Abstract
This study explored drug transporter expression levels and their impact on clinical response to imatinib and second-generation tyrosine kinase inhibitors (TKIs) in imatinib- resistant chronic myeloid leukemia (CML). Imatinib-resistant chronic phase CML patients treated with dasatinib (n=10) and nilotinib (n=12) were enrolled. The mRNA expression of the OCT-1, ABCG2, and ABCB1 genes was quantified by using paired bone marrow samples obtained before administering imatinib and at the point of detecting imatinib resistance (just before starting second-generation TKIs). The expression levels of OCT-1 and ABCG2 were lower in follow-up than in imatinib-naïve samples. ABCB1 revealed highly variable expression levels before and after imatinib treatment. In addition, median ABCB1 expression in follow-up samples was lower in patients achieving complete cytogenetic response or major molecular response during imatinib treatment than in failed patients. Higher ABCG2 expression in imatinib-exposed samples showed a negative impact on optimal response to dasatinib. Patients with higher ABCG2 expression in imatinib-exposed samples also had shorter progression- free survival with dasatinib treatment. However, no significant correlation was found between these drug transporter expression levels in imatinib-naïve or imatinib- exposed samples and responses to nilotinib. In imatinib-resistant CML, OCT-1 and ABCG2 mRNA expression decreased after imatinib treatment. Patients with higher ABCG2 expression in imatinib-exposed samples showed poor treatment outcome with dasatinib. On the other hand, a higher expression level of ABCB1 in imatinib-exposed samples did not affect second-generation TKI responses but was correlated with poor imatinib responses.
Keywords: ABCB1 protein, ABCG2 protein, Myeloid leukemia, Imatinib
INTRODUCTION
Imatinib mesylate (imatinib), a potent BCR-ABL tyrosine kinase inhibitor (TKI), has been widely used for newly developed chronic myeloid leukemia (CML).1 However, the failure rate in achieving optimal response to imatinib reaches up to 20% to 30%.2 For these patients, second-generation TKIs (dasatinib or nilotinib) may be used as alternatives. About half of patients failing to achieve optimal response to imatinib can be rescued with second-generation TKIs. However, resistance to these second-generation TKIs also occurs.3,4,5,6
BCR-ABL1 tyrosine kinase domain mutations are a main mechanism of imatinib resistance and account for 40% to 50% of all cases of imatinib resistance.7,8 Other BCR-ABL-independent mechanisms in imatinib resistance remain unclear. One mechanism of resistance to imatinib is known to be associated with drug transporters. Higher plasma imatinib trough levels is associated with optimal response in CML patients.9 TKIs including imatinib are transported by influx or efflux pumps; hence, these drug transporters have been postulated to influence TKI responses.
Human organic cation transporter 1 transporter (OCT-1, or SLC22A1) induces imatinib influx into cells. In clinical studies, the activity of OCT-1 and the mRNA expression of OCT-1 correlate well with the imatinib response rate in the aspect of molecular response (MR) and overall survival.10,11,12 Dose escalation of imatinib may overcome OCT-1 transporter activity in patients with correspondingly low imatinib plasma levels; however, the transport of newly developed second-generation TKIs, dasatinib or nilotinib, is not mediated by OCT-1.13
In addition, imatinib is a substrate for the adenosine triphosphate binding cassette (ABC) transporters, ATP binding cassette B1 (ABCB1) and ABCG2 (breast cancer resistance protein, or BCRP). Accordingly, ABCB1 and ABCG2 might be influencing pharmacokinetics and intracellular or systemic levels of imatinib. Both ABCB1 and ABCG2 contribute to resistance by extruding imatinib from hematopoietic cells.14,15 Recently, there have been several reports that intracellular levels of nilotinib and dasatinib are influenced by the efflux ABC transporters such as ABCB1 or ABCG2.13,16,17 In these studies, ABCB1 and ABCG2 mRNA expression revealed higher levels in dasatinib-resistant cell lines, indicating an important role of drug efflux transporters on second-generation TKI resistance.17,18 However, most of these studies were performed in vitro, and gene expression levels were usually assessed at a single time point.
In this study, we evaluated the mRNA expression levels of OCT-1, ABCG2, and ABCB1 before and after imatinib exposure in imatinib-resistant CML and analyzed the changes in drug transporter expression levels. We also explored the relationship between the expression of these genes and treatment outcomes of imatinib and second-generation TKIs.
MATERIALS AND METHODS
1. Patients and treatment protocol
Twenty-four adult CML patients (aged 34-72 years) who showed imatinib resistance as a first-line therapy were enrolled and their treatment data were analyzed retrospectively. All patients were diagnosed as having chronic phase CML before imatinib treatment in accordance with the 2008 World Health Organization criteria.19 Two patients who showed TKD mutations during imatinib treatment were excluded from this study. Informed consent was provided for all enrolled patients. The study design was approved by the institutional review boards. All 22 patients were administered imatinib (400 mg/day) for more than 12 months. Median treatment duration with imatinib was 26.9 months (range, 12.4-85.4 months). Shortly after imatinib resistance was detected, 10 and 12 patients started dasatinib (100 mg/day) and nilotinib (600 mg/day) treatment as a second-line therapy, respectively. Bone marrow samples were obtained twice, before imatinib treatment and at the point of detecting resistance to imatinib (before starting second-generation TKIs).
