Abstract
MicroRNA (miRNA) pathways have been implicated in stem cell regulation. This study investigated the molecular effects of propofol on adipocyte stem cells (ASCs) by analyzing RNA expression arrays. Human ASCs were isolated by use of a liposuction procedure. ASCs were treated with saline, 50 µM propofol, or 100 µM propofol in culture media for 3 hours. After the isolation of total RNA, the expression of 76 miRNAs was evaluated with peptide nucleic acid-miRNA array analysis through denaturation and hybridization processes. Treatment with 50 µM propofol resulted in significant down-regulation of expression of 18 miRNAs and upregulation of expression of 25 miRNAs; 100 µM propofol resulted in significant downregulation of expression of 14 miRNAs and upregulation of expression of 29 miRNAs. The lowest expression was seen for miR-204, which was 0.07-fold with 50 µM propofol and 0.18-fold with 100 µM propofol. The highest expression was seen for miR-208b, which was 11.23-fold with 50 µM propofol and 11.20-fold with 100 µM propofol. Expression patterns of miRNAs were not significantly different between 50 µM and 100 µM propofol treatment. The results of this study suggest that propofol is involved in altering the miRNA expression level in human ASCs. Additional research is necessary to establish the functional effect of miRNA alteration by propofol.
Keywords: microRNA, Propofol, Stem cell
INTRODUCTION
MicroRNA (miRNA) is an intrinsic RNA molecule that is made up of 20 to 25 nucleotides.1 miRNA regulates cell growth, differentiation, and apoptosis by negatively controlling post-transcriptional gene expression.2 In addition, miRNA plays a pivotal role in homeostasis and regulates biological and pharmacological alterations in stem cells.3 In recent studies, the variable expression of miRNA has been shown to play an important role in human pathologies such as cancer, viral diseases, and metabolic disorders.3,4
Because adipose stem cells (ASCs) can differentiate into other cells and can be easily obtained through a simple isolation method,5 they have a diverse spectrum of potential clinical implications. ASCs may offer great clinical utility in regenerative therapies and wound healing in various diseases.6
Propofol is widely used for sedation and clinical anesthesia. Efforts have been made to explain the molecular mechanisms of the effects of propofol on human cellular responses such as inhibition of inflammation and protection against oxidative stress.7 A recent report found that propofol anesthesia affects miRNA expression in rat liver.8 However, no studies have reported on changes in miRNA expression caused by treatment of human ASCs with propofol.
In this study, we hypothesized that expression of miRNA in ASCs would be affected by propofol treatment. Thus, we evaluated the effect of propofol on the miRNA expression profile in adipocyte-derived adult stem cells. We also analyzed the relationship between propofol concentration and miRNA expression.
MATERIALS AND METHODS
1. Isolation and culture of stem cells
Adipose-derived mesenchymal stem cells (courtesy of Dr. YI Yang)9 were isolated by use of a liposuction procedure. 10 Stromal vascular fraction (SVF) cells were obtained from adipose tissue (AT) by a modified enzyme-dissociating method.9 In short, after washing and cutting of the AT, the tissues were digested with type I collagenase. To separate SVF cells from mature adipocytes, tissue lysates were centrifuged at 300 ×g for 10 minutes. The pellet was resuspended in Dulbecco's modified Eagle medium (DMEM) containing 2% bovine serum (Hyclone, Provo, UT), and the suspension was filtered through 250-µm mesh (Millipore, Seoul, Korea) and then centrifuged at 300 ×g for 10 min. Resuspension was done in 154 mM NH4Cl. The final pellet was resuspended in a growth culture medium composed of a DMEM:Ham's F12 (1:1) mixture supplemented with 10% fetal bovine serum and a penicillin/streptomycin antibiotic mixture. The pellet was placed onto a tissue culture plate. To eliminate nonadherent cells, the plates were washed twice with Dulbecco's phosphate-buffered saline for 24 hours. The growth culture medium was replaced. Cells were subcultured at 80% confluence after the first plating. This initial passage was regarded as passage 1. Upon reaching 80% confluence, cells were detached by using Trypsin-EDTA solution.
