Background: CaMKII autophosphorylates at Thr286 in cell-free lysates supplemented with glucose-6-phosphate (G6P).
Results: The B55β and C subunits of PP2A interact with and dephosphorylate CaMKII at novel sites following addition of G6P.
Conclusion: B55β/PP2A is critical for stimulating CaMKII in the presence of G6P.
Significance: This work identifies a novel role for a specific phosphatase complex in controlling CaMKII.
Keywords: Ca2+/Calmodulin-dependent Protein Kinase II (CaMKII), Metabolism, Mutagenesis, Phosphatase, Phosphorylation, Caspase 2
Abstract
High levels of metabolic activity confer resistance to apoptosis. Caspase-2, an apoptotic initiator, can be suppressed by high levels of nutrient flux through the pentose phosphate pathway. This metabolic control is exerted via inhibitory phosphorylation of the caspase-2 prodomain by activated Ca2+/calmodulin-dependent protein kinase II (CaMKII). We show here that this activation of CaMKII depends, in part, on dephosphorylation of CaMKII at novel sites (Thr393/Ser395) and that this is mediated by metabolic activation of protein phosphatase 2A in complex with the B55β targeting subunit. This represents a novel locus of CaMKII control and also provides a mechanism contributing to metabolic control of apoptosis. These findings may have implications for metabolic control of the many CaMKII-controlled and protein phosphatase 2A-regulated physiological processes, because both enzymes appear to be responsive to alterations in glucose metabolized via the pentose phosphate pathway.
Introduction
Apoptosis is a programmed form of cell death executed by caspase proteases. Multiple studies have implicated caspase-2 in cell stress-induced apoptosis (i.e. via DNA damage, endoplasmic reticulum stress, or heat shock) (1–5). Acting upstream of mitochondria in the intrinsic pathway (6), caspase-2 leads to cleavage of the pro-apoptotic Bcl-2 family member, Bid, to promote mitochondrial outer membrane permeabilization (7, 8).
In the Xenopus egg extract system, caspase-2 has also been tied to metabolic control of apoptosis (9–11). We have reported that caspase-2 is important for recapitulating apoptotic events in this system and that its activity can be modulated by controlling the metabolic status of the egg extracts. Specifically, incubation of extracts at room temperature reduced levels of pentose phosphate pathway (PPP)-generated3 NADPH, and supplementation of extracts with NADPH or PPP stimulatory glucose-6-phosphate (G6P) greatly delayed caspase-2 activation and ensuing apoptotic events (9). Biochemical analyses revealed that metabolic inhibition of caspase-2 was caused by inhibitory phosphorylation within the caspase-2 prodomain at Ser135 (Xenopus numbering). Using kinase inhibitors and immunodepletions, we found that this phosphorylation was catalyzed by the Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) and that CaMKII activity was elevated following G6P or NADPH treatment of extracts (9).
Four highly similar isoforms exist of CaMKII, which is an important mediator of many Ca2+-induced signaling pathways (12–15). Each isoform contains a catalytic domain near the N terminus, an autoregulatory domain, and a C-terminal association domain (16). When inactive, pseudosubstrate sequences bind and inhibit the catalytic domains (17). Ca2+/CaM binding disrupts catalytic and autoinhibitory domain interaction, activating the kinase and allowing access to an autophosphorylation site (Thr286, α isoform) (18). Once activated, within the holoenzyme, one subunit phosphorylates an adjacent subunit at Thr286 when both are bound to Ca2+/CaM (19). Once phosphorylated on Thr286, the Ca2+/CaM off-rate drops over 1000-fold, stabilizing CaMKII activity (20). Therefore, the autophosphorylation of Thr286 can be used as an indicator of CaMKII activation. Following Ca2+/CaM dissociation, Thr(P)286 CaMKII remains active, and further autophosphorylation occurs at Thr305, Thr306, and Ser314 (21, 22).
Recently, the Nutt laboratory reported that CoA, generated in Xenopus egg extracts in the presence of abundant nutrients, binds to and activates CaMKII (23). We show here that nutrient-driven CaMKII activation additionally requires release of a “brake.” Specifically, we identify two novel sites of CaMKII phosphorylation (Thr393/Ser395 on the Xenopus γ isoform L subunit and Thr371/Ser373 on the human homolog) located within the association domain, whose phosphorylation falls in the presence of high G6P levels. Dephosphorylation of these sites, catalyzed by protein phosphatase 2A (PP2A), is necessary (albeit not sufficient) for metabolic activation of CaMKII. In addition, nutrient-driven PP2A targeting to CaMKII is driven by metabolically regulated interaction of CaMKII with the PP2A targeting subunit B55β. Furthermore, this mechanism of CaMKII regulation is conserved in mammalian cells. Together, these findings provide insight into metabolic control of apoptosis and define a new mechanism for controlling CaMKII, a protein critical for cell signaling in response to multiple stimuli.
EXPERIMENTAL PROCEDURES
Preparation of Xenopus Egg Extracts and Nutrient Treatment
Xenopus egg extracts were prepared as previously described (24). G6P was prepared as a 1 m solution in water. Extracts were prepared at 4 °C, treated with G6P at a final concentration of 20 mm, and incubated at room temperature.
Cell Culture and Nutrient Treatment
HEK 293T cells were grown in DMEM with 10% FBS medium at 37 °C. Before nutrient treatment, cells were starved with glucose-free DMEM with 10% dialyzed FBS medium containing no d-glucose and sodium pyruvate at 37 °C for 12 h and then treated with or without 25 mm d-glucose (Sigma) for another 12 h. Cells were lysed in 50 mm Tris, pH 7.5, 150 mm NaCl, 1 mm DTT, and 1% Nonidet P-40 with 5 μg/ml aprotonin/leupeptin and 100 μm PMSF and phosphatase inhibitors (PhosSTOP Phosphatase Inhibitor Mixture Tablets from Roche, 20×) on ice.
siRNA Transfection
Lipofectamine RNAiMAX (Invitrogen) was used for siRNA transfection. PP2A-B55β siRNA was purchased from Santa Cruz Biotechnology to knock down B55β in 293T cells. Control siRNA was designed to target a nonmammalian protein, firefly luciferase (5′-CGUACGCGGAAUACUUCGA-3′).