Treatment responses to imatinib and second-generation TKIs were determined on the basis of standard recommendations: the European LeukemiaNet20 and National Comprehensive Cancer Network guidelines.21 Briefly, complete hematologic response was defined as a white blood cell count <10×109/L; basophils <5%; an absence of myelocytes, promyelocytes, or myeloblasts in peripheral blood; platelet counts <450×109/L; and no palpable spleen. Cytogenetic response (CyR) was defined as the ratio of Philadelphia chromosomes (Ph+) in bone marrow cell metaphases after TKI treatment to those before treatment (eg, complete cytogenetic response, or CCyR: no Ph+ metaphases). Molecular response (MR) was defined as the ratio of BCR-ABL1 transcripts by real-time quantitative polymerase chain reaction (qPCR) after TKI treatment to those before treatment (eg, major molecular response, or MMR: BCR-ABL1/ABL1≤0.1% with the international scale).20 The definition of primary imatinib resistance was an absence of a complete hematologic response by 3-6 months, any CyR by 6 months, major CyR by 12 months, or CCyR by 18 months. Disease progression following prior response to imatinib was defined as secondary resistance.21
Optimal response with second-generation TKIs was defined as BCR-ABL1≤10% or Ph+<65% at 3 months, BCR-ABL1≤10% or Ph+<35% at 6 months, BCR-ABL1 <1% or Ph+ 0 at 12 months, and then BCR-ABL1≤0.1% at any time. Failure with second-generation TKIs was defined as an absence of complete hematologic response or Ph+>95% or developing new mutations at 3 months, BCR-ABL1>10% or Ph+>65% or newly developed mutations at 6 months, or BCR-ABL1>10% or Ph+>35% or the occurrence of new mutations at 6 months. Loss of complete hematologic response, CCyR/partial CyR (PCyR) or MMR, developing clonal chromosome abnormalities in Ph+ cells, or new mutations were also considered as failure.20
2. Expression of drug transporters
Genomic DNA was obtained from diagnostic bone marrow cryopreserved mononuclear cells with a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA). To analyze mRNA expression of OCT-1, ABCG2, and ABCB1, RNA was obtained from at least 1×106 cells with TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). QuantiTect Reverse Transcription Kit (Qiagen) was used for cDNA synthesis. PCR amplification was performed with the following primers: OCT-1, (forward) 5'-CTG AGC TGT ACC CCA CAT TCG-3', (reverse) 5'-CCA ACA CCG CAA ACA AAA TGA-3'22; ABCG2, (forward) 5'-AGA TGG GTT TCC AAG CGT TCA T-3', (reverse) 5'-CCA GTC CCA GTA CGA CTG TGA CA-(3)'23; ABCB1, (forward) 5'-AGA CAT GAC CAG GTA TGC CTA T-(3)', (reverse) 5'-AGC CTA TCT CCT GTC GCA TTA-3'22 (GeneWorks Pty. Ltd., Adelaide, SA, Australia). qPCR was performed by use of a Rotor Gene 3000 real-time PCR thermal cycler (Corbett Biosciences, San Francisco, CA, USA) with a Rotor-Gene SYBR Green PCR Kit (Qiagen). mRNA expression of BCR [(forward) 5'-CCT TCG ACG TCA ATA ACA ACG AT-3', (reverse) 5'-CCT GCG ATG GCG TTC AC-3')]22 was used for an assessment of the expression ratio of these three drug transporters.
3. Statistical analysis
Fisher's exact test (categorical value) and Mann-Whitney U test (continuous data) were used for between-group comparisons. For comparison of gene expression levels between imatinib-naïve and imatinib-exposed samples, Wilcoxon signed-rank test was used. Survival was assessed through the use of Kaplan-Meier survival curves and estimates. The log-rank test was used for comparison of survival distributions between groups. p<0.05 was regarded to have statistical significance. All statistical analyses were carried out through the use of SPSS software (version 18.0, SPSS Inc., Chicago, IL).
RESULTS
1. Patient's characteristics
A total of 22 imatinib-resistant patients (aged 34-72 years) without TKD mutation were analyzed in this study (Table 1). Sokal's risk evaluation at diagnosis showed that 12 (54.5%) were high risk, 8 (36.4%) were intermediate risk, and 2 (9.1%) were low risk. Median treatment duration of imatinib was 26.9 months (range, 12.4-85.4 months). During imatinib treatment, 6 (27.3%) and 3 (13.6%) patients achieved CCyR and MMR, respectively. A total of 13 (59.1%) and 9 (40.9%) patients switched to second-generation TKIs owing to primary and secondary imatinib resistance, respectively.
TABLE 1.
