Background: Mammalian olfaction has circadian rhythm, and glycosylation plays critical roles in the olfactory system.
Results: α1–2-Fucosylation increases during the nighttime in axons of secondary olfactory neurons in WT but not in Clock mutant mice.
Conclusion: Rhythmic α1–2-fucosylation governed by clock genes is a potential mechanism of circadian olfaction.
Significance: Glycosylation in the central nervous system is circadian.
Keywords: Circadian Rhythm, Clock Gene, Glycosylation, Lectin, Olfaction
Abstract
The circadian clock regulates various behavioral and physiological rhythms in mammals. Circadian changes in olfactory functions such as neuronal firing in the olfactory bulb (OB) and olfactory sensitivity have recently been identified, although the underlying molecular mechanisms remain unknown. We analyzed the temporal profiles of glycan structures in the mouse OB using a high-density microarray that includes 96 lectins, because glycoconjugates play important roles in the nervous system such as neurite outgrowth and synaptogenesis. Sixteen lectin signals significantly fluctuated in the OB, and the intensity of all three that had high affinity for α1–2-fucose (α1–2Fuc) glycan in the microarray was higher during the nighttime. Histochemical analysis revealed that α1–2Fuc glycan is located in a diurnal manner in the lateral olfactory tract that comprises axon bundles of secondary olfactory neurons. The amount of α1–2Fuc glycan associated with the major target glycoprotein neural cell adhesion molecule (NCAM) varied in a diurnal fashion, although the mRNA and protein expression of Ncam1 did not. The mRNA and protein expression of Fut1, a α1–2-specific fucosyltransferase gene, was diurnal in the OB. Daily fluctuation of the α1–2Fuc glycan was obviously damped in homozygous Clock mutant mice with disrupted diurnal Fut1 expression, suggesting that the molecular clock governs rhythmic α1–2-fucosylation in secondary olfactory neurons. These findings suggest the possibility that the molecular clock is involved in the diurnal regulation of olfaction via α1–2-fucosylation in the olfactory system.
Introduction
Endogenous oscillators control various behavioral and physiological circadian rhythms in most living organisms ranging from bacteria to humans. The olfactory bulb (OB)2 has recently been identified as a circadian oscillator that mediates daily changes in mammalian olfaction, and levels of olfactory sensitivity in rodents are far higher during the early nighttime than the daytime (1, 2). The firing of secondary olfactory neurons of the OB (3), as well as the suprachiasmatic nucleus (4), habenular nucleus (5), cerebellum (6), and hippocampus (7) is circadian. The molecular mechanisms generating circadian olfaction remain obscure, although diurnal fluctuations have been found among connexins, AMPA receptors, and monoamines in the OB (8, 9).
Several molecular findings suggest that the periodic expression of clock genes drives the circadian oscillator in various tissues. Basic helix-loop-helix/Per-Arnt-Sim (PAS) transcription factors such as CLOCK, NPAS2, and BMAL1 are positive regulators of an autoregulatory transcription-translation feedback loop of the molecular circadian clock (10). Clock was the first clock gene to be identified in vertebrates by forward mutagenesis using N-ethyl-N-nitrosourea in a behavioral screening. The Clock allele is truncated and causes a deletion of 51 amino acids, but the mutation does not have a significant effect on the N-terminal basic helix-loop-helix and PAS domains, leaving CLOCK dimerization and DNA binding intact (10). Hundreds of circadian clock-controlled genes that regulate an impressive diversity of biological processes in peripheral tissues have been identified (11, 12). Granados-Fuentes et al. (2) showed that canonical clock genes are involved in the regulation of circadian olfactory sensitivity in mice, but the underlying mechanisms remain unknown.
Glycosylation affects the functional properties of proteins as well as lipids and regulates several cellular functions in various tissues. Glycosylation plays critical roles in neuronal formation such as neurite outgrowth and synaptogenesis in the OB (13), and the abundance and location of glycoconjugate moieties vary among developmental stages in the main olfactory bulb (14) and in the accessory olfactory bulb (AOB), which is the primary center of the vomeronasal system (15). We postulated that glycoconjugates in the OB regulate circadian rhythms in olfaction, because several recent studies have found that glycosylation is involved in regulation of the circadian clock (16, 17).
We evaluated temporal changes in glycan structures in the OB using a high-density lectin microarray, which is a useful platform for glycan analysis (18). Lectins are proteins that bind with high affinity to specific glycan structures (19). We then investigated diurnal variations of the α1–2-fucose (α1–2Fuc) glycan, because all three lectins that had high affinity for this glycan in our microarray significantly fluctuated in a diurnal manner. Histochemical analysis using Ulex europaeus agglutinin-I (UEA-I), which detects α1–2Fuc glycan, confirmed circadian variation of α1–2Fuc glycan in axon bundles of secondary olfactory neurons. Real-time PCR and Western blotting revealed diurnal mRNA and protein expression of Fut1, respectively, in the OB. Daily fluctuations in α1–2Fuc glycan and Fut1 expression were severely dampened in Clock mutant mice, suggesting the possibility that the molecular clock is involved in diurnal regulation of olfaction via α1–2-fucosylation in the OB.
EXPERIMENTAL PROCEDURES
Animals
Male C57BL/6NCrSlc and ICR mice (Japan SLC), as well as Clock mutant mice on an ICR background (20) were maintained under a 12-h light:12-h dark cycle (lights on at 08:00 as zeitgeber time (ZT) 0) at a controlled ambient temperature of 24 ± 1 °C. This study proceeded in accordance with the guidelines for the Care and Use of Laboratory Animals at the National Institute of Advanced Industrial Science and Technology (AIST), and all procedures were approved by the Animal Care and Use Committee at AIST (approval number 2013-020).
