells treated with naringenin (IC50: 70 μg/mL); (h) MDA-MB-468 cells treated with a mixture of kaempferol and naringenin (IC50: 44 μg/mL). Scale bars: 100 μM.
4. Discussion
Phytochemical analysis of kaempferol and naringenin in leaf extracts from five locations was evaluated and shown in Figure 1. The highest concentrations of kaempferol and naringenin were found in leaf extracts from Mersing with 1591.80 mg/kg and 444.35 mg/kg, respectively. Positive correlations were observed between kaempferol and naringenin in all leaf extracts when analysed using the Pearson method. In addition, there was a significant difference in the kaempferol and naringenin distribution contents of leaf extracts from five different locations.
The cytotoxicity profile of J. gendarussa leaf extracts from five different locations and flavonoids (kaempferol, naringenin, and a mixture of kaempferol and naringenin) against MDA-MD-231, MDA-MB-468, and CHO cells are shown in Table 1. The inhibitory effects of all leaf extracts against breast cancer cell lines were decreased in a dose dependent manner, and these trends are consistent with previous studies [10, 42, 45, 46]. The IC50 values of the leaf extract from Mersing (40 μg/mL) showed weak cytotoxicity, followed by leaf extracts from Skudai (61 μg/mL), Batu Pahat (250 μg/mL), Muar (275 μg/mL), and Pulai (275 μg/mL) against MDA-MB-231 breast cancer cells. IC50 values of the leaf extract from Mersing (23 μg/mL) showed weak cytotoxicity, followed by leaf extracts from Muar (160 μg/mL), Skudai (259 μg/mL), Batu Pahat (299 μg/mL), and Pulai (398 μg/mL) against MDA-MB-468 cell lines. The percent cell viability of leaf extracts and flavonoids was compared to the control (untreated cell). The results demonstrate that there was a significant difference in IC50 values of each leaf extract against MDA-MB-231 and MDA-MB-468 cell lines (Tables 2 and 3). Because both flavonoids were present in high concentrations in leaf extracts, it is suggested that cytotoxic effects were mainly due to the presence of these flavonoids in elucidating tumour suppressive effects.
Table 2.
Percentage viability of MDA-MB-231 cells in leaf extracts from five different locations.
| Concentration (μg/mL) | 7.81 | 15.63 | 31.25 | 62.5 | 125 | 250 | 500 | 1000 |
|---|---|---|---|---|---|---|---|---|
| Leaf extract from Mersing |
60.25 ± 0.06*** | 54.35 ± 0.05*** | 55.95 ± 0.08*** | 33.91 ± 0.07*** | 24.08 ± 0.02*** | 23.78 ± 0.02*** | 17.60 ± 0.03*** | 29.85 ± 0.08** |
|
| ||||||||
| Leaf extract from Muar |
79.26 ± 0.07* | 72.89 ± 0.07* | 71.35 ± 0.08* | 70.34 ± 0.07* | 63.49 ± 0.09* | 54.58 ± 0.04** | 8.29 ± 0.01*** | 11.54 ± 0.02*** |
|
| ||||||||
| Leaf extract from Skudai |
67.41 ± 0.03** | 64.76 ± 0.01*** | 55.77 ± 0.03*** | 50.44 ± 0.03*** | 49.61 ± 0.01*** | 47.07 ± 0.04*** | 8.42 ± 0.01*** | 11.65 ± 0.01*** |
|
| ||||||||
| Leaf extract from Batu Pahat |
82.67 ± 0.07* | 78.96 ± 0.02** | 77.20 ± 0.02* | 60.89 ± 0.01*** | 58.22 ± 0.01*** | 50.59 ± 0.01*** | 11.40 ± 0.01*** | 10.28 ± 0.01*** |
|
| ||||||||
| Leaf extract from Pulai |
74.61 ± 0.08** | 73.06 ± 0.07** | 69.24 ± 0.09* | 66.06 ± 0.10** | 65.44 ± 0.10* | 60.58 ± 0.07* | 53.23 ± 0.03** | 16.71 ± 0.02*** |
Values are mean ± STDEV for 3 replicates * P < 0.05, ** P < 0.01, and *** P < 0.001 compared to control (untreated cell).
