Abstract
Purpose
Although previous studies have focused on risk factors for osteoarthritis, there is some debate on this issue. Furthermore, associated factors with arthritic symptom (arthralgia) have not been sufficiently investigated, despite its clinical importance in the management of osteoarthritis. This study was performed to examine the risk factors for osteoarthritis and the contributing factors to current arthritic pain in older adults.
Materials and Methods
The Fourth Korean National Health and Nutrition Examination Surveys was conducted in 2009. Therein, 720 males and 1008 females aged 65 years and older were included. Comprehensive data on habitual, socioeconomic, medical, nutritional, and psychological factors were collected along with the presence of osteoarthritis and arthritic pain. After univariate analysis, binary logistic regression analysis was performed to identify risk factors for osteoarthritis and contributing factors to current arthritic pain.
Results
Age (p=0.005), female gender (p<0.001), higher body mass index (BMI) (p<0.001), and osteoporosis (p<0.001) were significant risk factors for osteoarthritis, while higher education level (p=0.025) was a protective factor for osteoarthritis. Higher BMI (p=0.047), lack of weekly moderate intensity activity (p<0.001), and unfavorable subjective health status (p<0.001) were significant factors contributing to current arthritic pain among subjects with osteoarthritis. Both osteoarthritis and current arthritic pain adversely affected health related quality of life.
Conclusion
Higher BMI, lack of weekly moderate intensity activity, and unfavorable subjective health status were significant factors contributing to current arthritic pain. More attention needs to be paid to psychiatric effects on osteoarthritis and joint related pain.
Keywords: Osteoarthritis, joint related pain, risk factors, older adults
INTRODUCTION
Reflecting the impact of osteoarthritis on our lives, numerous studies have set out to investigate the risk factors of the disease.1,2,3,4,5,6,7,8,9,10,11 However, studies have shown somewhat inconsistent or often conflicting results in terms of body mass index (BMI), activity, and the involved anatomic structures.4,12,13,14 This has provoked the necessity of a more comprehensive study to include as many factors as possible to rule out the effect of known and unknown confounding factors in these studies.
Furthermore, there is little known about the contributing factors to current joint-related pain in patients with osteoarthritis, which is the most important symptom caused by osteoarthritis. Joint-related pain usually triggers those with osteoarthritis to seek medical or surgical treatment. Therefore, we need to focus on investigating the contributing factors related to joint-related pain, along with associated factors in osteoarthritis.
We hypothesized that there are various factors associated with osteoarthritis and current arthritic pain in the elderly Korean population. This study was performed to investigate the associated factors for osteoarthritis and the contributing factors to current arthritic pain in older adults using the Fourth Korean National Health and Nutrition Examination Surveys (KNHNES IV) 2009 data.
MATERIALS AND METHODS
Subjects
This cross-sectional study was based on the comprehensive data acquired from the KNHNES IV 2009. The data were publicly available from the Korean Centers for Disease Control and Prevention. The surveys were undertaken from community populations and selected from stratified multistage probability samples of Korean households representing the civilian, non-institutionalized population. The surveys included a health interview survey, a health behavior survey, a health examination survey, and a nutrition survey. In total, 12722 people were invited and 10533 responded, with a response rate of 82.8%.15,16 Of these, the data of the older adults who are aged 65 years and older were included in this study. The Institutional Review Board of Seoul National University Bundang Hospital exempted the need for approval of this study because it did not involve the possibility of violating human rights.
The health interview survey and the health behavior survey included the following: 1) demographics (age, gender, education level, socioeconomic status, and marital status), 2) health habits (smoking, sleeping hours, and weight change during the past one year), 3) medical diseases (past history and current symptom), 4) activity level using short-form international physical activity questionnaire, 5) health related quality of life (evaluated by using EuroQoL), and 6) psychological aspects (subjective health status using a Likert scale, suicidal ideation, stress, and depressive mood).
The health examination survey included height, weight, waist circumference, BMI, blood test (fasting blood sugar, total cholesterol, HDL cholesterol, LDL cholesterol, triglyceride, glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, hemoglobin, hematocrit, ferritin, blood urea nitrogen, white blood cell count, red blood cell count, vitamin D, alkaline phosphatase, and parathyroid hormone, and urine test [urine acidity (pH), urine specific gravity, cotinine, urine creatinine, and urine sodium].
The nutrition survey analyzed the nutritional ingredients of the foods that the subjects had taken the day before using the Food Composition Table developed by the National Rural Resources Development Institute (7th revision), based on a 24-hour dietary recall questionnaire administered by a dietician. The ingredients included total mass of food, total calories, total water, protein, fat, carbohydrates, calcium, phosphorus, iron, sodium, potassium, vitamin A, carotene, retinol, thiamine (vitamin B1), riboflavin (vitamin B2), niacin (vitamin B3), and vitamin C.
