Abstract
The present study investigated the possible role of miR-21, a miRNA that has known prosurvival function, in poor outcomes in the elderly following traumatic brain injury compared to adults. Controlled cortical impact injury was induced in adult (5–6 months) and aged (22–24 months) C57/BL6 mice. miR-21 and four of its targets (PDCD4, TIMP3, RECK, PTEN) were analyzed at 1, 3, 7 days post injury in samples of injured cortex using real-time PCR analysis. Basal miR-21 expression was higher in the aged brain than in the adult brain. In the adult brain,miR-21 expression increased in response to injury, with the maximum increase 24 hours after injury followed by a gradual decrease, returning to baseline 7 days post-injury. In contrast, in aged mice, miR21 showed no injury response, and expression of miR-21 target genes (PTEN, PDCD4, RECK, TIMP3) was up-regulated at all post injury time points, with a maximal increase at 24 hours post injury. Based on these results, we conclude that the diminished miR21 injury response in the aged brain leads to up-regulation of its targets, with the potential to contribute to the poor prognosis following TBI in aging brain. Therefore, strategies aimed at up-regulation of miR-21 and/or down regulation of its targets might be useful in improving outcomes in the elderly following TBI.
Keywords: Aging, miR-21, PDCD4, PTEN, RECK, Traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) is a complex and heterogeneous disorder that will surpass many diseases as a major cause of death and disability by the year 2020 (Hyder et al., 2007). It is a particularly serious problem in the geriatric population and in the last decade, there has been a 21% increase in TBI events in individuals over the age of 65 (Adekoya et al., 2002). Age is also an important factor influencing prognosis after TBI. In the elderly the outcomes are notoriously poor, recovery is incomplete and unsatisfactory, and the mortality and functional disability rates are twice those of younger patients (Jacobs, 2003). Worsened outcomes after TBI in the elderly suggest that aging alters the brain’s responses to injury. Our previous studies have demonstrated that several aspects of the response to brain injury are altered in aged mice. Behavioral outcomes are worse, inflammatory responses to injury are increased and neuroprotective responses are decreased (Anderson et al., 2009; Onyszchuk et al., 2008b; Sandhir and Berman, 2010; Sandhir et al., 2004, 2008). Recently, we have shown that there is increased blood brain barrier permeability in the aged brain following injury which is accompanied by increased matrix metalloproteinase-9 (MMP-9) activation and decreased blood brain barrier repair responses (Lee et al., 2012). In particular, expression of several pro-inflammatory mediators is increased (Sandhir et al., 2004). All of these changes are likely to result from altered gene and protein expression patterns after injury. Recently, small non-coding RNAs have been shown to have wide ranging effects on the translation of many proteins/function of cells (Tran and Hutvagner, 2013). One mechanism that could produce changes in many downstream events is the alteration in microRNA (miR) expression following CNS trauma.
miRNAs are small, non-coding RNAs that regulate gene expression at the post-transcriptional level (Bushati and Cohen, 2007). miRNAs are important regulators of diverse biological processes such as cell differentiation, growth, proliferation and apoptosis (Tran and Hutvagner, 2013). Recent studies have demonstrated that miRNAs are highly expressed in the nervous system (Petri et al., 2014). miRNAs have been shown to play key role in neurogenesis and neurodegeneration (Im and Kenny, 2012). Accumulating evidence suggests that miRNAs are altered in various neurological disorders such as Parkinson’s disease (Filatova et al., 2012), Alzheimer’s disease (Delay et al., 2012), stroke (Yin et al., 2014), and Down’s syndrome (Xu et al., 2013). Microarray analysis has revealed several miRNAs are dysregulated following TBI (Lei et al., 2009). Of the miRNAs that are altered following TBI, miR-21 has been shown to be globally and consistently up-regulated after TBI. Further, Rendell et al (Redell et al., 2011) have also observed increased expression of miR-21 along with altered expression of its targets after TBI. miR-21 plays a key role in a plethora of biological functions that might influence outcome of TBI. Therefore, the present study has been designed to examine the role of miR-21 and its targets [phosphatase and tensin homolog (PTEN); programmed cell death 4 (PDCD4); reversion-inducing-cysteine-rich protein with kazal motifs (RECK); and tissue inhibitor of metalloproteinases-3 (TIMP3)] in poor outcomes following TBI in aged mice.