Table 3.
Percentage viability of MDA-MB-468 cells in leaf extracts from five different locations.
| Concentration (μg/mL) | 7.81 | 15.63 | 31.25 | 62.5 | 125 | 250 | 500 | 1000 |
|---|---|---|---|---|---|---|---|---|
| Leaf extract from Mersing |
92.63 ± 0.04 | 76.53 ± 0.02* | 14.21 ± 0.02*** | 7.13 ± 0.01*** | 3.78 ± 0.01*** | 3.63 ± 0.01*** | 3.38 ± 0.01*** | 6.91 ± 0.01*** |
|
| ||||||||
| Leaf extract from Muar |
79.53 ± 0.03** | 74.33 ± 0.02*** | 69.20 ± 0.09* | 75.0 ± 0.03** | 63.7 ± 0.01* | 22.63 ± 0.02*** | 3.03 ± 0.01*** | 2.49 ± 0.01*** |
|
| ||||||||
| Leaf extract from Skudai |
98.03 ± 0.082 | 101.67 ± 0.021 | 97.87 ± 0.016 | 82.9 ± 0.021** | 77.0 ± 0.014*** | 52.33 ± 0.045** | 4.83 ± 0.002*** | 4.43 ± 0.01*** |
|
| ||||||||
| Leaf extract from Batu Pahat |
96.26 ± 0.02 | 92.06 ± 0.03* | 91.23 ± 0.09 | 89.0 ± 0.04* | 102.46 ± 0.06 | 64.61 ± 0.08* | 7.07 ± 0.01*** | 6.19 ± 0.01*** |
|
| ||||||||
| Leaf extract from Pulai |
92.96 ± 0.03 | 88.68 ± 0.03* | 87.69 ± 0.02** | 86.4 ± 0.01** | 82.6 ± 0.04*** | 77.53 ± 0.04** | 36.8 ± 0.07** | 8.12 ± 0.01*** |
Values are mean ± STDEV for 3 replicates * P < 0.05, ** P < 0.01, and *** P < 0.001 compared to control (untreated cell).
Table 1 also shows the ability of kaempferol, naringenin, and a mixture of kaempferol and naringenin to inhibit the proliferation of breast cancer cell lines in this study. However, kaempferol showed weak cytotoxicity, with IC50 values of approximately 34 μg/mL (MDA-MB-231) and 23 μg/mL (MDA-MB-468). This was followed by naringenin, with IC50 values of approximately 238 μg/mL (MDA-MB-231) and 70 μg/mL (MDA-MB-468). The mixture of flavonoids also showed weak cytotoxicity, with IC50 values of approximately 43 μg/mL (MDA-MB-231) and 44 μg/mL (MDA-MB-468). It is proposed that kaempferol associated highest cytotoxicity against breast cancer cell lines compared to naringenin and a mixture of kaempferol and naringenin. Table 4 shows that there was a significant difference between the control with MDA-MB-231 and MDA-MB-468 treated cells for IC50 values of flavonoids, except for kaempferol against MDA-MD-231. The leaf extracts and flavonoids also showed low cytotoxicity toward CHO cells (Table 1). This indicates a lack of selectivity in the cytotoxicity between cancer and normal cells by the leaf extracts and flavonoids [47].
Table 4.
