Abstract
Purpose
To compare the accuracy of liver fat quantification using a three-echo chemical shift-encoded magnetic resonance imaging (MRI) technique without and with correction for confounders with spectroscopy (MRS) as the reference standard.
Materials and Methods
Fifty patients (23 women, mean age 56.6 ± 13.2 years) with fatty liver disease were enrolled. Patients underwent T2-corrected single-voxel MRS and a three-echo chemical shift-encoded gradient echo (GRE) sequence at 3.0T. MRI fat fraction (FF) was calculated without and with T2* and T1 correction and multispectral modeling of fat and compared with MRS-FF using linear regression.
Results
The spectroscopic range of liver fat was 0.11%–38.7%. Excellent correlation between MRS-FF and MRI-FF was observed when using T2* correction (R2=0.96). With use of T2* correction alone, the slope was significantly different from 1 (1.16 ± 0.03, P < 0.001) and the intercept was different from 0 (1.14% ± 0.50%, P < 0.023). This slope was significantly different than 1.0 when no T1 correction was used (P=0.001). When T2*, T1, and spectral complexity of fat were addressed, the results showed equivalence between fat quantification using MRI and MRS (slope: 1.02 ± 0.03, P=0.528; intercept: 0.26% ± 0.46%, P=0.572).
Conclusion
Complex three-echo chemical shift-encoded MRI is equivalent to MRS for quantifying liver fat, but only with correction for T2* decay and T1 recovery and use of spectral modeling of fat. This is necessary because T2* decay, T1 recovery, and multispectral complexity of fat are processes which may otherwise bias the measurements.
Keywords: fatty liver, magnetic resonance imaging, chemical shift-encoded imaging, magnetic resonance spectroscopy
Nonalcoholic Fatty Liver disease (NAFLD) is the most common chronic liver disease. Fatty liver is present in 20%–30% of the population (1, 2) and in as many as 10% of all children (3). Individuals with fatty liver disease have an increased risk of developing progressive steatohepatitis and chronic late complications such as cirrhosis, liver failure, and hepatocellular carcinoma (2, 4–6). NAFLD is a feature of the metabolic syndrome and has been shown to be an independent risk factor for cardiovascular diseases (7, 8). Further, it has been implicated as a possible cause of type II diabetes mellitus (4), and in a 5-year observation, individuals with fatty liver disease and its sequelae were found to have 26% higher healthcare costs (9, 10).
The diagnosis of NAFLD currently relies on liver biopsy and histopathologic evaluation, which is considered the clinical gold standard for the quantitative assessment of hepatic steatosis. Unfortunately, liver biopsy is subject to sampling errors resulting from the heterogeneous distribution of liver fat (11, 12). Further, biopsy is invasive, expensive, and associated with complications (13).
A reliable noninvasive test for routine clinical quantification of fatty liver is urgently needed. Such a test must be reproducible, accurate, precise, and widely accessible. None of the available techniques meets all of these criteria. Magnetic resonance spectroscopy (MRS) is currently regarded as the reference standard for the noninvasive quantification of fatty liver (14). Preclinical and clinical studies have shown T2-corrected single-voxel 1H MRS to be a reliable method with excellent results in estimating liver fat (15). Limitations of MRS include its lack of anatomic information and the need for advanced post-processing by trained individuals.
Another promising noninvasive modality is complex chemical shift-encoded magnetic resonance imaging (MRI)—an emerging method that has undergone important refinement in recent years. Correction algorithms have been introduced to address known confounding factors such as relaxation processes (T1, T2*), the spectral complexity of fat, and noise bias (16–20). Correction for all factors that affect MR signal intensity permits estimation of the proton density fat fraction (PDFF) (21, 22). The full set of correction algorithms is not currently available on most clinical MRI scanners, making it difficult to compare results obtained on scanners from different manufacturers.
The equivalence of liver fat quantification with chemical shift-encoded MRI and MRS has been demonstrated in previous work at both 1.5T and 3T using a magnitude-based six-echo 2D acquisition technique (23, 24) and also using a complex-based six-echo 3D acquisition technique (9). Both the magnitude-based and complex-based methods correct for T2* decay and use low flip angles to avoid T1 bias in fat quantification. Acquisition of six echoes increases scan time and limits the achievable spatial resolution for imaging of the entire liver during a single breath-hold. Further, acquisition of T1-independent images with very low flip angles (typically 5° at 1.5T and 3° at 3T) results in lower signal-to-noise ratio (SNR). T1 correction enables the use of a short TR and large flip angle and consequently increases the SNR or reduces scan time.