Plasmids and Protein Preparation
Xenopus CaMKIIγ was amplified by PCR using the primers 5′-GGGGAATTCATGGCCACTACCCAGACTTGCACC-3′ and 5′-GGGCTCGAGTCACTGGAGAGGGGCTGCTGGTGC-3′. Purified PCR products were cloned into the EcoRI and XhoI sites of pENTR3C. The QuikChange site-directed mutagenesis kit (Stratagene) was used to generate point mutants in CaMKIIγ in pENTR3C. The T393A/S395A primers were 5′-CAGATGGGATAAAAGGATCAGCAGAGGCTTGCAACACCACCACTGAAG-3′ and its complement. The T393D/S395D primers were 5′-CAGATGGGATAAAAGGATCAGACGAGGATTGCAACACCACCACTGAAG-3′ and its complement. The Gateway LR Clonase II enzyme mix (Invitrogen) was used to transfer CaMKIIγ wild type and mutants from pENTR3C into pDEST24. Bacmid DNAs were produced by transforming pDEST24 plasmids into MAX Efficiency DH10Bac Chemically Competent Escherichia coli (Invitrogen) and transfected into SF9 cells using Cellfectin II reagent (Invitrogen).
Antibodies, Immunoprecipitation, and Immunodepletion
The following antibodies were used in this study: anti-CaMKII Thr(P)286 (Abcam), anti-CaMKII (BD Transduction Laboratories), anti-PP2A catalytic subunit (Millipore), anti-PP5 (BD Transduction Laboratories), anti-PPP2R1A (Abcam), anti-PPP2R2B (Abcam), anti-pan B56 (Millipore), and anti-GST (Santa Cruz Biotechnology). Proteins were measured by Western blot using LI-COR Biosciences Odyssey software or ECL method with HRP secondary antibody.
For immunoprecipitation, two micrograms of anti-CaMKIIα (Sigma) or Mouse control IgG were incubated with 20 μl of Dynabead protein G (Invitrogen) slurry overnight at 4 °C. 100 μl of Xenopus egg extracts treated with or without G6P were incubated with beads for 1 h at 4 °C. Beads were washed four times with wash buffer (described below) and eluted with SDS-PAGE sample buffer. Samples were resolved by SDS-PAGE for immunoblotting.
The wash buffer for immunoprecipitation: 300 mm NaCl and 0.1% Triton X-100 (Sigma) in 1× egg lysis buffer (10 mm HEPES, 50 mm KCl, 2.5 mm MgCl2, 1 mm DTT, and 0.25 m sucrose, pH 7.7).
For immunodepletion, 10 μg of PPP2R2B antibody (0.25 μg/μl) or rabbit control IgG were incubated with 100 μl of Dynabead protein A (Invitrogen) overnight at 4 °C. Beads were washed and divided into three equal parts. 90 μl of Xenopus egg extracts were incubated with beads for 1 h at 4 °C. This step was repeated three times.
Phospho-antibody Purification
The following peptides were synthesized to generate and purify phospho-antibody against Thr393 and Ser395 of Xenopus CaMKIIγ: [H]-VHNATDGIKGSTESCN-[NH2] (non-phospho-peptide), [H]-VHNATDGIKGS-Thr(P)-ESCN-[NH2] (Thr(P)393 peptide), and [H]-VHNATDGIKGSTE-Ser(P)-CN-[NH2] (Ser(P)395 peptide). Sera were run through a column of non-phospho-peptide conjugated with UltraLink Biosupport (Thermo Scientific) and were then purified on a column of phospho-peptide.
Gel Filtration Chromatography
Xenopus egg extracts were treated with G6P or water and co-treated with okadaic acid or DMSO. After incubation at room temperature for 4 h, the extracts were centrifuged at 200,000 × g and were fractionated on Superose 6 (GE Healthcare Life Sciences).
Nano-Flow Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry (LC-MS/MS) Analysis
Xenopus egg extracts were treated with G6P or water, and CaM-Sepharose was added (Agilent Technologies). After incubation, the CaM-Sepharose beads were collected by centrifugation and washed four times with 1× egg lysis buffer containing 500 mm NaCl and 0.5% Triton X-100 and then eluted with RapiGest SF Surfactant (Waters).
Following buffer exchange into 50 mm ammonium bicarbonate, pH 8.0, samples were subjected to a standardized reduction/alkylation procedure followed by overnight trypsin digestion according to the in-solution tryptic digestion protocol established by the Duke Center for Genomic and Computational Biology. Peptides were then either analyzed directly by LC-MS/MS analysis or subjected to a phosphopeptide enrichment with a 200 μl of TiO2 Protea Tip (Protea Bio) as per the manufacturer's recommended protocol. Samples were then subjected to LC-MS/MS analysis using a Waters NanoAquity UPLC equipped with a 1.7-μm BEH130 C18 75-μm inner diameter × 250-mm reversed phase column employing a 90-min gradient at 300 nl/min from 5% acetonitrile, 0.1% formic acid to 40% acetonitrile, 0.1% formic acid. Eluting peptides were analyzed on a Thermo LTQ-Orbitrap XL mass spectrometer set to acquire a precursor MS scan in the Orbitrap from m/z 400–2000 with r = 60,000 at m/z 400 and a target AGC setting of 1e6 ions. MS/MS spectra of the five most abundant precursor ions were acquired either in the Orbitrap with r = 7500 at m/z with a target AGC setting of 2e5 ions for nonenriched samples or in the ion trap with a target AGC setting of 1e3 for enriched samples. Max fill times were set to 1000 ms for full MS scans and either 500 ms for Orbitrap MS/MS scans or 250 ms for ion trap MS/MS scans with minimum MS/MS triggering thresholds of 5000 counts. For all experiments, fragmentation occurred in the LTQ linear ion trap with a CID energy setting of 35%, and a dynamic exclusion of 60 s was employed for previously fragmented precursor ions.
Raw LC-MS/MS data files were processed in Mascot distiller (Matrix Science) and then submitted to independent Mascot searches (Matrix Science) against an Trembl database (v 40.14 Xenopus laevis taxonomy, 12,530 forward entries) containing both forward and reverse entries of each protein. Search tolerances for LTQ-Orbitrap XL data were 5 ppm for precursor ions and 0.02 Da for Orbitrap product ions or 0.8 Da for ion trap product ions using full trypsin specificity. Carbamidomethylation (+57.0214 Da on Cys) was set as a fixed modification, whereas oxidation (+15.9949 Da on Met) and phosphorylation (+79.9663 Da on Ser, Thr, and Tyr) were considered variable modifications. All searched spectra were imported into Scaffold (v4.0, Proteome Software), and mascot ion scoring thresholds of 26 (p 0.05 Mascot identity score was 14.0) were set to achieve a false discovery rate of 0.0%. Probability of correct phosphorylation modification localization to a specific Ser, Thr, or Tyr residue was performed by submitting each MS/MS spectrum to the AScore algorithm with AScores of >15 or >19, indicating a >90% or >99% probability of correct localization, respectively. Relative quantitation was performed in Skyline (v1.4.1, University of Washington) by applying the full MS precursor extracted ion chromatogram function to integrate and measure peak areas (area under the curve) of each identified phosphopeptides. Reported area under the curve measurements for each phosphopeptide were the sum of the monoisotopic peak extracted ion chromatogram, as well as the second and third isotopomer extracted ion chromatogram. To adjust for slight variations in starting CaMKII prior to TiO2 enrichment, the average area under the curve of three nonphosphorylatable (i.e. did not contain a STY) peptides from the nonenriched LC-MS samples were used to generate a correction factor.