Definition of groups and subgroups
The questionnaire regarding osteoarthritis included presence of osteoarthritis diagnosed by a physician and presence of current arthritic pain. Based on these questions, the subjects were categorized into the osteoarthritis group and non-osteoarthritis group, and the osteoarthritis group was further subgrouped into current arthritic pain and no-current arthritic pain groups.
Statistical analysis
A descriptive analysis was performed for all the variables, including the mean and standard deviation or frequency. Data normality was tested using the Kolmogorov-Smirnov test. Student's t-test was utilized for comparison of continuous variables and chi-square test was used for comparison of categorical variables between the two groups.
The demographic data and medial diseases were compared and analyzed between the osteoarthritis and non-osteoarthritis groups. The variables (continuous variables) that were significantly different or associated (categorical variables) between the groups were subsequently included in the binary logistic regression analysis to investigate the significant associated factors for osteoarthritis.
A subgroup analysis of the osteoarthritis group was performed based on current arthritic pain. The variables that were significantly different or associated between the current and no-current arthritic pain subgroups were included in the binary logistic regression analysis to investigate the significant contributing factors to the current arthritic pain.
Each subscale of the EuroQoL and EuroQoL visual analogue scale (VAS) was compared between the osteoarthritis and non-osteoarthritis groups, as well as between the current and no-current arthritic pain subgroups.
All statistical analyses were performed using SPSS software, version 18.0 (IBM Corporation, Armonk, NY, USA), with statistical significance set at p<0.05.
RESULTS
Data from 1728 subjects aged >65 years were selected from the overall dataset. Of these subjects, 58 with incomplete data were excluded, and 1670 subjects were finally included in the data analysis. Mean age of the subjects was 72.7 years (SD 5.7, range from 65 years to 95 years), and there were 720 males and 1008 females. Of these, 476 were diagnosed with osteoarthritis.
In comparing demographics and medical diseases between the osteoarthritis group and non-osteoarthritis group, there were significant differences in gender (p<0.001), height (p<0.001), waist circumference (p<0.001), BMI (p<0.001), education level (p<0.001), marital status (p<0.001), the amount of previous smoking (p<0.001), hypertension (p<0.001), and osteoporosis (p<0.001) (Table 1).
Table 1.
Comparison of Demographics and Medial Diseases between the Osteoarthritis Group and Non-Osteoarthritis Group
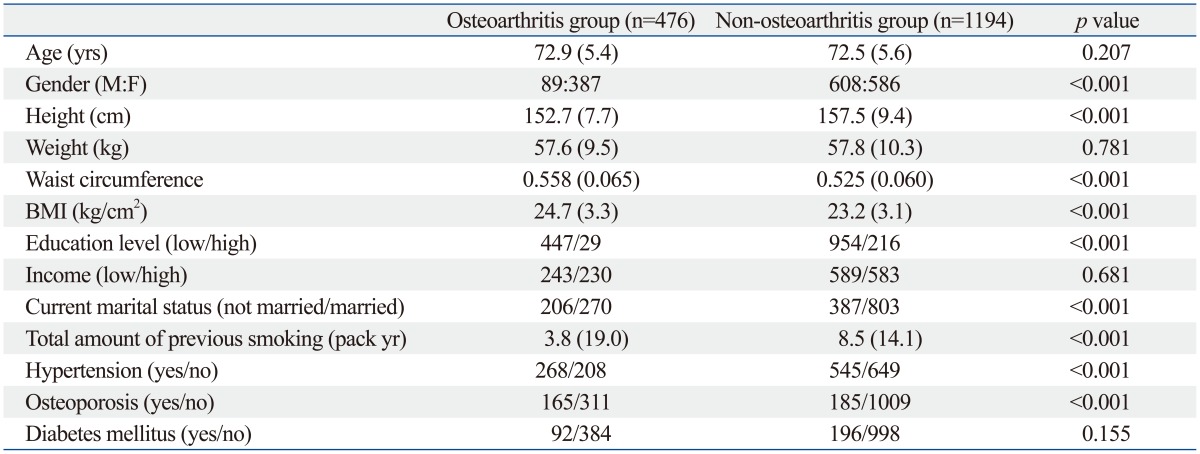
BMI, body mass index.
Data are presented as mean (SD). Waist circumference was normalized by dividing with height.
