Abstract
Transient receptor potential cation channel subfamily M member 7 (TRPM7) is a bi-functional protein comprising a TRP ion channel segment linked to an α-type protein kinase domain. Genetic inactivation of TRPM7 revealed its central role in magnesium metabolism, cell motility, proliferation and differentiation. TRPM7 is associated with anoxic neuronal death, cardiac fibrosis and tumor progression highlighting TRPM7 as a new drug target. Recently, several laboratories have independently identified pharmacological compounds inhibiting or activating the TRPM7 channel. The recently found TRPM7 modulators were used as new experimental tools to unravel cellular functions of the TRPM7 channel. Here, we provide a concise overview of this emerging field.
Keywords: TRPM7, TRPM6, TRP channel, α-kinase, magnesium, calcium
1. Functional Roles of TRPM7
TRPM7 is a plasma membrane protein that contains a transmembrane ion channel segment linked to a cytosolic α-type serine/threonine protein kinase domain as illustrated in Figure 1 [1,2,3,4,5]. It is commonly accepted that the overall architecture of the pore-forming segment of TRPM7 channels is analogous to that of tetrameric potassium channels. The channel domain of TRPM7 comprises six transmembrane helixes (Figure 1). A stretch of amino acids between 5th and 6th helices contains a predicted pore helix followed by a predicted pore loop (Figure 1). Like in potassium channels, it is assumed that the pore loops of four channel subunits contribute to a common ion selectivity filter. Among all ion channels, only TRPM7 and its homologous protein TRPM6 are known as channels covalently fused to kinase domains [1,6,7,8,9,10,11]. TRPM7 is a ubiquitously expressed protein and endogenous TRPM7 currents were detected in all cells investigated so far [12,13,14,15].
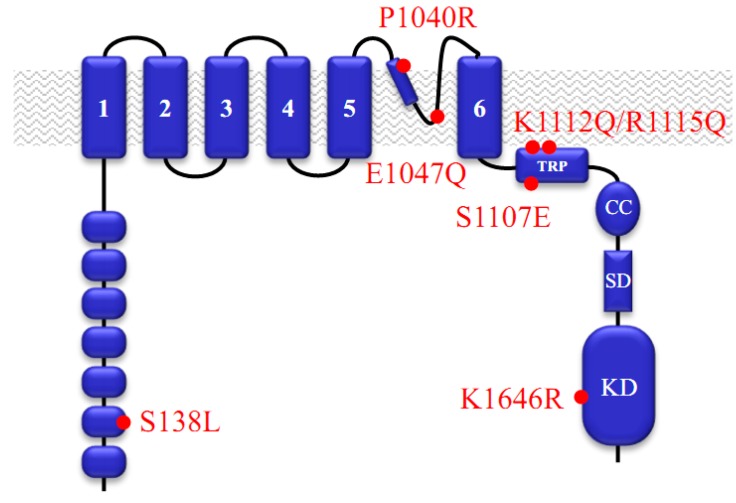
Figure 1.
Domain topology of the murine kinase-coupled channel Transient receptor potential cation channel subfamily M member 7 (TRPM7).
The plasma membrane channel segment of TRPM7 comprises six transmembrane helices (1–6). A short stretch between the 5 and 6 helices contains a predicted pore forming loop and pore helix [16]. A large cytosolic N-terminus of TRPM7 contains a set of domains that are highly conserved among the TRPM gene family and resemble ankyrin repeats as revealed by 3D modeling [11]. A C-terminus of TRPM7 contains a highly conserved transient receptor potential (TRP) domain, a coiled-coil (CC) domain, a kinase substrate domain (SD) and a kinase domain (KD). Red dots indicate the location of point mutations that were highly instrumental in probing of TRPM7 functions. Mutations S138L and P1040R correspond to TRPM6 missense mutations causing an inherited disorder in humans known as hypomagnesemia and secondary hypocalcemia (HSH) [9,17]. S138L disrupts assembly of TRPM7 channel complexes [9], whereas P1040R results in a dominant-negative channel subunit [17]. E1047 is a negatively charged residue located in a ‘selectivity’ filter of the TRPM7 channel pore and E1047Q mutation results in an active channel permeable to monovalent cations and impermeable to divalent ions like Ca2+ and Mg2+ [16,18] recapitulating the characteristic feature of the taste-signaling TRPM5 channel [19,20]. The S1107E mutation produces a constitutively active channel insensitive to intracellular Mg2+ and PIP2 [21]. Positively charged residues K1112 and R1115 were suggested to be required for PIP2 dependent gating of TRPM7 and, consequently, the K1112Q/R1115Q double mutation ablates TRPM7 currents [22]. K1646 is a highly conserved residue located in the catalytic site of the kinase domain [4] and the K1646R mutation is sufficient to block the kinase function of TRPM7 (‘kinase-dead’ mutation) [23,24].