Percentage viability of MDA-MB-231 and MDA-MB-468 cells in kaempferol, naringenin, and a mixture of kaempferol and naringenin.
| Cells | Concentration (μg/mL) | 3.91 | 7.81 | 15.63 | 31.25 | 62.5 | 125 | 250 | 500 |
|---|---|---|---|---|---|---|---|---|---|
| MDA-MB-231 | Kaempferol | 82.16 ± 0.06 | 81.07 ± 0.06*** | 61.48 ± 1.59** | 50.96 ± 0.09*** | 36.45 ± 0.04* | 27.45 ± 0.06 | 43.52 ± 0.02* | 77.37 ± 3.30* |
| Naringenin | 85.53 ± 0.06* | 100.23 ± 0.06 | 87.13 ± 0.07 | 69.35 ± 0.02*** | 93.79 ± 0.07 | 83.97 ± 0.04* | 47.91 ± 0.04** | 13.48 ± 0.01*** | |
| Mixture of kaempferol and naringenin | 72.42 ± 0.11 | 66.94 ± 0.11** | 62.17 ± 0.64*** | 56.10 ± 0.12* | 27.15 ± 0.34*** | 26.37 ± 0.03*** | 23.42 ± 0.11*** | 23.32 ± 0.22*** | |
|
| |||||||||
| MDA-MB-468 | Kaempferol | 96.00 ± 0.07 | 74.82 ± 0.02** | 53.99 ± 0.03*** | 31.64 ± 0.03*** | 17.67 ± 0.02*** | 12.73 ± 0.01*** | 7.05 ± 0.01*** | 11.33 ± 0.03*** |
| Naringenin | 85.62 ± 2.03 | 94.26 ± 2.30 | 117.71 ± 0.07* | 112.18 ± 3.26 | 58.08 ± 1.51* | 13.54 ± 0.02*** | 3.24 ± 0.01*** | 3.94 ± 0.01*** | |
| Mixture of kaempferol and naringenin | 92.02 ± 0.11 | 89.79 ± 0.01*** | 83.99 ± 0.98 | 70.82 ± 0.04*** | 29.38 ± 0.04*** | 16.44 ± 0.02*** | 13.34 ± 0.03*** | 13.33 ± 0.06*** | |
Values are mean ± STDEV for 3 replicates * P < 0.05, ** P < 0.01, and *** P < 0.001 compared to control (untreated cell).
However, the current study also has contradictory results. It is shown in Figures 1, 2, and 3 that treated cells showed more prominent growth inhibition and shrinkage of the cells when compared to untreated cells that remained confluent. Many factors may have influenced these contradictory results. The plant source, environmental and geographic conditions, cell lines, and seeding number used in this study were completely different from those used in published works [48–50]. Thus, the results presented in this study were not totally in agreement with published [40, 43, 44] statements of IC50 values ranging toward crude extracts. Moreover, different plant extracts exhibited different effects on the proliferation of cells according to properties of the compounds [48]. This was because selectivity could be due to the sensitivities of cell lines against the active compounds in crude extracts that have a specific response [51, 52]. Overall, J. gendarussa leaf extracts and flavonoids were considered to hold promising anticancer effects on MDA-MB-231 and MDA-MB-468 cells.
The results of J. gendarussa leaf extract from Mersing showed less of an effect against MDA-MB-231 compared to MDA-MB-468 (Table 1). This suggests that the effects of active compounds, particularly flavonoids, on MDA-MB-231 are less cytotoxic compared to those on MDA-MB-468 cell lines. MDA-MB-231 is an oestrogen receptor (ER-negative) cell line that contains more than one cell population and is highly aggressive, invasive, and poorly differentiated from human breast cancer cell lines [53, 54]. MDA-MB-468 cells were most resistant to hyperacetylation and DNA degradation by drug treatments. This suggests that the MDA-MB-468 cell line has a phenotypic difference from and is less invasive than MDA-MB-231 [4]. In a previous study, T. crispa and M. calabura methanolic leaf extracts produced IC50 values of approximately 52.5 μg/mL and more than 100 μg/mL, respectively [10, 42]. However, J. gendarussa leaf extract from Mersing showed an IC50 value of 40 μg/mL, exhibiting higher toxicity compared to other leaf extracts. It is suggested that J. gendarussa leaf extract from Mersing has cytotoxicity potential against MDA-MB-231 cells compared to other plant leaf extracts.