In binary logistic regression analysis, the variables that were significantly different between the osteoarthritis and non-osteoarthritis groups were included except for height and waist circumference because these two were represented by BMI. Age was included in logistic regression analysis since it is a well known risk factor for osteoarthritis. The binary logistic regression analysis showed that older age (p=0.005), female gender (p<0.001), higher BMI (p<0.001), and osteoporosis (p<0.001) were significant factors associated with osteoarthritis, while higher education level (p=0.025) was a significant protective factor for osteoarthritis (Table 2).
Table 2.
Binary Logistic Regression Analysis Evaluating Associated Factors for Osteoarthritis
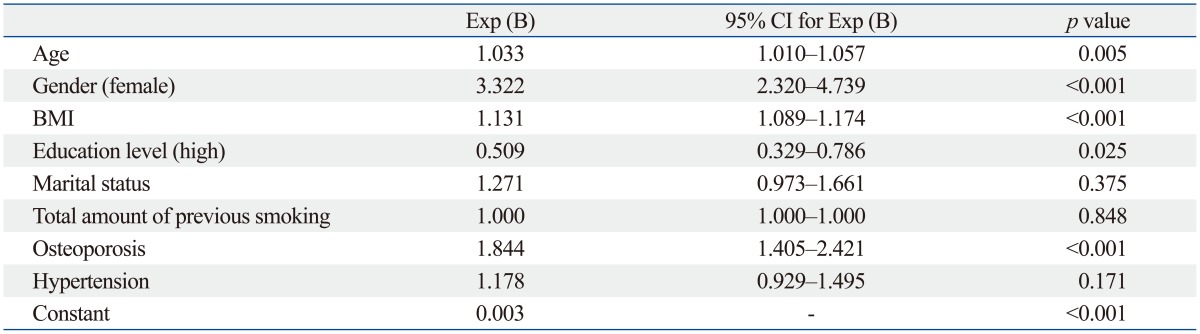
BMI, body mass index; CI, confidence interval.
Among the osteoarthritis group, current arthritic pain subgroup (n=450) and no-current arthritic pain subgroup (n=26) showed significant differences in BMI (p=0.031), proportion of hypertension (p=0.022), weekly moderate intensity activity hours (p=0.033), stress (p=0.048), and subjective health status (p<0.001). There were no significantly different laboratory tests and nutrition intake between the current arthritic pain and no-current arthritic pain subgroups (Table 3).
Table 3.
Comparison of Demographics, Current Health Habits, Current Laboratory Tests, and Current Nutrition Intake between the Current Pain Subgroup and No-Current Pain Subgroup within the Osteoarthritis Group