Genetic ablation of TRPM7 in cultured cells revealed that TRPM7 regulates cellular Mg2+ levels [9,24,25,26], cell motility [27,28,29,30,31,32,33,34], proliferation/cell survival [1,24,26,35], differentiation [36,37], mechanosensitivity [28,38,39] and exocytosis [40]. Furthermore, it was suggested that TRPM7 plays a role in anoxic neuronal death [41], hypertension [42], neurodegenerative disorders [43,44], atrial fibrillation, cardiac fibrosis [45] and tumor growth/progression [46,47,48,49,50,51,52,53]. Genetic association studies in humans revealed that TRPM7 may be implicated in myocardial repolarization [54]. Experiments with Trpm7 gene deficient mice and zebrafish and genetic association studies in humans showed that TRPM7 is required for early embryonic development [25,55,56,57], thymopoiesis [55], morphogenesis of the kidney [57], cardiac rhythmicity [58], cardiac repolarization [59] and systemic Mg2+ homeostasis [25] - though the latter finding remains controversial [55].
Our mechanistic understanding of the functional interplay between TRPM7 kinase and channel moieties is still in its infancy. In vitro, TRPM7 kinase is able to phosphorylate serine/threonine residues of annexin A1 [60], myosin II isoforms [61], eEF2-k [62] and PLCγ2 [63]. Furthermore, multiple residues located in a ‘substrate’ segment of TRPM7 are potential autophosphorylation targets of the kinase domain [64,65]. Recently, it was shown that the TRPM7 kinase domain can be cleaved by caspases during Fas-receptor stimulation in immune cells [66]. The truncated channel exhibited substantially higher activity and potentiated Fas-receptor signaling [66]. In another study, the cleaved TRPM7 kinase domain was found in multiple tissues and cell lines. The mechanism of TRPM7 cleavage was not established. Interestingly, the portion of TRPM7 containing the channel domain is eliminated, whereas the released kinase domain is able to translocate into the cell nucleus and phosphorylates histones to modulate the chromatin covalent modification landscape [67]. However, the physiological relevance of these findings remains to be elucidated. Along these lines, Kaitsuka et al. [23] have recently shown that mice carrying a point mutation in the catalytic site of the TRPM7 kinase domain (‘kinase-dead’ knock-in mutation, Figure 1) display an unaltered lifespan as well as normal Ca2+ and Mg2+ serum levels and do not develop obvious pathophysiologic phenotypes.
The channel segment of TRPM7 forms a constitutively active ion channel that is highly selective for divalent cations such as Zn2+, Ca2+ and Mg2+ [1,2,67,68]. It has been hypothesized that influx of all these cations is relevant for the physiological role of TRPM7 [1,2,68]. Mutagenesis of the pore-forming sequence of TRPM7 allowed for the identification of specific residues that contribute to the ‘selectivity filter’ of the channel pore (Figure 1) [16,18]. In contrast, molecular mechanisms underlying TRPM7 channel gating are still a matter of debate. The prevailing models are mainly resting upon two findings. First, perfusion of cells with an Mg2+ free internal solution induces TRPM7 currents implying that intracellular Mg2+ (either free Mg2+ or Mg2+-ATP) may be a physiological negative regulator of the channel [1,69,70]. Experiments with the ‘kinase-dead’ knock-in mutation (Figure 1) or a channel variant lacking the whole kinase domain led to the concept that the kinase domain modifies the sensitivity of the TRPM7 channel to Mg2+ and Mg2+-ATP [24,69]. However, Hofmann et al. have shown recently that the TRP domain plays a key role in Mg2+ dependent gating of TRPM7 since a point mutation of a conserved serine residue in the TRP domain (Figure 1) is sufficient to create a constitutively active TRPM7 channel insensitive to intracellular Mg2+ [21].
The second model is predicated on the observation that the TRPM7 channel is tightly regulated by the plasma membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) [71]. Consequently, stimulation of phospholipase C (PLC)-coupled G protein-coupled receptors (GPCRs) causes depletion of membrane PIP2 and, subsequently, inactivation of TRPM7 currents even in the absence of Mg2+ [71]. Kozak et al. [72] hypothesized that internal Mg2+ interacts directly with negatively charged PIP2 to interfere with the gating process of TRPM7. Recently, Xie et al. [22] reported that neutralization of basic residues in the TRP domain (Figure 1) leads to non-functional or dysfunctional TRPM7 with dampened regulation by PIP2 suggesting that the TRP domain may interact with PIP2.