Lectins and Antibodies
Lectins from natural sources (58 lectins, see supplemental Table S1) were purchased from J-OIL MILLS, Vector Laboratories, EY Laboratories, and Seikagaku Corporation, and 38 recombinant lectins (supplemental Table S1) were prepared as described (21). Biotinylated UEA-I was purchased from Sigma (catalog number L8262). A mouse monoclonal antibody against neural cell adhesion molecule (NCAM) and secondary antibody were purchased from Santa Cruz (123A8, sc-59864) and the Jackson Laboratory (115-036-003), respectively, for Western blotting and immunoprecipitation. Another mouse monoclonal antibody against NCAM and secondary antibody were purchased from LifeSpan Bioscience (ERIC-1, LS-C49053-100) and the Jackson Laboratory (115-066-003), respectively, for immunohistochemistry. A rabbit polyclonal antibody against fucosyltransferase 1 (FUT1) and secondary antibody were purchased from Aviva Systems Biology (ARP44222) and GE Healthcare (NA934), respectively.
Lectin Microarray Production
Lectin microarrays were produced as described (21) with a minor modification. Briefly, 96 lectins were dissolved in spotting solution (0.5 mg/ml each) and spotted onto epoxysilane-coated glass slides in triplicate using a MicroSys4000 non-contact microarray printing robot (Genomic Solution). Lectins immobilized on the slides were incubated with blocking reagent N102 (NOF Co.) and stored at 4 °C. Spot quality and the reproducibility of the microarrays were confirmed before use as described (21).
Lectin Microarray Analysis
Male C57BL/6NCrSlc mice (age, 26 weeks; n = 3 per group) were killed by cervical dislocation at 10:00 (ZT2) and 22:00 (ZT14), and the OB and liver (as control) were homogenized. Hydrophobic fractions isolated from these sources using the CelLytic MEM Protein Extraction kit (Sigma) as described by the manufacturer were labeled with fluorescent Cy3 monoreactive dye (GE Healthcare), and excess Cy3 was removed by desalting through columns containing Sephadex G-25 (GE Healthcare). The protein concentration was adjusted to 2 μg/ml with PBS-T (10 mm PBS, pH 7.4, 140 mm NaCl, 2.7 mm KCl, and 1% Triton X-100), then the hydrophobic fractions were labeled with Cy3 NHS ester (GE Healthcare), diluted with probing buffer (25 mm Tris-HCl, pH 7.5, 140 mm NaCl, 2.7 mm KCl, 1 mm CaCl2, 1 mm MnCl2, and 1% Triton X-100) to 0.5 μg/ml, applied to the lectin microarray, and left overnight. After washing with probing buffer, images were acquired using an evanescent field-activated GlycoStation Reader 1200 fluorescence scanner (GP BioSciences). Fluorescence signals emitted by each spot were quantified using Array Pro Analyzer version 4.5 (Media Cybernetics), and the background value was subtracted. Lectin signals from triplicate spots were averaged and normalized to the mean value of the 96 lectins immobilized on the microarray.
Western Blotting
Male ICR and Clock mutant mice (age, 11 weeks; n = 3 per group) were killed by cervical dislocation at 10:00 (ZT2), 16:00 (ZT8), 22:00 (ZT14), and 4:00 (ZT20). Proteins were extracted from OB homogenates using extraction buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% Triton X-100, 5 mm EDTA and protease inhibitor mixture; Roche Diagnostics). Whole protein extracts (20 μg/lane) resolved by SDS-PAGE (10% polyacrylamide) were blotted onto PVDF membranes and processed for UEA-I detection or for anti-NCAM or anti-FUT1 immunoreaction.
The membranes were incubated with 3% powdered skim milk in PBS followed by biotinylated UEA-I (1 μg/ml) or anti-NCAM antibody (1:200) in PBS for 1 h. Bound anti-NCAM antibody was reacted with biotinylated secondary antibody (1:5000) in PBS for 1 h. The biotinylated substances were reacted with the avidin-biotin complex reagent (Vector Laboratories) for 30 min. These complexes were stained using 0.02% 3,3′-diaminobenzidine tetrahydrochloride (DAB) dissolved in 50 mm Tris-HCl containing 0.006% H2O2 for 1 min (rapid DAB staining) or detected using ImmunoStar LD (Wako Pure Chemicals). The negative control comprised UEA-I that had been pre-absorbed with 0.5 m l-fucose.
Other membranes were incubated with Block Ace (Dainippon Pharmaceutical) in PBS followed by anti-FUT1 antibody (0.25 μg/ml) in PBS for 1 h. The bound antibody was reacted with secondary antibody (1:5000) in PBS for 1 h and detected using ImmunoStar LD. The amounts of α1–2Fuc glycan, NCAM, and FUT1 were normalized relative to the amount of β-actin.
Immunoprecipitation
Male ICR mice (age 11 weeks; n = 3–4 per group) were killed by cervical dislocation at 10:00 (ZT2), 16:00 (ZT8), 22:00 (ZT14), and 4:00 (ZT20). Proteins (100 μg) extracted from OB were incubated with anti-NCAM antibody (5 μg) and Protein A/G-agarose (Santa Cruz) overnight at 4 °C. After washing with extraction buffer, the pellets were resolved by SDS-PAGE (7.5% polyacrylamide), blotted onto PVDF membranes, and reacted with biotinylated UEA-I (1 μg/ml). Bound UEA-I was reacted with the avidin-biotin complex reagent and detected using ImmunoStar LD. The amount of α1–2Fuc glycan was normalized relative to that of NCAM.
