Abstract
The aim of this study was to evaluate the risk factors for distant metastasis (DM) as a primary site of failure in early-stage breast cancer. Data from 294 patients diagnosed with pathologic stage I or II breast cancer between January 2000 and December 2005 were reviewed retrospectively. Median follow-up duration was 81.0 months (range, 18-135 months). The total number of patients with DM without evidence of locoregional recurrence was 20 and the median time between surgery and DM was 29 months (range, 9-79 months). Median survival time was 38 months (range, 22-77 months) after operation. HER-2 positivity (p=0.015), T stage of tumor (p=0.012), and number of involved lymph nodes (p=0.008) were significant predictors of DM in the univariable analysis. Number of involved lymph nodes [p=0.005, hazards ratio (HR): 1.741; 95% confidence interval (CI): 1.178-2.574] and HER-2 positivity (p=0.018, HR: 2.888; 95% CI: 1.201-6.941) had a statistically significant effect on DM-free survival in the multivariable analysis. A cautious evaluation may be helpful when patients with risk factors for DM have symptoms implying the possibility of DM. To reduce DM, applying intensive therapy is needed after curative surgery for patients with high risk for DM.
Keywords: Breast neoplasm, Neoplasm metastasis, Risk factors
INTRODUCTION
Breast cancer is known to have a relatively favorable survival rate in comparison with other cancers.1 In Korea, 5-year relative survival rates were reported as 89.9% during 2004-2008 for breast cancer patients.2 However, many patients developed relapse and the most frequent type of relapse in breast cancer was reported as distant metastasis (DM).3 In early-stage breast cancer, the 10-year cumulative incidence of DM after breast-conserving surgery was reported as 7.1%,4 and low-risk patients with node-negative small tumors have an approximately 10% to 25% risk of developing DM.1 Even in early-stage cancer, DM is a well-known cause of disease-related mortality. The previous metastasis model suggested that specific accessional genomic events result in the tumor having invasive characteristics and that the metastasis arises in the last steps of tumorigenesis.5 However, many reports have indicated that metastasis occurs earlier during tumor progression. Also, it is suggested that the progenitors for DM must be disseminated to metastatic sites before resection of the primary breast cancer.6,7 Thus, we need to be concerned about the chance of DM even for patients diagnosed with early-stage breast cancer.
After DM as the initial treatment failure, the 5- and 10-year survival rates for early-stage breast cancer are reported to be 22% and 9%, respectively.8 Early detection and intervention of metastatic foci can increase the quality of life in breast cancer patients. Often, however, the occurrence of metastasis is not as closely tracked in patients with early-stage breast cancer as in patients with advanced stage cancer. A need exists to define high-risk groups for metastasis in early-stage breast cancer. In addition, individualized therapy for the particular group with high risk for DM would produce more benefit from adjuvant treatment and reduce the chance for adverse effects by unnecessary treatment.9 This study was performed to determine the risk factors for DM as a primary recurrent site in early-stage breast cancer.
MATERIALS AND METHODS
1. Study subjects
Data were reviewed retrospectively for 320 patients who were diagnosed with pathologic stage I or II breast cancer (in accordance with the American Joint Committee on Cancer, 7th edition) without a history of neoadjuvant chemotherapy or other malignancy and who underwent operation or chemotherapy between January 2000 and December 2005. Among the 320 patients, 17 patients who developed locoregional recurrence (LR) as a first site of relapse and 9 patients who were diagnosed with LR and DM concurrently were excluded. The remaining 294 patients were enrolled. Every patient underwent a curative operation. Median follow-up duration was 81.0 months (range, 18 to 135 months).
2. Statistical analysis
We retrospectively reviewed the possible predictive factors for DM as a primary site of recurrence in each patient. Twenty patients who had DM as a first site of recurrence were compared with 274 patients who had no evidence of disease.