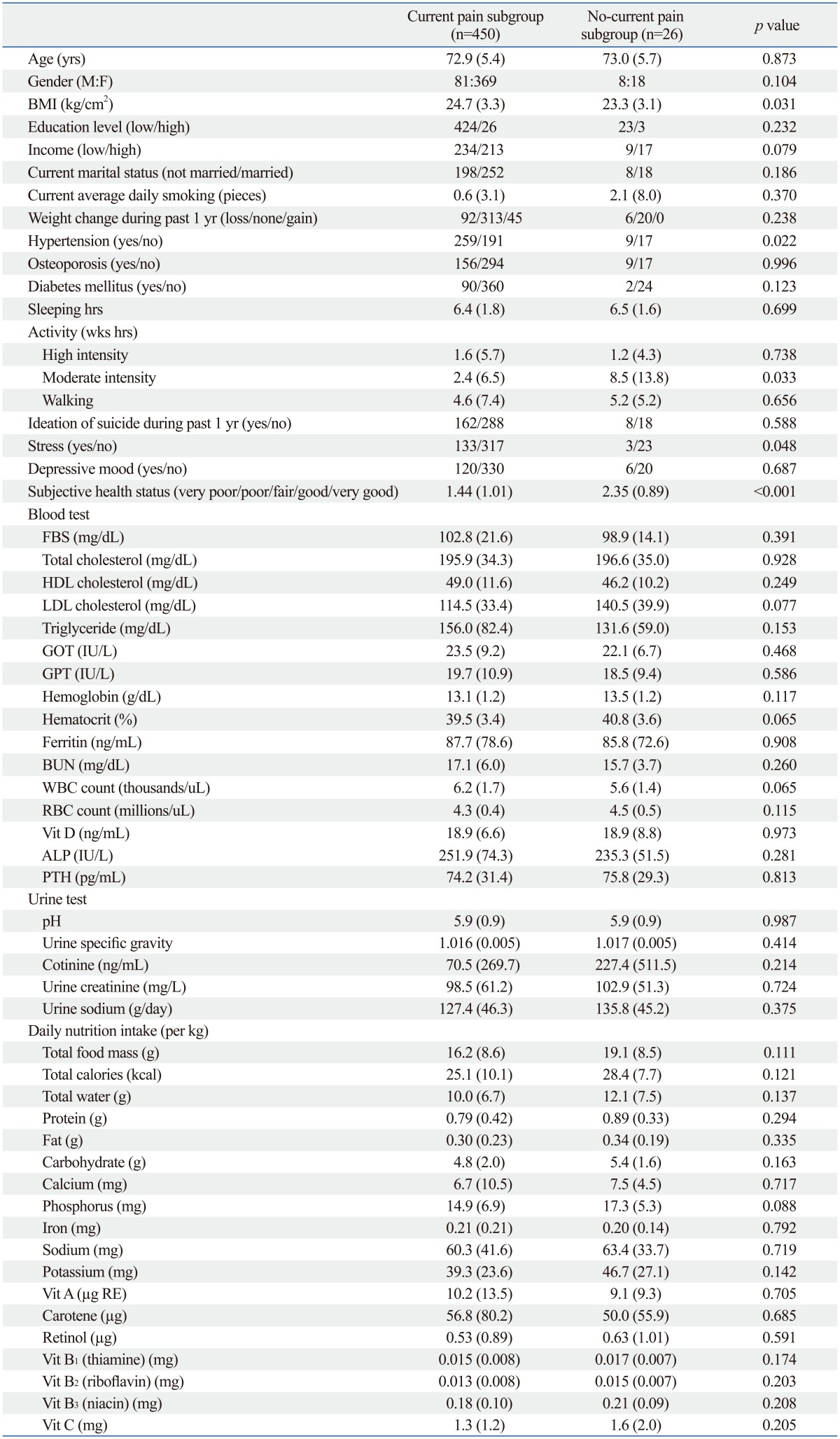
BMI, body mass index; FBS, fasting blood sugar; Vit, vitamin; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; BUN, blood urea nitrogen; WBC, white blood cell; RBC, red blood cell; ALP, alkaline phosphatase; PTH, parathyroid hormone.
Data are presented as mean (SD). Waist circumference was normalized by dividing with height. Nutrition intake was normalized by dividing with body weight.
Binary logistic regression analysis showed that higher BMI (p=0.047), less weekly moderate intensity activity (p<0.001), and unfavorable subjective health status (p<0.001) were significant factors contributing to current arthritic pain among the subjects with osteoarthritis (Table 4).
Table 4.
Binary Logistic Regression Analysis for Factors Contributing to Current Joint Related Pain in Osteoarthritis Group
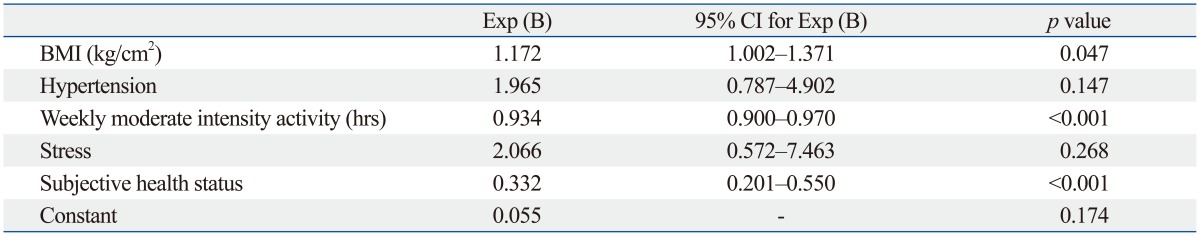
BMI, body mass index; CI, confidence interval.
Both osteoarthritis and current arthritic pain adversely affected health related quality of life (Table 5 and 6). However, the self-care subscale of EuroQoL was not significantly different according to the presence of current arthritic pain.
Table 5.
Comparison of Health-Related Quality of Life between the Osteoarthritis Group and Non-Osteoarthritis Group
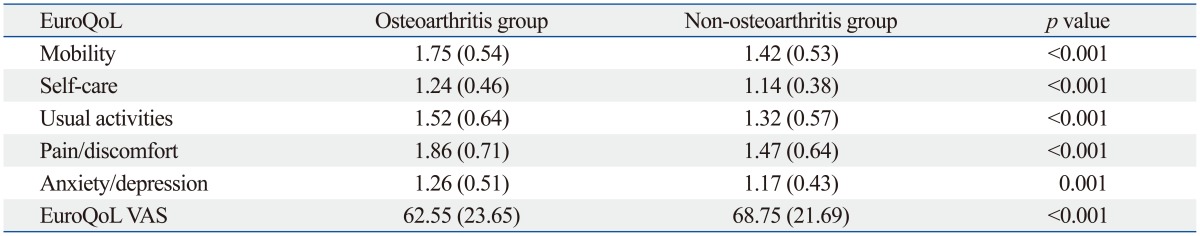
VAS, visual analogue scale.