2. Pharmacological Compounds Inhibiting the TRPM7 Channel
Because of the pivotal role of the TRPM7 channel in physiology and pathophysiology, there is a pressing need to identify pharmacological compounds allowing to acutely probe TRPM7 channel versus kinase activity. Efforts of several laboratories resulted in the independent identification of an array of small organic compounds behaving as blockers of the TRPM7 channel as summarized in Table 1 and Figure 2a.
Table 1.
Organic compounds inhibiting TRPM7 channel.
| Compound | IC50 (μM) * | Description of the block | Reference |
|---|---|---|---|
| 2-APB | 174 | Reversible | [73,74] |
| Spermine | 2.3 † | Reversible, voltage dependent | [75] |
| SKF-96365 | n.d. | Tested only at 20 μM | [75] |
| Nafamostat | 617 | Reversible, voltage dependent | [76] |
| Carvacrol | 306 | Reversible | [77] |
| NDGA | n.d. | Tested only at 10 and 20 μM | [78] |
| AA861 | n.d. | Tested only at 10 and 40 μM | [78] |
| MK886 | n.d. | Tested only at 10 μM | [78] |
| Waixenicin A | 7.0 | Irreversible, [Mg2+]i dependent | [79] |
| NS8593 | 1.6 | Reversible, [Mg2+]i dependent | [80] |
| Quinine | n.d | Reversible, tested only at 30 μM | [80] |
| CyPPA | n.d | Tested only at 30 μM | [80] |
| Dequalinium | n.d | Tested only at 30 μM | [80] |
| SKA31 | n.d | Tested only at 30 μM | [80] |
| UCL 1684 | n.d | Tested only at 30 μM | [80] |
| Sphingosine | 0.6 | Reversible | [81] |
| FTY720 | 0.7 | Reversible | [81] |
* IC50 values were shown for recombinant TRPM7 currents measured in the absence of internal Mg2+. † The dose-dependent effect of spermine was evaluated on endogenous TRPM7 currents in divalent-free external solution. n.d. - not determined.
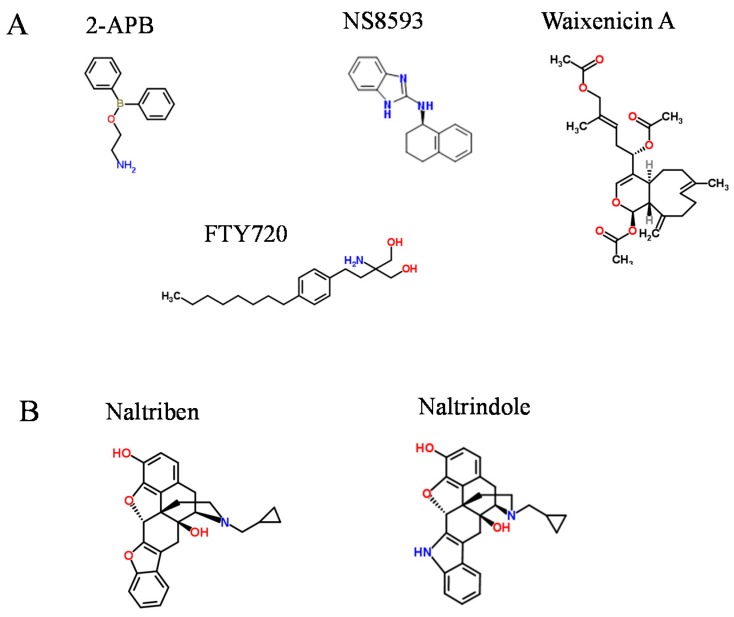
Figure 2.
Chemical structures of modulators of the TRPM7 channel. (A) A subset of broadly used inhibitors of the TRPM7 channel; (B) A newly identified activator of the TRPM7 channel, naltriben, and the related inactive compound naltrindole.
The list of TRPM7 inhibitors comprises a group of non-specific channel blockers such as spermine, SKF-96365 and 2-aminoethyl diphenylborinate (2-APB), natural metabolites including waixenicin A, quinine and sphingosine and an array of drug-like synthetic compounds (Table 1). 2-APB (Figure 2a) reversibly blocked the endogenous TRPM7 channel in Jurkat T cells [73]. The inhibitory effect of 2-APB was characterized further with recombinant TRPM7 protein [74]. Extracellular spermine blocked endogenous TRPM7 currents in rat basophilic leukemia (RBL) cells with an IC50 value of 2.3 µM, and 20 µM SKF-96365 was sufficient for complete inactivation of TRPM7 in RBL cells [75]. It has been proposed that 2-APB does not act on TRPM7 directly, but rather inhibits the channel by means of intracellular acidification [82]. The broad spectrum serine protease inhibitor and anticoagulant nafamostat mesylate inhibited the TRPM7 channel with an IC50 of 617 µM [76]. Carvacrol [77] and several 5-lipoxygenase inhibitors (NDGA, AA861 and MK886) blocked TRPM7 currents in the high µM range [78].