2. Materials and methods
2.1. Animals
C57BL/6 male mice were obtained from Charles River (Wilmington, MA) from the contracted colony maintained by the National Institute on Aging (NIA) in a specific pathogen-free (SPF) barrier facility. Two age groups of mice were used: 5–6 months (adult) and 22–24 months (aged). Body weights of the mice ranged between 28 and 32 grams. Animals were housed individually in the AAALAC-accredited Laboratory Animal Resources (LAR) of the University of Kansas Medical Center on a 12:12 h light/dark cycle, with free access to standard rodent diet (8604, Harlan Teklad Laboratories, Madison, WI) and water. All the procedures were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee, and adhered to the ‘NIH Guide for the Care and Use of Laboratory Animals’. A total 48 mice were used were used in the study (adult n = 24 and aged n = 24).
2.2. Traumatic brain injury
The injury device and surgical procedures used for administration of controlled cortical impact (CCI) injury were performed as previously described (Onyszchuk et al., 2007). Briefly, mice were anesthetized with 4% isoflurane in 78% nitrogen, and 21% oxygen, placed in a stereotactic frame and maintained on 1–2% isoflurane with mask ventilation and spontaneous breathing. Breathing and temperature were monitored throughout surgery. After midline incision, craniotomy (approximately 3.5 mm diameter) was made lateral to the mid-sagittal suture, with center coordinates: AP = 0, ML = +2.0 rostral to bregma. The mouse was subjected to a moderate TBI by the controlled cortical impact (CCI) device, with tip 3.0mmin diameter, compression depth of 1.0 mm, velocity of 1.5m/sec, and contact time of 85 ms. The tip contact area included motor (M1, M2) and sensory (S1FL, S1HL) cortical areas. After surgery the scalp was reapproximated and sutured, anesthesia was discontinued and animals allowed to recover in a temperature-controlled environment. The animals were sacrificed 1, 3, 7 days post-injury. There was no mortality in any of the groups.
2.3. RNA isolation
Animals were anesthetized and decapitated, brains were removed and placed in RNAlater (Ambion, Austin, TX) for preservation of RNA. Ipsilateral cortex was dissected into a coronal block extending 3mm rostral and 3 mm caudal from the injury epicenter using a mouse brain matrix (Harvard Apparatus, Holliston, MA). This block was further dissected into a 6 mm wide sample centered on the injury epicenter. The tissue sampled by this method is comparable in adult and aged mice because they demonstrate the same lesion volume following TBI using this injury paradigm (Onyszchuk et al., 2008a). Total RNA was prepared using mirVana miRNA Isolation kit (Ambion, Austin, TX). Purity and concentration of the samples were assessed using a Bioanalyzer 2100 (Agilent, Santa Clara CA).
2.4. miRNAs assays
The levels of miR-21 and sno-202 were analyzed by Taqman microRNA assay kits (Invitrogen, Carlsbad, CA, USA) as described by the manufacturer using ABI 7500 Real-time PCR system (Applied Biosystems, Foster City, CA, USA). sno-202 was used as normalization control (Brattelid et al., 2011). Relative gene expression was determined by the delta-delta CT method (Pfaffl et al., 2002).