Histochemical Analysis
Male ICR and Clock mutant mice (age, 12 weeks; n = 3 per group) were anesthetized at 10:00 (ZT2) and 22:00 (ZT14) with an intraperitoneal injection of pentobarbital (0.20 mg/g body weight), sacrificed by cardiac perfusion with 4% paraformaldehyde fixative, and histochemically processed as described (22) with a minor modification. Briefly, the OB was routinely embedded in paraffin and cut sagittally into 5-μm thick sections, which were deparaffinized, rehydrated, and incubated with 0.3% H2O2 in methanol followed by 3% normal goat serum. The sections were incubated with biotinylated UEA-I (20 μg/ml) or anti-NCAM antibody (1:50) overnight in PBS at 4 °C. The sections that had been incubated with anti-NCAM antibody were reacted with biotinylated secondary antibody (1:200) in PBS for 1 h. Those with biotinylated complexes were reacted with the avidin-biotin complex reagent and colored with DAB. The negative control comprised UEA-I that had been pre-absorbed with 0.05–0.5 m l-fucose.
Quantitation of Histochemical Staining Intensity
Gray scale images were inversed and measured using ImageJ (National Institutes of Health) software. A lower intensity threshold was adopted for negative regions on sections. Mean signal intensity was quantified within the glomerular layer of the AOB and the lateral olfactory tract, which contains most axons of secondary olfactory neurons.
Real-time RT-PCR
Male ICR and Clock mutant mice (age, 12 weeks; n = 4–5 per group) were killed by cervical dislocation at 10:00 (ZT2), 16:00 (ZT8), 22:00 (ZT14), and 4:00 (ZT20). Total RNA was extracted from the OB using RNAiso Plus (Takara Bio), and cDNA was synthesized using PrimeScript RT reagent kits (Takara Bio). Real-time RT-PCR proceeded using SYBR Premix ExTaq II (Takara Bio) and a LightCycler (Roche Diagnostics). The reaction conditions were 95 °C for 10 s followed by 45–55 cycles of 95 °C for 5 s, 57 °C for 10 s, and 72 °C for 10 s. Table 1 shows the sequences of the primer pairs. The amount of target mRNA was normalized relative to that of Gapdh mRNA.
TABLE 1.
Primers used in the present study
| Genes | Sequence | Predicted product | |
|---|---|---|---|
| bp | |||
| Ncam1 | Fa | AGAGGACGGGAACTCCATCA | 222 |
| R | GCTGCCTTGGATTTTCCTTG | ||
| Fut1 | F | ACCTGCTGAAGTCCCCAGTG | 210 |
| R | CAGGTTGGATGAAGGCTTGG | ||
| Fut2 | F | AGTCAAGGGGAGGGAGAACG | 207 |
| R | GCCAGGGCTACAGAAGTGGA | ||
| Fuca1 | F | ACTGGCAGAGCTTGGACTCG | 163 |
| R | GTCATGAAGCGCTGGTAGGC | ||
| Fuca2 | F | ATGCAAACCAGTGGGCAGAT | 241 |
| R | TCCAGAAAGAGCGGATGGAA | ||
| Fuk | F | GGACCTCTGCCTACGTCCAC | 212 |
| R | GCTGAATCCACTCAGGGTTG | ||
| Fpgt | F | GTGTTTGGAAAGCCTCTGTGG | 197 |
| R | GCCTCATGTTTGAGGGGAAA | ||
| Gmds | F | CTGGATGCCAAACGAGACTG | 159 |
| R | GGTTTTTCCGATGTGCATGA | ||
| Tsta3 | F | GTGGAGCCCATCATCCTCTC | 185 |
| R | AAACGGAAGTCGGGCAAGTA |
a F, forward; R, reverse.
RESULTS
Diurnal Variation of Glycan Structures Determined Using a High-density Lectin Microarray
Signals emitted by 16 of the 96 lectins differed between day and night (Student's t test, p < 0.05, n = 3; see supplemental Table S1). Signals from 1 and 15 of the lectins increased at ZT2 and ZT14, respectively (Fig. 1A). These results suggested that the abundance of several glycan structures in the mouse OB varies in a diurnal manner.
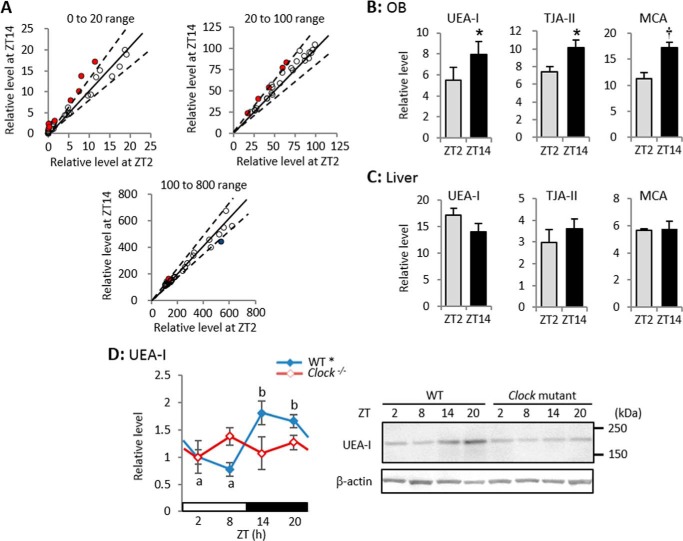
FIGURE 1.
Lectin microarray and Western blotting with UEA-I show diurnal variation of α1–2Fuc glycan in OB. A, correlations between relative signal levels of 96 lectins at ZT2 and ZT14. After each lectin signal is normalized to mean value of 96 lectins, relative levels of 37 lectins are between 0 and 20 (upper left), those of 28 lectins are between 20 and 100 (upper right), and those of 31 lectins are between 100 and 800 (bottom). Dashed lines, range of critical region (p < 0.05, Student's t test, n = 3). Relative levels of 15 lectins are higher at ZT14 (red dots) and that of a single lectin is higher at ZT2 (blue dot). See also supplemental Table S1. B and C, relative signal levels of three lectins with high affinity for α1–2Fuc glycan in OB (B) and liver (C). Values are shown as mean ± S.E. *, p < 0.05; †, p < 0.01, Student's t test, n = 3. D, Western blotting with UEA-I in WT and Clock mutant mice. *, p < 0.05, one-way ANOVA, n = 4–5. Different characters indicate significant differences, p < 0.05, Tukey-Kramer test.