Based on the collected data, kaempferol showed the highest cytotoxicity against MDA-MB-468, followed by MDA-MB-231 and naringenin. These results are consistent with other studies showing weak inhibition of naringenin by other flavonoids [55]. A previous study reported that flavonoids with hydroxyl substituents at the 4′ and 7 positions were invariably oestrogenic, and an additional hydroxyl group at the 5th position increased estrogenic activity [56]. The present study supports this claim [56]. Previous work also demonstrated that naringenin showed a stronger oestrogenicity when tested on BT-474 human breast cancer cell lines [57]. It is plausible to suggest that both flavonoids contribute strong oestrogenic potency to the inhibition of oestrogen-independent breast cancer cells, MDA-MB-231 and MDA-MB-468.
Table 1 also shows the cytotoxicity of J. gendarussa leaf extracts, flavonoids, and tamoxifen on a normal cell line (CHO). CHO cells were a positive control used for comparison with the cytotoxicity activity on MDA-MB-231 and MDA-MB-468 breast cancer cell lines. Comparisons of J. gendarussa leaf extracts, flavonoids, and tamoxifen were performed in terms of IC50 values between breast cancer and normal cell lines. Tamoxifen was demonstrated to be cytotoxic to CHO cell lines (IC50 < 20 μg/mL) in this study. Although the IC50 values of leaf extracts and flavonoids were not as low as tamoxifen, they had low toxicity against CHO cells. Due to its high toxicity in CHO cells, the continuous use of tamoxifen can cause adverse side effects [58]. If these results also occur in vivo, these leaf extracts would be considered safe for human consumption and could be used for further toxicity and clinical studies. Hence, the use of leaf extracts and flavonoids as anticancer agents in combination with other therapeutic drugs may reduce the adverse effects of drugs. Therefore, more comprehensive studies involving animal and clinical investigations are required.
5. Conclusion
In conclusion, J. gendarussa leaf extract from Mersing and kaempferol were considered cytotoxic against MDA-MB-231 and MDA-MB-468 compared to other leaf extracts and naringenin. Leaf extract from Mersing showed high contents of kaempferol and naringenin compared to other leaf extracts when quantified using GC-FID. Our results suggest that there is a correlation between the presence of kaempferol in the leaf extract from Mersing with the level of cytotoxicity against both breast cancer cell lines. These data will be beneficial to other researchers and validate the potential use of J. gendarussa leaves as novel anticancer agents.
Acknowledgments
The authors thank Universiti Teknologi Malaysia (UTM) and the Ministry of Education Malaysia (MOE) (MyPhD) for financial support and Animal Tissue Culture, Faculty of Biosciences and Medical Engineering for lab facilities.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Kummalue T., O-charoenrat P., Jiratchariyakul W., Chanchai M., Pattanapanyasat K., Sukapirom K., Iemsri S. Antiproliferative effect of Erycibe elliptilimba on human breast cancer cell lines. Journal of Ethnopharmacology. 2007;110(3):439–443. doi: 10.1016/j.jep.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Registry N. C. Malaysia Cancer Statistics—Data and Figure 2007. Malaysia Ministry of Health; 2007. [Google Scholar]