2.5. miR-21 targets
Specific quantitative assays for mRNAs for miR-21 targets were carried out using SYBR Green dye. The sequences of all the primers pairs used are given in Table 1. RNA samples were treated with DNase to eliminate potential genomic DNA contamination. Complementary DNA (cDNA) was synthesized by using 1 µg total RNA from each sample and random hexamers in a Taqman reverse transcription reaction (Applied Biosystems, Foster City, CA, USA). 10 ng cDNA and gene-specific primers were added to SYBR Green PCR Master Mix (SYBR Green I Dye, AmpliTaq-DNA polymerase, dNTPs mixture dUTP and optimal buffer components (Applied Biosystems, Foster City, CA), and subjected to PCR amplification (one cycle at 50 °C for 2 min, one cycle at 95 °C for 10 min, and 40 cycles at 95 °C for 15 s and 60 °C for 1 min). Real time PCR was performed on an ABI 7500 Real-time PCR system (Applied Biosystems, Foster City, CA, USA). The data were collected and analyzed with Sequence Detection Software 2.0 (Applied Biosystems, Foster City, CA). The resulting amplicon products were visualized on an agarose gel to verify size and specificity of RT-PCR reaction. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene. Relative gene expression was determined using the delta-delta CT method (Pfaffl et al., 2002).
Table 1.
Primer sequences used for real-time RT-PCR.
| Gene | Primer sequence | Position | Amplicon (bp) | |
|---|---|---|---|---|
| PTEN (NM 008960) | sense | 5′- AGT-TTG-TGG-TCT-GCC-AGC-TAA -3′ | 1509–1529 | 219 |
| antisense | 5′- AGG-TTT-CCT-CTG-GTC-CTG-GTA -3′ | 1707–1727 | ||
| PDCD4 (NM011050) | sense | 5′-GTG-TAT-GAT-GTG-GAA-GAG-GTG-G -3′ | 678–699 | 95 |
| antisense | 5′-TCA-TCC-AGG-GGC-AAA-ACT-ACA-G -3′ | 751–772 | ||
| RECK (NM007679) | sense | 5′- CTC-CAG-CAG-TCT-CCC-GTC-AT-3′ | 2884–2903 | 120 |
| antisense | 5′-GTT-GTG-GGT-GGT-CAG-GGT-CTA -3′ | 2983–3003 | ||
| TIMP3 (NM011595) | sense | 5′- CCT-TTG-GCA-CTC-TGG-TCT-ACA -3′ | 609–629 | 209 |
| antisense | 5′- GTC-CCA-CCT-CTC-CAC-AAA-GTT -3′ | 797–817 | ||
| GAPDH (XM001478544) | sense | 5′-ATG-ACA-TCA-AGA-AGG-TGG-TG-3′ | 839–858 | 177 |
| antisense | 5′-CAT-ACC-AGG-AAA-TGA-GCT-TG-3′ | 996–1015 |
2.6. Statistical analysis