Data are presented as mean (SD). Higher scores are more unfavorable in each subscale of EuroQoL and more favorable in EuroQoL VAS.
Table 6.
Comparison of Health-Related Quality of Life between the Current Pain Subgroup and the No-Current Pain Subgroup within the Osteoarthritis Group
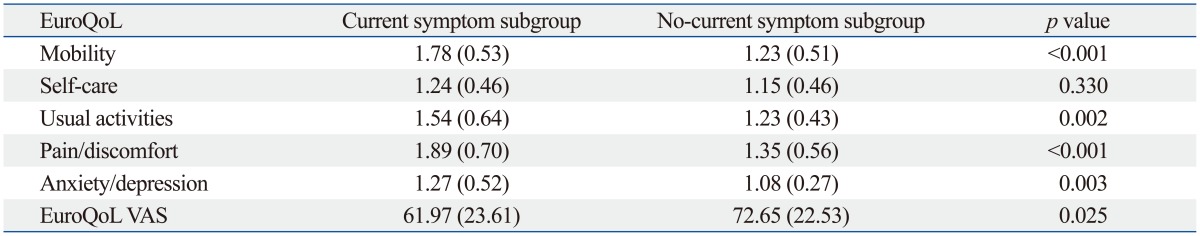
VAS, visual analogue scale.
Data are presented as mean (SD). Higher scores are more unfavorable in each subscale of EuroQoL and more favorable in EuroQoL VAS.
DISCUSSION
This study investigated the associated factors for osteoarthritis and contributing factors to current arthritic pain in community-based older population. Older age (p=0.005), female gender (p<0.001), higher BMI (p<0.001), and osteoporosis (p<0.001) were found to be significant factors associated with osteoarthritis, while higher education level (p=0.025) was a significant protective factor against osteoarthritis. Higher BMI (p=0.047), less weekly moderate intensity activity (p<0.001), and unfavorable subjective health status (p<0.001) were found to be significant contributing factors to current arthritic pain among the older adults with osteoarthritis. Presence of osteoarthritis and current arthritic pain adversely affected health related quality of life.
Female gender, age, and BMI are well known risk factors for osteoarthritis, as shown in previous studies,2,3,4,6,7,9,11,14 as well as in this study. A low level of education was also found to be a significant factor associated with osteoarthritis from our data. We do not know if subjects with a lower level of education were involved in work requiring more physical labor or joint loading, although it is a possible explanation since overload on the joints could be a risk factor for osteoarthritis.1,5,8,17
Osteoporosis was another factor associated with osteoarthritis in this study. The relationship between osteoarthritis and osteoporosis has been somewhat controversial. There is a clinical implication that osteoarthritis and osteoporosis might have opposite effects on each other,18,19 as an increased physical load on the skeletal structure is thought to be a risk factor for osteoarthritis, while also a protective factor for osteoporosis.1,2,5,8,17,20 On the other hand, age and female gender are concurrent risk factors for both osteoarthritis and osteoporosis.2,20,21,22 Our logistic regression model showed that osteoporosis is an associated factor for osteoarthritis, when adjusted for age and gender, which concurred with previous studies.23,24 A recent trial showed that osteoporosis medication improved arthritic pain but failed to prevent structural progression of hip osteoarthritis.25 Therefore, this issue is still inconclusive and needs further investigation.
Higher BMI was another significant factor associated with osteoarthritis and a contributing factor to current arthritic pain at the same time. Overweight is thought to cause osteoarthritis by increasing the load exerted on the joints.26,27 However, it is also known to be associated with hand osteoarthritis, where being overweight does not necessarily cause increased joint load.28,29,30 Previous studies have suggested that systemic and metabolic factors associated with overweight might play a role in inflammatory processes.31,32 Weight control is believed to be a treatable issue for both osteoarthritis and current arthritic pain. In our data, no subject in the no-current arthritic pain subgroup showed weight gain during the past 1 year (Table 3), which supports this concept.
The amount of moderate intensity activity on a weekly basis was found to be significantly associated with current arthritic pain among the subjects with osteoarthritis. Although it was difficult to ascertain whether the reduced weekly amount of moderate activity in symptomatic subgroup was the cause or the result in this cross-sectional study, previous studies have suggested that an appropriate physical activity is beneficial for functioning.33,34,35 We believe that moderate intensity activity is more beneficial for maintaining muscle mass than walking, and does not cause joint overload, compared with high intensity activity. Therefore, moderate intensity activity could be cautiously recommended for those with osteoarthritis to reduce their arthritis-related joint pain; a well-designed longitudinal study is needed to clarify this issue.