DM was defined as radiologic changes confirmed by at least 1 of the 4 imaging procedures: magnetic resonance imaging, computed tomography, positron emission tomography, and whole-body bone scan. We defined metastasis-free survival as the period from the date of operation to the date of radiologic confirmation of metastasis. We calculated follow-up duration from the date of surgery to the date of the last visit to a hospital.
Kaplan-Meier survival analysis with the log-rank test was used to analyze potential risk factors for DM in early-stage breast cancer. Multivariate analysis was performed by use of the Cox proportional hazard model with the forward stepwise method to evaluate the risk factors for DM. Analysis was executed by use of SPSS, version 19.0 (SPSS Inc, IBM Corp, Armonk, NY).
RESULTS
Among 158 patients with T1N0 disease, 36 patients (23%) were HER-2 (human epidermal growth factor receptor 2) positive. Of 40 patients with T1N1 disease, 6 patients (15%) were HER-2 positive. The number of patients with T2N0 disease was 51, and 17 patients (33%) showed HER-2 positivity. The number of patients with T2N1 disease was 39, and 15 patients (42%) were HER-2 positive. Six patients were diagnosed with T3N0 disease, and 1 patient (17%) showed HER-2 positivity. Further characteristics of the 294 patients are described in Tables 1 and 2.
TABLE 1.
Clinical characteristics of patients and methods of treatments
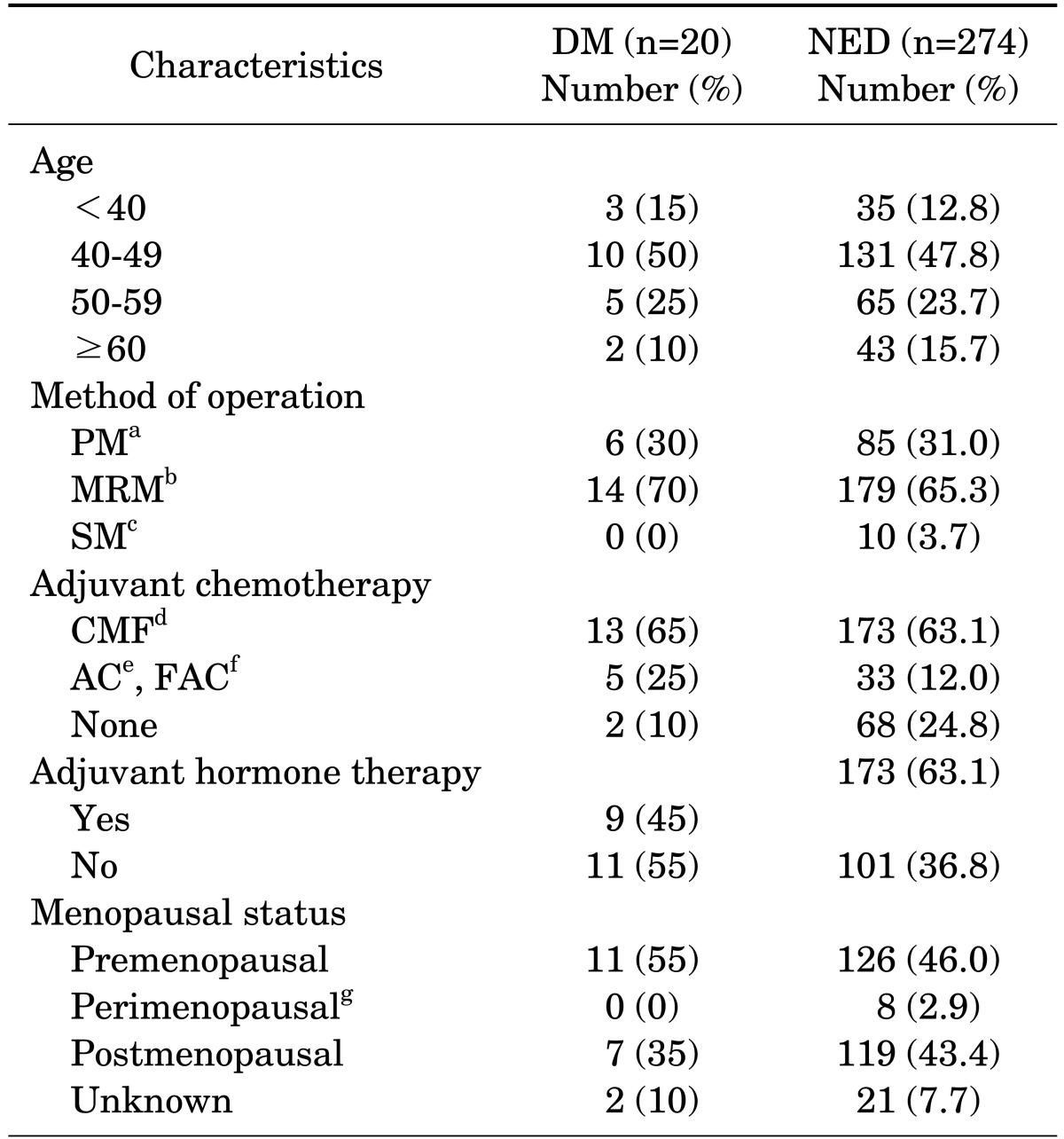