Significant group differences were determined by a one-way analysis of variance (ANOVA), followed by a post-hoc analysis using the Student–Newman–Keuls test. In all cases, p value of < 0.05 was considered significant. Results were expressed as the mean ± standard error of mean (S.E.M).
3. Results
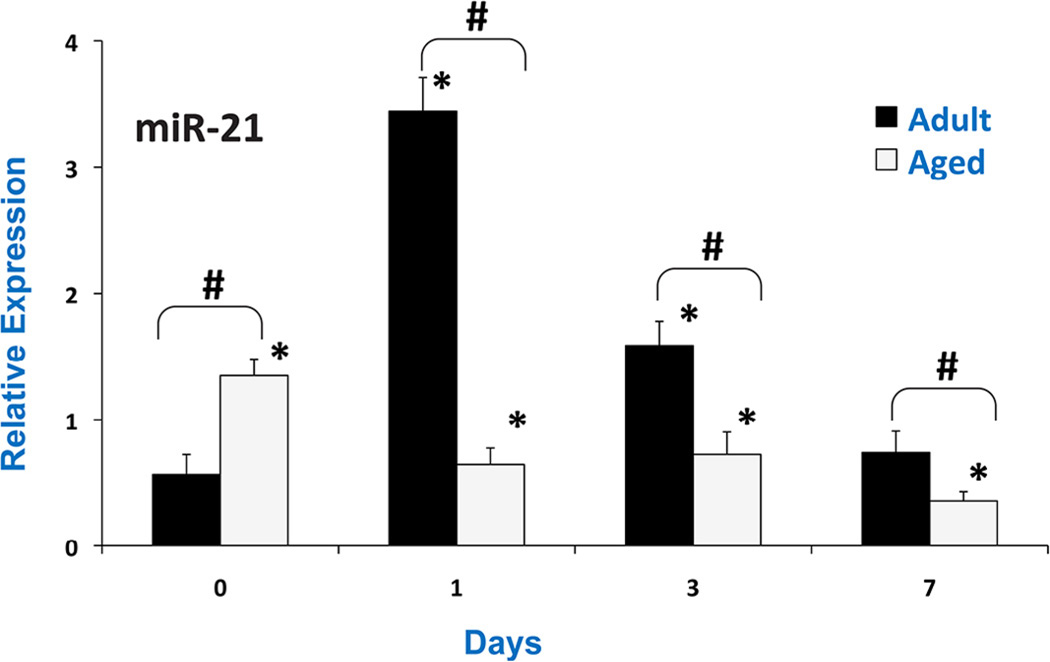
miR-21, a special miRNA that has known which can increase cell proliferation and reduce apoptotic cell death by regulating cell cycle and apoptosis-related genes, was investigated following controlled cortical impact injury in adult (5–6 months) and aged (22–24 months) mice. miR-21 and four of its targets were analyzed at 1, 3, 7 days post injury in samples of injured cortex using real-time PCR analysis. A significantly higher basal expression of miR-21 was observed in the aged mice brain compared to adult (Fig. 1). In addition, miR-21 levels increased following TBI in the adult mice, with the maximum increase at 24 hours post-injury. However, in the aged mice miR-21 expression was decreased in brain after TBI.
Fig. 1.
Relative miR-21 expression in pericontusional cortex after CCI in adult and aged mice analyzed by real-time RT-PCR at days 1, 3 and 7 post-injury. The real time reactions were performed in triplicate for miR-21 and sno202 used as a housekeeping control. The relative expression was calculated using delta delta CT method. Values are mean ± S.E.M. (N = 6/group). # indicates significant difference between injured vs. controls (p < 0.05), * indicates significant difference from aged vs. adult animals (p < 0.05).
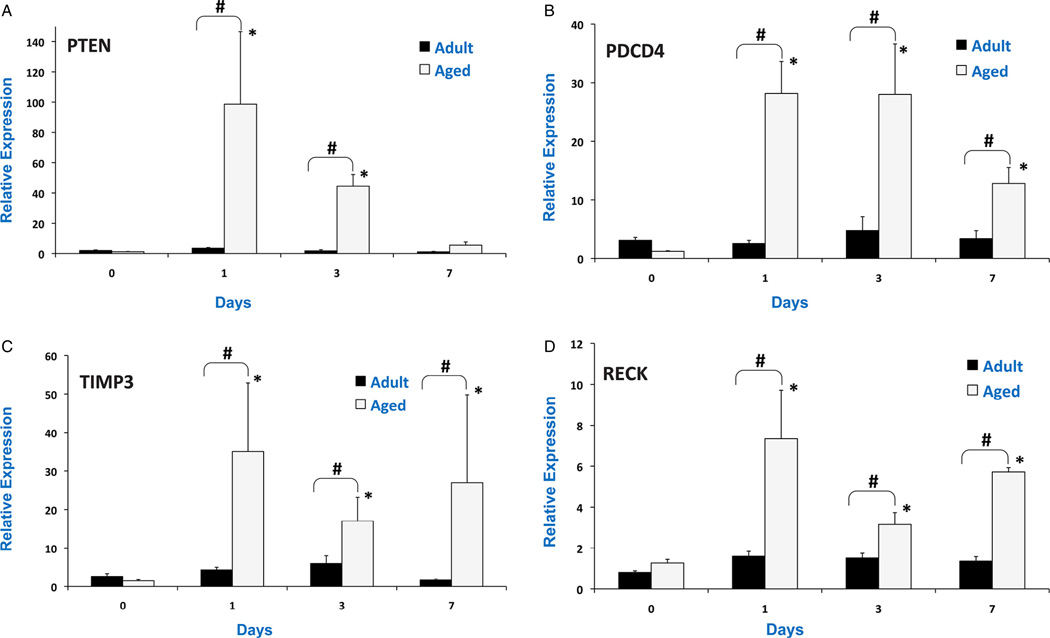
miR-21 regulates cellular survival, invasiveness and apoptosis through specific targets that include PTEN, PDCD4, RECK and TIMP3. It was observed that miR-21 targets were highly up-regulated after TBI in aged mice (Fig. 2). PTEN mRNA was up-regulated by 3.8 fold in the adult brain 24 hours post-injury. However, the expression was 98 fold higher in the aged brain. PCDC4 was not up-regulated in the adult brain, but was up-regulated by 28 fold in the aged brain 24 hours post-injury. TIMP3 and RECK were up-regulated by 20 fold and 6 fold in the aged brain 24 hours injury, whereas no significant effect was observed in adult brain post-injury. The results suggest that miR-21 response was blunted in the aged brain following TBI which resulted in up-regulation of mRNA targets.