Kinase Assay
40 μl of glutathione-Sepharose containing 4 μg of recombinant GST tagged caspase-2 prodomain or GST-only fusion proteins were incubated in B55β-depleted or undepleted egg extracts together with 20 μCi of [γ-32P]ATP, treated by 20 mm G6P or water, at 30 °C for 1 h. Samples were washed, eluted with SDS-PAGE sample buffer, and resolved by SDS-PAGE for autoradiography.
RESULTS
CaMKII Activity Is under Metabolic Control
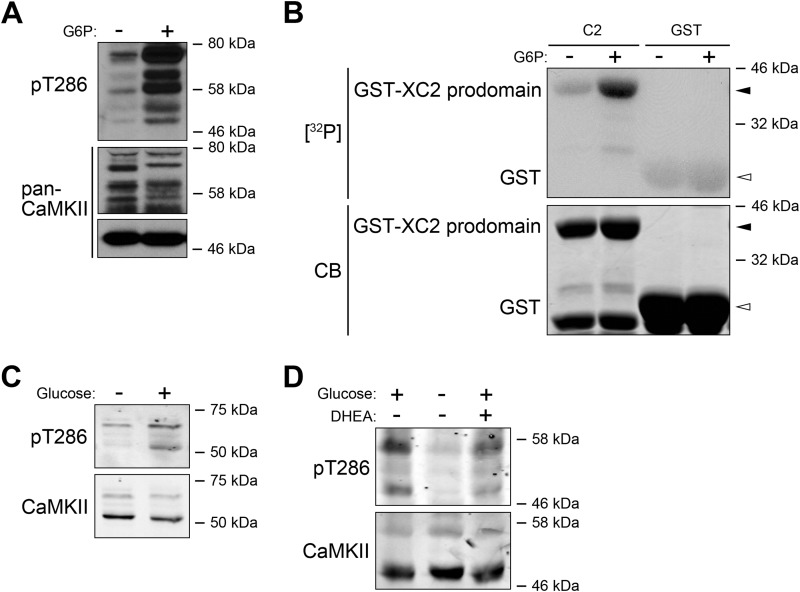
As we reported previously, treatment of Xenopus egg extracts with G6P should elevate the kinase activity of CaMKII (9). We first tested the autophosphorylation of Thr286, an indicator of CaMKII activation. By directly treating egg extracts with or without G6P, we discovered that G6P increased phosphorylation of Thr286 on CaMKII, a known site of CaMKII autophosphorylation (Fig. 1A; note that the multiple phosphorylated bands are likely due to multiple isoforms and allelic variants in the pseudotetraploid X. laevis). Although the total CaMKII antibody used here recognized predominantly the α isoform, with increased exposure time, additional CaMKII bands became evident, corresponding to multiple bands of Thr286 phosphorylation. This suggests that the observed increase in Thr286 phosphorylation following G6P treatment is likely generalizable to multiple CaMKII isoforms. Additionally, as we reported previously, the Xenopus caspase-2 prodomain (which we showed to be a CaMKII substrate (9)) added to egg extracts was more heavily phosphorylated in the presence of G6P, again consistent with the notion that the kinase activity of CaMKII is up-regulated by G6P (Fig. 1B). As also reported, CaMKII can physically bind the caspase-2 prodomain, which is stimulated by G6P treatment (25). Interestingly, an up-regulation of Thr286 phosphorylation was also observed in human 293T cells after glucose starvation and resupplementation (Fig. 1C). As we reported in Xenopus egg extracts, the increased activity of CaMKII depended on the activity of the PPP; glucose-induced Thr286 phosphorylation was reduced by co-treatment of 293T cells with dehydroepiandrosterone, an allosteric inhibitor of glucose-6-phosphate dehydrogenase, suggesting that the regulation of Thr286 phosphorylation by glucose is also through the PPP (Fig. 1D).
FIGURE 1.
CaMKII activation is sensitive to metabolic status. A, Xenopus egg extracts were treated with or without G6P for 0. 5 h at room temperature and analyzed for CaMKII Thr286 autophosphorylation using a Thr(P)286 antibody. Note that the middle and bottom panels are from two different films as the CaMKII antibody recognized CaMKIIα (∼50 kDa) much more strongly than the other isoforms, so detection of this isoform and the others required very different exposures. B, GST tagged Xenopus caspase-2 prodomain or GST bound to glutathione-Sepharose was incubated with Xenopus egg extracts supplemented with [γ-32P]ATP and treated with or without G6P. Samples were resolved by SDS-PAGE and detected by autoradiography. CB, Coomassie Blue. C, glucose-starved 293T cells treated with or without glucose (25 mm) were lysed and analyzed for CaMKII Thr286 autophosphorylation. D, glucose-starved 293T cells treated with or without glucose and with or without dehydroepiandrosterone (DHEA) were lysed and analyzed for CaMKII Thr286 autophosphorylation.
The Phosphorylation Status of CaMKII Is Altered by G6P Treatment of Egg Extracts
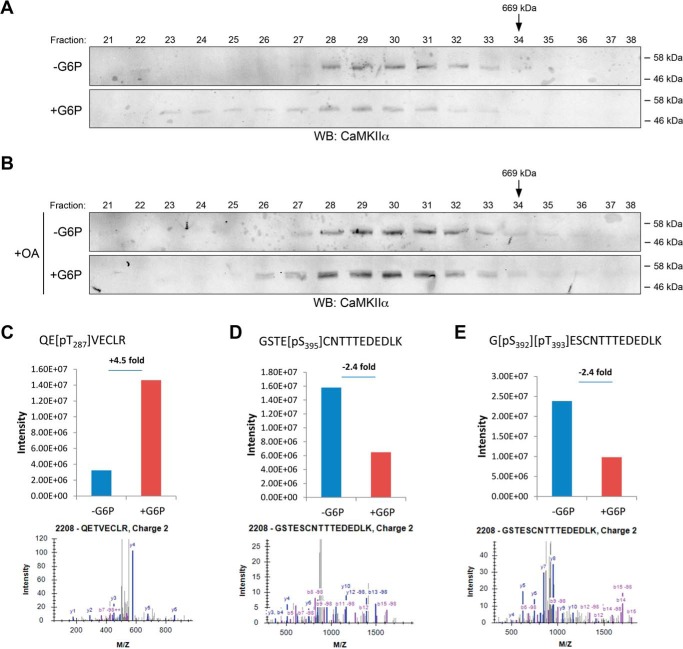
Although phosphorylation of Thr286 on CaMKII is stabilized following G6P treatment (25), it was not determined whether other modifications of CaMKII, upstream of activation, are modulated by metabolism. To examine the status of CaMKII in G6P-treated egg extracts, we resolved G6P-treated and untreated extracts by gel filtration and examined the profile of CaMKII fractionation. As shown in Fig. 2A, the apparent molecular weight of the CaMKII complex was increased following G6P treatment. It is possible that this G6P-induced shift in the CaMKII fractionation profile is caused by the association of additional proteins with CaMKII, because even in the untreated extract, the molecular weight of CaMKII was above 600,000, consistent with the dodecameric (potentially active) form; note that the shift might also be caused by the incorporation of other, higher molecular weight CaMKII isoforms into the CaMKII holoenzyme.