Among 96 lectins, UEA-I, Trichosanthes japonica agglutinin-II and Momordica charantia agglutinin specifically reacted with α1–2Fuc glycan, and all three reacted more intensely at ZT14 than at ZT2 (Fig. 1B) although the signal intensity was identical between ZT14 and ZT2 in liver extracts (Fig. 1C). Western blotting using UEA-I also showed significant diurnal variation in the mouse OB (one-way ANOVA, p < 0.05, n = 3; Fig. 1D). These results suggest that α1–2Fuc glycan is expressed in a diurnal and tissue-specific manner in the OB, and that more is expressed during the nighttime.
Hericium erinaceum agglutinin, Agaricus bisporus agglutinin, Artocarpus integrifolia agglutinin (Jacalin), Maclura pomifera agglutinin, and recombinant A. bisporus agglutinin that have high affinity for galactose β1–3 N-acetylgalactosamine (Galβ1–3GalNAc) glycan, and Griffonia simplicifolia lectin-I A4, Dolichos biflorus agglutinin, and Wisteria floribunda agglutinin that have high affinity for GalNAc, reacted more intensely at ZT14 than at ZT2 (supplemental Table S1). Therefore, Galβ1–3GalNAc and α1–2Fuc glycans appear to similarly fluctuate in a diurnal manner. On the other hand, Datura stramonium agglutinin that specifically reacts with N-acetylglucosamine β1–6 mannose (GlcNAcβ1–6Man), reacted intensely at ZT2 (supplemental Table S1), suggesting that GlcNAcβ1–6Man glycan fluctuates in an opposing manner to α1–2Fuc glycan. Although previous studies have shown that polysialic acid binding to NCAM has diurnal variation in the suprachiasmatic nucleus (23, 24), the signal intensity of 13 lectins in the OB that had high affinity for sialic acid was identical between day and night.
Diurnal Variation of α1–2Fuc Glycan in Axon Bundles of Secondary Olfactory Neurons
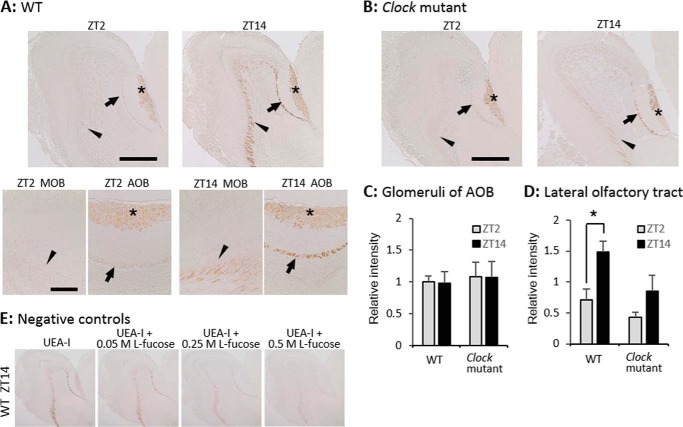
Histochemical analysis showed that UEA-I reacted with glomeruli in the AOB, the lateral olfactory tract that comprises axon bundles of secondary olfactory neurons, and cell bodies of these neurons (Fig. 2A). The UEA-I reaction with the lateral olfactory tract (Fig. 2A, arrows and arrowheads) was significant at ZT14, but weak at ZT2 (Student's t test, p < 0.05, n = 3; Fig. 2D), whereas this reaction with glomeruli in the AOB (Fig. 2A, asterisks) was identical between these time points (Student's t test, p = 0.92, n = 3; Fig. 2C). Pre-absorption with l-fucose dose dependently inhibited the UEA-I reaction (Fig. 2E). These findings agree with the results of the lectin microarray and suggest that α1–2Fuc glycan is more abundant in axons of secondary olfactory neurons during the early nighttime than in the morning.
FIGURE 2.
Diurnal variation of α1–2Fuc glycan in lateral olfactory tract is controlled by clock genes. A, sagittal sections of OB with UEA-I staining obtained at ZT2 and ZT14 from WT mice. Upper and lower bars, 500 and 150 μm, respectively. B, UEA-I staining at ZT2 and ZT14 in Clock mutant mice. Bar, 500 μm. *, glomerular layer of AOB; arrows and arrowheads, lateral olfactory tract. C and D, signal intensity within the glomerular layer of AOB (C) and lateral olfactory tract (D). Values are shown as mean ± S.E. *, p < 0.05, Student's t test, n = 3. E, UEA-I pre-absorption with l-fucose.