In this study, we evaluate a complex three-echo 3D acquisition (which can be acquired rapidly with high spatial resolution) using a larger flip angle (9° , resulting in higher SNR relative to low-flip angle imaging) postprocessing to correct for the resulting T1 bias (ie, a T1-corrected approach). This T1-corrected three-echo technique is used to quantify liver fat content and compare the results with single-voxel 1H MRS as the reference standard. In addition, this study investigates how fat quantification by chemical shift-encoded MRI as specified above is affected by correction for relevant confounding factors: T2* relaxation, T1, and the spectral complexity of fat.
MATERIALS AND METHODS
This prospective study was approved by the local Ethics Committee of the University of Greifswald. Prior to enrollment in the study, patients provided written informed consent for the MRI examination and storage of their data. Unenhanced MR images of the upper abdomen were acquired solely for the purpose of this study.
Patients
Between September 2011 and March 2012, 50 patients with a sonographic diagnosis of fatty liver underwent noncontrast-enhanced MRI of the upper abdomen (1H MRS and chemical shift-encoded MRI). There were 23 women and 27 men with a mean age of 56.6 ± 13.2 years (men, 51.8 ± 11.6 years; women, 62.2 ± 13.0 years). The sonographic diagnosis of fatty liver was based on the subjective visual identification of increased echogenicity of the liver relative to the renal cortex of the right kidney. Patients with general contraindications to MRI (pacemaker, non-MRI-compatible implants, etc.) or patients who refused consent were not included in the study.
The upper abdomen of each subject was imaged at 3T (Magnetom Verio, Siemens Healthcare, Erlangen, Germany) using a 12-channel body phased-array coil system. The standardized MRI protocol included two investigational pulse sequences: a three-echo chemical shift-encoded sequence and a T2-corrected multiecho single-voxel 1H MRS sequence (15).
Chemical Shift Sequence and Offline Reconstruction
The 3D chemical shift gradient echo (GRE) sequence used for complex-based water-fat separation was acquired in the axial plane. Imaging was performed during breath-hold using the following parameters: TR/TE1/TE2/TE3=6.51/1.22/2.45/4.90 msec; flip angle=9°; averages=1; echo train length=1; bandwidth=± 914 Hz/pixel; imaging matrix=288 × 125, interpolated to 576 × 384; field of view (FOV)=450 × 300 mm; parallel acquisition (GRAPPA) with an acceleration factor of 2 and 24 references/line, slice partial Fourier factor of 6/8; slice thickness=3.0 mm. Total acquisition time was 16 seconds.
Calculations to correct for confounding factors were carried out offline using the MatLab software package (v. 7.12.0 (R2011a), MathWorks, Natick, MA). The raw image data (all three TEs) were used to decompose the liver signal into its fat and water signal components in order to obtain fat and water images. The fat fraction (FF) was calculated as follows:
FF = fat/(fat + water)
The phase information of the water and fat vectors of the raw data was stored in the basic sequence to resolve fat-water ambiguities (25). In this way, it was possible to display fat fractions over the full range of 0%–100%. Noise bias was eliminated by noise correction (17). To investigate the influence of different confounding factors on liver fat quantification—T2* effects, T1 effects, and spectral complexity of fat—we determined the fat fraction under different conditions: No correction (FF); Correction for T2* effects (FFT2*) (19); Correction for T2* and T1 effects (FFT2*T1) (17, 26); Correction for T2* and T1 effects and multipeak fat modeling (PDFF) (21). T1 correction was performed assuming a T1 of water of 809 msec and a T1 of fat of 382 msec (26).
Multiecho T2-Corrected Single-Voxel 1H MRS
The T2-corrected single-voxel multiecho (HISTO) 1H MRS technique (WIP-599, Siemens Healthcare) has been evaluated for reliability in quantifying fat in preclinical and clinical investigations (15). During a single breath-hold of 15 seconds, a spin-echo sequence (STEAM: stimulated echo acquisition mode) with a repeated TR of 3000 msec was acquired with readout at five defined echo times (12, 24, 36, 48, 72 msec) using a flip angle of 90° and a bandwidth of 1200 Hz. Voxel size was 30 × 30 × 30 mm. The long TR minimizes T1 effects. Analysis of the relaxation curves for the different TEs included the spectral peaks of fat. T2 correction was used to reduce other undesirable effects such as those of iron overload.
Prior to each acquisition, shimming was performed to reduce magnetic field in homogeneities. This was done with the patient breathing freely and took about 2 min. The spectroscopy voxel was placed in a standardized manner in liver segment VII, avoiding large vessels and large bile ducts.
An MR diagnosis of fatty liver was made if the PDFF measured by MRS exceeded 5.56% (14).
Data Analysis
Fat fractions determined with chemical shift-encoded MRI without and with application of the different corrections described above were analyzed by a reader with 8 years of experience in liver MRI (J.P.K.). The analysis was conducted separately for chemical shift-encoded MRI and 1H MRS.