Patient characteristics (n=22)
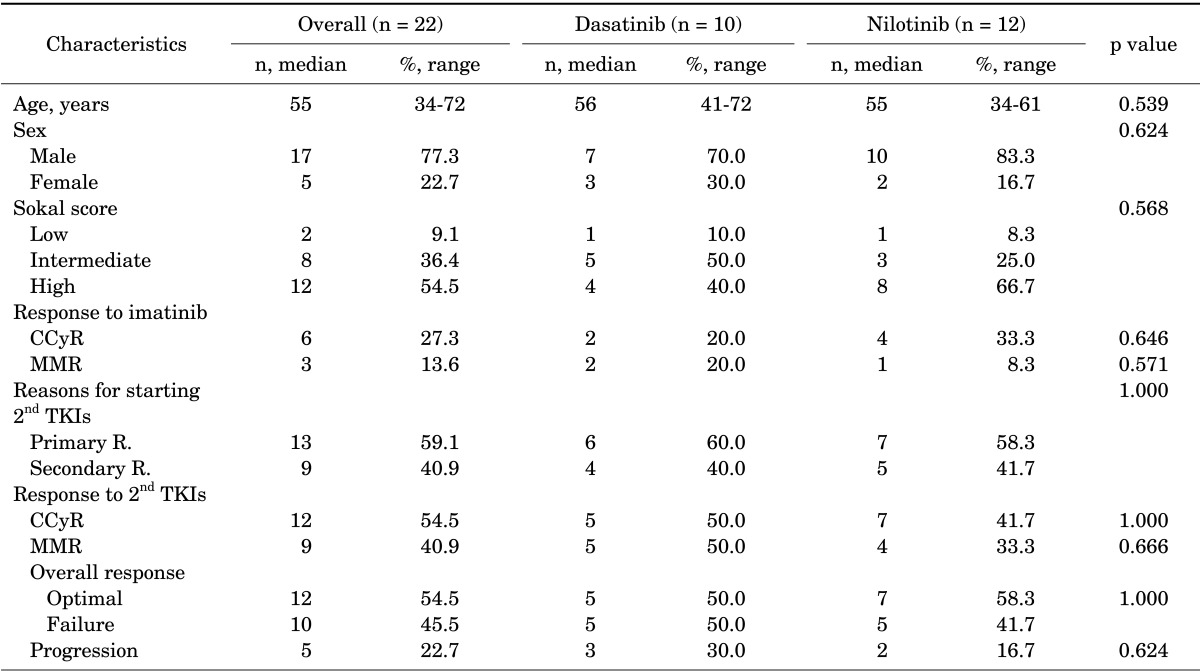
CCyR: complete cytogenetic response, MMR: major molecular response, 2nd TKI: second-generation tyrosine kinase inhibitors, R.: resistance.
With salvage therapy with second-generation TKIs, 54.5% and 40.9% of the imatinib-resistant patients had CCyR and MMR. Of the patients with imatinib resistance, 54.5% of the patients with imatinib resistance could have optimal response with second-generation TKIs (Table 1). No significant differences were found in baseline characteristics or outcome between dasatinib- and nilotinibtreated patients. Median follow-up time with second-generation TKIs was 51.2 months (range, 8.4-107.5 months).
2. Changes in drug transporter expression levels and treatment outcomes with imatinib
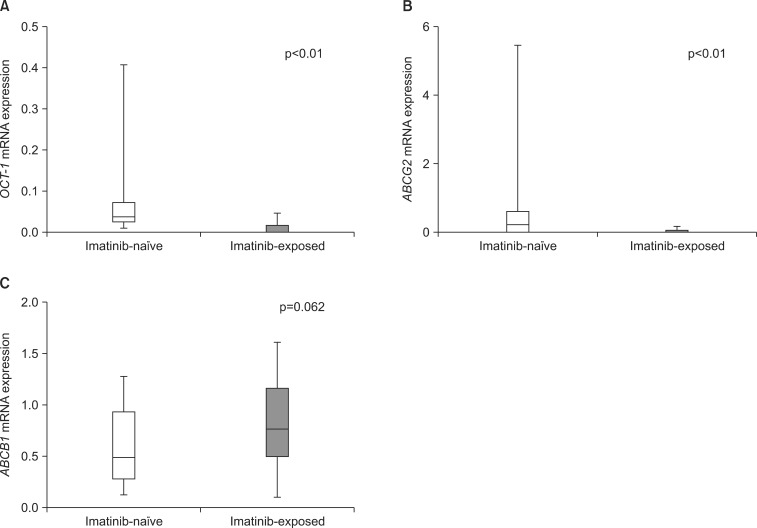
The mRNA expression of the three drug transporters before administering imatinib and at the point of detecting imatinib resistance was analyzed. By use of pair-wise analysis, OCT-1 expression was significantly reduced in samples obtained at the point of imatinib resistance compared with the expression in imatinib-naïve samples (median, 0.3965 vs. 0.0099, p<0.01). In addition, ABCG2 expression was also significantly decreased after imatinib treatment (0.2192 vs. 0.0282, p<0.01). Median mRNA expression of ABCB1 in imatinib-exposed samples was higher than that in imatinib-naïve samples; however, the difference was not statistically significant (0.5042 vs. 0.7785, p=0.062). In addition, ABCB1 expression levels were highly variable compared with OCT-1 and ABCG2 in imatinib-exposed samples (Fig. 1).
FIG. 1.
mRNA expression of OCT-1 (A), ABCG2 (B), and ABCB1 (C) before and after imatinib exposure (n=22). Each column represents the median (range) of three independent experiments performed in duplicate at each time point.