FIGURE 2.
Modification of CaMKII by metabolism. A, Xenopus egg extracts were treated with or without G6P for 4 h at room temperature and centrifuged at 200,000 × g. Cytosolic extracts were fractionated by gel filtration chromatography, and fractions were immunoblotted for CaMKII. B, Xenopus egg extracts treated with or without G6P and okadaic acid (OA) were fractionated and analyzed as in A. C–E, post-translational modifications of CaMKII were identified by MS analysis. Upper panels, change of phosphorylation with or without G6P. Lower panels, tandem mass spectra used for identification and localization of phosphorylation modifications on phosphopeptide containing corresponding site. C, Thr287 phosphorylation levels with or without G6P. D, Ser395 phosphorylation levels with or without G6P. E, Thr393 phosphorylation levels with or without G6P. WB, Western blot.
To further investigate the mechanism of CaMKII activation, we looked for post-translational modifications of CaMKII induced by G6P treatment. Endogenous CaMKII was precipitated from extracts treated with either G6P or buffer using CaM-Sepharose. These precipitates were then analyzed by mass spectrometry (MS). The predominant isoform identified was CaMKIIγ L subunit. MS analysis identified several phosphorylation sites on CaMKII that were responsive to G6P addition. As expected, we observed an increase in phosphorylated Thr287 (Thr286 on CaMKIIα) phosphorylation (Fig. 2C). More importantly, as shown in Fig. 2 (D and E), we identified two sites on CaMKII, Thr393 and Ser395, whose phosphorylation was decreased in response to G6P treatment. These data suggested that regulated dephosphorylation of these two sites could influence G6P-induced CaMKII activation.
Based on these observations, we also performed the gel filtration experiment described in Fig. 2A with or without the phosphatase inhibitor, okadaic acid. Although G6P treatment alone induced an upshift in the apparent molecular weight of CaMKII, as expected, co-treatment with the phosphatase inhibitor okadaic acid largely abrogated this up-shift, suggesting that some okadaic acid-inhibitable phosphatase(s) might be required for the observed G6P-induced CaMKII molecular weight upshift (Fig. 2B). Combined with the MS analysis result, these data suggested the possible involvement of some phosphatase(s) in G6P-induced CaMKII activation.
G6P Treatment Increases Binding of PP2A to CaMKII
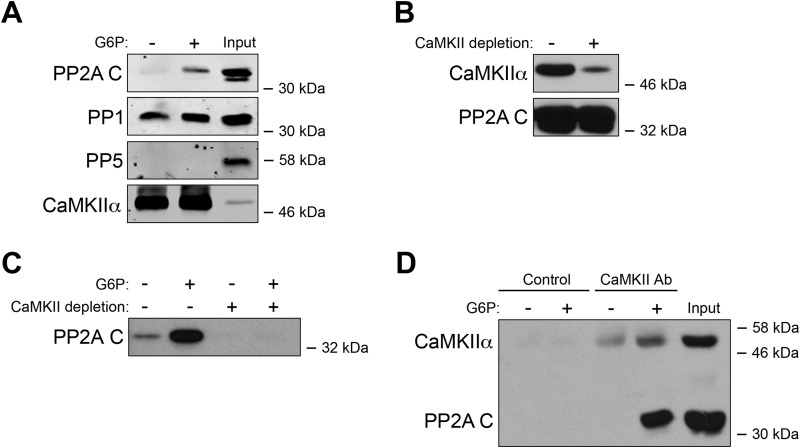
To determine whether a phosphatase is involved in CaMKII activation, CaM-Sepharose was used to pull down endogenous CaMKII from Xenopus egg extracts treated with or without G6P. Precipitates were immunoblotted for PP1, PP2A, and PP5. Although there was constitutive binding between PP1 and CaMKII, consistent with a previous report (26), this association did not appear to be regulated metabolically (Fig. 3A). Only PP2A exhibited increased interaction with CaM-Sepharose following G6P treatment. PP2A did not bind directly to CaM in the extract because prior removal of all CaM-associated proteins, including CaMKII (via CaM-Sepharose), did not affect total PP2A levels but prevented G6P-inducible binding of PP2A to CaM-Sepharose (Fig. 3, B and C). Furthermore, endogenous PP2A could be co-immunoprecipitated with anti-CaMKII antibody at greater levels in the presence of G6P (Fig. 3D; note that G6P treatment appeared to increase background CaMKII immunoprecipitation in the presence of G6P, but the PP2A interaction was elevated specifically in the presence of G6P). Together, these data strongly suggest that CaMKII interacts with PP2A, and this interaction is up-regulated by G6P-stimulated metabolism.
FIGURE 3.
CaMKII-PP2A interactions are regulated by G6P. A, CaM-Sepharose was dipped into Xenopus egg extracts that had been treated with or without G6P for 0.5 h at room temperature and incubated for 1 h at 4 °C. Beads were retrieved by centrifugation and analyzed for the presence of candidate phosphatases by immunoblotting. B, CaM-Sepharose was incubated with Xenopus egg extracts for 1 h at 4 °C and then removed by centrifugation. This process was repeated three times. Depleted and undepleted extracts were analyzed by CaMKII or PP2A immunoblotting. C, CaM-Sepharose was incubated with either CaMKII-depleted or undepleted extracts described in B treated with or without G6P. Beads were retrieved by centrifugation and analyzed by PP2A C subunit immunoblotting. D, CaMKII antibody or control IgG coupled to protein G beads was dipped into Xenopus egg extracts treated with or without G6P, incubated for 1 h at 4 °C, and retrieved for CaMKII or PP2A C immunoblotting.