- 3.Jones E. L., Prosnitz L. R., Dewhirst M. W., et al. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clinical Cancer Research. 2004;10(13):4287–4293. doi: 10.1158/1078-0432.CCR-04-0133. [DOI] [PubMed] [Google Scholar]
- 4.Tate C. R., Rhodes L. V., Segar H. C., Driver J. L., Pounder F. N., Burow M. E., Collins-Burow B. M. Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat. Breast Cancer Research. 2012;14(79):1–15. doi: 10.1186/bcr3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cailleau R., Mackay B., Young R. K., Reeves W. J., Jr. Tissue culture studies on pleural effusions from breast carcinoma patients. Cancer Research. 1974;34(4):801–809. [PubMed] [Google Scholar]
- 6.Engel J. B., Schally A. V., Halmos G., Baker B., Nagy A., Keller G. Targeted cytotoxic bombesin analog AN-215 effectively inhibits experimental human breast cancers with a low induction of multi-drug resistance proteins. Endocrine-Related Cancer. 2005;12(4):999–1009. doi: 10.1677/erc.1.01022. [DOI] [PubMed] [Google Scholar]
- 7.Cailleau R., Olive M., Cruciger Q. V. J. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978;14(11):911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- 8.Figueroa A. C., Soria E. A., Cantero J. J., et al. Cytotoxic activity of Thelesperma megapotamicum organic fractions against MCF-7 human breast cancer cell line. Journal of Cancer Therapy. 2012;3:103–109. [Google Scholar]
- 9.Rajkapoor B., Jayakar B., Murugesh N. Antitumor activity of Indigofera aspalathoides on Ehrlich ascites carcinoma in mice. Indian Journal of Pharmacology. 2004;36(1):38–40. [Google Scholar]
- 10.Zakaria Z. A., Mohamed A. M., Jamil N. S. M., Rofiee M. S., Hussain M. K., Sulaiman M. R., Teh L. K., Salleh M. Z. In vitro antiproliferative and antioxidant activities of the extracts of Muntingia calabura leaves. The American Journal of Chinese Medicine. 2011;39(1):183–200. doi: 10.1142/S0192415X11008749. [DOI] [PubMed] [Google Scholar]
- 11.Thomas T. D., Yoichiro H. In vitro propagation for the conservation of a rare medicinal plant Justicia gendarussa Burm. f. by nodal explants and shoot regeneration from callus. Acta Physiologiae Plantarum. 2010;32(5):943–950. doi: 10.1007/s11738-010-0482-1. [DOI] [Google Scholar]
- 12.Janarthanam B., Sumathi E. In vitro regeneration of Justicia gendarussa Burm. f. Libyan Agriculture Research Center Journal International. 2010;1(5):284–287. [Google Scholar]
- 13.Prajogo E. W. B., Ifadotunnikmah F., Febriyanti A. P., et al. Effect of Justicia gendarussa Burm.f. leaves water fraction on male rabbit liver and renal fuction (Sub acute toxicity test of Justicia gendarussa Burm.f. leaves water fraction as male contraceptive agent) Veterinaria Medika. 2008;1(3):79–82. [Google Scholar]
- 14.Patel S. S., Zaveri M. N. Cytotoxic activity to find bioactive compound from Justicia gendarussa using brine shrimp lethality assay. Asian Journal of Traditional Medicines. 2012;7(3):102–108. [Google Scholar]
- 15.Periyanayagam K., Umamaheswari B., Suseela L., Padmini M., Ismail M. Evaluation of antiangiogenic effect of the leaves of Justicia gendarussa (Burm. f) (Acanthaceae) by chrio allontoic membrane method. American Journal of Infectious Diseases. 2009;5(3):187–189. doi: 10.3844/ajidsp.2009.187.189. [DOI] [Google Scholar]
- 16.Krishna K. L., Mehta T. A., Patel J. A. In-vitro hepatoprotective activity of Justicia gendarussa stem on isolated rat hepatocytes. Pharmacologyonline. 2010;2:9–13. [Google Scholar]