Several small conductance Ca2+-activated K+ channel inhibitors such as the antimalarial plant alkaloid quinine, CyPPA, dequalinium, NS8593, SKA31 and UCL1684 also act as potent blockers of TRPM7 currents [80]. The most potent compound NS8593 (Figure 2a) inhibited the TRPM7 channel in an Mg2+-dependent mode with an IC50 of 1.6 µM. Furthermore, NS8593 suppresses TRPM7-dependent motility of HEK293 cells [80]. Epithelial–mesenchymal transition (EMT) in breast cancer cells is a Ca2+ dependent processes. Studies based on an RNAi silencing approach in combination with NS8593 highlighted a role of the TRPM7 channel in this process [83]. Recently, Siddiqui et al. [84] took advantage of NS8593 and showed that TRPM7 critically contributes to the ability of microglia cells to migrate and invade in anti-inflammatory states. In addition, Schilling et al. [85] employed NS8593 to demonstrate that the TRPM7 channel is required for proliferation and polarization of macrophages towards an anti-inflammatory phenotype.
Waixenicin A (Figure 2a), a natural terpenoid of the soft coral Sarcothelia edmondsoni inactivated TRPM7 currents in an Mg2+ dependent manner with an IC50 of 7 µM in the absence of internal Mg2+ [79]. Moreover, waixenicin A was found to be efficient in suppression of TRPM7-dependent proliferation of RBL cells [79]. More recently, Kim et al. [86] employed waixenicin A to elucidate the functional role of TRPM7 in interstitial cells of Cajal and found that this terpenoid inhibits endogenous TRPM7 currents leading to a block of pacemaker activity of interstitial cells [86]. Waixenicin A also inhibits the growth and survival of the human gastric and breast adenocarcinoma cells (AGS and MCF-7, respectively) suggesting that TRPM7 may turn out to be a novel therapeutic target in gastric and breast cancer [86]. Yet, other researchers [87] used waixenicin A to demonstrate that TRPM7 regulates actomyosin contractility and invadosome formation in N1E-115 mouse neuroblastoma cells.
Sphingosine, the core building block of sphingolipids in the plasma membrane and its synthetic homolog FTY720 (Figure 2a) inactivated the TRPM7 channel with IC50’s of 0.6 µM and 0.7 µM, respectively [81]. Sphingosine and FTY720 were able to suppress TRPM7-dependent motility of HEK293 cells [81], pacemaker activity of interstitial cells of Cajal [88], and polarization of macrophages [85].
To summarize, several potent inhibitors of the TRPM7 channel with IC50 values in the low µM range have been identified. Pharmacological targeting in conjunction with genetic silencing of TRPM7 or comparative analysis of effects induced by structurally unrelated TRPM7 blockers are promising experimental strategies to uncover hitherto unrecognized cellular functions of TRPM7.
3. Drug-Like Compounds Acting as Activators of the TRPM7 Channel
Recently our group has identified a set of small molecules serving as TRPM7 channel agonists [21]. We implemented a Ca2+ imaging-based assay to screen for activators of recombinant TRPM7 and identified 20 drug-like compounds (Table 2) with different structural backbones that can stimulate TRPM7-mediated Ca2+ influx and TRPM7 currents [21]. Among the latter compounds, we studied naltriben (Figure 2b) in greater detail [21]. Naltriben reversibly activates recombinant and native TRPM7 channels without prior depletion of intracellular Mg2+ and even under conditions of low PIP2. The calculated EC50 value was about 20 µM. The stimulatory effect of 50 µM naltriben was not observed when testing several TRP channels like TRPM2, TRPM8 and TRPV1. Furthermore, we showed that naltriben interfered with the inhibitory effect of NS8593 on TRPM7 currents in a competitive fashion. Our experiments with TRPM7 variants carrying mutations in the pore, TRP and kinase domains suggested that the site of TRPM7 activation by naltriben is most likely located in the TRP domain [21]. Naltriben functions as an antagonist of δ-opioid receptors [89]. It shows high structural similarity to other broadly used opioid receptor antagonists, with naltrindole (Figure 2b) most closely resembling naltriben. Of note, we observed that neither naltrindole, nor more distantly related analogs of naltriben like naltrexone and morphine were able to induce TRPM7 currents [21]. Taken together, we proposed that naltriben represents a positive gating modulator of the TRPM7 channel.
Table 2.