PM: partial mastectomy, MRM: modified radical mastectomy, SM: simple mastectomy, aEvery patient received external beam radiotherapy, bThree patients in DM group and 6 patients in NED group received external beam radiotherapy, cTwo patients received external beam radiotherapy, dCyclophophamide, methotrexate and 5-fluorouracil, eDoxorubicin and cyclophosphamide, f5-fluorouracil, doxorubicin and cyclophosphamide, gIrregular menstruation in 40s and 50s with history of menstruation within 3 months before curative therapy.
TABLE 2.
Pathologic characteristics of patients
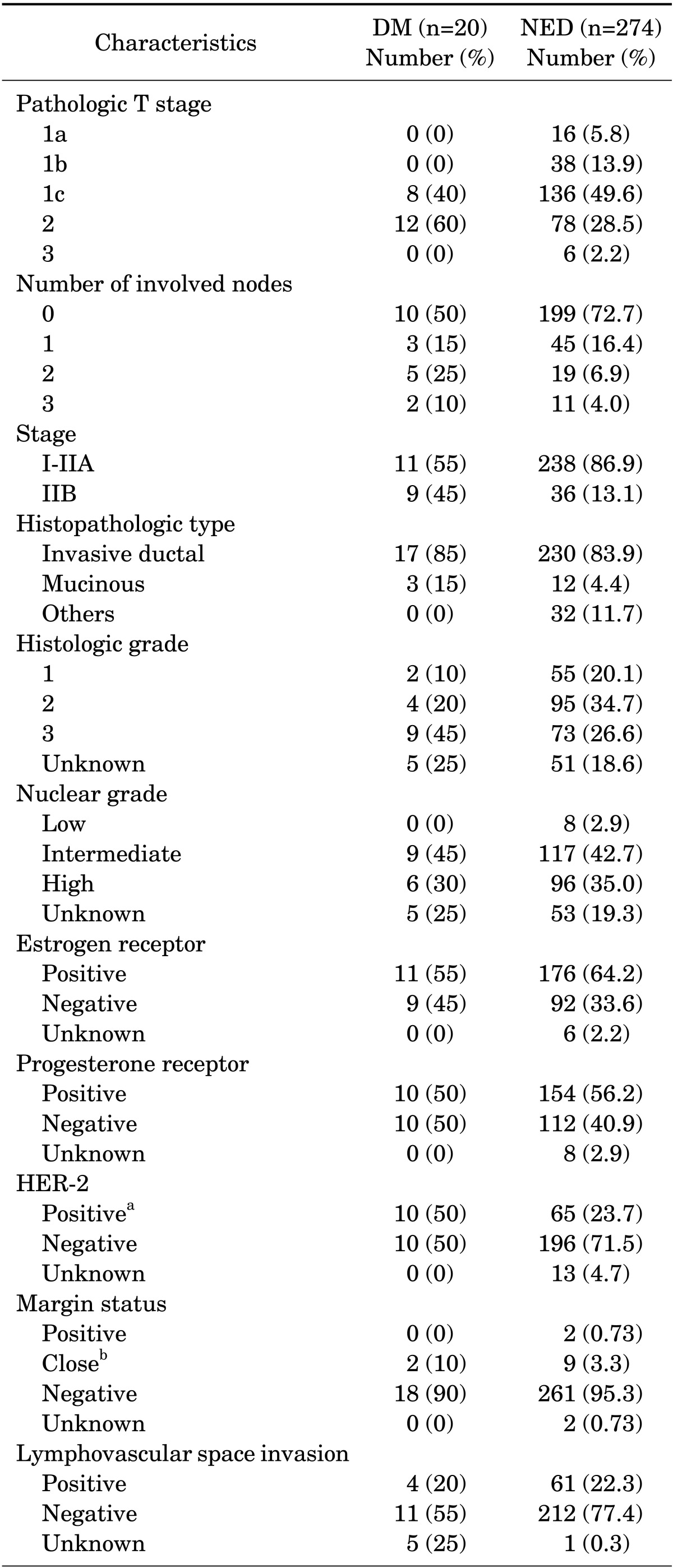
HER 2: human epidermal growth factor receptor 2, aThe results of immunohistochemistry 3+ or more than 2 copies of the HER-2 gene found in test using Fluorescence In Situ Hybridization, bDefined as microscopic identification of tumor cell within 2 mm from resection margin.
No patients with HER-2 positivity received trastuzumab. The 5-year overall survival rate was 89.8%, and the 5-year DM-free survival rate was 88.1%.
The total number of cases of DM without evidence of LR was 20 and the median elapsed time from surgery to DM was 29 months (range, 9-79 months). Twelve patients died, and the median survival time was 38 months (range, 22-77 months) after the operation and 12 months (range, 1-61 months) after the diagnosis of DM. Three patients who developed DM were not followed up.