Diurnal Variation of α1–2Fuc Glycan Does Not Depend on the Amount of Main Core Protein NCAM
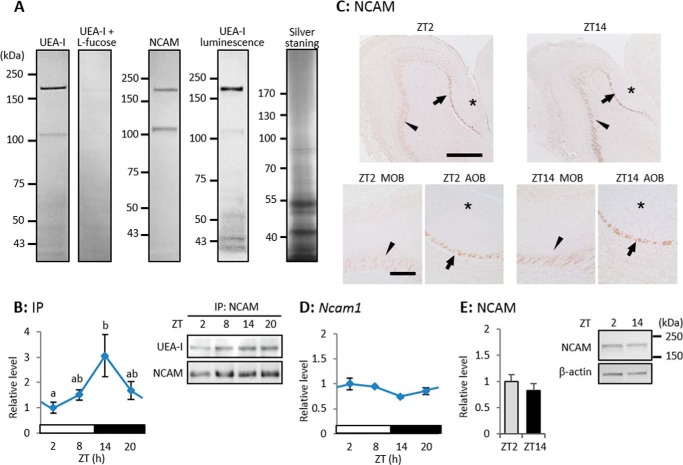
Murrey et al. (25) found using UEA-I affinity chromatography that 32 proteins including NCAM are α1–2-fucosylated in the OB, and Pestean et al. (26) reported that NCAM is the main glycoprotein with α1–2Fuc glycan in the OB. We again confirmed using Western blotting, immunoprecipitation, and histochemical means that the main core protein of α1–2Fuc glycan in the OB of WT mice is NCAM. Western blotting using UEA-I and rapid DAB staining detected only two bands with a molecular mass range that was similar to that of NCAM (about 180 and 110 kDa; Fig. 3A), although several UEA-I-positive bands were detected using luminescence (Fig. 3A). NCAM protein mainly consists of different proportions of three isoforms that are defined according to their molecular weight as NCAM-180, -140 and -120, at various developmental stages (27), and NCAM-180 and -120 seemed to be mainly α1–2-fucosylated in the adult OB. Immunoprecipitated NCAM reacted with UEA-I, with an intensity that varied in a diurnal manner (one-way ANOVA, p < 0.05, n = 3–4), peaking at ZT14 (Fig. 3B). At both ZT2 and ZT14, anti-NCAM antibody significantly reacted with the lateral olfactory tract, but not with glomeruli in the AOB (Fig. 3C). The mRNA expression of Ncam1 did not vary (one-way ANOVA, p = 0.40, n = 4 -5; Fig. 3D), and the amount of NCAM protein was identical between ZT2 and ZT14 (Student's t test, p = 0.46 n = 3; Fig. 3E). These results suggested that α1–2-fucosylation associated with the major target protein NCAM is diurnal, and that diurnal variation of the α1–2Fuc glycan in the OB does not depend on the amount of NCAM.
FIGURE 3.
α1–2-Fucosylation of NCAM is diurnal in OB, whereas expression levels of Ncam1 mRNA and NCAM protein are not. A, Western blotting of the OB extract visualized using rapid DAB staining for UEA-I, anti-NCAM immunostaining, UEA-I luminescence, and silver staining. B, immunoprecipitation of NCAM with UEA-I detection. Values are shown as mean ± S.E., p < 0.05, one-way ANOVA, n = 3–4. Different characters indicate significant differences, p < 0.05, Tukey-Kramer test. C, sagittal sections of OB with anti-NCAM immunostaining. *, glomerular layer of AOB; arrows and arrowheads, lateral olfactory tract. Upper and lower bars = 500 and 150 μm, respectively. D, Ncam1 mRNA expression level. Values are shown as mean ± S.E., and there is no significant difference, one-way ANOVA, n = 3. E, NCAM protein expression level. Values are shown as mean ± S.E., and there is no significant difference, Student's t test, n = 3.
Diurnal Expression of Fut1 mRNA and FUT1 Protein
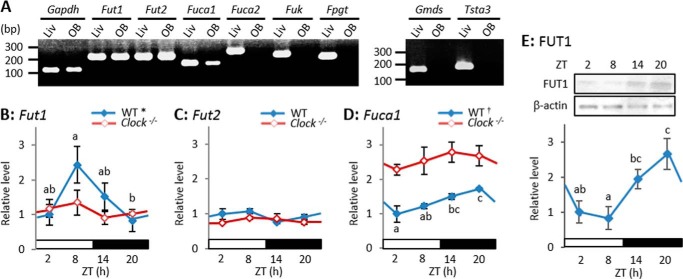
We used RT-PCR to assess the mRNA expression of eight enzymes that are involved in α1–2Fuc glycan metabolism. We confirmed that all applied primer sets could amplify each target gene, because we detected the PCR products of all of these enzymes in liver extracts with the predicted base pairs (Fig. 4A, Table 1). Although these eight enzymes play important roles in mammalian cells (28), only Fut1, Fut2, and Fuca1 transcripts were detected in the OB (Fig. 4A). Both Fut1 and Fut2 are α1–2-specific fucosyltransferase genes and Fuca1 is a lysosomal α-fucosidase gene that generates fucose residues as a substrate for fucosylation by salvage pathways (28). These three enzymes are apparently critical for α1–2-fucosylation in the OB, and thus we used real-time RT-PCR to evaluate temporal changes in these three genes.
FIGURE 4.
Expression levels of Fut1 mRNA and FUT1 protein in OB are diurnal. A, messenger RNA expression of eight enzymes associated with α1–2Fuc glycan metabolism in OB and liver. See predicted base pairs in Table 1. B–D, Fut1 (B), Fut2 (C), and Fuca1 (D) mRNA expression levels. Values are shown as mean ± S.E. *, p < 0.05; †, p < 0.01, one-way ANOVA, n = 4–5. Different characters indicate significant differences, p < 0.05, Tukey-Kramer test. E, FUT1 protein expression levels in WT mice. Values are shown as mean ± S.E. p < 0.05, one-way ANOVA, n = 3. Different characters indicate significant differences, p < 0.05, Tukey-Kramer test.