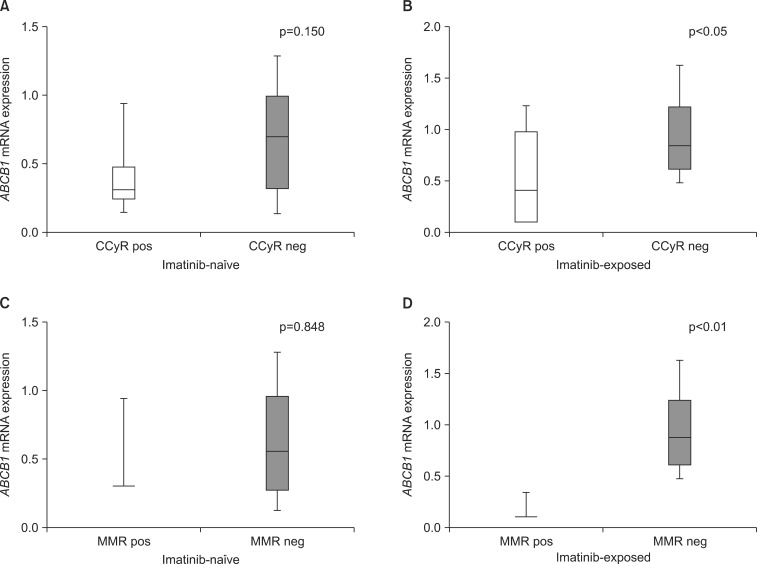
The mRNA expression of the three drug transporters in imatinib-naïve samples did not significantly affect responses to imatinib. On the other hand, ABCB1 expression in imatinib-exposed samples was significantly lower in patients achieving CCyR (0.4131 vs. 0.8440, p<0.05) and MMR (0.1141 vs. 0.8761, p<0.01) than in other patients (Table 2, Fig. 2). Conversely, the mRNA expression of ABCG2 in imatinib-exposed samples was slightly lower in patients achieving CCyR (p=1.000) and MMR (p=0.510) during imatinib treatment; however, the difference was not significant. The expression values of these three drug transporters in both imatinib-naïve and imatinib-exposed samples did not affect overall imatinib-resistant patterns.
TABLE 2.
mRNA expression of drug transporters according to the treatment outcomes of imatinib (n=22)
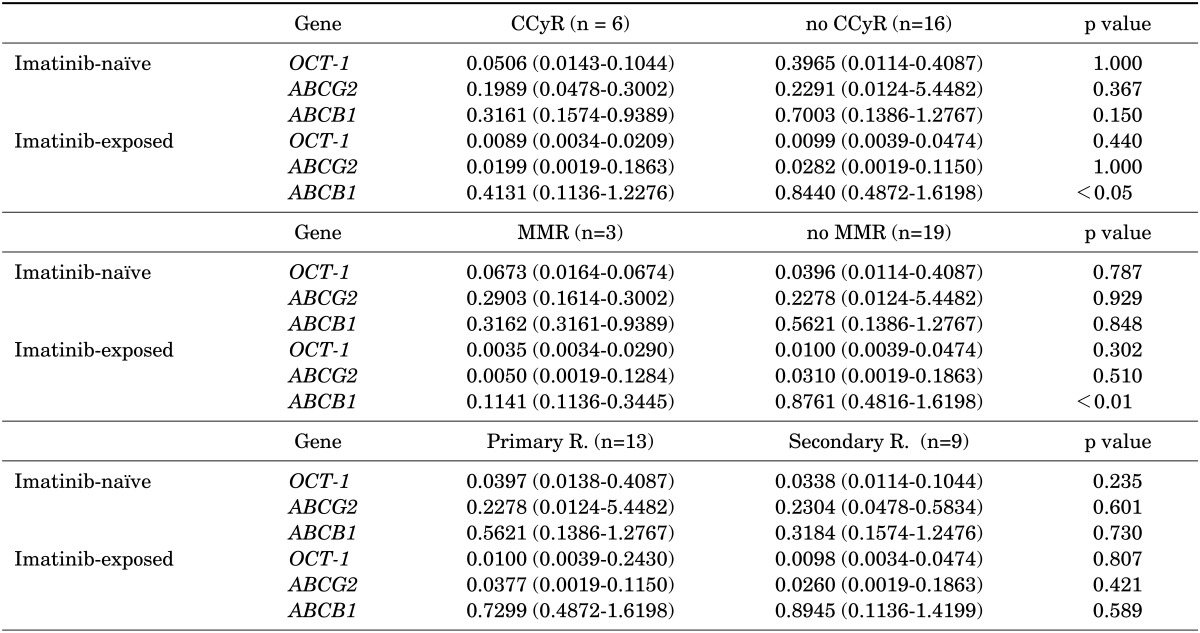
Each column represents the median (range) of three independent experiments performed in duplicate at each time point. CCyR: complete cytogenetic response, MMR: major molecular response, R.: resistance.
FIG. 2.
ABCB1 mRNA expression levels according to the treatment outcomes of imatinib (n=22). ABCB1 mRNA expression levels before (A) and after (B) imatinib treatment in patients achieving CCyR or no CCyR. ABCB1 mRNA expression levels before (C) and after imatinib treatment (D) in patients achieving MMR or no MMR. CCyR: complete cytogenetic response, MMR: major molecular response, pos: positive, neg: negative.