The most common site of primary DM was bone (11 patients), and spine (7 patients) was the most frequently involved site among cases of bone metastasis. The pelvic bone (3 patients) was the next most frequent site. Among patients with visceral organ metastasis, 2 patients had primary lung metastasis and 2 patients had primary liver metastasis. One patient was diagnosed with liver and lung metastasis at the same time. Both skeletal and visceral metastasis was found in 2 patients, and distant lymph nodes were the primary site of metastasis in 2 patients. After the occurrence of DM, new sites of metastasis were found in 8 patients, and the interval to subsequent metastasis was 5 to 13 months. Only 4 patients had a single site of DM and the other 16 patients developed multiple metastatic foci during follow-up. Subsequent central nervous system metastasis developed in 2 patients.
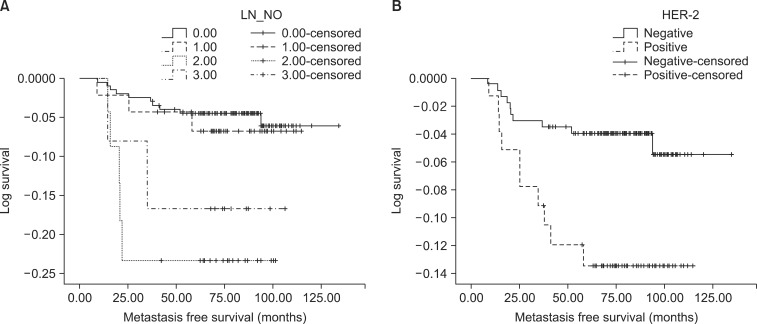
According to the results of the Kaplan-Meier survival analysis, a higher pT stage of tumor (p=0.008), increased number of involved lymph nodes (p=0.008), and HER-2 positivity (p=0.015) were all significant risk factors for DM in early-stage breast cancer (Fig. 1). The adjuvant chemotherapy group had a shorter metastasis-free survival (p=0.041; Table 3). Nineteen patients (8.4%) in the chemotherapy group and 1 patient (1.4%) who did not receive chemotherapy developed metastasis. The ratios of stage 1, 2A, and 2B were 44.4%, 36.9%, and 18.7% in the chemotherapy group and 87.0%, 8.7%, and 4.3% for patients who did not receive chemotherapy, respectively.