The mRNA expression of Fut1 varied in a diurnal manner (one-way ANOVA, p < 0.05, n = 4–5), peaking late in the day (ZT8) and reaching the nadir late in the night (ZT20) (Fig. 4B), whereas that of Fut2 did not (Fig. 4C). The mRNA expression of Fuca1 varied in a diurnal fashion (one-way ANOVA, p < 0.01, n = 4–5), peaking late at night (ZT20) and reaching the nadir in the morning (ZT2) (Fig. 4D). The amount of FUT1 protein also varied in a diurnal manner (one-way ANOVA, p < 0.05, n = 3), peaking at ZT20 and reaching the nadir at ZT8 (Fig. 4E). These findings suggest that FUT1 expression that fluctuates according to the time of day results in diurnal variation in the abundance of α1–2Fuc glycan.
Diurnal Variation of α1–2Fuc Glycan Depends on the Molecular Clock
Homozygous Clock mutant mice were examined using Western blotting, histochemistry, and real-time RT-PCR to determine whether the molecular clock is involved in diurnal variation of α1–2Fuc glycan in axons of secondary olfactory neurons. Diurnal variation of the UEA-I reaction with OB extracts was abolished in Clock mutant mice (one-way ANOVA, p = 0.52, n = 3; Fig. 1D). The UEA-I reaction with the lateral olfactory tract in Clock mutant mice was obviously weak at both ZT2 and ZT14 (Fig. 2B) and identical between at ZT2 and ZT14 (Student's t test, p = 0.29, n = 3; Fig. 2D), whereas that with glomeruli in the AOB was significant at both ZT2 and ZT14 as in WT mice (Fig. 2C). Real-time RT-PCR revealed that diurnal mRNA expression of Fut1 was completely abolished (one-way ANOVA, p = 0.63, n = 4–5) at a low level in the OB of Clock mutant mice (Fig. 4B), whereas significant diurnal expression of Fuca1 disappeared (one-way ANOVA, p = 0.56, n = 4–5) and continued at a high level in the OB of Clock mutant mice (Fig. 4D). High levels of Fuca1 expression appear to increase the number of fucose residues, which might result from a deficit in α1–2Fuc due to the low level of Fut1 expression in Clock mutant mice. These findings suggest that the molecular clock controls diurnal variation of the abundance of α1–2Fuc glycan by regulating Fut1 expression.
DISCUSSION
Olfaction is more sensitive during the period of active onset in the early night, than during the day in mice (2), and secondary olfactory neurons generate circadian rhythm in the activity (1, 3). Glycosylation affects the functional properties of many proteins, and glycoconjugates in the OB play several important roles in neuronal formation including neurite outgrowth and synaptogenesis (13). Therefore, we postulated that glycan structures fluctuate from day to night in the mouse OB. Here, we showed that α1–2-fucosylation of NCAM fluctuates in a diurnal manner in axons of secondary olfactory neurons, and that such fluctuation is apparently governed by the molecular clock via rhythmic expression of the Fut1 gene.
Lectin microarrays provide a useful platform for the exhaustive and precise analysis of minuscule differences in glycan structures between two specimens (18). The present results of such microarray indicated that the abundance of several glycan structures, including α1–2Fuc glycan, has diurnal variation in the mouse OB. The findings of several studies suggest that α1–2Fuc glycan mediates neuronal functions, such as learning and memory, as well as neuronal morphology, including neurite outgrowth and synaptic plasticity (29–35). The inhibition of α1–2-fucosylation caused by 2-deoxy-d-galactose incorporation delays neurite outgrowth and synaptic plasticity in the rat hippocampus (29, 30). Mice that are deficient in FUT1 exhibit developmental defects in neurons that express NCAM in the OB (25). We showed that the abundance of α1–2Fuc glycan fluctuates in the lateral olfactory tract, which mostly contains axons of secondary olfactory neurons. This finding suggests that the efficiency of transmission between secondary and higher neurons might be subject to diurnal variations in the olfactory system via changes in the degree of synaptic plasticity. Many fucosylated glycoproteins are transported in axons, and those that are synthesized arrive at neuronal endings within a few hours via rapid axonal transport (36). These properties of the axonal transport of fucosylated proteins apparently permit diurnal variation of α1–2Fuc glycan in axon bundles.
Murrey et al. (25) found using UEA-I affinity chromatography that 32 proteins including NCAM are α1–2-fucosylated in the OB, and Pestean et al. (26) reported that NCAM is the main glycoprotein with α1–2Fuc glycan in the OB. We showed that the abundance of α1–2Fuc glycan associated with NCAM is diurnal, although the expression of Ncam1 mRNA and NCAM protein does not vary. NCAM functions in neurite outgrowth and synaptic formation, especially in the OB and hippocampus (37), and it is a highly glycosylated protein with multiple types of glycan (38, 39). NCAM-180 contributes to the maintenance of synaptic formation (39), and OB development is defective in NCAM-180 knock-out (40), as in FUT1 knock-out (25) mice. Therefore, the part of α1–2Fuc glycan function described above might reflect the regulation of NCAM proteins.
Müller et al. (41) showed that fucosylated glycoproteins are abundant and synthesized de novo in the OB, and that the OB absorbs exogenous fucose residues more frequently than other areas, indicating rapid metabolic turnover of fucosylated glycoproteins in the OB. We found that the expression of Fut1 mRNA and FUT1 protein significantly fluctuated according to the time of day, and that the molecular clock apparently controls the diurnal expression of Fut1 in the OB. Granados-Fuentes et al. (2) reported that clock molecules regulate circadian rhythm of olfactory sensitivity, although the molecular mechanism remains unknown. Because hundreds of circadian clock-controlled genes regulate an impressive diversity of biological processes (11, 12), the molecular clock-regulated diurnal expression of Fut1 might play an important role in olfactory sensitivity. Although diurnal variations of α1–2Fuc glycan regulated by the molecular clock is a potential mechanism for circadian rhythms in the activity of secondary olfactory neurons, further studies are needed to elucidate whether it is involved directly in circadian rhythm of olfactory sensitivity.