3. Expression of drug transporters and treatment outcomes following second-generation TKIs
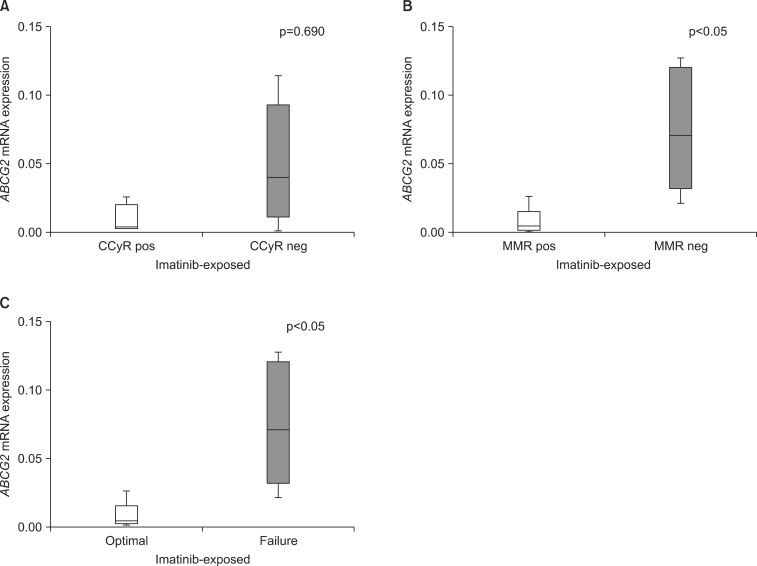
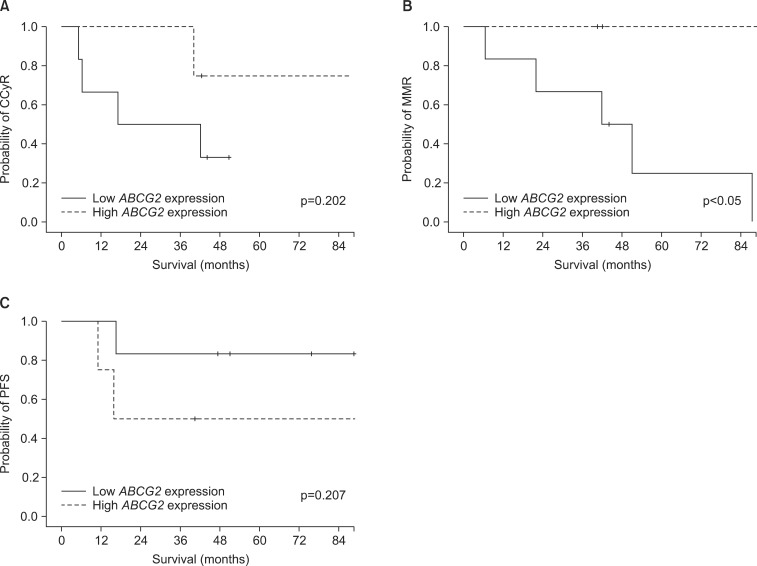
In dasatinib-treated patients (n=10), the mRNA expression levels of the three drug transporters in imatinib-naïve samples were not significantly associated with response rates. Also, OCT-1 and ABCB1 mRNA expression levels in imatinib-exposed samples did not affect the treatment outcome of dasatinib. Conversely, at the point of imatinib resistance, median mRNA expression levels of ABCG2 were significantly lower in patients achieving MMR (0.0050 vs. 0.0722, p<0.05) than in patients with failed response to dasatinib (Table 3A, Fig. 3). In terms of overall response to dasatinib, patients with lower ABCG2 expression levels in imatinib-exposed samples demonstrated a higher rate of optimal response (0.0050 vs. 0.0722, p<0.05). Furthermore, patients with higher ABCG2 expression after imatinib needed a significantly longer time to achieve MMR (median; not applicable vs. 43.9 months, p<0.05) with dasatinib than did those with lower ABCG2 expression after imatinib treatment (Fig. 4). Patients with higher ABCG2 expression after imatinib also showed shorter progression-free survival than did those with lower ABCG2 expression after imatinib; however, there was no statistical significance (p=0.207, Fig. 4). On the other hand, there was no significant correlation between the expression levels of the three drug transporters before or after imatinib and clinical outcome in nilotinib-treated patients (n=12; Table 3B).
TABLE 3.