- 17.Sharma K. K., Saikia R., Kotoky J., Kalita J. C., Devi R. Antifungal activity of Solanum melongena L, Lawsonia inermis L. and Justicia gendarussa B. against dermatophytes. International Journal of PharmTech Research. 2011;3(3):1635–1640. [Google Scholar]
- 18.Uddin M. R., Sinha S., Hossain M. A., Kaisar M. A., Hossain M. K., Rashid M. A. Chemical and biological investigations of Justicia gendarussa (Burm. F) Dhaka University Journal of Pharmaceutical Sciences. 2011;10(1):53–57. [Google Scholar]
- 19.Fazaludeen M. F., Manickam C., Ashankyty I. M. A., et al. Synthesis and characterizations of gold nanoparticles by Justicia gendarussa Burm F leaf extract. Journal of Microbiology and Biotechnology Research. 2012;2(1):23–34. [Google Scholar]
- 20.Saha M. R., Debnath P. C., Rahman M. A., Islam M. A. U. Evaluation of in vitro anthelmintic activities of leaf and stem extracts of Justicia gendarussa . Bangladesh Journal of Pharmacology. 2012;7(1):50–53. doi: 10.3329/bjp.v7i1.10558. [DOI] [Google Scholar]
- 21.Subramanian N., Jothimanivannan C., Moorthy K. Antimicrobial activity and preliminary phytochemical screening of Justicia gendarussa (Burm. f.) against human pathogens. Asian Journal of Pharmaceutical and Clinical Research. 2012;5(3):229–233. [Google Scholar]
- 22.Sudhanandh V. S., Arjun J. K., Faisal A. K., et al. In-vitro antibacterial screening of selected folklore Indian medicinal plants with few clinical pathogens. Indian Journal of Pharmaceutical Education and Research. 2012;46(2):174–178. [Google Scholar]
- 23.Kowsalya D., Sankaranarayanan S. Efficacies of bactericidal Justicia gendarussa extract inhibiting protein synthesis against methicilin resistant Staphylococcus aureus . IOSR Journal of Pharmacy and Biological Sciences. 2012;4(2):32–41. [Google Scholar]
- 24.Mustafa R. A., Hamid A. A., Mohamed S., Bakar F. A. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. Journal of Food Science. 2010;75(1):28–35. doi: 10.1111/j.1750-3841.2009.01401.x. [DOI] [PubMed] [Google Scholar]
- 25.Prajogo E. B., Guliet D., Ferreira Queiroz E., et al. Isolation of male antifertility compound in n-butanol fraction of Justicia gendarussa Burm. F. leaves. Folia Medica Indonesiana. 2009;45(1):28–31. [Google Scholar]
- 26.Chakravarty A. K., Dastidar P. P. G., Pakrashi S. C. Simple aromatic amines from Justicia gendarussa. 13C NMR spectra of the bases and their analogues. Tetrahedron. 1982;38(12):1797–1802. doi: 10.1016/0040-4020(82)80253-6. [DOI] [Google Scholar]
- 27.Ratnasooriya W. D., Deraniyagala S. A., Dehigaspitiya D. C. Antinociceptive activity and toxicological study of aqueous leaf extract of Justicia gendarussa Burm. F. in rats. Pharmacognosy Magazine. 2007;3(11):145–155. [Google Scholar]
- 28.Ayob Z., Saari N. H. M., Samad A. A. In vitro propagation and flavonoid contents in local Justicia gendarussa Burm. F. Proceedings of the 11th International Annual Symposium on Sustainability Science and Management; July 2012; Terengganu, Malaysia. pp. 403–409. [Google Scholar]
- 29.Ayob Z., Abd Samad A., Mohd Bohari S. P. Cytotoxicity activities in local Justicia gendarussa crude extracts against human cancer cell lines. Jurnal Teknologi (Sciences and Engineering) 2013;64(2):45–52. doi: 10.11113/jt.v64.2043. [DOI] [Google Scholar]