First, a region of interest (ROI) was placed in liver segment VII in the source images (ie, without correction for T2* and T1 effects and without multipeak fat modeling). ROI sizes varied between 1.5 and 3 cm2. The ROIs were placed avoiding large vessels, visible partial volume effects, and motion artifacts. All measurements were performed using the commercial Osirix software package (v. 3.8.1, 64bit, Pixmeo Sarl, Bernex, Switzerland).
The ROIs placed in the uncorrected images were transferred to the other series using the copy-and-paste function. In this way, ROIs were placed in identical locations in all series analyzed.
Statistics
All data are presented as means and standard deviations. Linear regression analysis was performed to compare the fat fractions determined with chemical shift-encoded MRI using different corrections with those obtained by T2-corrected single-voxel 1H MRS. Perfect agreement was defined as a regression line slope of 1.0 and regression line intercept at 0%. Differences in slope from 1.0 and intercept from 0% tested for significance using a Student’s t-test, assuming significance at P < 0.05. In addition, the two MRI-based techniques were compared using Bland–Altman plots (27).
Data were stored in Excel (V14.1.4, Microsoft, Redmond, WA). Statistical analysis was performed using Stata 12.0 (College Station, TX).
RESULTS
All MRI examinations (1H MRS and complex chemical shift-encoded MRI) were completed successfully in all study patients (Fig. 1). The mean liver fat content determined by spectroscopy was 12.6% ± 9.4% with a range of 0.1%–38.7%.
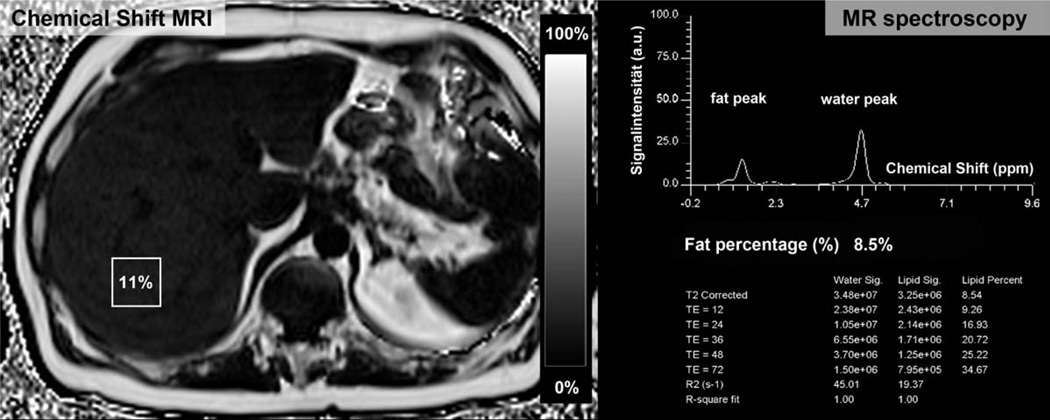
Figure 1.
Comparison of the chemical shift-encoded fat fraction (left side) and MRS (right side). The fraction map calculated from the chemical shift-encoded sequence allows quantification of liver fat content in each pixel of the liver and provides anatomical information of the entire organ. In this way chemical shift imaging avoids sampling errors arising from a heterogeneous distribution of liver fat and also avoid vessels, bile ducts, or masses. In contrast, T2 corrected 1H MRS can quantify liver fat content in a defined voxel.
Eleven of the 50 patients (22%) with a sonographic diagnosis of fatty liver had a liver fat content below the threshold of 5.56% defined for PDFF measured by MRS.