FIG. 1.
Kaplan-Meier log survival plot displaying metastasis free survival. (A) Metastasis free survival according to number of involved lymph node (log-rank test, p=0.008). (B) Metastasis free survival according to HER-2 positive (log-rank test, p=0.015).
TABLE 3.
Results of the univariate analysis for distant metastasis without locoregional recurrence
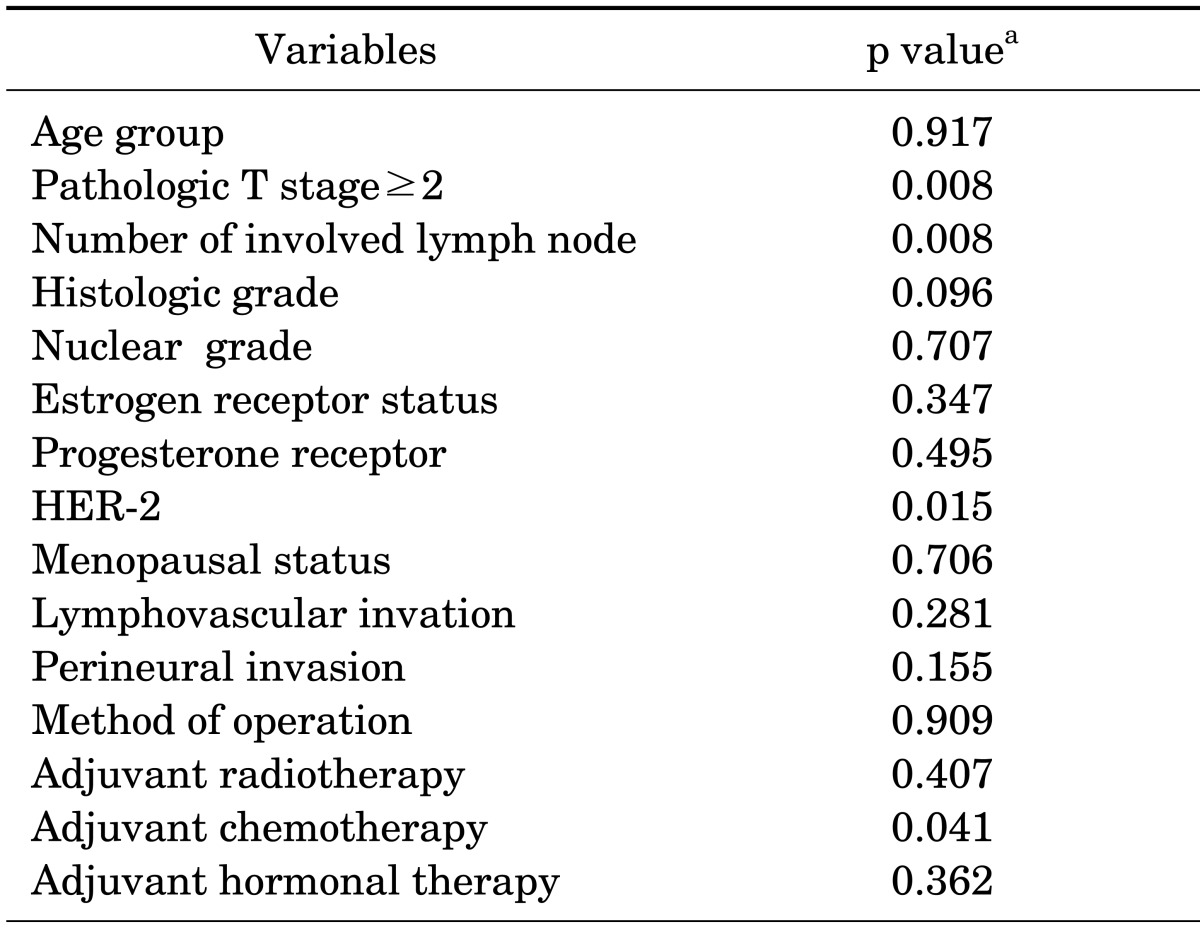
HER-2: human epidermal growth factor receptor-2. aAnalyzed using Kaplan-Meier survival analysis by the means of log-rank test.