B55β Targets PP2A to Regulate CaMKII Activation
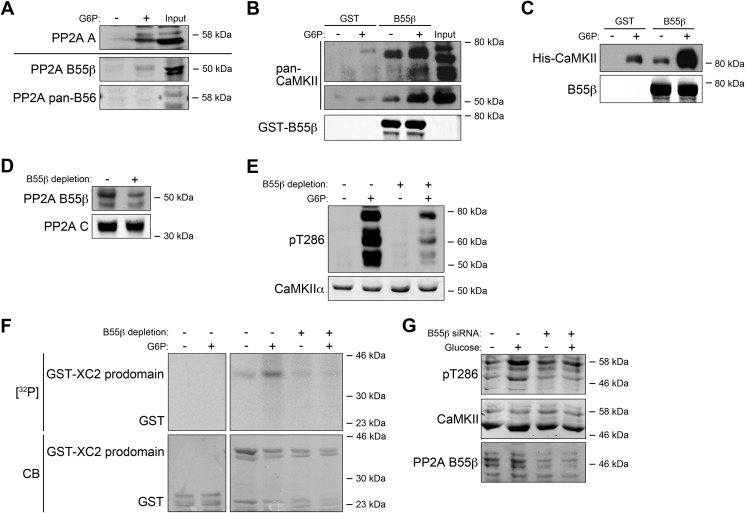
Functional PP2A is a multiprotein complex containing a catalytic (C) subunit, a scaffolding (A) subunit, and a regulatory (B) subunit. The B subunit typically determines substrate specificity. Four different B subunit families have been identified: B (PR55), B′ (B56), B″ (PR72), and B‴ (PR93). The B55 and B56 families have been implicated in cell proliferation/death in several settings (27, 28). We identified a B subunit subtype in the PR55 family, B55β whose interaction with CaM-Sepharose was up-regulated upon G6P treatment. B56 isoforms were unaffected (Fig. 4A). In agreement, G6P could stimulate the interaction of recombinant GST-tagged B55β protein with multiple CaMKII isoforms (Fig. 4B). In addition, recombinant CaMKIIγ (the isoform identified in the MS analysis) was added into egg extracts. Although G6P treatment increased background protein-protein interactions (GST alone could pull down some CaMKII protein), we were still able to see that G6P could significantly stimulate the interaction between B55β and the recombinant CaMKIIγ protein (Fig. 4C). Taken together, these data suggest that a complex containing CaMKII, PP2A C subunit, A subunit, and B55β, forms in the presence of abundant nutrients, potentially contributing to the metabolic activation of CaMKII, and that this increased protein complex formation stimulated by G6P seems to occur with multiple CaMKII isoforms.
FIGURE 4.
B55β regulates CaMKII activation. A, CaM-Sepharose was incubated with Xenopus egg extracts treated with or without G6P and incubated for 1 h at 4 °C. Beads were retrieved by centrifugation and analyzed by immunoblotting for PP2A regulatory subunits. B, GST-B55β or GST bound to glutathione-Sepharose was incubated for 1 h at 4 °C with Xenopus egg extracts treated with or without G6P. Beads were retrieved by centrifugation and analyzed for CaMKII or B55β immunoblotting. Note that the top and middle panels are from two different films with different exposures as the CaMKII antibody recognized CaMKIIα much more strongly than the other isoforms. C, Xenopus CaMKIIγ WT protein expressed from baculoviral vectors in SF9 cells was added into Xenopus egg extracts for 0.5 h at room temperature, and then the extracts were treated with or without G6P for 0.5 h at room temperature and incubated with GST-B55β or GST bound to glutathione-Sepharose for 1 h at 4 °C. The beads were retrieved by centrifugation and analyzed for CaMKII or B55β immunoblotting. D, B55β antibody or control rabbit IgG bound to Dynabead-linked protein A was incubated with Xenopus egg extracts for 1 h at 4 °C and removed with a magnet (repeated three times). Extracts were analyzed by B55β and PP2A C subunit immunoblotting. E, the B55β-depleted or undepleted extract was treated with or without G6P for 0.5 h and analyzed by pT286 immunoblotting. F, GST tagged Xenopus caspase-2 prodomain or GST bound to glutathione-Sepharose was incubated with B55β-depleted or undepleted Xenopus egg extracts supplemented with [γ-32P]ATP and treated with or without G6P. The samples were resolved by SDS-PAGE and detected by autoradiography. CB, Coomassie Blue. G, B55β-specific or control siRNA-treated 293T cells were glucose-starved for 12 h and then incubated with or without 25 mm glucose. The lysates were analyzed by immunoblotting for pT286, CaMKII, and B55β.
To determine whether B55β was required for the observed increased Thr286 phosphorylation of CaMKII following G6P treatment, we immunodepleted B55β from egg extracts and monitored G6P-induced alterations in CaMKII phosphorylation. As shown in Fig. 4D, B55β was largely depleted from egg extracts without significant depletion of the PP2A C subunit. The depleted and undepleted extracts were treated with or without G6P, and the Thr286 autophosphorylation of CaMKII was monitored. Compared with undepleted extracts, the depleted extracts showed significantly diminished Thr286 autophosphorylation in the presence of G6P (Fig. 4E). In addition, by using the GST-tagged Xenopus caspase-2 prodomain as a substrate, we were able to monitor the ability of B55β-depleted and undepleted extracts to phosphorylate caspase-2, treated with or without G6P. We found that the undepleted extracts could phosphorylate caspase-2 in the presence of G6P, whereas this phosphorylation was largely diminished by B55β depletion (Fig. 4F). Because Thr286 autophosphorylation and caspase-2 phosphorylation were both indicators of CaMKII activation, both measures indicated diminished CaMKII activation in the B55β-depleted extracts, suggesting that B55β is critical for G6P-driven CaMKII activation in the Xenopus egg extract system. In addition, the fact that the immunodepletion of B55β diminished multiple Thr286 autophosphorylation bands suggests that B55β can regulate multiple CaMKII isoforms (Fig. 4E). Moreover, when endogenous B55β was knocked down by B55β siRNA in 293T cells, the glucose-driven stimulation of CaMKII Thr286 autophosphorylation was suppressed, suggesting that the role of B55β in regulating CaMKII is evolutionarily conserved (Fig. 4G).