Studies have suggested that nutrition plays a role in osteoarthritis.36,37,39 For example, antioxidants were found to reduce the progression of osteoarthritis, although they did not significantly reduce the initiation of osteoarthritis.37 In addition, some nutritional factors have been found to exhibit a relationship with the inflammatory process and modulating chronic pain.39,40,41 However, this study failed to reveal the role of nutrition intake in current arthritis pain among older adults with osteoarthritis. Our data on nutritional intake were based on the recall of food intake during the past day. A more intensive and detailed study design would be needed to investigate the relationship between osteoarthritis and nutritional state.
In the present study, the presence of osteoarthritis and current arthritic pain adversely affected health-related quality of life in older subjects. The subjects with osteoarthritis showed a 6.2 lower EuroQoL VAS score than those without osteoarthritis (p<0.001), and those with current arthritic pain showed 10.68 lower EuroQoL VAS score than those without pain (p=0.025). In addition, subjective health status significantly affected current arthritic pain in subjects with osteoarthritis. Previous studies have reported an association between osteoarthritis and psychosocial factors, such as depression and catastrophizing,42,43,44 which supports our study results. Therefore, more attention needs to be paid to psychiatric causes and outcomes of osteoarthritis and arthritis related joint pain.
This study has some limitations that need to be addressed. First, the information on the medical conditions of the participants were based on self-reports. The validity of self-reported medical conditions is not established for our study cohort. Second, current medication, alternative therapy, or other treatment for osteoarthritis was not included in the data analysis, which could have affected the presence of osteoarthritis-associated pain.
In conclusion, older age, female gender, higher BMI, and osteoporosis were significant factors associated with osteoarthritis, while higher education level was a protective factor for osteoarthritis in South Korean older adults. Higher BMI, less weekly moderate intensity activity, and unfavorable subjective health status were significant contributing factors to current arthritic pain. More attention needs to be paid to psychiatric effects on osteoarthritis and joint related pain, and a more detailed study is needed to address this issue.
ACKNOWLEDGEMENTS
The authors wish to thank Hyun Mi Kim, BS, and Chong Bum Chang, MD for technical support and advice.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Wang Y, Simpson JA, Wluka AE, Teichtahl AJ, English DR, Giles GG, et al. Is physical activity a risk factor for primary knee or hip replacement due to osteoarthritis? A prospective cohort study. J Rheumatol. 2011;38:350–357. doi: 10.3899/jrheum.091138. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura A, Hasegawa M, Kato K, Yamada T, Uchida A, Sudo A. Risk factors for the incidence and progression of radiographic osteoarthritis of the knee among Japanese. Int Orthop. 2011;35:839–843. doi: 10.1007/s00264-010-1073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton CB. Obesity as a risk factor for osteoarthritis: mechanical versus metabolic. Med Health R I. 2004;87:201–204. [PubMed] [Google Scholar]
- 4.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, Macera CA, Davis DR, Ainsworth BE, Troped PJ, Blair SN. Physical activity and self-reported, physician-diagnosed osteoarthritis: is physical activity a risk factor? J Clin Epidemiol. 2000;53:315–322. doi: 10.1016/s0895-4356(99)00168-7. [DOI] [PubMed] [Google Scholar]
- 6.Hart DJ, Doyle DV, Spector TD. Incidence and risk factors for radiographic knee osteoarthritis in middle-aged women: the Chingford Study. Arthritis Rheum. 1999;42:17–24. doi: 10.1002/1529-0131(199901)42:1<17::AID-ANR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 7.Slemenda C, Heilman DK, Brandt KD, Katz BP, Mazzuca SA, Braunstein EM, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41:1951–1959. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Imeokparia RL, Barrett JP, Arrieta MI, Leaverton PE, Wilson AA, Hall BJ, et al. Physical activity as a risk factor for osteoarthritis of the knee. Ann Epidemiol. 1994;4:221–230. doi: 10.1016/1047-2797(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 9.Carman WJ, Sowers M, Hawthorne VM, Weissfeld LA. Obesity as a risk factor for osteoarthritis of the hand and wrist: a prospective study. Am J Epidemiol. 1994;139:119–129. doi: 10.1093/oxfordjournals.aje.a116974. [DOI] [PubMed] [Google Scholar]