Organic compounds activating TRPM7 channel [21].
| Compound | EC50 (μM) | Description of the Effect |
|---|---|---|
| Naltriben | 20.7 | Reversible, [Mg2+]i independent |
| Clozapine | n.d | Tested only at 30–50 μM |
| Proadifen | n.d | Tested only at 30–50 μM |
| Doxepin | n.d | Tested only at 30–50 μM |
| A3 hydrochloride | n.d | Tested only at 30–50 μM |
| Mibefradil | n.d | Tested only at 30–50 μM |
| U-73343 | n.d | Tested only at 30–50 μM |
| CGP-74514A | n.d | Tested only at 30–50 μM |
| Metergoline | n.d | Tested only at 30–50 μM |
| L-733,060 | n.d | Tested only at 30–50 μM |
| A-77636 | n.d | Tested only at 30–50 μM |
| ST-148 | n.d | Tested only at 30–50 μM |
| Clemastine | n.d | Tested only at 30–50 μM |
| Desipramine | n.d | Tested only at 30–50 μM |
| Sertraline | n.d | Tested only at 30–50 μM |
| Methiothepin | n.d | Tested only at 30–50 μM |
| NNC 55–0396 | n.d | Tested only at 30–50 μM |
| Prochlorperazine | n.d | Tested only at 30–50 μM |
| Nortriptyline | n.d | Tested only at 30–50 μM |
| Loperamide | n.d | Tested only at 30–50 μM |
These investigations underscore significant experimental advantages of TRPM7 agonists. TRPM7 carries very small divalent cation-selective inward currents at physiological membrane potentials. Therefore, a commonly used approach to quantify TRPM7 channel activity relies on fairly large monovalent outward cation currents (usually Cs+) measured at artificially high positive membrane potentials (+100 mV) upon depletion of intracellular Mg2+. These experimental results, however, can hardly be correlated with TRPM7-mediated influx of divalent cations at physiological membrane potentials in the presence of internal Mg2+ and Mg-ATP. In contrast, naltriben allows for the recording of TRPM7 currents without chelation of intracellular Mg2+. Furthermore, naltriben is well suited to monitor TRPM7 activity using Ca2+ imaging techniques that are easily adaptable to screen for new TRPM7 modulators, and in experiments with freshly isolated/primary cells that are difficult to culture or problematic to assess by the patch-clamp technique. Finally, it will be interesting to study whether activation of TRPM7 currents would impact the function of the TRPM7 kinase.
4. Conclusions/Outlook
In the recent past, several research groups identified a set of small organic modulators of the TRPM7 channel. These research efforts resulted in new compounds allowing for the first time to probe TRPM7 currents in native tissues under physiological conditions. The identified molecules have the potential to serve as lead structures for the development of high-affinity in vivo drugs targeting TRPM7. Drugs specifically acting on the TRPM7 kinase are not available yet. In the future, an additional rewarding line of research will be the identification of specific drugs acting on the TRPM7 kinase moiety to decipher TRPM7 channel versus kinase function in cellular physiology and pathophysiology.
Acknowledgments
Thomas Gudermann and Vladimir Chubanov were supported by the Deutsche Forschungsgemeinschaft (grants CH1181/1-1; TRP 152/1). Sebastian Schäfer was supported by the Förderprogramm für Forschung und Lehre Fellowship (FöFoLe) of the LMU, Munich.
Author contributions
Vladimir Chubanov, Sebastian Schäfer, Silvia Ferioli and Thomas Gudermann wrote the review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Nadler M.J., Hermosura M.C., Inabe K., Perraud A.L., Zhu Q., Stokes A.J., Kurosaki T., Kinet J.P., Penner R., Scharenberg A.M., et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 2.Runnels L.W., Yue L., Clapham D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 3.Ryazanov A.G., Pavur K.S., Dorovkov M.V. Alpha-kinases: A new class of protein kinases with a novel catalytic domain. Curr. Biol. 1999;9:R43–R45. doi: 10.1016/S0960-9822(99)80006-2. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi H., Matsushita M., Nairn A.C., Kuriyan J. Crystal structure of the atypical protein kinase domain of a trp channel with phosphotransferase activity. Mol. Cell. 2001;7:1047–1057. doi: 10.1016/S1097-2765(01)00256-8. [DOI] [PubMed] [Google Scholar]