- 30.Zhang Y., Chen A. Y., Li M., Chen C., Yao Q. Ginkgo biloba extract kaempferol inhibits cell proliferation and induces apoptosis in pancreatic cancer cells. Journal of Surgical Research. 2008;148(1):17–23. doi: 10.1016/j.jss.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavia-Saiz M., Busto M. D., Pilar-Izquierdo M. C., Ortega N., Perez-Mateos M., Muñiz P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. Journal of the Science of Food and Agriculture. 2010;90(7):1238–1244. doi: 10.1002/jsfa.3959. [DOI] [PubMed] [Google Scholar]
- 32.Kim K.-T., Yeo E.-J., Moon S.-H., Cho S.-G., Han Y.-S., Nah S.-Y., Paik H.-D. Inhibitory effects of naringenin, kaempherol, and apigenin on cholesterol biosynthesis in HepG2 and MCF-7 cells. Food Science and Biotechnology. 2008;17(6):1361–1364. [Google Scholar]
- 33.Tu Y.-C., Lian T.-W., Yen J.-H., Chen Z.-T., Wu M.-J. Antiatherogenic effects of kaempferol and rhamnocitrin. Journal of Agricultural and Food Chemistry. 2007;55(24):9969–9976. doi: 10.1021/jf0717788. [DOI] [PubMed] [Google Scholar]
- 34.Susanti D., Sirat H. M., Ahmad F., Ali R. M., Aimi N., Kitajima M. Antioxidant and cytotoxic flavonoids from the flowers of Melastoma malabathricum L. Food Chemistry. 2007;103(3):710–716. doi: 10.1016/j.foodchem.2006.09.011. [DOI] [Google Scholar]
- 35.Park J.-H., Lee J.-W., Paik H.-D., et al. Cytotoxic effects of 7-O-butyl naringenin on human breast cancer MCF-7 cells. Food Science and Biotechnology. 2010;19(3):717–724. doi: 10.1007/s10068-010-0101-3. [DOI] [Google Scholar]
- 36.Kanno S.-I., Tomizawa A., Hiura T., Osanai Y., Shouji A., Ujibe M., Ohtake T., Kimura K., Ishikawa M. Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice. Biological and Pharmaceutical Bulletin. 2005;28(3):527–530. doi: 10.1248/bpb.28.527. [DOI] [PubMed] [Google Scholar]
- 37.Kamuhabwa A., Nshimo C., De Witte P. Cytotoxicity of some medicinal plant extracts used in Tanzanian traditional medicine. Journal of Ethnopharmacology. 2000;70(2):143–149. doi: 10.1016/S0378-8741(99)00161-0. [DOI] [PubMed] [Google Scholar]
- 38.Sarju N., Samad A., Ghani M., Ahmad F. Detection and quantification of Naringenin and kaempferol in Melastoma decemfidum extracts by GC-FID and GC-MS. Acta Chromatographica. 2012;24(2):221–228. doi: 10.1556/AChrom.24.2012.2.5. [DOI] [Google Scholar]
- 39.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad R., Ali A. M., Israf D. A., Ismail N. H., Shaari K., Lajis N. H. Antioxidant, radical-scavenging, anti-inflammatory, cytotoxic and antibacterial activities of methanolic extracts of some Hedyotis species. Life Sciences. 2005;76(17):1953–1964. doi: 10.1016/j.lfs.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 41.Pallant J. SPSS Survival Manual. New York, NY, USA: McGraw-Hill; 2007. [Google Scholar]
- 42.Ibahim M. J., Wan-Nor I. W. M. Z., Narimah A. H. H., Nurul A. Z., Siti-Nur S. S. A. R., Froemming G. A. Anti-proliperative and antioxidant effects of Tinospora crispa (Batawali) Biomedical Research. 2011;22(1):57–62. [Google Scholar]
- 43.Geran R. I., Greenberg H. M., McDonald M., et al. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemotherapy Reports. 1972;33:1–17. [Google Scholar]
- 44.Mohamed S. M., Ali A. M., Rahmani M., Wiart C., Dhaliwal J. S., Yusoff K. Apoptotic and necrotic cell death manifestations in leukemic cells treated with methylgerambullin a sulphone from Glycosmis calcicola . Journal of Biochemistry, Molecular Biology and Biophysics. 2000;4(4):253–261. [Google Scholar]