- 10.Duncan IJ, Hurst NP, Disney A, Sebben R, Milazzo SC. Is chronic renal failure a risk factor for the development of erosive osteoarthritis? Ann Rheum Dis. 1989;48:183–187. doi: 10.1136/ard.48.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper C, Inskip H, Croft P, Campbell L, Smith G, McLaren M, et al. Individual risk factors for hip osteoarthritis: obesity, hip injury, and physical activity. Am J Epidemiol. 1998;147:516–522. doi: 10.1093/oxfordjournals.aje.a009482. [DOI] [PubMed] [Google Scholar]
- 12.Reijman M, Hazes JM, Pols HA, Koes BW, Bierma-Zeinstra SM. Acetabular dysplasia predicts incident osteoarthritis of the hip: the Rotterdam study. Arthritis Rheum. 2005;52:787–793. doi: 10.1002/art.20886. [DOI] [PubMed] [Google Scholar]
- 13.Juhakoski R, Heliövaara M, Impivaara O, Kröger H, Knekt P, Lauren H, et al. Risk factors for the development of hip osteoarthritis: a population-based prospective study. Rheumatology (Oxford) 2009;48:83–87. doi: 10.1093/rheumatology/ken427. [DOI] [PubMed] [Google Scholar]
- 14.Lohmander LS, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Engström G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann Rheum Dis. 2009;68:490–496. doi: 10.1136/ard.2008.089748. [DOI] [PubMed] [Google Scholar]
- 15.Yang S, Hwang JS, Park HK, Lee HS, Kim HS, Kim EY, et al. Serum lipid concentrations, prevalence of dyslipidemia, and percentage eligible for pharmacological treatment of Korean children and adolescents; data from the Korea National Health and Nutrition Examination Survey IV (2007-2009) PLoS One. 2012;7:e49253. doi: 10.1371/journal.pone.0049253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo KH, Kim YS, Sheen SS, Park JH, Hwang YI, Kim SH, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the fourth Korean National Health and Nutrition Examination Survey, 2008. Respirology. 2011;16:659–665. doi: 10.1111/j.1440-1843.2011.01951.x. [DOI] [PubMed] [Google Scholar]
- 17.Toivanen AT, Heliövaara M, Impivaara O, Arokoski JP, Knekt P, Lauren H, et al. Obesity, physically demanding work and traumatic knee injury are major risk factors for knee osteoarthritis--a population-based study with a follow-up of 22 years. Rheumatology (Oxford) 2010;49:308–314. doi: 10.1093/rheumatology/kep388. [DOI] [PubMed] [Google Scholar]
- 18.Dequeker J, Aerssens J, Luyten FP. Osteoarthritis and osteoporosis: clinical and research evidence of inverse relationship. Aging Clin Exp Res. 2003;15:426–439. doi: 10.1007/BF03327364. [DOI] [PubMed] [Google Scholar]
- 19.Dequeker J, Goris P, Uytterhoeven R. Osteoporosis and osteoarthritis (osteoarthrosis). Anthropometric distinctions. JAMA. 1983;249:1448–1451. [PubMed] [Google Scholar]
- 20.Wright NC, Riggs GK, Lisse JR, Chen Z Women's Health Initiative. Self-reported osteoarthritis, ethnicity, body mass index, and other associated risk factors in postmenopausal women-results from the Women’s Health Initiative. J Am Geriatr Soc. 2008;56:1736–1743. doi: 10.1111/j.1532-5415.2008.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietschmann P, Rauner M, Sipos W, Kerschan-Schindl K. Osteoporosis: an age-related and gender-specific disease--a mini-review. Gerontology. 2009;55:3–12. doi: 10.1159/000166209. [DOI] [PubMed] [Google Scholar]
- 22.Melton LJ., 3rd The prevalence of osteoporosis: gender and racial comparison. Calcif Tissue Int. 2001;69:179–181. doi: 10.1007/s00223-001-1043-9. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Stannus OP, Aitken D, Cicuttini F, Antony B, Jones G, et al. Cross-sectional and longitudinal associations between systemic, subchondral bone mineral density and knee cartilage thickness in older adults with or without radiographic osteoarthritis. Ann Rheum Dis. 2014;73:2003–2009. doi: 10.1136/annrheumdis-2013-203691. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, Harvey WF, Price LL, Paulus JK, Dawson-Hughes B, McAlindon TE. Relationship of bone mineral density to progression of knee osteoarthritis. Arthritis Rheum. 2013;65:1541–1546. doi: 10.1002/art.37926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishii T, Tamura S, Shiomi T, Yoshikawa H, Sugano N. Alendronate treatment for hip osteoarthritis: prospective randomized 2-year trial. Clin Rheumatol. 2013;32:1759–1766. doi: 10.1007/s10067-013-2338-8. [DOI] [PubMed] [Google Scholar]