- 5.Fleig A., Chubanov V. TRPM7. Handb. Exp. Pharmacol. 2014;222:521–546. doi: 10.1007/978-3-642-54215-2_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlingmann K.P., Waldegger S., Konrad M., Chubanov V., Gudermann T. TRPM6 and TRPM7—gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta. 2007;1772:813–821. doi: 10.1016/j.bbadis.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Schlingmann K.P., Weber S., Peters M., Nejsum L.N., Vitzthum H., Klingel K., Kratz M., Haddad E., Ristoff E., Dinour D., et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 8.Walder R.Y., Landau D., Meyer P., Shalev H., Tsolia M., Borochowitz Z., Boettger M.B., Beck G.E., Englehardt R.K., Carmi R., et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat. Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 9.Chubanov V., Waldegger S., y Schnitzler M.M., Vitzthum H., Sassen M.C., Seyberth H.W., Konrad M., Gudermann T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. USA. 2004;101:2894–2899. doi: 10.1073/pnas.0305252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryazanov A.G. Elongation factor-2 kinase and its newly discovered relatives. FEBS Lett. 2002;514:26–29. doi: 10.1016/S0014-5793(02)02299-8. [DOI] [PubMed] [Google Scholar]
- 11.Chubanov V., Gudermann T. TRPM6. Handb. Exp. Pharmacol. 2014;222:503–520. doi: 10.1007/978-3-642-54215-2_20. [DOI] [PubMed] [Google Scholar]
- 12.Penner R., Fleig A. The Mg2+ and Mg2+-nucleotide-regulated channel-kinase TRPM7. Handb. Exp. Pharmacol. 2007:313–328. doi: 10.1007/978-3-540-34891-7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paravicini T.M., Chubanov V., Gudermann T. TRPM7: A unique channel involved in magnesium homeostasis. Int. J. Biochem. Cell Biol. 2012;44:1381–1384. doi: 10.1016/j.biocel.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Runnels L.W. TRPM6 and TRPM7: A Mul-TRP-PLIK-cation of channel functions. Curr. Pharm. Biotechnol. 2010;12:42–53. doi: 10.2174/138920111793937880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bates-Withers C., Sah R., Clapham D.E. TRPM7, the Mg2+ inhibited channel and kinase. Adv. Exp. Med. Biol. 2011;704:173–183. doi: 10.1007/978-94-007-0265-3_9. [DOI] [PubMed] [Google Scholar]
- 16.Mederos y Schnitzler M., Waring J., Gudermann T., Chubanov V. Evolutionary determinants of divergent calcium selectivity of TRPM channels. FASEB J. 2008;22:1540–1551. doi: 10.1096/fj.07-9694com. [DOI] [PubMed] [Google Scholar]
- 17.Chubanov V., Schlingmann K.P., Waring J., Heinzinger J., Kaske S., Waldegger S., Mederos y Schnitzler M., Gudermann T. Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J. Biol. Chem. 2007;282:7656–7667. doi: 10.1074/jbc.M611117200. [DOI] [PubMed] [Google Scholar]
- 18.Li M., Du J., Jiang J., Ratzan W., Su L.T., Runnels L.W., Yue L. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J. Biol. Chem. 2007;282:25817–25830. doi: 10.1074/jbc.M608972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann T., Chubanov V., Gudermann T., Montell C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr. Biol. 2003;13:1153–1158. doi: 10.1016/S0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- 20.Kaske S., Krasteva G., Konig P., Kummer W., Hofmann T., Gudermann T., Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann T., Schafer S., Linseisen M., Sytik L., Gudermann T., Chubanov V. Activation of TRPM7 channels by small molecules under physiological conditions. Pflugers. Arch. 2014 doi: 10.1007/s00424-014-1488-0. [DOI] [PubMed] [Google Scholar]
- 22.Xie J., Sun B., Du J., Yang W., Chen H.C., Overton J.D., Runnels L.W., Yue L. Phosphatidylinositol 4,5-bisphosphate (pip(2)) controls magnesium gatekeeper TRPM6 activity. Sci. Rep. 2011;1:146. doi: 10.1038/srep00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaitsuka T., Katagiri C., Beesetty P., Nakamura K., Hourani S., Tomizawa K., Kozak J.A., Matsushita M. Inactivation of TRPM7 kinase activity does not impair its channel function in mice. Sci. Rep. 2014;4:5718. doi: 10.1038/srep05718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz C., Perraud A.L., Johnson C.O., Inabe K., Smith M.K., Penner R., Kurosaki T., Fleig A., Scharenberg A.M. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/S0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 25.Ryazanova L.V., Rondon L.J., Zierler S., Hu Z., Galli J., Yamaguchi T.P., Mazur A., Fleig A., Ryazanov A.G. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat. Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahni J., Scharenberg A.M. TRPM7 ion channels are required for sustained phosphoinositide 3-kinase signaling in lymphocytes. Cell Metab. 2008;8:84–93. doi: 10.1016/j.cmet.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su L.T., Agapito M.A., Li M., Simonson W.T., Huttenlocher A., Habas R., Yue L., Runnels L.W. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J. Biol. Chem. 2006;281:11260–11270. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei C., Wang X., Chen M., Ouyang K., Song L.S., Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark K., Langeslag M., van Leeuwen B., Ran L., Ryazanov A.G., Figdor C.G., Moolenaar W.H., Jalink K., van Leeuwen F.N. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng X., Cai C., Wu J., Cai S., Ye C., Chen H., Yang Z., Zeng H., Shen Q., Zou F. TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway. Cancer Lett. 2013;333:96–102. doi: 10.1016/j.canlet.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui T.A., Lively S., Vincent C., Schlichter L.C. Regulation of podosome formation, microglial migration and invasion by Ca2+-signaling molecules expressed in podosomes. J. Neuroinflam. 2012;9:250. doi: 10.1186/1742-2094-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuras Z., Yun Y.H., Chimote A.A., Neumeier L., Conforti L. KCA3.1 and TRPM7 channels at the uropod regulate migration of activated human T cells. PLoS One. 2012;7:e43859. doi: 10.1371/journal.pone.0043859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su L.T., Liu W., Chen H.C., Gonzalez-Pagan O., Habas R., Runnels L.W. TRPM7 regulates polarized cell movements. Biochem. J. 2011;434:513–521. doi: 10.1042/BJ20101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J.P., Luan Y., You C.X., Chen X.H., Luo R.C., Li R. TRPM7 regulates the migration of human nasopharyngeal carcinoma cell by mediating Ca2+ influx. Cell Calcium. 2010;47:425–432. doi: 10.1016/j.ceca.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Chen K.H., Xu X.H., Liu Y., Hu Y., Jin M.W., Li G.R. TRPM7 channels regulate proliferation and adipogenesis in 3T3-L1 preadipocytes. J. Cell. Physiol. 2013;229:60–67. doi: 10.1002/jcp.24417. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z., Wang M., Fan X.H., Chen J.H., Guan Y.Y., Tang Y.B. Upregulation of TRPM7 channels by angiotensin II triggers phenotypic switching of vascular smooth muscle cells of ascending aorta. Circ. Res. 2012;111:1137–1146. doi: 10.1161/CIRCRESAHA.112.273755. [DOI] [PubMed] [Google Scholar]
- 37.Abed E., Martineau C., Moreau R. Role of melastatin transient receptor potential 7 channels in the osteoblastic differentiation of murine MC3T3 cells. Calcif Tissue Int. 2011;88:246–253. doi: 10.1007/s00223-010-9455-z. [DOI] [PubMed] [Google Scholar]
- 38.Numata T., Shimizu T., Okada Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell. Physiol. Biochem. 2007;19:1–8. doi: 10.1159/000099187. [DOI] [PubMed] [Google Scholar]
- 39.Oancea E., Wolfe J.T., Clapham D.E. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ. Res. 2006;98:245–253. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- 40.Brauchi S., Krapivinsky G., Krapivinsky L., Clapham D.E. TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane. Proc. Natl. Acad. Sci. USA. 2008;105:8304–8308. doi: 10.1073/pnas.0800881105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aarts M., Iihara K., Wei W.L., Xiong Z.G., Arundine M., Cerwinski W., MacDonald J.F., Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/S0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 42.Touyz R.M. Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: Implications in hypertension. Am J. Physiol. Heart Circ. Physiol. 2008;294:H1103–H1118. doi: 10.1152/ajpheart.00903.2007. [DOI] [PubMed] [Google Scholar]
- 43.Hermosura M.C., Nayakanti H., Dorovkov M.V., Calderon F.R., Ryazanov A.G., Haymer D.S., Garruto R.M. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two guamanian neurodegenerative disorders. Proc. Natl. Acad. Sci. USA. 2005;102:11510–11515. doi: 10.1073/pnas.0505149102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseveleki V., Rubio R., Vamvakas S.S., White J., Taoufik E., Petit E., Quackenbush J., Probert L. Comparative gene expression analysis in mouse models for multiple sclerosis, alzheimer’s disease and stroke for identifying commonly regulated and disease-specific gene changes. Genomics. 2010;96:82–91. doi: 10.1016/j.ygeno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du J., Xie J., Zhang Z., Tsujikawa H., Fusco D., Silverman D., Liang B., Yue L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ. Res. 2010;106:992–1003. doi: 10.1161/CIRCRESAHA.109.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guilbert A., Gautier M., Dhennin-Duthille I., Haren N., Sevestre H., Ouadid-Ahidouch H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am. J. Physiol. Cell Physiol. 2009;297:C493–C502. doi: 10.1152/ajpcell.00624.2008. [DOI] [PubMed] [Google Scholar]