- 45.Chong H. Z., Rahmat A., Yeap S. K., Md Akim A., Alitheen N. B., Othman F., Gwendoline-Ee C. L. In vitro cytotoxicity of Strobilanthes crispus ethanol extract on hormone dependent human breast adenocarcinoma MCF-7 cell. BMC Complementary and Alternative Medicine. 2012;12, article 35 doi: 10.1186/1472-6882-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subarnas A., Diantini A., Abdulah R., et al. Antiproliferative activity of primates-consumed plants against MCF-7 human breast cancer cell lines. E3 Journal of Medical Research. 2012;1(4):38–43. [Google Scholar]
- 47.Armania N., Yazan L. S., Ismail I. S., Foo J. B., Tor Y. S., Ishak N., Ismail N., Ismail M. Dillenia suffruticosa extract inhibits proliferation of human breast cancer cell lines (MCF-7 and MDA-MB-231) via Induction of G2/M arrest and apoptosis. Molecules. 2013;18(11):13320–13339. doi: 10.3390/molecules181113320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Barazanjy R. K., Dizaye K., AL-Asadye A. Cytotoxic and cytogenetic effects of Salvia officinalis on different tumor cell lines. Middle East Journal of International Medicine. 2013;6(4):15–25. [Google Scholar]
- 49.Alsemari A., Alkhodairy F., Aldakan A., Al-Mohanna M., Bahoush E., Shinwari Z., Alaiya A. The selective cytotoxic anti-cancer properties and proteomic analysis of Trigonella Foenum-Graecum . BMC Complementary and Alternative Medicine. 2014;14, article 114 doi: 10.1186/1472-6882-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han J., Talorete T. P., Yamada P., Isoda H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology. 2009;59(1):45–53. doi: 10.1007/s10616-009-9191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirana C., Record I. R., McIntosh G. H., Jones G. P. Screening for antitumor activity of 11 species of Indonesian zingiberaceae using human MCF-7 and HT-29 cancer cells. Pharmaceutical Biology. 2003;41(4):271–276. doi: 10.1076/phbi.41.4.271.15673. [DOI] [Google Scholar]
- 52.Joshi C. G., Gopal M., Byregowda S. M. Cytotoxic activity of Tragia involucrata. Linn. extracts. American-Eurasian Journal of Toxicological Sciences. 2011;3(2):67–69. [Google Scholar]
- 53.Liu H., Zang C., Fenner M. H., Possinger K., Elstner E. PPARγ ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Research and Treatment. 2003;79(1):63–74. doi: 10.1023/A:1023366117157. [DOI] [PubMed] [Google Scholar]
- 54.Yin K. B. The mesenchymal-like phenotype of the MDA-MB-231 cell line, breast cancer—focusing tumor microenvironment, stem cells and metastasis. In: Gunduz P. M., editor. Breast Cancer—Focusing Tumor Microenvironment, Stem cells and Metastasis. Vol. 18. InTech; 2011. pp. 385–402. [Google Scholar]
- 55.Le Bail J. C., Varnat F., Nicolas J. C., Habrioux G. Estrogenic and antiproliferative activities on MCF-7 human breast cancer cells by flavonoids. Cancer Letters. 1998;130(1-2):209–216. doi: 10.1016/S0304-3835(98)00141-4. [DOI] [PubMed] [Google Scholar]
- 56.So F. V., Guthrie N., Chambers A. F., et al. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen. Cancer Letters. 1997;112:127–133. doi: 10.1016/s0304-3835(96)04557-0. [DOI] [PubMed] [Google Scholar]
- 57.Rosenberg Zand R. S., Jenkins D. J. A., Diamandis E. P. Steroid hormone activity of flavonoids and related compounds. Breast Cancer Research and Treatment. 2000;62(1):35–49. doi: 10.1023/A:1006422302173. [DOI] [PubMed] [Google Scholar]
- 58.Lazzeroni M., Serrano D., Dunn B. K., et al. Oral low dose and topical tamoxifen for breast cancer prevention: modern approaches for an old drug. Breast Cancer Research. 2012;14, article 214 doi: 10.1186/bcr3233. [DOI] [PMC free article] [PubMed] [Google Scholar]