mRNA expression of drug transporters according to the treatment outcomes of (A) dasatinib and (B) nilotinib
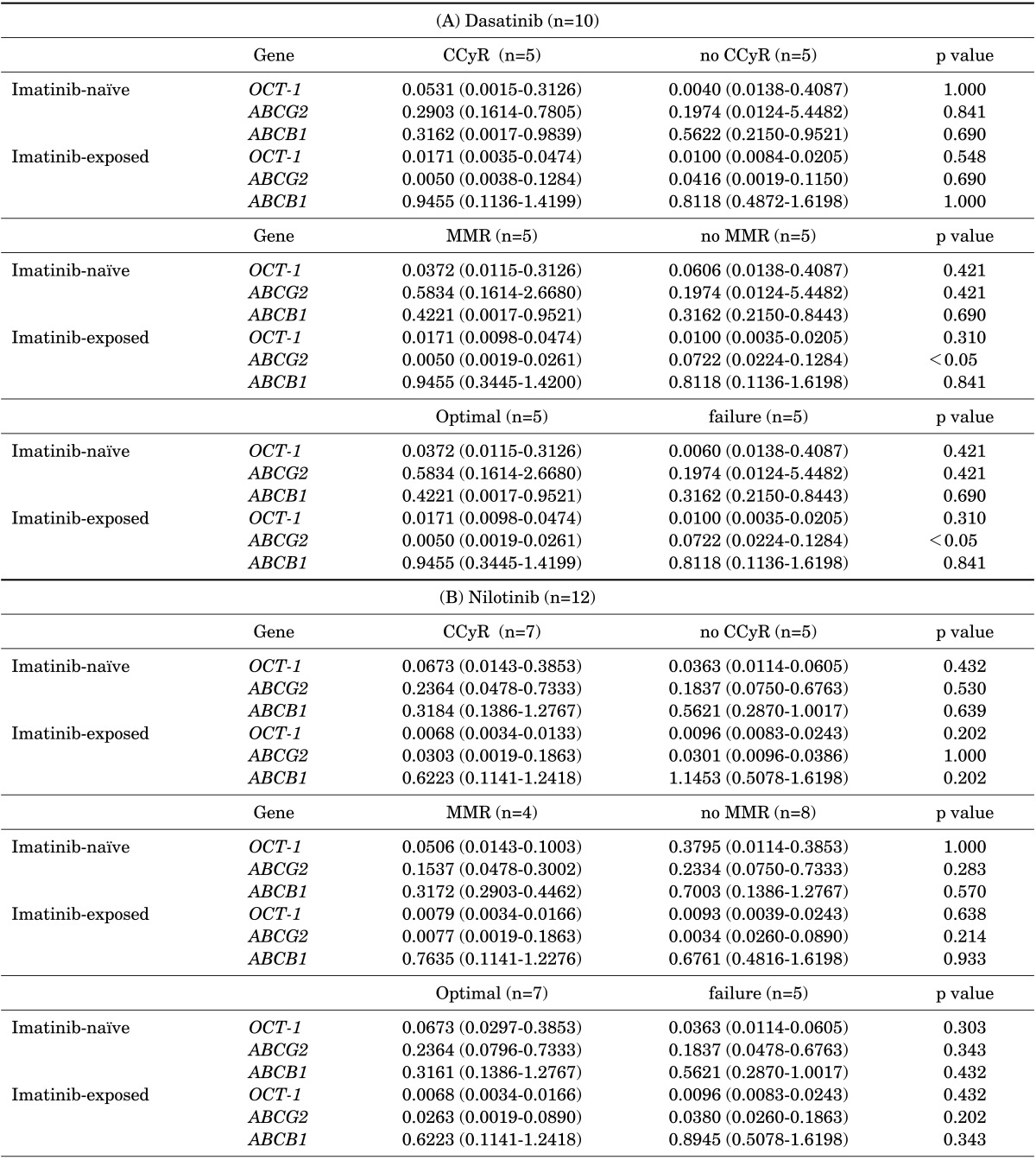
Each column represents the median (range) of three independent experiments performed in duplicate at each time point. CCyR: complete cytogenetic response, MMR: major molecular response.
FIG. 3.
ABCG2 mRNA expression levels in imatinib-exposed samples according to the treatment outcomes of dasatinib (n=10). ABCG2 mRNA expression levels in imatinib-exposed samples in patients achieving CCyR and no CCyR (A), MMR or no MMR (B), and optimal response or failure (C). CCyR: complete cytogenetic response, MMR: major molecular response, pos: positive, neg: negative.
FIG. 4.
Time to achieving optimal responses and PFS according to the ABCG2 mRNA expression after dasatinib treatment. (A) Time to achieving CCyR. (B) Time to achieving MMR. (C) PFS with dasatinib treatment. PFS: progression-free survival, CCyR: complete cytogenetic response, MMR: major molecular response.
DISCUSSION
Chronic imatinib exposure may change the expression patterns of drug transporters, which can affect the treatment outcome of imatinib. In studies in vitro, these drug transporter expression patterns are diverse depending on the cell type, imatinib concentration, and imatinib treatment duration.17,24 One in vitro study demonstrated increased OCT-1 expression levels with increasing doses of imatinib.17 In that study, prolonged exposure to imatinib also induced expression of both ABCG2 and ABCB1 in imatinib- resistant CML cells. However, with time, the overexpression of ABCB1 progressively declined to baseline expression levels, whereas the overexpression of ABCG2 was maintained throughout the study. Another in vitro study using intestinal epithelial Caco2 cells revealed that prolonged exposure to imatinib affected the expression patterns of genes potentially involved in drug transport. In that study, imatinib exposure induced ABCBG2 and ABCB1 expression, and chronic imatinib administration to Caco2 cells reduced the intracellular imatinib concentration, suggesting up-regulation of ABCB1 and ABCG2 drug efflux transporters.25
There have been few in vivo clinical studies of the relationship between these drug transporter expression levels and clinical outcomes. Furthermore, these in vivo studies offered conflicting results. One study showed that the mean pre-imatinib OCT-1 expression level was eight times higher in imatinib responders than in nonresponders.26 In that study, the ABCB1 and ABCG2 expression levels doubled in bone marrow mononuclear cells in nonresponders with imatinib treatment. On the contrary, another study revealed that long-term imatinib exposure did not induce the ABC transporters.27
In our study, OCT-1 and ABCG2 mRNA expression showed a significant change after imatinib treatment. At the time point of imatinib resistance, mRNA expression levels of OCT-1 were significantly decreased compared with those before imatinib exposure. ABCG2 expression also decreased after imatinib treatment. Conversely, ABCB1 expression was slightly elevated after long-term imatinib exposure, but there was no statistical significance to this finding. Interestingly, these ABCB1 expression levels showed significant variation after imatinib-exposed samples in imatinib-resistant patients. A recent study also demonstrated that there was considerable diversity in the drug transporter expression levels including ABCB1 in imatinib- resistant patients, whereas the expression levels remained constant during follow-up samples in imatinib responders.28 Furthermore, the drug transporter expression levels in imatinib-naïve samples in imatinib-resistant patients did not affect the treatment outcome of imatinib in our study. Only higher ABCB1 expression levels in imatinib-exposed samples showed a negative impact on imatinib responses. Most other studies have evaluated the drug transporter expression levels using single time point samples usually collected at diagnosis to compare clinical outcomes with TKIs; however, these drug expression levels in imatinib-resistant patients were not stable. Therefore, sequential monitoring of these drug transporter expressions may be warranted in patients treated with TKIs.