Supplementary Material
Acknowledgments
We thank Jinko Murakami and Kayo Suzuki for lectin microarray production, and Keiko Hiemori for lectin microarray analysis.
This work was supported in part by Grant-in-Aid for Scientific Research (C) KAKENHI 25350179 (to K. O.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.

This article contains supplemental Table S1.
- OB
- olfactory bulb
- α1–2Fuc
- α1–2-fucose
- AOB
- accessory olfactory bulb
- FUT1
- fucosyltransferase 1
- Galβ1–3GalNAc
- galactose β1–3 N-acetylgalactosamine
- GlcNAcβ1–6Man
- N-acetylglucosamine β1–6 mannose
- NCAM
- neural cell adhesion molecule
- PAS
- Per-Arnt-Sim
- UEA-I
- Ulex europaeus agglutinin-I
- ZT
- Zeitgeber time
- FUT1
- fucosyltransferase 1
- DAB
- 3,3′-diaminobenzidine tetrahydrochloride
- ANOVA
- analysis of variance.
REFERENCES
- 1. Granados-Fuentes D., Tseng A., Herzog E. D. (2006) A circadian clock in the olfactory bulb controls olfactory responsivity. J. Neurosci. 26, 12219–12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Granados-Fuentes D., Ben-Josef G., Perry G., Wilson D. A., Sullivan-Wilson A., Herzog E. D. (2011) Daily rhythms in olfactory discrimination depend on clock genes but not the suprachiasmatic nucleus. J. Biol. Rhythms 26, 552–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Granados-Fuentes D., Saxena M. T., Prolo L. M., Aton S. J., Herzog E. D. (2004) Olfactory bulb neurons express functional, entrainable circadian rhythms. Eur. J. Neurosci. 19, 898–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aujard F., Herzog E. D., Block G. D. (2001) Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neuroscience 106, 255–261 [DOI] [PubMed] [Google Scholar]
- 5. Zhao H., Rusak B. (2005) Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience 132, 519–528 [DOI] [PubMed] [Google Scholar]
- 6. Mordel J., Karnas D., Pévet P., Isope P., Challet E., Meissl H. (2013) The output signal of Purkinje cells of the cerebellum and circadian rhythmicity. PLoS One 8, e58457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Munn R. G., Bilkey D. K. (2012) The firing rate of hippocampal CA1 place cells is modulated with a circadian period. Hippocampus 22, 1325–1337 [DOI] [PubMed] [Google Scholar]
- 8. Corthell J. T., Fadool D. A., Trombley P. Q. (2012) Connexin and AMPA receptor expression changes over time in the rat olfactory bulb. Neuroscience 222, 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corthell J. T., Stathopoulos A. M., Watson C. C., Bertram R., Trombley P. Q. (2013) Olfactory bulb monoamine concentrations vary with time of day. Neuroscience 247, 234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buhr E. D., Takahashi J. S. (2013) Molecular components of the mammalian circadian clock. Handb. Exp. Pharmacol. 217, 3–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panda S., Antoch M. P., Miller B. H., Su A. I., Schook A. B., Straume M., Schultz P. G., Kay S. A., Takahashi J. S., Hogenesch J. B. (2002) Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 [DOI] [PubMed] [Google Scholar]
- 12. Ueda H. R., Chen W., Adachi A., Wakamatsu H., Hayashi S., Takasugi T., Nagano M., Nakahama K., Suzuki Y., Sugano S., Iino M., Shigeyoshi Y., Hashimoto S. (2002) A transcription factor response element for gene expression during circadian night. Nature 418, 534–539 [DOI] [PubMed] [Google Scholar]
- 13. Plendl J., Sinowatz F. (1998) Glycobiology of the olfactory system. Acta Anat. 161, 234–253 [DOI] [PubMed] [Google Scholar]
- 14. Tisay K. T., St. John J. A., Key B. (2002) Expression of specific glycoconjugates in both primary and secondary olfactory pathways in BALB/C mice. J. Comp. Neurol. 443, 213–225 [DOI] [PubMed] [Google Scholar]
- 15. Salazar I., Sánchez Quinteiro P. (2003) Differential development of binding sites for four lectins in the vomeronasal system of juvenile mouse: from the sensory transduction site to the first relay stage. Brain Res. 979, 15–26 [DOI] [PubMed] [Google Scholar]
- 16. Kaasik K., Kivimäe S., Allen J. J., Chalkley R. J., Huang Y., Baer K., Kissel H., Burlingame A. L., Shokat K. M., Ptáček L. J., Fu Y. H. (2013) Glucose sensor O-GlcNAcylation coordinates with phosphorylation to regulate circadian clock. Cell Metab. 17, 291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li M. D., Ruan H. B., Hughes M. E., Lee J. S., Singh J. P., Jones S. P., Nitabach M. N., Yang X. (2013) O-GlcNAc signaling entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab. 17, 303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirabayashi J., Yamada M., Kuno A., Tateno H. (2013) Lectin microarrays: concept, principle and applications. Chem. Soc. Rev. 42, 4443–4458 [DOI] [PubMed] [Google Scholar]
- 19. Damjanov I. (1987) Lectin cytochemistry and histochemistry. Lab. Invest. 57, 5–20 [PubMed] [Google Scholar]
- 20. Oishi K., Miyazaki K., Ishida N. (2002) Functional CLOCK is not involved in the entrainment of peripheral clocks to the restricted feeding: entrainable expression of mPer2 and BMAL1 mRNAs in the heart of Clock mutant mice on Jcl:ICR background. Biochem. Biophys. Res. Commun. 298, 198–202 [DOI] [PubMed] [Google Scholar]