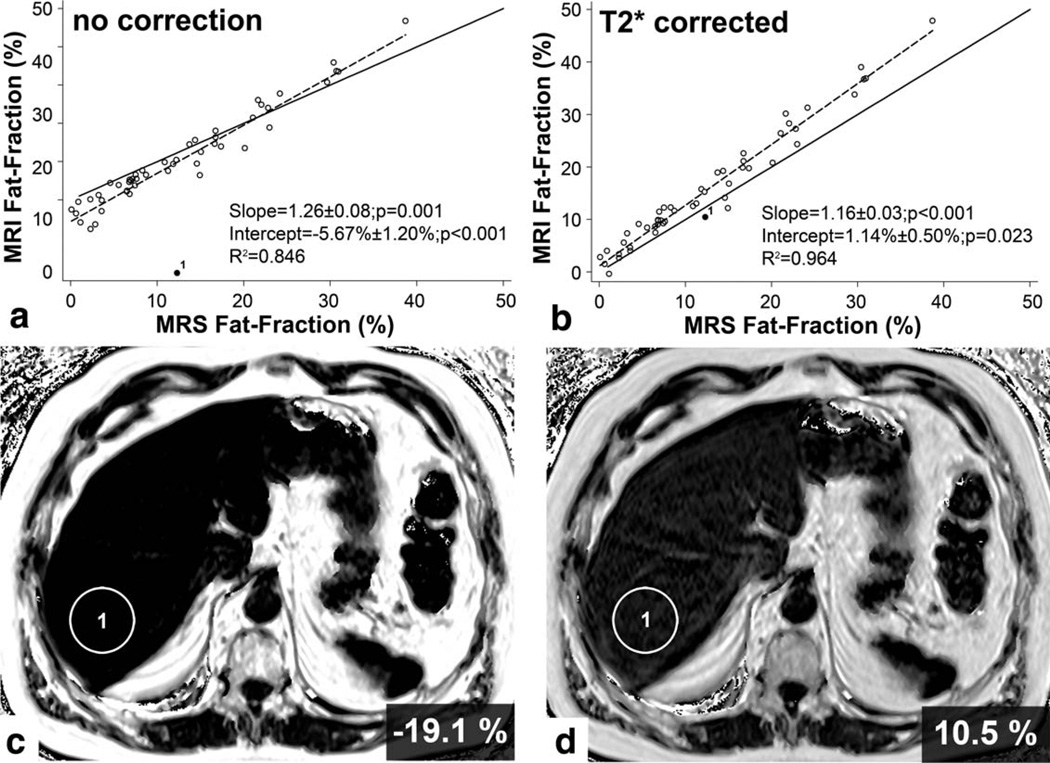
Chemical shift-encoded MRI without any correction showed good correlation with 1H MRS with R2=0.846. The mean fat content was 10.3% ± 12.8%. There was significant deviation from the line of unity (1.26 ± 0.08, P=0.001). The intercept was negative (−5.67% ± 1.20%) (P < 0.001). Bland–Altman analysis revealed a mean difference of −2.37% (−13.22%; 8.47%) between the two techniques. There was one outlier (case without agreement) with false-negative diagnosis of fatty liver (Fig. 2a,c). In this case, MRI showed signs of iron overload with a T2* of 4.2 msec.
Figure 2.
Comparison of fat fractions from MR spectroscopy (MRS Fat-Fraction) and chemical shift imaging (MRI Fat-Fraction) without any correction (a) and with T2* correction (b). Without any corrections it is not possible to quantify liver fat, especially in cases of liver iron overload (c). One patient with iron overload is depicted as point 1. In subjects with iron overload, the fat fraction will be estimated incorrectly if T2* effects are not corrected. With T2* correction, both techniques are comparable; however, there is a significant difference in the slope and intercept for both techniques. D: Fat quantification in a subject with liver iron overload (point 1) is more accurate when corrected for T2* decay.
Correction for T2* decay eliminated this outlier and also resulted in improved correlation between the two techniques (R2=0.964). However, linear regression still showed a significant difference with the slope for the chemical shift-encoded technique suggesting overestimation of fat fraction (1.16 ± 0.03, P < 0.001). The intercept was partially corrected (1.14% ± 0.50%) with a difference that was still significant (P=0.023) (Figs. 2d, 3a). The systematic deviation between MRS and T2*-corrected chemical shift-encoded MRI was 3.14% (−1.85%; 8.13%). Further, mean fat corrected for T2* calculated from the T2*-corrected reconstructions was 29.3 ± 15.0 msec with a range of 4.2–95.2 msec.
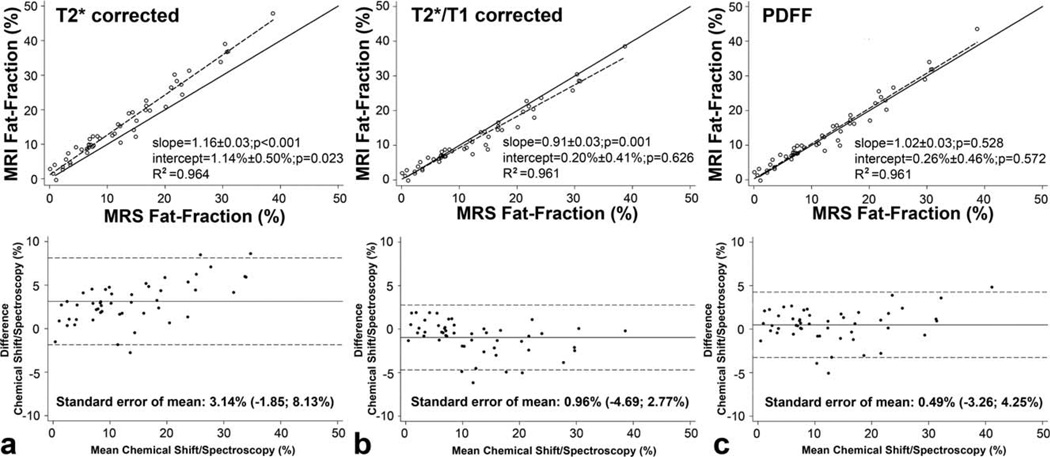
Figure 3.