All the covariates that were statistically significant in the univariate analysis were analyzed for the multivariate analysis. Values were missing for 14 cases (4.8%). Lymph node status was unknown for 1 case and HER-2 status was missing for 13 cases. A listwise deletion approach was used to handle missing data. The remaining 280 cases were enrolled in the multivariate analysis. HER-2 positivity [hazard ratio (HR): 2.88; 95% confidence interval (CI): 1.20-6.92, p=0.018] and number of involved lymph nodes (HR: 1.74; 95% CI: 1.18-2.57, p=0.006) had a statistically significant effect on DM-free survival (Table 4). The 5-year overall survival rate for the HER-2 positive and HER-2 negative group was 86.7% and 91.3%, respectively.
TABLE 4.
Results of the multivariate analysis for distant metastasis without locoregional recurrence
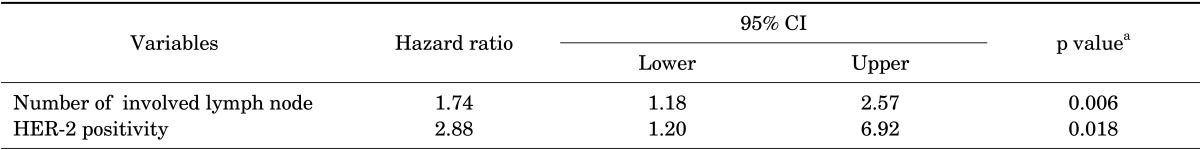
HER-2: human epidermal growth factor receptor-2. aAnalyzed using Cox proportional hazard model with forward stepwise method.
DISCUSSION
Many reports have suggested that LR is related to DM. Local recurrence within a short interval from surgery was reported as a significant prognostic factor for poor DM-free survival,10,11 and isolated LR was reported to increase the probability of DM.12,13 In some patients, however, DM is the primary site of disease recurrence. Mansell et al.3 reported that in a study of early-stage breast cancer, LR was 1.1% and DM was 4.8% for women with positive estrogen receptor and postmenopausal status during 2.5 years of follow-up. For patients who developed distant spread as a primary site of treatment failure, there is no time to give systemic therapies to prevent distant spread after local recurrence. Therefore, a need exists to define high-risk groups of DM without LR so that intensive adjuvant therapy can be given when the initial diagnosis is confirmed. Identifying high-risk groups for DM also helps the practitioner pay careful attention when symptoms that suggest the possibility of metastasis appear in this group. Therefore, we compared patients who developed DM as the only site of treatment failure with patients who had no evidence of disease. HER-2 positivity and the number of involved lymph nodes were significant predictors of DM without LR in the multivariable analysis.