Fig. 2.
Relative expression of miR-21 targets: PTEN mRNA (A), PDCD4 mRNA (B), TIMP3 mRNA (C) and RECK mRNA (D) in pericontusional cortex after CCI in adult and aged mice analyzed by real-time RT-PCR at days 1, 3 and 7 post-injury. The real time reactions were performed in triplicate for target mRNAs and GAPDH used as a housekeeping control. The relative expression was calculated using delta delta CT method. Values are mean ± S.E.M. (N = 6/group). # indicates significant difference between injured vs. controls (p < 0.05), * indicates significant difference from aged vs. adult animals (p < 0.05).
4. Discussion
miR-21 has been demonstrated to play an important role in diverse biological and pathological processes, including cell survival, apoptosis and inflammation (Van Wynsberghe et al., 2011). Increased basal expression of miR-21 was observed in aged mice brain compared to adult brain. This is in agreement with the study that miR-21 is increased in aging heart (Zhang et al., 2012). miR-21 levels increased following TBI in the adult mice, with the maximum at 24 hours post-injury, whereas, in the aged mice miR-21 levels decreased after TBI. The increase in expression of miR-21 has been reported following TBI (Redell et al., 2011) and spinal cord injury (Bhalala et al., 2012). miR-21 has also been reported to be involved in the process of wound healing (Wang et al., 2012). Further, diminished expression of miR-21 is associated with delay in wound healing (Wang et al., 2012). Astrocyte activation and reactive gliosis accompany most of the pathologies in the brain including TBI that involves hypertrophy followed by hyperplasia (Sandhir et al., 2008). It is believed that astrocyte activation and glial scar formation is beneficial. It has recently been shown that there is increased miR-21 expression following spinal cord injury correlates with astrocyte hypertrophy (Bhalala et al., 2012). A shift in properties of activated astrocytes from neuroprotective to neurotoxic types with morphological and phenotypic differences has been observed in aging brain. The diminished miR-21 response in aged brain might play a role in altering astrocyte activation after injury to shift the astrocytes from neuroprotective to a more proinflammatory state. It has also been observed that miR-21 is an axotomy-induced miRNA that enhances axon growth, suggesting that it is an important player in regulating growth pathways following peripheral nerve injury (Strickland et al., 2011). These findings suggest that miR-21 is a neuroprotective molecule in TBI (Buller et al., 2010) and neuroprotective astrocyte response is diminished in aged brain. The total lack of a miR-21 injury response in the aged brain is likely to contribute to poor outcomes by diminishing these neuroprotective responses.
miR-21 regulates many target genes that are involved in cellular survival, invasiveness and apoptosis through specific targets of miR-21 that include PTEN, PDCD4, RECK and TIMP3 (Gabriely et al., 2008; Shi et al., 2013). The findings from the present study indicate miR-21 targets were highly up-regulated after TBI in aged mice. The results suggest that increased miR-21 expression in adult brain negatively regulates its mRNA targets by formation of RNA-induced silencing complex (RISC complex). Since there was down regulation of miR-21 in the aged brain following TBI, the levels of its targets were high up-regulated. However, there is a possibility that protein expression of the targets may not increase by as many fold as mRNAs as the translational machinery may be compromised.