The relationship between MRI fat fraction and MRS fat fraction with (a) T2* correction, (b) T2* and T1 correction, and (c) T2*, T1 correction and multipeak spectral modeling of fat, ie, measurement of PDFF. a: Chemical shift-encoded imaging with T2* correction shows an excellent correlation with spectroscopy (R2 = 0.96). However, the fat fraction calculated from the chemical shift imaging technique will be overestimated in comparison to spectroscopy. b: With use of T2* and T1 correction, the agreement between spectroscopy and chemical shift imaging is improved. However, using both corrections the chemical shift technique results in slight but significant underestimation compared to spectroscopy. c: PDFF demonstrates both excellent correlation (R2 = 0.961) and agreement (slope = 1.02 ± 0.03, P = 0.528 ; intercept = 0.26% ± 0.46%, P = 0.572) between MRS and the 3-echo high-flip angle 3D chemical shift-encoded fat quantification technique.
Combined correction for T2* and T1 effects resulted in underestimation of liver fat by complex chemical-shift-encoded MRI, while correlation was unchanged (R2=0.961). There was a significant difference in the slope (0.91 ± 0.03, P=0.001). Combined T2* and T1 correction led to normalization of the intercept (0.20% ± 0.41%, P=0.626) (Fig. 3b). Bland–Altman analysis shows a reduction of the mean systematic deviation (−0.96%; −4.69%; 2.77%).
Using both T2*/T1 correction and multipeak spectral modeling of fat to calculate a PDFF (22)) resulted in strong correlation between PDFF and 1H MRS (R2 = 0.961). Unlike T2* correction alone and combined T2*/T1 correction without multipeak fat modeling, PDFF showed no significant difference in slope from 1.0 (1.02 ± 0.03, P=0.528) and intercept from 0.0 (0.26% ± 0.46%, P=0.572), demonstrating statistical agreement between MRI and MRS when all confounding factors are corrected (Fig. 3c). Bland–Altman analysis revealed a further reduction of the systematic deviation to 0.49% (−3.26%; 4.25%).
DISCUSSION
MRI is increasingly being used for the detection and quantification of fatty liver. To become widely used in clinical practice, an MRI method for quantification of liver fat must be reproducible, meaning that measurements must be accurate across platforms and MRI equipment and robust to use of different imaging parameters (protocol) and techniques. Our results demonstrate that complex chemical shift-encoded MRI with three echoes and a relatively large flip angle provides accurate estimates of liver fat content only if relaxation effects are accounted for (T2* and T1 correction) and if multipeak spectral modeling of fat is employed. In conjunction with earlier investigations that have validated chemical shift-encoded fat quantification techniques with different acquisition protocols (eg, 6-echo technique, 2D) and on different platforms, our results suggest that chemical shift-encoded fat quantification is accurate across platforms and protocols as long as all relevant confounding factors are accounted for.
Ultrasonography, with its wide availability and low cost, has been used as a screening tool for fatty liver (28). Unfortunately, the sonographic diagnosis of fatty liver is subjective and has high interobserver variability (29). A standardized ultrasound technique for quantifying liver fat content is currently undergoing preclinical evaluation; however, this method is neither established nor accepted clinically (30). Available data confirm that the subjective nature of sonographic fat estimates is associated with a high rate of false-positive results. In our study, ultrasound had a specificity of only 78% with a 22% false-positive rate. This rate is in agreement with published data (31).
Other techniques such as 1H MRS yield accurate and comparable quantitative estimates of liver fat (32–34). Unfortunately, single-voxel methods provide no anatomic information and sample only a small portion of the liver. Since steatosis is well known to be heterogeneous, these methods provide incomplete evaluation. Incomplete sampling may also degrade the repeatability (precision) of MRS since it is difficult to coregister MRS voxels with past exams, particularly if studies are done at different sites or if prior studies are not available for comparison. Further, volumetric imaging methods have the potential to quantify the total volume of fat within the liver. The clinical utility of total fat content compared to fat concentration alone is unknown.
Chemical shift-encoded imaging is a simple MRI technique for quantifying liver fat (35). Without correction for confounding factors, this technique is available on all MRI systems. Correction of T2* decay, however, is theoretically necessary because high tissue T2* causes signal loss and leads to errors in calculating the fat fraction (19, 36). The magnitude of the error increases with the magnitude of T2*. Theoretical considerations and practical results indicate that T2* reflects the liver iron content (37–39). In up to 40% of patients with NAFLD, an association has been found between liver fat and iron overload (40, 41). In patients with fatty liver and concomitant iron overload, a double-echo technique (ie, one without T2* correction) would not allow accurate quantification of liver fat content (20, 42). Our results for the three-echo chemical shift-encoded technique without T2* correction confirm that errors in calculated fat fraction occur not only when T2* is high but also when T2* is normal (intercept of −5.67%, P < 0.001). Since T2* varies between patient populations (28.1 ± 7.1 msec with a range of 13.6–45.9 msec (43) vs. 29.3 ± 15.0 msec with a range of 4.2–95.2 msec in our study), it is necessary to correct for the effects of T2*. Given that physiologic T2* variation can impair the accuracy of liver fat estimates, we conclude that accurate fat quantification with chemical shift-encoded MRI requires correction for T2*.