HER-2 is known to have a role in increasing the growth and life-span of the primary tumor and also plays a part in disease dissemination. HER-2 is also known to increase metastatic cell mobility and to contribute to angiogenesis.6,14 The prevalence of HER-2 positivity is documented as 20% to 30% of breast cancer.9,15 Tsutsui et al.16 reported that HER-2 positivity turned out to be related to shorter disease-free survival and shorter overall survival. Also, the coexistence of negative estrogen receptor (ER) and positive HER-2 status showed the worst outcome in a subgroup analysis. Nguyen et al.17 reported that basal type [negative for ER, progesterone receptor (PR), and HER-2 receptor: adjusted HR=2.3; p=0.035] and luminal B type (positive ER and PR, HER-2 positive: adjusted HR=2.9; p=0.007) were significant risk factors for DM. In a study of T1NO patients, HER-2 type (negative ER and PR, HER-2 positive) had a higher risk of LR and DM compared with luminal A type (positive ER or PR, HER-2 negative).18 In other reports, however, HER-2 type was not a statistically significant predictor of a short distant disease-free survival in early-stage breast cancer patients who underwent breast-conserving therapy.19,20 In this study, HER-2 positivity (both HER-2 type and luminal B type) was a prognostic factor for DM without LR in stage I and II breast cancer. Many reports support the aggressiveness of triple negative and HER-2 positive breast cancer.16,17,18,21 However, the pattern of recurrence and outcome is not consistent among studies.19,20 Our results support the characteristics of HER-2, which is prone to develop metastasis in the early period of the disease course.
In our study, no patients underwent targeted therapy with trastuzumab. Thus, the comparison between the incidence of DM without LR and disease-free survival according to the usage of trastuzumab in the HER-2 positive group could not be performed. National Comprehensive Cancer Network (NCCN) guidelines version 2.2014 suggest adding adjuvant chemotherapy and trastuzumab after operation for patients with HER-2 positivity in cases with a tumor larger than 1 cm or the involvement of lymph nodes. However, trastuzumab was only administered since late 2005 in the studied institution. Thus, no patient had adjuvant targeted therapy with trastuzumab, which might have affected the rate of DM without LR and the prognosis of the patients in our study.
In this study, no patient who developed DM without LR had T1a or T1b disease. Although careful follow-up with many patients is needed to confirm the incidence of DM without LR in T1a and T1b patients, considering the cardiac toxicity and other potential toxicities of trastuzumab, our results support that it would be reasonable to omit trastuzumab in T1aN0 and T1bN0 patients.
Jatoi et al.22 reported that nodal metastasis is a marker of an aggressive phenotype. Furthermore, lymph node status at the time of diagnosis in breast cancer is known to be related to a poor outcome after relapse.23 These findings support that lymph node involvement at the time of diagnosis is not a reflection of tumor chronology, but an indicator of tumors with aggressive biological behavior. In our study, even 1 to 3 axillary lymph node metastases were related with poorer distant-disease-free survival. The aggressive nature of lymph node-involved disease needs to be considered compared with node-negative disease even of the same stage.
The number of patients with DM without LR was small and was a limitation of this study. Also, we could not analyze the effect of HER-2 positivity for DM-free survival after targeted therapy. However, our study adds information about the high-risk features of the HER-2 positive subgroup with a tumor size of more than 1 cm for DM without LR.
In conclusion, the HER-2 positive subtype and increased number of involved lymph nodes are associated with a high risk of DM without LR in early-stage breast cancer. In these patients, immediate evaluation is needed when symptoms implying the possibility of DM appear. To reduce DM, intensive adjuvant therapy is needed after curative treatments.
Footnotes
None declared.