In this experiment, the 2nd to 5th passages of ASCs were used. All procedures used equipment similar to that used in our former study.11
2. Propofol treatment
For propofol treatment, we used concentrations of 50 µM and 100 µM propofol. ASCs were treated with 50 µM or 100 µM propofol for 3 hours. After 3 hours, the propofol was removed. The cells were collected after washing with 5 ml media without fetal bovine serum and were carefully put into 1.5-ml tubes. The tubes were then frozen to prevent RNA lysis.
3. miRNA extraction
We extracted total RNA from each sample with the reagent Trizol (Life Technologies, Carlsbad, CA, USA). We extracted low molecular weight, enriched RNA from 50 µg total RNA by use of a mirVana miRNA extraction kit (Ambion, Inc., Austin, TX, USA). Quantification of extracted RNA was analyzed with an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). RNA was analyzed with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
4. miRNA expression profiling
In this experiment, owing to the stronger binding affinity and study expression aspect of miRNA, we used peptide nucleic acid miRNA array kits (Panazene, Daejeon, Korea) according to the manufacturer's protocol.
1) Denaturation and hybridization
The miRNA denatured mixture (400 ng miRNA and 15 µL RNase-free water) was cultured at 95℃ in a circulating water bath for 5 minutes and then moved to ice immediately. A total of 85 µL of hybridization mixture and the 15 µL of denatured mixture were combined for hybridization. The mixture was hybridized for 4 hours at 55℃ in a humid chamber.
To wash the microarray slides, the 20X washing buffer was diluted up to 250 ml of 1X washing buffer. The 1X washing buffer was added with a magnetic stir bar to a glass jar. After removal of the slide chambers, the microarray slides were loaded with a holder and immersed in the 1X washing buffer in the jar. Washed microarray slides were spun dry at 1,000 rpm for 5 minutes.
2) Ligation reaction:
We prepared a ligation mixture of 10 µL 10X T4 RNA ligase buffer, 2 µL 0.1% BSA, 3 µL pCp-Cy3, 1 µL T4 RNA ligase (10 U/µL), and 84 µL RNase-free water. We set the slide chambers on hybridized microarray slides and added 100 µL of the ligation mixtures to each well. The slides were cultured at 37℃ in a humid chamber for 2 hours and then washed immediately.
3) Scanning and image analysis
We scanned the hybridized arrays by use of an Axon GenePix 4000B scanner (Molecular Devices Corporation, Sunnyvale, CA, USA). We also collected the median spot intensities by use of Axon GenePix 4.0 (Molecular Devices, Sunnyvale, CA, USA). Analyses of expression profiling were performed in Gene-Spring 7.0 (Agilent Technologies, Santa Clara, CA, USA). We considered an alteration of miRNA expression to be significant when it was more than 1.5-fold or less than 0.5-fold.
5. miRNA target predictions
We predicted human targets of variable miRNA expression through use of the public web-based prediction tools TargetScan, PicTar, and MiRBase Target.