Our analysis shows excellent correlation for the three-echo technique with T2* correction. This contradicts earlier assumptions that adequate T2* correction requires the use of more than three echoes (44). In our opinion, the echo times employed have a much greater impact than the number of echoes used. Longer echo times are adequate in patients with normal liver iron, while shorter echo times are preferable when there is rapid T2* decay due to iron overload. Further investigations are needed to determine the optimal number of echoes and the best echo times for adequate T2* quantification and estimation of liver fat.
T1 recovery effects can impact fat quantification when a GRE sequence is used (17). This error arises because fat has shorter T1 relaxation time than water, resulting in higher signal intensity on GRE images (26) and overestimation of relative fat content. The degree of overestimation depends on the amount of fat present (17). T1 effects can be minimized during data acquisition using a low flip angle (17) or corrected during postprocessing (26). During imaging, T1 effects can be minimized by using a long TR (at the cost of acquisition time) or a low flip angle (at the cost of SNR) (17). With the relatively high flip angle of 9° used for acquisition of the 3D GRE sequence in our study, the observed bias in estimating the fat fraction was expected. As theoretically predicted, this technique led to overestimation of fat content without T1 correction. With T1 correction, this overestimation was eliminated. Note that correction for T1 recovery assumes that the values of T1 of the water and fat components are known. Although further work is needed to test this assumption, the close correlation and agreement in this study strongly suggest that this assumption is valid. It should also be noted that T1 correction methods may fail after the administration of gadolinium contrast medium, and precontrast imaging should be performed. With postcontrast imaging, low flip angle acquisitions may be preferred.
Fat has a complex MR signal spectrum that consists of multiple spectral components, which are seen as multiple distinct spectral peaks at different resonance frequencies (0.9, 1.3, 2.1, 4.2, 5.3 ppm). The relative amplitude is highest for the main fat peak at 1.3 ppm. For comparison, the water peak appears at 4.7 ppm (20, 45–47). The main fat peak originates from hydrogen protons in the methylene group (CH2) and constitutes ~70% of the total fat content (47). The spectral complexity of fat is often ignored by imaging techniques that model fat as a single resonant peak. With these methods, a considerable fraction of the fat signal will be incorrectly assigned to water and the fat fraction will be underestimated. Our results confirm that methods modeling fat as a single peak underestimate liver fat compared with 1H MRS and that the degree of underestimation depends on the amount of liver fat present. Available data and our findings confirm that the bias inherent in single-peak models can be eliminated by multipeak fat modeling, both in theory (18) and in practice (9, 16, 44).
In summary, our study demonstrates that the combination of T2* correction, T1 correction, and multipeak fat modeling is needed for accurate and reliable quantification of liver fat, yielding close agreement with MRS (slope=1.02 ± 0.03: P=0.528; intercept=0.26 ± 0.46 P=0.572). In a study conducted at 1.5T (Signa Hdx, GE), Meisamy et al (9) found similar results for determination of the PDFF compared with MRS (slope: P=0.77; intercept: P=0.19). However, they used a six-echo acquisition technique and a lower flip angle (5° ) to minimize T1 effects. Yokoo et al (23) also investigated the correlation between MRS and 2D chemical shift imaging and investigated how liver fat estimates are affected by the number of echoes acquired. They compared a six-echo and a three-echo method to an MRS reference. For the three-echo technique they found a slope near (although statistically different from) 1.0 (0.978; P=0.015) and an intercept nearly 0.0 (−0.002%; P=0.081). For the six-echo technique they found a slope (0.982; P=0.073) and intercept (−0.0007; P=0.591) nearly 1.0 and 0.0, respectively. The authors avoided T1 effects using a non-T1-weighted sequence with a combination of a 10° flip angle and a long TR of 125 msec and more. They did not investigate how liver fat estimates are influenced by T1 bias. The results of Yokoo et al might be influenced by noise effects resulting from the acquisition of non-T1-weighted images. Further, in an intraindividual comparison conducted by Kang et al (48), PDFF estimates at 1.5T (Symphony, Siemens) and 3T (Signa Excite HD, GE) were found to be highly correlated.