PTEN, a tumor suppressor gene that plays role in survival pathway downstream of neuronal growth factors, is a target of miR-21. It has been reported that PTEN inhibition improves neurological recovery following TBI and stroke (Ding et al., 2013; Zhao et al., 2013). A recent study has shown up-regulation of miR-21 reduced neuronal apoptosis via promoting the PTEN-Akt signaling pathway in in vitro TBI model (Han et al., 2014). Furthermore, PTEN inhibition has been reported to promote regenerative outgrowth of adult peripheral axons. The decreased PTEN expression in adult brain following TBI therefore may contribute to improved outcome following TBI, whereas higher expression of PTEN in aged brain following TBI may contribute to higher neuronal loss (Onyszchuk et al., 2008a).
PDCD4 is a miR-21 target involved in reduced cell proliferation and increased apoptosis. The reduced expression of PDCD4 mRNA in adult brain may promote glial cell proliferation. Reduced expression of PDCD4 has also been reported in glioblastomas (Chen et al., 2008). These results suggest that miR-21 dysregulation contributes to increased gliosis and altered inflammatory responses in the aging brain (Onyszchuk et al., 2008; Sandhir et al., 2008; Bi et al., 2009).
miR-21 also controls expression of RECK and TIMP3, key regulators of matrix metalloproteinases (Gabriely et al., 2008). Increased expression of miR-21 leads to down regulation of RECK and TIMP3 in adult brain. We have previously reported higher matrix metalloproteinase activity in aged brain following TBI leading to increased BBB permeability. The increased expression of RECK and TIMP3 in aged brain following TBI might be compensatory response to increased MMP activation that is responsible for disruption of blood brain barrier. Recombinant TIMP3 has been shown to inhibit BBB permeability caused by TBI (Menge et al., 2012). In a study by Wang et al. (2010) up-regulation of RECK in transient cerebral ischemia has been implicated in protection of ECM/tissue integrity and promotion of functional recovery.
It has been reported that serum and other body fluids contain sufficiently stable miRNA signatures with half-life more than 12 days (Brase et al., 2010). The profiles of circulating miRNAs have been explored in a variety of studies aiming at the identification of novel non-invasive biomarkers (Guay and Regazzi, 2013; Rao et al., 2013). Thus, circulating miR-21 may have the potential to be used as a biomarker for the diagnosis and prognosis of TBI.
Based on these results, we conclude that the diminished mi-R21 response in the aged brain leads to up-regulation of its targets, contributing to the poor prognosis following TBI in aging brain. Therefore, strategies aimed at up-regulation of miR-21 and down regulation its targets might be useful in improving outcomes in the elderly following TBI. A recent study has suggested that upregulation of miR-21 in neurons by transfecting with miR-21 agomir reduces apoptosis in an in vitro model of TBI which is mediated through PTEN-AKT signaling (Han et al., 2014).
Acknowledgements
This work was supported by the National Institute of Aging, University of Kansas Alzheimer’s Disease Center and Steve Palermo Endowment.
Abbreviations
- miR
microRNA
- RECK
reversion-inducing-cysteine-rich protein with kazal motifs
- TIMP3
tissue inhibitor of metalloproteinases-3
- PDCD4
programmed cell death 4
- PTEN
phosphatase and tensin homolog
Footnotes
Conflict of interest
None.