RESULTS
1. Measurement of RNA quality
The ratio of RNA quality for control and treated cells by use of the ribosomal RNA ratio was 28s/18s >1, OD260/OD230 >2.0, and OD260/OD280 >1.8. RIN (RNA integrity number) values were 8.0-10.0.12
2. miRNA expression profile of adipose stem cells treated with 50 uM propofol
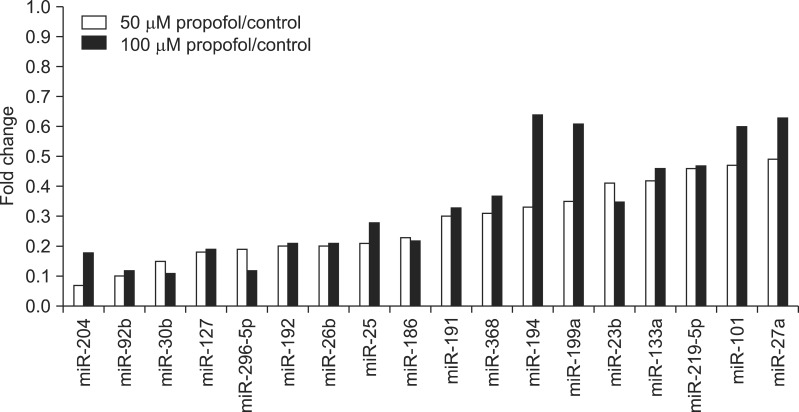
Microarray analysis of 76 miRNAs revealed significant downregulation of 18 miRNAs and upregulation of 25 miRNAs. Nine miRNAs (miR-204, -92b, -30b, -127, -296-5p, -192, -26b, -25, and -186) showed 0.07- to 0.23-fold differences from control. Nine miRNAs (miR-191, -368, -194, -199a, -23b, -133a, -219-5p, -101, and -27a) revealed 0.30- to 0.49-fold differences from control (Fig. 1).
FIG. 1.
Downregulated miRNAs after treatment of adipocyte stem cells with propofol. miRNAs displayed decreased by more than 50% with treatment. Each bar is the average of two adipocyte stem cells.
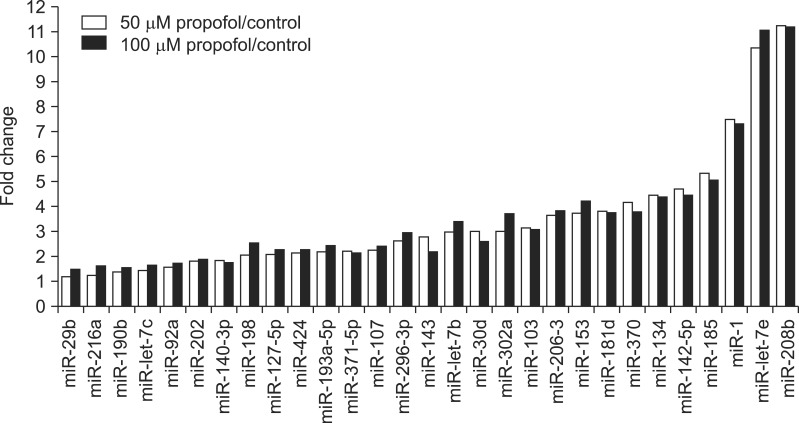
Twelve miRNAs (miR-92a, -202, -140-3p, -198, -127-5p, -424, -193a-5p, -371-5p, -107, -296-3p, -143, and let-7b) showed 1.57- to 2.98-fold differences from controls. Nine miRNAs (miR-30d, -303a, -103, -206-3, -153, -181d, -370, -134, and -142-5p) showed 3.00- to 4.70-fold differences from controls. Four miRNAs (miR-185, -1, let-7e, and -208b) had 5.31- to 11.23-fold differences from controls (Fig. 2).
FIG. 2.
Upregulated miRNAs after treatment of adipocyte stem cells with propofol. miRNAs displayed more than a 1.5-fold increase after treatment. Each bar represents the average of two adipocyte stem cells.
3. miRNA expression profile of adipose stem cells treated with 100 uM propofol
Microarray analysis with 76 miRNAs revealed significant downregulation of 14 miRNAs and upregulation of 29 miRNAs. Five miRNAs (miR-191, -368, -23b, -133a, and -219-5p) showed 0.30- to 0.49-fold differences from controls. Nine miRNAs (miR-204, -92b, -30b, -127, -296-5p, -192, -26b, -25, and -186) showed 0.11- to 0.28-fold differences from controls (Fig. 1).