Taken together, these studies and our own suggest that all confounding factors must be taken into account (T2* correction, T1 correction, and multipeak fat modeling) to obtain the PDFF—the only biomarker that provides consistent estimates of liver fat across platforms and parameters. In our work we have shown that T1 correction can be performed even if acquiring T1-weighted images. Finally, our results also indicate that adequate T2* correction can be accomplished with the use of only three echoes.
This study has some limitations. We deliberately did not compare chemical shift-encoded MRI with histopathology since earlier results indicate that, while there is excellent correlation between histopathology and MRI, absolute fat content estimates are different because MRI and histology measure fundamentally different metrics (22). To directly compare histologic fat determination with the results of noninvasive MRI techniques, it is necessary to perform calibration (49). For this calibration step to be applicable across platforms and parameters, the noninvasive techniques must yield consistent results. This underlines the clinical relevance of our study. The second limitation is that 1H MRS is subject to sampling error, meaning that the reference standard may have yielded liver fat estimates that are not representative of the whole liver. We minimized this error by colocalizing the MRS voxel with the MR images in the same segment. Another limitation is that the echo times are not equally spaced in the three-echo sequence used in this study. It may be possible to use shorter echo times (especially a second opposed-phase instead of a second in-phase echo time) and reduce overall scan time. The number of echoes and echo times necessary for accurate fat estimates should be optimized in a future study.
In conclusion, complex three-echo 3D chemical shift-encoded MRI demonstrates excellent correlation and agreement with single-voxel 1H MRS for quantifying liver fat. Equivalence between the two noninvasive modalities is only obtained if chemical shift-encoded MRI is performed with correction for confounding relaxation processes (T2* and T1 effects) and use of spectral modeling of liver fat since these confounders can bias fat fraction measurement. These corrections can be applied by postprocessing after data acquisition.
Acknowledgments
This work is part of the research project Greifswald Approach to Individualized Medicine (GANI_MED). The GANI_MED consortium is funded by the German Federal Ministry of Education and Research (FKZ 03IS2061A) and the Ministry of Cultural Affairs of the Federal State of Mecklenburg – West Pomerania (UG 09 033).
REFERENCES
- 1.Harrison SA, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Liver Dis. 2004;8:861–879. ix. doi: 10.1016/j.cld.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 3.Schwimmer JB. Definitive diagnosis and assessment of risk for nonalcoholic fatty liver disease in children and adolescents. Semin Liver Dis. 2007;27:312–318. doi: 10.1055/s-2007-985075. [DOI] [PubMed] [Google Scholar]
- 4.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 5.Stadlmayr A, Aigner E, Steger B, et al. Nonalcoholic fatty liver disease: an independent risk factor for colorectal neoplasia. J Intern Med. 2011;270:41–49. doi: 10.1111/j.1365-2796.2011.02377.x. [DOI] [PubMed] [Google Scholar]
- 6.Hui JM, Kench JG, Chitturi S, et al. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38:420–427. doi: 10.1053/jhep.2003.50320. [DOI] [PubMed] [Google Scholar]
- 7.Schindhelm RK, Diamant M, Heine RJ. Nonalcoholic fatty liver disease and cardiovascular disease risk. Curr Diab Rep. 2007;7:181–187. doi: 10.1007/s11892-007-0030-6. [DOI] [PubMed] [Google Scholar]
- 8.Kim D, Choi S-Y, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–613. doi: 10.1002/hep.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meisamy S, Hines CDG, Hamilton G, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767–775. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumeister SE, Völzke H, Marschall P, et al. Impact of fatty liver disease on health care utilization and costs in a general population: a 5-year observation. Gastroenterology. 2008;134:85–94. doi: 10.1053/j.gastro.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Ratziu V, Bugianesi E, Dixon J, et al. Histological progression of non-alcoholic fatty liver disease: a critical reassessment based on liver sampling variability. Aliment Pharmacol Ther. 2007;26:821–830. doi: 10.1111/j.1365-2036.2007.03425.x. [DOI] [PubMed] [Google Scholar]
- 12.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 13.Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology. 1978;74:103–106. [PubMed] [Google Scholar]
- 14.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 15.Pineda N, Sharma P, Xu Q, Hu X, Vos M, Martin DR. Measurement of hepatic lipid: high-speed T2-corrected multiecho acquisition at 1H MR spectroscopy—a rapid and accurate technique. Radiology. 2009;252:568–576. doi: 10.1148/radiol.2523082084. [DOI] [PubMed] [Google Scholar]
- 16.Hines CDG, Yu H, Shimakawa A, et al. Quantification of hepatic steatosis with 3-T MR imaging: validation in ob/ob mice. Radiology. 2010;254:119–128. doi: 10.1148/radiol.09090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C-Y, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354–364. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 18.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60:1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, McKenzie CA, Shimakawa A, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging. 2007;26:1153–1161. doi: 10.1002/jmri.21090. [DOI] [PubMed] [Google Scholar]