References
- Adekoya N, Thurman DJ, White DD, Webb KW. Surveillance for traumatic brain injury deaths – United States, 1989–1998. MMWR Surveill. Summ. 2002;51:1–14. [PubMed] [Google Scholar]
- Anderson J, Sandhir R, Hamilton ES, Berman NE. Impaired expression of neuroprotective molecules in the HIF-1alpha pathway following traumatic brain injury in aged mice. J. Neurotrauma. 2009;26:1557–1566. doi: 10.1089/neu.2008.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, et al. microRNA-21 regulates astrocytic response following spinal cord injury. J. Neurosci. 2012;32:17935–17947. doi: 10.1523/JNEUROSCI.3860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Liu G, Yang R. MicroRNAs: novel regulators during the immune response. J. Cell. Physiol. 2009;218:467–472. doi: 10.1002/jcp.21639. [DOI] [PubMed] [Google Scholar]
- Brase JC, Wuttig D, Kuner R, Sultmann H. Serum microRNAs as non-invasive biomarkers for cancer. Mol. Cancer. 2010;9:306. doi: 10.1186/1476-4598-9-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattelid T, Aarnes EK, Helgeland E, Guvaag S, Eichele H, Jonassen AK. Normalization strategy is critical for the outcome of miRNA expression analyses in the rat heart. Physiol. Genomics. 2011;43:604–610. doi: 10.1152/physiolgenomics.00131.2010. [DOI] [PubMed] [Google Scholar]
- Buller B, Liu X, Wang X, Zhang RL, Zhang L, Hozeska-Solgot A, et al. MicroRNA-21 protects neurons from ischemic death. FEBS J. 2010;277:4299–4307. doi: 10.1111/j.1742-4658.2010.07818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N, Cohen SM. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu W, Chao T, Zhang Y, Yan X, Gong Y, et al. MicroRNA-21 down-regulates the expression of tumor suppressor PDCD4 in human glioblastoma cell T98G. Cancer Lett. 2008;272:197–205. doi: 10.1016/j.canlet.2008.06.034. [DOI] [PubMed] [Google Scholar]
- Delay C, Mandemakers W, Hebert SS. MicroRNAs in Alzheimer’s disease. Neurobiol. Dis. 2012;46:285–290. doi: 10.1016/j.nbd.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Ding J, Guo J, Yuan Q, Yuan F, Chen H, Tian H. Inhibition of phosphatase and tensin homolog deleted on chromosome 10 decreases rat cortical neuron injury and blood-brain barrier permeability, and improves neurological functional recovery in traumatic brain injury model. PLoS ONE. 2013;8:e80429. doi: 10.1371/journal.pone.0080429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatova EV, Alieva A, Shadrina MI, Slominsky PA. MicroRNAs: possible role in pathogenesis of Parkinson’s disease. Biochemistry (Mosc) 2012;77:813–819. doi: 10.1134/S0006297912080020. [DOI] [PubMed] [Google Scholar]
- Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, et al. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol. Cell. Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013;9:513–521. doi: 10.1038/nrendo.2013.86. [DOI] [PubMed] [Google Scholar]
- Han Z, Chen F, Ge X, Tan J, Lei P, Zhang J. miR-21 alleviated apoptosis of cortical neurons through promoting PTEN-Akt signaling pathway in vitro after experimental traumatic brain injury. Brain Res. 2014;1582:12–20. doi: 10.1016/j.brainres.2014.07.045. [DOI] [PubMed] [Google Scholar]
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. Neurorehabilitation. 2007;22:341–353. [PubMed] [Google Scholar]
- Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35:325–334. doi: 10.1016/j.tins.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DG. Special considerations in geriatric injury. Curr. Opin. Crit. Care. 2003;9:535–539. doi: 10.1097/00075198-200312000-00012. [DOI] [PubMed] [Google Scholar]