Sixteen miRNAs (miR-29b, -216a, -190b, -let-7c, -92a, -202, -140-3p, -198, -127-5p, -424, -193a-5p, -371-5p, -107, -296-3p, -143, and -30d) showed 1.5- to 2.96-fold differences from controls. Nine miRNAs (miR-let-7b, -302a, -103, -206-3, -153, -181d, -370, -134, and -142-5p) showed 3- to 5-fold differences from controls. Expression of mir-185 was 5.07-fold different from control and expression of miR-1, let-7e, and 208b was 7.31-, 11.05-, and 11.20-fold different from controls, respectively (Fig. 2).
DISCUSSION
The results of the present study showed that propofol affects the miRNA expression of ASCs. Treatment with 50 µM propofol significantly downregulated 18 miRNAs and upregulated 25 miRNAs in ASCs. Treatment with 100 µM propofol significantly downregulated 14 miRNAs and upregulated 29 miRNAs. The expression of miR-194, -199a, -101, and -125a was significantly decreased only by 50 µM propofol. The expression of miR-29b, -216a, -190b, and -let-7c was significantly upregulated only by 100 µM propofol. However, overall, the expression patterns and miRNA levels were not significantly different between treatment with 50 µM and 100 µM propofol. These results suggest that propofol induced an alteration in miRNA expression, although the effect was not dose-dependent.
Few studies have reported on the relationship between miRNA and anesthetic agents. Ishikawa et al.8 reported that different miRNA expression patterns in rat liver are induced by sevoflurane and propofol, with 46 miRNAs of 177 (26%) differentially expressed in response to anesthetic agents. After sevoflurane anesthesia, 16 miRNAs were significantly upregulated and 11 were significantly downregulated compared with controls. By contrast, after propofol anesthesia, 31 miRNAs were upregulated and 8 were downregulated. Thus, the miRNA expression patterns were particular to the anesthetic agent. Another study evaluated the changes in the miRNA expression profile caused by different sevoflurane concentrations in rat lungs (2.0% vs 4.0%).13 Anesthesia with 2.0% sevoflurane induced changes in miRNA expression that were not significantly different from changes after 4.0% sevoflurane. For general anesthesia of humans, 20 to 35 µM is a typical plasma propofol concentration.14 Although doses in vitro are not similar to plasma concentrations, the concentrations of propofol (50 and 100 µM) used in this study were relatively high.
Expression changes in some miRNAs are involved in diseases and cancer. In this study, the expression of several miRNAs decreased to more than half of the expression in the controls. In general, downregulation of miRNAs indicates inhibition or inactivation of gene function. For example, in this study, the miRNA with the lowest expression was miR-204 with 0.07-fold expression after 50 µM and 0.18-fold expression after 100 µM propofol. Downregulated miR-204 might inhibit differentiation of human-derived cardiomyocyte progenitor cells.15 In addition, miR-204 inhibits osteogenesis and promotes adipogenesis of mesenchymal progenitor cells.16 Recently, miR-133a was reported to be significantly reduced in several types of cancer.17 miR-26b and miR-127 are associated with tumorigenesis or cell proliferation.18 The downregulation of miR-192 is involved in lipogenesis and obesity.19 miR-186 and miR-709 may be associated with a cascade of molecular reactions that are important in the control of the stress response.20 Feng et al.21 reported that the expression of miR-27a and miR-331-5p is inversely related to drug-resistant factor expression and gradually increasing drug resistance in leukemia cell lines. Thus, deregulation of miR-331-5p and miR-27a could be related to recurrence of leukemia.