PP2A Dephosphorylates Ser395 on CaMKII in the Presence of G6P
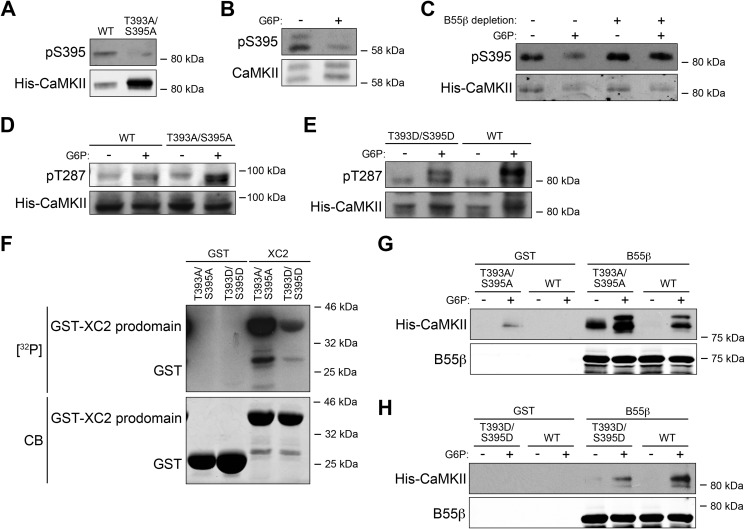
Because the MS analysis showed that Thr393 and Ser395 of CaMKII were dephosphorylated in the presence of G6P, we postulated that these sites might be targeted by B55β-PP2A. To assess this, we generated phospho-antibodies recognizing each of these sites on the Xenopus CaMKIIγ isoform. Although we were unable to produce a high quality antibody specific for phosphorylated Thr393, we were successful with Ser(P)395. As shown in Fig. 5A, WT CaMKIIγ protein (SF9, baculovirus produced) exhibited basal phosphorylation of Ser395. However, the S395A mutation greatly diminished antibody recognition, attesting to its phospho-specificity. To confirm that the phosphorylation level of Ser395 was down-regulated by G6P, we incubated Xenopus egg extract with or without G6P, precipitated endogenous CaMKII with CaM-Sepharose, and immunoblotted with the phospho-antibody. As shown in Fig. 5B, phosphorylation of Ser395 was down-regulated in the presence of G6P (note that the γ isoform is shown in the loading control as the Ser395 antibody was designed based on a γ phosphopeptide sequence). Additionally, when B55β was depleted from the Xenopus egg extract, the G6P effect was inhibited, strongly suggesting that Ser395 was dephosphorylated by the B55β-PP2A (Fig. 5C). Although the Ser395 antibody was unable to recognize the CaMKIIα isoform, because B55β immunodepletion could suppress the activation of all CaMKII isoforms, it is quite possible that Ser344 (the equivalent residue to Ser395 in the α isoform) is also dephosphorylated by B55β-PP2A.
FIGURE 5.
B55β-mediated Thr393/Ser395 dephosphorylation contributes to CaMKII activation. A, Xenopus WT and T393A/S395A mutant CaMKIIγ expressed from baculoviral vectors in SF9 cells were analyzed by Ser(P)395 immunoblotting. B, CaM-Sepharose was incubated with Xenopus egg extracts treated with or without G6P, retrieved by centrifugation, and analyzed by Ser(P)395 immunoblotting. C, B55β-depleted or undepleted Xenopus egg extracts with the addition of recombinant CaMKIIγ were treated with or without G6P. Recombinant CaMKIIγ was precipitated with CaM-Sepharose. The beads were retrieved by centrifugation and analyzed for Ser(P)395. D, CaMKIIγ WT or T393A/S395A mutant was added into Xenopus egg extracts for 0.5 h at room temperature, and then the extracts were treated with or without G6P and analyzed for pT287. E, CaMKIIγ WT or T393D/S395D mutant was treated and processed as in D. F, GST tagged Xenopus caspase-2 prodomain or GST bound to glutathione-Sepharose was incubated with Xenopus CaMKIIγ T393A/S395A or T393D/S395D mutants, calmodulin, and [γ-32P] ATP at 30 °C for 0.5 h. Samples were resolved by SDS-PAGE and detected by autoradiography. CB, Coomassie Blue. G, Xenopus CaMKIIγ WT or T393A/S395A mutant proteins were added into Xenopus egg extracts for 0.5 h at room temperature, and then the extracts were treated with or without G6P for 0.5 h at room temperature and incubated with GST-B55β or GST bound to glutathione-Sepharose for 1 h at 4 °C. The beads were retrieved by centrifugation and analyzed for CaMKII or B55β immunoblotting. H, Xenopus CaMKIIγ WT or T393D/S395D mutant proteins were added into Xenopus egg extracts for 0.5 h at room temperature, and then the extracts were treated with or without G6P for 0.5 h at room temperature and incubated with GST-B55β or GST bound to glutathione-Sepharose for 1 h at 4 °C. Beads were retrieved by centrifugation and analyzed for CaMKII or B55β immunoblotting.
The Phosphorylation Status of Thr393 and Ser395 Affects CaMKII Activation
To determine whether B55β-mediated dephosphorylation of Ser395 is critical for nutrient-driven activation of CaMKII, we first added CaMKIIγ WT, T393A/S395A mutant, and T393D/S395D mutant proteins into Xenopus egg extract, treated with or without G6P, and monitored Thr287 (Thr286 for CaMKIIα) autophosphorylation. As shown in Fig. 5D, after only 10 min of incubation with G6P, the T393A/S395A mutant exhibited higher phosphorylation of Thr287, compared with WT CaMKII. Consistent with these data, G6P-induced stimulation of CaMKII Thr287 autophosphorylation was significantly dampened by the T393D/S395D mutations (which may not perfectly mimic phosphorylation) (Fig. 5E). In addition, an in vitro kinase assay was performed, incubating CaMKIIγ T393A/S395A or T393D/S395D with calmodulin and the Xenopus caspase-2 prodomain. We found that the CaMKII T393A/S395A was more able than the T393D/S395D to phosphorylate the caspase-2 prodomain, consistent with the idea that the dephosphorylation of Thr393 and Ser395 is important for CaMKII activation (Fig. 5F). Finally, CaMKIIγ WT, T393A/S395A, and T393D/S395D were added into egg extracts, treated with or without G6P, and pulled down using GST-tagged B55β to monitor the interaction between B55β and CaMKII proteins. We found that the T393A/S395A mutant exhibited stronger interactions and that the T393D/S395D showed weaker interactions with B55β, compared with the WT CaMKII, suggesting that the dephosphorylation of Thr393 and Ser395 might stabilize the binding of B55β to CaMKII (Fig. 5, G and H). Collectively, these data suggest that nutrient status is communicated to CaMKII in part through the B55β/PP2A-mediated dephosphorylation of CaMKII, a novel locus for the control of CaMKII.