References
- 1.Rugo HS. The importance of distant metastases in hormone-sensitive breast cancer. Breast. 2008;17(Suppl 1):S3–S8. doi: 10.1016/S0960-9776(08)70002-X. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43:1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansell J, Monypenny IJ, Skene AI, Abram P, Carpenter R, Gattuso JM, et al. Patterns and predictors of early recurrence in postmenopausal women with estrogen receptor-positive early breast cancer. Breast Cancer Res Treat. 2009;117:91–98. doi: 10.1007/s10549-008-0291-z. [DOI] [PubMed] [Google Scholar]
- 4.Mell LK, Jeong JH, Nichols MA, Polite BN, Weichselbaum RR, Chmura SJ. Predictors of competing mortality in early breast cancer. Cancer. 2010;116:5365–5373. doi: 10.1002/cncr.25370. [DOI] [PubMed] [Google Scholar]
- 5.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 6.Freudenberg JA, Wang Q, Katsumata M, Drebin J, Nagatomo I, Greene MI. The role of HER2 in early breast cancer metastasis and the origins of resistance to HER2-targeted therapies. Exp Mol Pathol. 2009;87:1–11. doi: 10.1016/j.yexmp.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantel K, Cote RJ, Fodstad O. Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst. 1999;91:1113–1124. doi: 10.1093/jnci/91.13.1113. [DOI] [PubMed] [Google Scholar]
- 8.Lê MG, Arriagada R, Spielmann M, Guinebretière JM, Rochard F. Prognostic factors for death after an isolated local recurrence in patients with early-stage breast carcinoma. Cancer. 2002;94:2813–2820. doi: 10.1002/cncr.10572. [DOI] [PubMed] [Google Scholar]
- 9.Hayes DF, Thor AD. c-erbB-2 in breast cancer: development of a clinically useful marker. Semin Oncol. 2002;29:231–245. doi: 10.1053/sonc.2002.32899. [DOI] [PubMed] [Google Scholar]
- 10.Monteiro Grillo I, Jorge M, Marques Vidal P, Ortiz M, Ravasco P. The effect of locoregional recurrence on survival and distant metastasis after conservative treatment for invasive breast carcinoma. Clin Oncol (R Coll Radiol) 2005;17:111–117. doi: 10.1016/j.clon.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Komoike Y, Akiyama F, Iino Y, Ikeda T, Akashi-Tanaka S, Ohsumi S, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer. 2006;106:35–41. doi: 10.1002/cncr.21551. [DOI] [PubMed] [Google Scholar]
- 12.Touboul E, Buffat L, Belkacémi Y, Lefranc JP, Uzan S, Lhuillier P, et al. Local recurrences and distant metastases after breast-conserving surgery and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys. 1999;43:25–38. doi: 10.1016/s0360-3016(98)00365-4. [DOI] [PubMed] [Google Scholar]
- 13.Koscielny S, Tubiana M. The link between local recurrence and distant metastases in human breast cancer. Int J Radiat Oncol Biol Phys. 1999;43:11–24. doi: 10.1016/s0360-3016(98)00424-6. [DOI] [PubMed] [Google Scholar]
- 14.Hynes NE. Tyrosine kinase signalling in breast cancer. Breast Cancer Res. 2000;2:154–157. doi: 10.1186/bcr48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, et al. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435–2446. [PubMed] [Google Scholar]
- 16.Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of c-erbB2 expression in breast cancer. J Surg Oncol. 2002;79:216–223. doi: 10.1002/jso.10079. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 18.Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, et al. Prognosis in women with small (T1mic, T1a, T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast Cancer Res Treat. 2011;127:713–720. doi: 10.1007/s10549-011-1465-7. [DOI] [PubMed] [Google Scholar]
- 19.Millar EK, Graham PH, O'Toole SA, McNeil CM, Browne L, Morey AL, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27:4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 20.Noh JM, Choi DH, Huh SJ, Park W, Yang JH, Nam SJ, et al. Patterns of recurrence after breast-conserving treatment for early stage breast cancer by molecular subtype. J Breast Cancer. 2011;14:46–51. doi: 10.4048/jbc.2011.14.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol. 2006;37:1217–1226. doi: 10.1016/j.humpath.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Jatoi I, Hilsenbeck SG, Clark GM, Osborne CK. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol. 1999;17:2334–2340. doi: 10.1200/JCO.1999.17.8.2334. [DOI] [PubMed] [Google Scholar]
- 23.Rack B, Janni W, Gerber B, Strobl B, Schindlbeck C, Klanner E, et al. Patients with recurrent breast cancer: does the primary axillary lymph node status predict more aggressive tumor progression? Breast Cancer Res Treat. 2003;82:83–92. doi: 10.1023/B:BREA.0000003955.73738.9e. [DOI] [PubMed] [Google Scholar]