- 20.Bydder M, Yokoo T, Hamilton G, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26:347–359. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeder SB, Sirlin CB. Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2010;18:337–357. ix. doi: 10.1016/j.mric.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized mr-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36:1011–1014. doi: 10.1002/jmri.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoo T, Shiehmorteza M, Hamilton G, et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258:749–759. doi: 10.1148/radiol.10100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokoo T, Bydder M, Hamilton G, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T1. Radiology. 2009;251:67–76. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang JS, Taouli B, Salibi N, Hecht EM, Chin DG, Lee VS. Opposed-phase MRI for fat quantification in fat-water phantoms with 1H MR spectroscopy to resolve ambiguity of fat or water dominance. AJR Am J Roentgenol. 2006;187:W103–W106. doi: 10.2214/AJR.05.0695. [DOI] [PubMed] [Google Scholar]
- 26.de Bazelaire CMJ, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology. 2004;230:652–659. doi: 10.1148/radiol.2303021331. [DOI] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 28.Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007;189:W320–W323. doi: 10.2214/AJR.07.2123. [DOI] [PubMed] [Google Scholar]
- 30.Xia M-F, Yan H-M, He W-Y, et al. Standardized ultrasound hepatic/renal ratio and hepatic attenuation rate to quantify liver fat content: an improvement method. Obesity (Silver Spring) 2012;20:444–452. doi: 10.1038/oby.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061–1067. doi: 10.1016/j.jhep.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longo R, Pollesello P, Ricci C, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995;5:281–285. doi: 10.1002/jmri.1880050311. [DOI] [PubMed] [Google Scholar]
- 33.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–749. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazhar SM, Shiehmorteza M, Sirlin CB. Noninvasive assessment of hepatic steatosis. Clin Gastroenterol Hepatol. 2009;7:135–140. doi: 10.1016/j.cgh.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merkle EM, Nelson RC. Dual gradient-echo in-phase and opposed-phase hepatic MR imaging: a useful tool for evaluating more than fatty infiltration or fatty sparing. Radiographics. 2006;26:1409–1418. doi: 10.1148/rg.265055711. [DOI] [PubMed] [Google Scholar]
- 36.Hussain HK, Chenevert TL, Londy FJ, et al. Hepatic fat fraction: MR imaging for quantitative measurement and display—early experience. Radiology. 2005;237:1048–1055. doi: 10.1148/radiol.2373041639. [DOI] [PubMed] [Google Scholar]
- 37.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 38.Wood JC. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hankins JS, McCarville MB, Loeffler RB, et al. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood. 2009;113:4853–4855. doi: 10.1182/blood-2008-12-191643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonkovsky HL, Jawaid Q, Tortorelli K, et al. Non-alcoholic steatohepatitis and iron: increased prevalence of mutations of the HFE gene in non-alcoholic steatohepatitis. J Hepatol. 1999;31:421–429. doi: 10.1016/s0168-8278(99)80032-4. [DOI] [PubMed] [Google Scholar]
- 41.George DK, Goldwurm S, MacDonald GA, et al. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311–318. doi: 10.1016/s0016-5085(98)70482-2. [DOI] [PubMed] [Google Scholar]
- 42.Westphalen ACA, Qayyum A, Yeh BM, et al. Liver fat: effect of hepatic iron deposition on evaluation with opposed-phase MR imaging. Radiology. 2007;242:450–455. doi: 10.1148/radiol.2422052024. [DOI] [PubMed] [Google Scholar]
- 43.Schwenzer NF, Machann J, Haap MM, et al. T2* relaxometry in liver, pancreas, and spleen in a healthy cohort of one hundred twenty-nine subjects-correlation with age, gender, and serum ferritin. Invest Radiol. 2008;43:854–860. doi: 10.1097/RLI.0b013e3181862413. [DOI] [PubMed] [Google Scholar]
- 44.Reeder SB, Robson PM, Yu H, et al. Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging. 2009;29:1332–1339. doi: 10.1002/jmri.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276(5 Pt 1):E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 46.Thomsen C, Becker U, Winkler K, Christoffersen P, Jensen M, Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12:487–495. doi: 10.1016/0730-725x(94)92543-7. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton G, Yokoo T, Bydder M, et al. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed. 2011;24:784–790. doi: 10.1002/nbm.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang GH, Cruite I, Shiehmorteza M, et al. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging. 2011;34:928–934. doi: 10.1002/jmri.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuehn J-P, Evert M, Friedrich N, et al. Noninvasive quantification of hepatic fat content using three-echo Dixon magnetic resonance imaging with correction for T2* relaxation effects. Invest Radiol. 2011;46:783–789. doi: 10.1097/RLI.0b013e31822b124c. [DOI] [PubMed] [Google Scholar]