DISCUSSION
Although calcium is a central regulator of CaMKII, previous work from our lab has shown that the centrifugal removal of Ca2+ stores from Xenopus egg extracts does not impede G6P-mediated activation of CaMKII, suggesting that increased available Ca2+ does not underlie nutrient-dependent CaMKII activation. Studies of CaMKII regulation have largely focused on Thr286 autophosphorylation after Ca2+/CaM binding. Indeed, G6P treatment of egg extract impairs PP1-mediated dephosphorylation of Thr286 (25). However, this is unlikely to drive CaMKII activation because this phosphorylation is a result of activation. It has also been reported that CoA generation is increased downstream of G6P addition to egg extracts (though the mechanism underlying this increase is not clear) and that CoA can directly bind to and activate CaMKII. We have now identified an additional metabolically regulated break to CaMKII activation that must be lifted for G6P to robustly activate CaMKII via dephosphorylation of Thr393/Ser395.
In addition to Thr393/Ser395 phosphorylation, MS analysis also identified several previously uncharacterized sites with increased phosphorylation in the presence of G6P: Ser311, Ser326, Ser333, and Thr421 (data not shown). Although we have not yet investigated these sites, they may contribute to full CaMKII activation. The T393D/S395D mutant of CaMKII is less potently activated by G6P than WT CaMKII, consistent with a requirement for phosphorylation of additional sites for full activation. Furthermore, the T393A/S395A mutant is not spontaneously active, so there must be additional metabolic input for full activation, likely CoA (the high levels of exogenous CoA used in the published experiments may have forced activation of CaMKII despite the brake (23)).
Effects of G6P-stimulated Dephosphorylation on CaMKII
Interestingly, most of the sites whose phosphorylation was altered by nutrient status are located in the association domain of CaMKII, responsible for self-association (29, 30). However, our gel filtration results suggest that oligomerization itself is not affected by phosphorylation. Rather, G6P appears to shift the molecular weight of the full holoenzyme, suggesting association of additional factors. Importantly, the molecular weight shift was largely abrogated by treatment with okadaic acid, consistent with the idea that dephosphorylation of Thr393/Ser395 might be required. It is attractive to speculate that Thr393/Ser395 dephosphorylation might allow binding of additional regulatory factors to the CaMKII association domain. Interestingly, one variant of CaMKIIγ (CaMKIIγG-2) contains within its association domain a targeting sequence essential for ERK activation and contractility of smooth muscle cells, suggesting that the association domain can perform roles other than self-association (31).
Regulation of Thr393/Ser395 Phosphorylation
Our data demonstrate a previously unknown role for the B55β targeting subunit of PP2A in regulating CaMKII. Often, the B subunit not only targets PP2A to the correct substrate(s) but also serves as a key locus of regulation. For example, Chk1-mediated phosphorylation B56 can inhibit PP2A-mediated dephosphorylation and activation of the Cdc25 mitotic regulator during DNA damage checkpoint signaling (27). Although it is possible that the CamKII-B55β association is regulated at the level of CaMKII modification, we speculate that B55β may be differentially modified in a nutrient-dependent manner to alter targeting of PP2A to CaMKII. Such a modification could be sensitive to CoA levels.
Thr393 and Ser395 are phosphorylated in the egg extract before any treatment. Therefore, Xenopus egg extracts must contain a kinase(s) directed against these sites. Because Thr393/Ser395 phosphorylation decreases prior to CaMKII activation, these residues are clearly not autophosphorylation sites. The relevant kinase is not currently known but may provide an additional locus of metabolic regulation if its activity is high in the presence of abundant nutrients and low upon their depletion. Future experiments will be directed toward the identification and characterization of kinase(s) directed toward these sites.
Metabolic Regulation of CaMKII in Other Signaling Pathways
CaMKII is ubiquitously expressed and has a diverse array of substrates. For example, CaMKIIγ regulates the contractility of smooth muscle cells, and reduced contractile force has been observed to be associated with altered metabolism. Although this decrease in force has been associated with impaired Ca2+ flux, metabolically controlled dephosphorylation of Thr393 and Ser395 might also control the activation of CaMKIIγ in smooth muscle cells. It will be interesting to determine whether these sites play a similar role in regulating the highly abundant CaMKIIα isoform in neurons, which is important for long term potentiation. Similarly, B55β may control CaMKII in other physiological paradigms of regulation or metabolic control, warranting further investigation in other physiological settings.
Acknowledgments
We thank Dr. M. Arthur Moseley for supporting the MS analysis and Dr. Christopher Freel for assistance with all aspects of figure preparation.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 GM080333.
- PPP
- pentose phosphate pathway
- CaMKII
- calmodulin-dependent protein kinase II
- CaM
- calmodulin
- PP2A
- protein phosphatase 2A
- G6P
- glucose-6-phosphate.