- 21. Tateno H., Toyota M., Saito S., Onuma Y., Ito Y., Hiemori K., Fukumura M., Matsushima A., Nakanishi M., Ohnuma K., Akutsu H., Umezawa A., Horimoto K., Hirabayashi J., Asashima M. (2011) Glycome diagnosis of human induced pluripotent stem cells using lectin microarray. J. Biol. Chem. 286, 20345–20353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kondoh D., Yamamoto Y., Nakamuta N., Taniguchi K., Taniguchi K. (2010) Lectin histochemical studies on the olfactory epithelium and vomeronasal organ in the Japanese striped snake, Elaphe quadrivirgata. J. Morphol. 271, 1197–1203 [DOI] [PubMed] [Google Scholar]
- 23. Prosser R. A., Rutishauser U., Ungers G., Fedorkova L., Glass J. D. (2003) Intrinsic role of polysialylated neural cell adhesion molecule in photic phase resetting of the mammalian circadian clock. J. Neurosci. 23, 652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glass J. D., Watanabe M., Fedorkova L., Shen H., Ungers G., Rutishauser U. (2003) Dynamic regulation of polysialylated neural cell adhesion molecule in the suprachiasmatic nucleus. Neuroscience 117, 203–211 [DOI] [PubMed] [Google Scholar]
- 25. Murrey H. E., Ficarro S. B., Krishnamurthy C., Domino S. E., Peters E. C., Hsieh-Wilson L. C. (2009) Identification of the plasticity-relevant fucose-α(1–2)-galactose proteome from the mouse olfactory bulb. Biochemistry 48, 7261–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pestean A., Krizbai I., Böttcher H., Párducz A., Joó F., Wolff J. R. (1995) Identification of the Ulex europaeus agglutinin-I-binding protein as a unique glycoform of the neural cell adhesion molecule in the olfactory sensory axons of adults rats. Neurosci. Lett. 195, 117–120 [DOI] [PubMed] [Google Scholar]
- 27. Rønn L. C., Hartz B. P., Bock E. (1998) The neural cell adhesion molecule (NCAM) in development and plasticity of the nervous system. Exp. Gerontol. 33, 853–864 [DOI] [PubMed] [Google Scholar]
- 28. Becker D. J., Lowe J. B. (2003) Fucose: biosynthesis and biological function in mammals. Glycobiology 13, 41R–53R [DOI] [PubMed] [Google Scholar]
- 29. Kalovidouris S. A., Gama C. I., Lee L. W., Hsieh-Wilson L. C. (2005) A role for fucose α(1–2) galactose carbohydrates in neuronal growth. J. Am. Chem. Soc. 127, 1340–1341 [DOI] [PubMed] [Google Scholar]
- 30. Murrey H. E., Gama C. I., Kalovidouris S. A., Luo W. I., Driggers E. M., Porton B., Hsieh-Wilson L. C. (2006) Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons. Proc. Natl. Acad. Sci. 103, 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pohle W., Acosta L., Rüthrich H., Krug M., Matthies H. (1987) Incorporation of [3H]fucose in rat hippocampal structures after conditioning by perforant path stimulation and after LTP-producing tetanization. Brain Res. 410, 245–256 [DOI] [PubMed] [Google Scholar]
- 32. Krug M., Jork R., Reymann K., Wagner M., Matthies H. (1991) The amnesic substance 2-deoxy-d-galactose suppresses the maintenance of hippocampal LTP. Brain Res. 540, 237–242 [DOI] [PubMed] [Google Scholar]
- 33. Krug M., Wagner M., Staak S., Smalla K. H. (1994) Fucose and fucose-containing sugar epitopes enhance hippocampal long-term potentiation in the freely moving rat. Brain Res. 643, 130–135 [DOI] [PubMed] [Google Scholar]
- 34. Matthies H., Staak S., Krug M. (1996) Fucose and fucosyllactose enhance in vitro hippocampal long-term potentiation. Brain Res. 725, 276–280 [DOI] [PubMed] [Google Scholar]
- 35. Lorenzini C. G., Baldi E., Bucherelli C., Sacchetti B., Tassoni G. (1997) 2-Deoxy-d-galactose effects on passive avoidance memorization in the rat. Neurobiol. Learn. Mem. 68, 317–324 [DOI] [PubMed] [Google Scholar]
- 36. Goodrum J. F., Morell P. (1984) Analysis of the apparent biphasic axonal transport kinetics of fucosylated glycoproteins. J. Neurosci. 4, 1830–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zamze S., Harvey D. J., Chen Y. J., Guile G. R., Dwek R. A., Wing D. R. (1998) Sialylated N-glycans in adult rat brain tissue: a widespread distribution of disialylated antennae in complex and hybrid structures. Eur. J. Biochem. 258, 243–270 [DOI] [PubMed] [Google Scholar]
- 38. Liedtke S., Geyer H., Wuhrer M., Geyer R., Frank G., Gerardy-Schahn R., Zähringer U., Schachner M. (2001) Characterization of N-glycans from mouse brain neural cell adhesion molecule. Glycobiology 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 39. Perlson E., Hendricks A. G., Lazarus J. E., Ben-Yaakov K., Gradus T., Tokito M., Holzbaur E. L. (2013) Dynein interacts with the neural cell adhesion molecule (NCAM180) to tether dynamic microtubules and maintain synaptic density in cortical neurons. J. Biol. Chem. 288, 27812–27824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Treloar H., Tomasiewicz H., Magnuson T., Key B. (1997) The central pathway of primary olfactory axons is abnormal in mice lacking the N-CAM-180 isoform. J. Neurobiol. 32, 643–658 [DOI] [PubMed] [Google Scholar]
- 41. Müller L., Mares V., Sýkorová J., Biesold D. (1985) Regional and cellular differences in fucosylation of glycomacromolecules in the mouse brain: a biochemical and autoradiographic study of early postnatal and adolescent animals. Neuroscience 14, 875–880 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.