Among the upregulated miRNAs in our study, the highest expression was seen for miR-208b, with 11.23-fold expression after treatment with 50 µM and 11.20-fold expression after treatment with 100 µM. Cardiac myocyteassociated miR-208b and miR-499 are overexpressed in the plasma of patients with acute myocardial infarction.22 miR-134 is known to be brain-specific and involved with the control of neuronal microstructure.23 Overexpression of miR-134 in vitro is related to reduced spine volume,23 whereas overexpression of miR-134 in vivo reduces total dendritic length.24 Other miRNAs, miR-153 and miR-198, are associated with parkinsonism and Alzheimer disease.25,26 Greco et al.27 demonstrated that miR-206-3 is associated with neurotransmitter regulation in developing neuronal cells. miR-30d has multiple functions. miR-30d~miR-30b and miR-23b~miR-27b~miR-24-1 are highly complex regulatory miRNA networks that contribute to the development of multi-drug resistance and the drug-resistant phenotype.28
Our study provides evidence that propofol treatment affects miRNA expression in human ASCs. Propofol is used as a sedative and hypnotic in clinical anesthesia. Although it had been reported that propofol causes many miRNA expression changes in rat liver, such findings had not yet been reported in human cells. Our results show that propofol, which is widely used in anesthesia, caused many miRNA expression changes in human ASCs. These changes in miRNA expression are anticipated to play an important role in distinctive gene-expression changes with propofol. Thus, further experiments are needed to clarify the meaning of the changes in miRNA expression in ASCs after propofol treatment.
This study had some limitations. First, we used commercially available propofol containing preservatives or lipid emulsion. Recently, some studies have reported the development of a therapeutic formulation using chemically synthesized miRNA with a lipid-based delivery vehicle that blocks tumor growth in mouse models. This formulation has effectiveness when administered locally or systemically.29 Although in this study our purpose was not to define the effect of lipid emulsions on miRNAs, we did not consider the effect of the lipid emulsion. Second, we did not conduct a functional study of the candidate miRNAs in ASCs. The results of this study provide basic data on the screening miRNA profiles of ASCs treated with propofol and an understanding of the molecular mechanism of propofol. Therefore, the next step will be a necessary functional study of the miRNAs that are strongly altered by clinical propofol anesthesia.
In conclusion, our results suggest that propofol is involved in altering miRNA expression in human ASCs. In addition, this study could provide candidates for investigating the molecular mechanism of action of propofol.
Footnotes
None declared.
References
- 1.Tomari Y, Zamore PD. Perspective: machines for RNAi. Genes Dev. 2005;19:517–529. doi: 10.1101/gad.1284105. [DOI] [PubMed] [Google Scholar]
- 2.Shruti K, Shrey K, Vibha R. Micro RNAs: tiny sequences with enormous potential. Biochem Biophys Res Commun. 2011;407:445–449. doi: 10.1016/j.bbrc.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 3.Gambari R, Fabbri E, Borgatti M, Lampronti I, Finotti A, Brognara E, et al. Targeting microRNAs involved in human diseases: a novel approach for modification of gene expression and drug development. Biochem Pharmacol. 2011;82:1416–1429. doi: 10.1016/j.bcp.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta. 2010;1803:1231–1243. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 6.Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res. 2014;163:399–408. doi: 10.1016/j.trsl.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Gu Y, Shao Z, Luo J, Tan Z. Propofol protects against hydrogen peroxide-induced oxidative stress and cell dysfunction in human umbilical vein endothelial cells. Mol Cell Biochem. 2010;339:43–54. doi: 10.1007/s11010-009-0368-y. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa M, Tanaka S, Arai M, Genda Y, Sakamoto A. Differences in microRNA changes of healthy rat liver between sevoflurane and propofol anesthesia. Anesthesiology. 2012;117:1245–1252. doi: 10.1097/ALN.0b013e3182746676. [DOI] [PubMed] [Google Scholar]