The resistance mechanisms to second-generation TKIs are still unclear. The intracellular levels of nilotinib29 and dasatinib13 are not affected by OCT-1 activity. Some previous studies reported that the intracellular levels of nilotinib and dasatinib were influenced by the activity of the efflux ABC drug transporters such as ABCG2 and ABCB1. An in vitro study demonstrated that OCT-1 activity was not correlated with the intracellular dasatinib concentration; however, the efflux of dasatinib was mediated by both drug efflux transporters, ABCG2 and ABCB1.18 Nilotinib is also known to be a substrate of ABCG2 or ABCB1. However, a recent experiment revealed that nilotinib may be transported by ABCB1, but does not interact strongly with ABCG2.24,30 In the current study, patients achieving the optimal response including MMR with dasatinib had lower ABCG2 expression levels in imatinib-exposed samples compared with failed patients. Furthermore, higher ABCG2 expression in imatinib-exposed samples also showed a trend of shorter progression-free survival in patients treated with dasatinib. However, nilotinib-treated patients were not affected by the expression levels of the three drug transporters before and after imatinib exposure, indicating that there are different interactions with nilotinib and drug efflux transporters. In addition, both drugs demonstrated no significant relationship between drug transporter expression levels in imatinib-naïve samples and clinical outcomes, suggesting that the drug transporter expression levels may be assessed just before starting treatment with second-generation TKIs.
In conclusion, OCT-1 and ABCG2 mRNA expression significantly decreased after imatinib treatment in imatinib-resistant CML patients. The ABCB1 expression level in imatinib-exposed samples was significantly higher in poor imatinib responders. On the other hand, a higher expression level of ABCG2 in imatinib-exposed samples was associated with poor treatment outcome in dasatinibtreated patients.
ACKNOWLEDGEMENTS
This study was supported by a grant (CRI10014-1) from the Chonnam National University Hospital Biomedical Research Institute.
Footnotes
None declared.
References
- 1.Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 2.Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. IRIS Investigators. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 3.Russo Rossi A, Breccia M, Abruzzese E, Castagnetti F, Luciano L, Gozzini A, et al. Outcome of 82 chronic myeloid leukemia patients treated with nilotinib or dasatinib after failure of two prior tyrosine kinase inhibitors. Haematologica. 2013;98:399–403. doi: 10.3324/haematol.2012.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim AR, Clark RE, Holyoake TL, Byrne J, Shepherd P, Apperley JF, et al. Second-generation tyrosine kinase inhibitors improve the survival of patients with chronic myeloid leukemia in whom imatinib therapy has failed. Haematologica. 2011;96:1779–1782. doi: 10.3324/haematol.2011.049759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bixby D, Talpaz M. Mechanisms of resistance to tyrosine kinase inhibitors in chronic myeloid leukemia and recent therapeutic strategies to overcome resistance. Hematology Am Soc Hematol Educ Program. 2009:461–476. doi: 10.1182/asheducation-2009.1.461. [DOI] [PubMed] [Google Scholar]
- 6.Giles FJ, Abruzzese E, Rosti G, Kim DW, Bhatia R, Bosly A, et al. Nilotinib is active in chronic and accelerated phase chronic myeloid leukemia following failure of imatinib and dasatinib therapy. Leukemia. 2010;24:1299–1301. doi: 10.1038/leu.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabbour E, Kantarjian H, Jones D, Talpaz M, Bekele N, O'Brien S, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20:1767–1773. doi: 10.1038/sj.leu.2404318. [DOI] [PubMed] [Google Scholar]
- 8.Soverini S, Colarossi S, Gnani A, Rosti G, Castagnetti F, Poerio A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006;12:7374–7379. doi: 10.1158/1078-0432.CCR-06-1516. [DOI] [PubMed] [Google Scholar]
- 9.Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109:3496–3499. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- 10.Eechoute K, Sparreboom A, Burger H, Franke RM, Schiavon G, Verweij J, et al. Drug transporters and imatinib treatment: implications for clinical practice. Clin Cancer Res. 2011;17:406–415. doi: 10.1158/1078-0432.CCR-10-2250. [DOI] [PubMed] [Google Scholar]