- Lee P, Kim J, Williams R, Sandhir R, Gregory E, Brooks WM, et al. Effects of aging on blood brain barrier and matrix metalloproteases following controlled cortical impact in mice. Exp. Neurol. 2012;234:50–61. doi: 10.1016/j.expneurol.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei P, Li Y, Chen X, Yang S, Zhang J. Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res. 2009;1284:191–201. doi: 10.1016/j.brainres.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Menge T, Zhao Y, Zhao J, Wataha K, Gerber M, Zhang J, et al. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci. Transl. Med. 2012;4:161ra150. doi: 10.1126/scitranslmed.3004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G, Al-Hafez B, He YY, Bilgen M, Berman NE, Brooks WM. A mouse model of sensorimotor controlled cortical impact: characterization using longitudinal magnetic resonance imaging, behavioral assessments and histology. J. Neurosci. Methods. 2007;160:187–196. doi: 10.1016/j.jneumeth.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J. Neurotrauma. 2008a;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- Petri R, Malmevik J, Fasching L, Akerblom M, Jakobsson J. miRNAs in brain development. Exp. Cell Res. 2014;321:84–89. doi: 10.1016/j.yexcr.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Benito E, Fischer A. MicroRNAs as biomarkers for CNS disease. Front. Mol. Neurosci. 2013;6:39. doi: 10.3389/fnmol.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redell JB, Zhao J, Dash PK. Altered expression of miRNA-21 and its targets in the hippocampus after traumatic brain injury. J. Neurosci. Res. 2011;89:212–221. doi: 10.1002/jnr.22539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Berman NE. Age-dependent response of CCAAT/enhancer binding proteins following traumatic brain injury in mice. Neurochem. Int. 2010;56:188–193. doi: 10.1016/j.neuint.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Puri V, Klein RM, Berman NE. Differential expression of cytokines and chemokines during secondary neuron death following brain injury in old and young mice. Neurosci. Lett. 2004;369:28–32. doi: 10.1016/j.neulet.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp. Neurol. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Zhang J, Qian X, Han L, Zhang K, Chen L, et al. AC1MMYR2, an inhibitor of dicer-mediated biogenesis of Oncomir miR-21, reverses epithelial-mesenchymal transition and suppresses tumor growth and progression. Cancer Res. 2013;73:5519–5531. doi: 10.1158/0008-5472.CAN-13-0280. [DOI] [PubMed] [Google Scholar]
- Strickland IT, Richards L, Holmes FE, Wynick D, Uney JB, Wong LF. Axotomy-induced miR-21 promotes axon growth in adult dorsal root ganglion neurons. PLoS ONE. 2011;6:e23423. doi: 10.1371/journal.pone.0023423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran N, Hutvagner G. Biogenesis and the regulation of the maturation of miRNAs. Essays Biochem. 2013;54:17–28. doi: 10.1042/bse0540017. [DOI] [PubMed] [Google Scholar]
- Van Wynsberghe PM, Chan SP, Slack FJ, Pasquinelli AE. Analysis of microRNA expression and function. Methods Cell Biol. 2011;106:219–252. doi: 10.1016/B978-0-12-544172-8.00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Imamura Y, Ishibashi R, Chandana EP, Yamamoto M, Noda M. The Reck tumor suppressor protein alleviates tissue damage and promotes functional recovery after transient cerebral ischemia in mice. J. Neurochem. 2010;115:385–398. doi: 10.1111/j.1471-4159.2010.06933.x. [DOI] [PubMed] [Google Scholar]
- Wang T, Feng Y, Sun H, Zhang L, Hao L, Shi C, et al. miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am. J. Pathol. 2012;181:1911–1920. doi: 10.1016/j.ajpath.2012.08.022. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li W, Liu X, Chen H, Tan K, Chen Y, et al. Identification of dysregulated microRNAs in lymphocytes from children with Down syndrome. Gene. 2013;530:278–286. doi: 10.1016/j.gene.2013.07.055. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Hamblin M, Chen YE. Non-coding RNAs in cerebral endothelial pathophysiology: emerging roles in stroke. Neurochem. Int. 2014;77C:9–16. doi: 10.1016/j.neuint.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Azhar G, Wei JY. The expression of microRNA and microRNA clusters in the aging heart. PLoS ONE. 2012;7:e34688. doi: 10.1371/journal.pone.0034688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Qu Y, Wu J, Cao M, Ferriero DM, Zhang L, et al. PTEN inhibition prevents rat cortical neuron injury after hypoxia-ischemia. Neuroscience. 2013;238:242–251. doi: 10.1016/j.neuroscience.2013.02.046. [DOI] [PubMed] [Google Scholar]