REFERENCES
- 1. Cheung H. H., Lynn Kelly N., Liston P., Korneluk R. G. (2006) Involvement of caspase-2 and caspase-9 in endoplasmic reticulum stress-induced apoptosis: a role for the IAPs. Exp. Cell Res. 312, 2347–2357 [DOI] [PubMed] [Google Scholar]
- 2. Robertson J. D., Enoksson M., Suomela M., Zhivotovsky B., Orrenius S. (2002) Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J. Biol. Chem. 277, 29803–29809 [DOI] [PubMed] [Google Scholar]
- 3. Tu S., McStay G. P., Boucher L. M., Mak T., Beere H. M., Green D. R. (2006) In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nat. Cell Biol. 8, 72–77 [DOI] [PubMed] [Google Scholar]
- 4. Ho L. H., Taylor R., Dorstyn L., Cakouros D., Bouillet P., Kumar S. (2009) A tumor suppressor function for caspase-2. Proc. Natl. Acad. Sci. U.S.A. 106, 5336–5341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parsons M. J., McCormick L., Janke L., Howard A., Bouchier-Hayes L., Green D. R. (2013) Genetic deletion of caspase-2 accelerates MMTV/c-neu-driven mammary carcinogenesis in mice. Cell Death Differ. 20, 1174–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lassus P., Opitz-Araya X., Lazebnik Y. (2002) Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 297, 1352–1354 [DOI] [PubMed] [Google Scholar]
- 7. Guo Y., Srinivasula S. M., Druilhe A., Fernandes-Alnemri T., Alnemri E. S. (2002) Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J. Biol. Chem. 277, 13430–13437 [DOI] [PubMed] [Google Scholar]
- 8. Bonzon C., Bouchier-Hayes L., Pagliari L. J., Green D. R., Newmeyer D. D. (2006) Caspase-2-induced apoptosis requires bid cleavage: a physiological role for bid in heat shock-induced death. Mol. Biol. Cell 17, 2150–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nutt L. K., Margolis S. S., Jensen M., Herman C. E., Dunphy W. G., Rathmell J. C., Kornbluth S. (2005) Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell 123, 89–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nutt L. K., Buchakjian M. R., Gan E., Darbandi R., Yoon S. Y., Wu J. Q., Miyamoto Y. J., Gibbons J. A., Andersen J. L., Freel C. D., Tang W., He C., Kurokawa M., Wang Y., Margolis S. S., Fissore R. A., Kornbluth S. (2009) Metabolic control of oocyte apoptosis mediated by 14-3-3ζ-regulated dephosphorylation of caspase-2. Dev. Cell 16, 856–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andersen J. L., Thompson J. W., Lindblom K. R., Johnson E. S., Yang C. S., Lilley L. R., Freel C. D., Moseley M. A., Kornbluth S. (2011) A biotin switch-based proteomics approach identifies 14-3-3ζ as a target of Sirt1 in the metabolic regulation of caspase-2. Mol. Cell 43, 834–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waxham M. N., Malenka R. C., Kelly P. T., Mauk M. D. (1993) Calcium/calmodulin-dependent protein kinase II regulates hippocampal synaptic transmission. Brain Res. 609, 1–8 [DOI] [PubMed] [Google Scholar]
- 13. Silva A. J., Stevens C. F., Tonegawa S., Wang Y. (1992) Deficient hippocampal long-term potentiation in α-calcium-calmodulin kinase II mutant mice. Science 257, 201–206 [DOI] [PubMed] [Google Scholar]
- 14. Rokolya A., Singer H. A. (2000) Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am. J. Physiol. Cell Physiol. 278, C537–C545 [DOI] [PubMed] [Google Scholar]
- 15. Sun P., Enslen H., Myung P. S., Maurer R. A. (1994) Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 8, 2527–2539 [DOI] [PubMed] [Google Scholar]
- 16. Kolodziej S. J., Hudmon A., Waxham M. N., Stoops J. K. (2000) Three-dimensional reconstructions of calcium/calmodulin-dependent (CaM) kinase IIα and truncated CaM kinase IIα reveal a unique organization for its structural core and functional domains. J. Biol. Chem. 275, 14354–14359 [DOI] [PubMed] [Google Scholar]
- 17. Colbran R. J., Smith M. K., Schworer C. M., Fong Y. L., Soderling T. R. (1989) Regulatory domain of calcium/calmodulin-dependent protein kinase: II. mechanism of inhibition and regulation by phosphorylation. J. Biol. Chem. 264, 4800–4804 [PubMed] [Google Scholar]
- 18. Colbran R. J., Fong Y. L., Schworer C. M., Soderling T. R. (1988) Regulatory interactions of the calmodulin-binding, inhibitory, and autophosphorylation domains of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 263, 18145–18151 [PubMed] [Google Scholar]
- 19. Hanson P. I., Meyer T., Stryer L., Schulman H. (1994) Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron 12, 943–956 [DOI] [PubMed] [Google Scholar]
- 20. Meyer T., Hanson P. I., Stryer L., Schulman H. (1992) Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science 256, 1199–1202 [DOI] [PubMed] [Google Scholar]
- 21. Patton B. L., Miller S. G., Kennedy M. B. (1990) Activation of type II calcium/calmodulin-dependent protein kinase by Ca2+/calmodulin is inhibited by autophosphorylation of threonine within the calmodulin-binding domain. J. Biol. Chem. 265, 11204–11212 [PubMed] [Google Scholar]
- 22. Hanson P. I., Schulman H. (1992) Inhibitory autophosphorylation of multifunctional Ca2+/calmodulin-dependent protein kinase analyzed by site-directed mutagenesis. J. Biol. Chem. 267, 17216–17224 [PubMed] [Google Scholar]
- 23. McCoy F., Darbandi R., Lee H. C., Bharatham K., Moldoveanu T., Grace C. R., Dodd K., Lin W., Chen S. I., Tangallapally R. P., Kurokawa M., Lee R. E., Shelat A. A., Chen T., Green D. R., Harris R. A., Lin S. H., Fissore R. A., Colbran R. J., Nutt L. K. (2013) Metabolic activation of CaMKII by coenzyme A. Mol. Cell 52, 325–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smythe C., Newport J. W. (1991) Systems for the study of nuclear assembly, DNA replication, and nuclear breakdown in Xenopus laevis egg extracts. Methods Cell Biol. 35, 449–468 [DOI] [PubMed] [Google Scholar]
- 25. McCoy F., Darbandi R., Chen S. I., Eckard L., Dodd K., Jones K., Baucum A. J., 2nd, Gibbons J. A., Lin S. H., Colbran R. J., Nutt L. K. (2013) Metabolic regulation of CaMKII protein and caspases in Xenopus laevis egg extracts. J. Biol. Chem. 288, 8838–8848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shields S. M., Ingebritsen T. S., Kelly P. T. (1985) Identification of protein phosphatase 1 in synaptic junctions: dephosphorylation of endogenous calmodulin-dependent kinase II and synapse-enriched phosphoproteins. J. Neurosci. 5, 3414–3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Margolis S. S., Perry J. A., Forester C. M., Nutt L. K., Guo Y., Jardim M. J., Thomenius M. J., Freel C. D., Darbandi R., Ahn J. H., Arroyo J. D., Wang X. F., Shenolikar S., Nairn A. C., Dunphy W. G., Hahn W. C., Virshup D. M., Kornbluth S. (2006) Role for the PP2A/B56delta phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell 127, 759–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Healy A. M., Zolnierowicz S., Stapleton A. E., Goebl M., DePaoli-Roach A. A., Pringle J. R. (1991) CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol. Cell. Biol. 11, 5767–5780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kolb S. J., Hudmon A., Ginsberg T. R., Waxham M. N. (1998) Identification of domains essential for the assembly of calcium/calmodulin-dependent protein kinase II holoenzymes. J. Biol. Chem. 273, 31555–31564 [DOI] [PubMed] [Google Scholar]
- 30. Shen K., Meyer T. (1998) In vivo and in vitro characterization of the sequence requirement for oligomer formation of Ca2+/calmodulin-dependent protein kinase IIα. J. Neurochem. 70, 96–104 [DOI] [PubMed] [Google Scholar]
- 31. Marganski W. A., Gangopadhyay S. S., Je H. D., Gallant C., Morgan K. G. (2005) Targeting of a novel Ca2+/calmodulin-dependent protein kinase II is essential for extracellular signal-regulated kinase-mediated signaling in differentiated smooth muscle cells. Circ. Res. 97, 541–549 [DOI] [PubMed] [Google Scholar]