- 11.White DL, Dang P, Engler J, Frede A, Zrim S, Osborn M, et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2010;28:2761–2767. doi: 10.1200/JCO.2009.26.5819. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Giannoudis A, Lane S, Williamson P, Pirmohamed M, Clark RE. Expression of the uptake drug transporter hOCT1 is an important clinical determinant of the response to imatinib in chronic myeloid leukemia. Clin Pharmacol Ther. 2008;83:258–264. doi: 10.1038/sj.clpt.6100268. [DOI] [PubMed] [Google Scholar]
- 13.Giannoudis A, Davies A, Lucas CM, Harris RJ, Pirmohamed M, Clark RE. Effective dasatinib uptake may occur without human organic cation transporter 1 (hOCT1): implications for the treatment of imatinib-resistant chronic myeloid leukemia. Blood. 2008;112:3348–3354. doi: 10.1182/blood-2007-10-116236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raaijmakers MH. ATP-binding-cassette transporters in hematopoietic stem cells and their utility as therapeutical targets in acute and chronic myeloid leukemia. Leukemia. 2007;21:2094–2102. doi: 10.1038/sj.leu.2404859. [DOI] [PubMed] [Google Scholar]
- 15.Burger H, van Tol H, Boersma AW, Brok M, Wiemer EA, Stoter G, et al. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004;104:2940–2942. doi: 10.1182/blood-2004-04-1398. [DOI] [PubMed] [Google Scholar]
- 16.Dohse M, Scharenberg C, Shukla S, Robey RW, Volkmann T, Deeken JF, et al. Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug Metab Dispos. 2010;38:1371–1380. doi: 10.1124/dmd.109.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gromicho M, Dinis J, Magalhães M, Fernandes AR, Tavares P, Laires A, et al. Development of imatinib and dasatinib resistance: dynamics of expression of drug transporters ABCB1, ABCC1, ABCG2, MVP, and SLC22A1. Leuk Lymphoma. 2011;52:1980–1990. doi: 10.3109/10428194.2011.584005. [DOI] [PubMed] [Google Scholar]
- 18.Hiwase DK, Saunders V, Hewett D, Frede A, Zrim S, Dang P, et al. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res. 2008;14:3881–3888. doi: 10.1158/1078-0432.CCR-07-5095. [DOI] [PubMed] [Google Scholar]
- 19.Vardiman JW, Melo JV, Baccarani M, Thiele J. Chronic myelogenous leukemia BCR-ABL1 positive. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lion: IARC; 2008. pp. 32–37. [Google Scholar]
- 20.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology, Chronic Myelogenous Leukemia. Version1. 2015. [Accessed August 28, 2014]. Available at: http://www.nccn.org/professionals/physician_gls/PDF/cml.pdf. [DOI] [PubMed]
- 22.Engler JR, Frede A, Saunders VA, Zannettino AC, Hughes TP, White DL. Chronic myeloid leukemia CD34+ cells have reduced uptake of imatinib due to low OCT-1 activity. Leukemia. 2010;24:765–770. doi: 10.1038/leu.2010.16. [DOI] [PubMed] [Google Scholar]
- 23.Herzog M, Storch CH, Gut P, Kotlyar D, Füllekrug J, Ehehalt R, et al. Knockdown of caveolin-1 decreases activity of breast cancer resistance protein (BCRP/ABCG2) and increases chemotherapeutic sensitivity. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:1–11. doi: 10.1007/s00210-010-0568-8. [DOI] [PubMed] [Google Scholar]
- 24.Eadie LN, Hughes TP, White DL. Interaction of the efflux transporters ABCB1 and ABCG2 with imatinib, nilotinib, and dasatinib. Clin Pharmacol Ther. 2014;95:294–306. doi: 10.1038/clpt.2013.208. [DOI] [PubMed] [Google Scholar]
- 25.Burger H, van Tol H, Brok M, Wiemer EA, de Bruijn EA, Guetens G, et al. Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther. 2005;4:747–752. doi: 10.4161/cbt.4.7.1826. [DOI] [PubMed] [Google Scholar]
- 26.Crossman LC, Druker BJ, Deininger MW, Pirmohamed M, Wang L, Clark RE. hOCT 1 and resistance to imatinib. Blood. 2005;106:1133–1134. doi: 10.1182/blood-2005-02-0694. [DOI] [PubMed] [Google Scholar]
- 27.Gardner ER, Sparreboom A, Verweij J, Figg WD. Lack of ABC transporter autoinduction in mice following long-term exposure to imatinib. Cancer Biol Ther. 2008;7:412–415. doi: 10.4161/cbt.7.3.5412. [DOI] [PubMed] [Google Scholar]
- 28.Gromicho M, Magalhães M, Torres F, Dinis J, Fernandes AR, Rendeiro P, et al. Instability of mRNA expression signatures of drug transporters in chronic myeloid leukemia patients resistant to imatinib. Oncol Rep. 2013;29:741–750. doi: 10.3892/or.2012.2153. [DOI] [PubMed] [Google Scholar]
- 29.Eadie L, Hughes TP, White DL. Nilotinib does not significantly reduce imatinib OCT-1 activity in either cell lines or primary CML cells. Leukemia. 2010;24:855–857. doi: 10.1038/leu.2010.7. [DOI] [PubMed] [Google Scholar]
- 30.Eadie LN, Saunders VA, Hughes TP, White DL. Degree of kinase inhibition achieved in vitro by imatinib and nilotinib is decreased by high levels of ABCB1 but not ABCG2. Leuk Lymphoma. 2013;54:569–578. doi: 10.3109/10428194.2012.715345. [DOI] [PubMed] [Google Scholar]