- 9.Yang YI, Kim HI, Shelby J, Choi MY, Jang SH, Kim JT, et al. Fibrin matrix-supported three-dimensional organ culture of adipose tissue for selective outgrowth, expansion, and isolation of adipose-derived stem cells. Acta Biomater. 2011;7:4109–4119. doi: 10.1016/j.actbio.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 11.Sung SH, Lee JG, Yu SB, Chang HK, Ryu SJ. The effects of lidocaine and procaine on microRNA expression of adipocyte-derived adult stem cells. Korean J Anesthesiol. 2012;62:552–557. doi: 10.4097/kjae.2012.62.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka S, Ishikawa M, Arai M, Genda Y, Sakamoto A. Changes in microRNA expression in rat lungs caused by sevoflurane anesthesia: a TaqMan® low-density array study. Biomed Res. 2012;33:255–263. doi: 10.2220/biomedres.33.255. [DOI] [PubMed] [Google Scholar]
- 14.Shafer A, Doze VA, Shafer SL, White PF. Pharmacokinetics and pharmacodynamics of propofol infusions during general anesthesia. Anesthesiology. 1988;69:348–356. doi: 10.1097/00000542-198809000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Xiao J, Liang D, Zhang H, Liu Y, Zhang D, Liu Y, et al. MicroRNA-204 is required for differentiation of human-derived cardiomyocyte progenitor cells. J Mol Cell Cardiol. 2012;53:751–759. doi: 10.1016/j.yjmcc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–364. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima S, Chiyomaru T, Kawakami K, Yoshino H, Enokida H, Nohata N, et al. Tumour suppressors miR-1 and miR-133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br J Cancer. 2012;106:405–413. doi: 10.1038/bjc.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji Y, He Y, Liu L, Zhong X. MiRNA-26b regulates the expression of cyclooxygenase-2 in desferrioxamine-treated CNE cells. FEBS Lett. 2010;584:961–967. doi: 10.1016/j.febslet.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 19.Chartoumpekis DV, Zaravinos A, Ziros PG, Iskrenova RP, Psyrogiannis AI, Kyriazopoulou VE, et al. Differential expression of micrornas in adipose tissue after long-term high-fat diet-induced obesity in mice. PLoS ONE. 2001;7:e34872. doi: 10.1371/journal.pone.0034872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babenko O, Golubov A, Ilnytskyy Y, Kovalchuk I, Metz GA. Genomic and epigenomic responses to chronic stress involve miRNA-mediated programming. PLoS One. 2012;7:e29441. doi: 10.1371/journal.pone.0029441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng DD, Zhang H, Zhang P, Zheng YS, Zhang XJ, Han BW, et al. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J Cell Mol Med. 2011;15:2164–2175. doi: 10.1111/j.1582-4934.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet. 2010;3:499–506. doi: 10.1161/CIRCGENETICS.110.957415. [DOI] [PubMed] [Google Scholar]
- 23.Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 24.Christensen M, Larsen LA, Kauppinen S, Schratt G. Recombinant Adeno-Associated Virus-Mediated microRNA Delivery into the Postnatal Mouse Brain Reveals a Role for miR-134 in Dendritogenesis in Vivo. Front Neural Circuits. 2010;3:16. doi: 10.3389/neuro.04.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouradian MM. MicroRNAs in Parkinson's disease. Neurobiol Dis. 2012;46:279–284. doi: 10.1016/j.nbd.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 26.Hansen T, Olsen L, Lindow M, Jakobsen KD, Ullum H, Jonsson E, et al. Brain Expressed microRNAs Implicated in Schizophrenia Etiology. PLoS ONE. 2007;2:e873. doi: 10.1371/journal.pone.0000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greco SJ, Rameshwar P. MicroRNAs regulate synthesis of the neurotransmitter substance P in human mesenchymal stem cell-derived neuronal cells. Proc Natl Acad Sci U S A. 2007;104:15484–15489. doi: 10.1073/pnas.0703037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husted S, Søkilde R, Rask L, Cirera S, Busk PK, Eriksen J, et al. MicroRNA expression profiles associated with development of drug resistance in Ehrlich ascites tumor cells. Mol Pharm. 2011;8:2055–2062. doi: 10.1021/mp200255d. [DOI] [PubMed] [Google Scholar]
- 29.Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D, et al. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther. 2011;19:1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]