- 26.Felson DT. Obesity and vocational and avocational overload of the joint as risk factors for osteoarthritis. J Rheumatol Suppl. 2004;70:2–5. [PubMed] [Google Scholar]
- 27.Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026–2032. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 28.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahaghin S, Bierma-Zeinstra SM, Koes BW, Hazes JM, Pols HA. Do metabolic factors add to the effect of overweight on hand osteoarthritis? The Rotterdam Study. Ann Rheum Dis. 2007;66:916–920. doi: 10.1136/ard.2005.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69:761–765. doi: 10.1136/ard.2008.106930. [DOI] [PubMed] [Google Scholar]
- 31.Stürmer T, Brenner H, Koenig W, Günther KP. Severity and extent of osteoarthritis and low grade systemic inflammation as assessed by high sensitivity C reactive protein. Ann Rheum Dis. 2004;63:200–205. doi: 10.1136/ard.2003.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfe F. The C-reactive protein but not erythrocyte sedimentation rate is associated with clinical severity in patients with osteoarthritis of the knee or hip. J Rheumatol. 1997;24:1486–1488. [PubMed] [Google Scholar]
- 33.Bennell KL, Egerton T, Wrigley TV, Hodges PW, Hunt M, Roos EM, et al. Comparison of neuromuscular and quadriceps strengthening exercise in the treatment of varus malaligned knees with medial knee osteoarthritis: a randomised controlled trial protocol. BMC Musculoskelet Disord. 2011;12:276. doi: 10.1186/1471-2474-12-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page CJ, Hinman RS, Bennell KL. Physiotherapy management of knee osteoarthritis. Int J Rheum Dis. 2011;14:145–151. doi: 10.1111/j.1756-185X.2011.01612.x. [DOI] [PubMed] [Google Scholar]
- 35.Egan BA, Mentes JC. Benefits of physical activity for knee osteoarthritis: a brief review. J Gerontol Nurs. 2010;36:9–14. doi: 10.3928/00989134-20100730-03. [DOI] [PubMed] [Google Scholar]
- 36.Sokoloff L, Mickelsen O. Dietary fat supplements, body weight and osteoarthritis in dba-2jn mice. J Nutr. 1965;85:117–121. doi: 10.1093/jn/85.1.117. [DOI] [PubMed] [Google Scholar]
- 37.McAlindon TE, Jacques P, Zhang Y, Hannan MT, Aliabadi P, Weissman B, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39:648–656. doi: 10.1002/art.1780390417. [DOI] [PubMed] [Google Scholar]
- 38.McAlindon TE, Felson DT, Zhang Y, Hannan MT, Aliabadi P, Weissman B, et al. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125:353–359. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz ER, Oh WH, Leveille CR. Experimentally induced osteoarthritis in guinea pigs: metabolic responses in articular cartilage to developing pathology. Arthritis Rheum. 1981;24:1345–1355. doi: 10.1002/art.1780241103. [DOI] [PubMed] [Google Scholar]
- 40.Wang ZB, Gan Q, Rupert RL, Zeng YM, Song XJ. Thiamine, pyridoxine, cyanocobalamin and their combination inhibit thermal, but not mechanical hyperalgesia in rats with primary sensory neuron injury. Pain. 2005;114:266–277. doi: 10.1016/j.pain.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 41.Jonas WB, Rapoza CP, Blair WF. The effect of niacinamide on osteoarthritis: a pilot study. Inflamm Res. 1996;45:330–334. doi: 10.1007/BF02252945. [DOI] [PubMed] [Google Scholar]
- 42.Wideman TH, Finan PH, Edwards RR, Quartana PJ, Buenaver LF, Haythornthwaite JA, et al. Increased sensitivity to physical activity among individuals with knee osteoarthritis: relation to pain outcomes, psychological factors, and responses to quantitative sensory testing. Pain. 2014;155:703–711. doi: 10.1016/j.pain.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 43.Herbert MS, Goodin BR, Pero ST, 4th, Schmidt JK, Sotolongo A, Bulls HW, et al. Pain hypervigilance is associated with greater clinical pain severity and enhanced experimental pain sensitivity among adults with symptomatic knee osteoarthritis. Ann Behav Med. 2014;48:50–60. doi: 10.1007/s12160-013-9563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holla JF, van der Leeden M, Knol DL, Roorda LD, van der Esch M, Voorneman RE, et al. The association of body-mass index and depressed mood with knee pain and activity limitations in knee osteoarthritis: results from the Amsterdam osteoarthritis cohort. BMC Musculoskelet Disord. 2013;14:296. doi: 10.1186/1471-2474-14-296. [DOI] [PMC free article] [PubMed] [Google Scholar]