- 47.Kim B.J., Park E.J., Lee J.H., Jeon J.H., Kim S.J., So I. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci. 2008;99:2502–2509. doi: 10.1111/j.1349-7006.2008.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang J., Li M.H., Inoue K., Chu X.P., Seeds J., Xiong Z.G. Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: Role in cell proliferation. Cancer Res. 2007;67:10929–10938. doi: 10.1158/0008-5472.CAN-07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanano T., Hara Y., Shi J., Morita H., Umebayashi C., Mori E., Sumimoto H., Ito Y., Mori Y., Inoue R. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J. Pharmacol. Sci. 2004;95:403–419. doi: 10.1254/jphs.FP0040273. [DOI] [PubMed] [Google Scholar]
- 50.Middelbeek J., Kuipers A.J., Henneman L., Visser D., Eidhof I., van Horssen R., Wieringa B., Canisius S.V., Zwart W., Wessels L.F., et al. TRPM7 is required for breast tumor cell metastasis. Cancer Res. 2012;72:4250–4261. doi: 10.1158/0008-5472.CAN-11-3863. [DOI] [PubMed] [Google Scholar]
- 51.Rybarczyk P., Gautier M., Hague F., Dhennin-Duthille I., Chatelain D., Kerr-Conte J., Pattou F., Regimbeau J.M., Sevestre H., Ouadid-Ahidouch H. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int. J. Cancer. 2012;131:E851–E861. doi: 10.1002/ijc.27487. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y.F., Chen Y.T., Chiu W.T., Shen M.R. Remodeling of calcium signaling in tumor progression. J. Biomed. Sci. 2013;20:23. doi: 10.1186/1423-0127-20-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao H., Chen X., Du X., Guan B., Liu Y., Zhang H. EGF enhances the migration of cancer cells by up-regulation of TRPM7. Cell Calcium. 2011;50:559–568. doi: 10.1016/j.ceca.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Arking D.E., Pulit S.L., Crotti L., van der Harst P., Munroe P.B., Koopmann T.T., Sotoodehnia N., Rossin E.J., Morley M., Wang X., et al. Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat. Genet. 2014;46:826–836. doi: 10.1038/ng.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin J., Desai B.N., Navarro B., Donovan A., Andrews N.C., Clapham D.E. Deletion of TRPM7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elizondo M.R., Arduini B.L., Paulsen J., MacDonald E.L., Sabel J.L., Henion P.D., Cornell R.A., Parichy D.M. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for TRPM7. Curr. Biol. 2005;15:667–671. doi: 10.1016/j.cub.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 57.Jin J., Wu L.J., Jun J., Cheng X., Xu H., Andrews N.C., Clapham D.E. The channel kinase, TRPM7, is required for early embryonic development. Proc. Natl. Acad. Sci. USA. 2012;109:E225–E233. doi: 10.1073/pnas.1120033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sah R., Mesirca P., Van den Boogert M., Rosen J., Mably J., Mangoni M.E., Clapham D.E. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc. Natl. Acad. Sci. USA. 2013;110:E3037–E3046. doi: 10.1073/pnas.1311865110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sah R., Mesirca P., Mason X., Gibson W., Bates-Withers C., Van den Boogert M., Chaudhuri D., Pu W.T., Mangoni M.E., Clapham D.E. Timing of myocardial TRPM7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation. 2013;128:101–114. doi: 10.1161/CIRCULATIONAHA.112.000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dorovkov M.V., Ryazanov A.G. Phosphorylation of annexin I by TRPM7 channel-kinase. J. Biol. Chem. 2004;279:50643–50646. doi: 10.1074/jbc.C400441200. [DOI] [PubMed] [Google Scholar]
- 61.Clark K., Middelbeek J., Lasonder E., Dulyaninova N.G., Morrice N.A., Ryazanov A.G., Bresnick A.R., Figdor C.G., van Leeuwen F.N. TRPM7 regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J. Mol. Biol. 2008;378:790–803. doi: 10.1016/j.jmb.2008.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perraud A.L., Zhao X., Ryazanov A.G., Schmitz C. The channel-kinase TRPM7 regulates phosphorylation of the translational factor EEF2 via EEF2-K. Cell Signal. 2011;23:586–593. doi: 10.1016/j.cellsig.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deason-Towne F., Perraud A.L., Schmitz C. Identification of ser/thr phosphorylation sites in the C2-domain of phospholipase c gamma2 (plcgamma2) using TRPM7-kinase. Cell Signal. 2012;24:2070–2075. doi: 10.1016/j.cellsig.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark K., Middelbeek J., Morrice N.A., Figdor C.G., Lasonder E., van Leeuwen F.N. Massive autophosphorylation of the ser/thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS One. 2008;3:e1876. doi: 10.1371/journal.pone.0001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsushita M., Kozak J.A., Shimizu Y., McLachlin D.T., Yamaguchi H., Wei F.Y., Tomizawa K., Matsui H., Chait B.T., Cahalan M.D., et al. Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/CHAK1. J. Biol. Chem. 2005;280:20793–20803. doi: 10.1074/jbc.M413671200. [DOI] [PubMed] [Google Scholar]
- 66.Desai B.N., Krapivinsky G., Navarro B., Krapivinsky L., Carter B.C., Febvay S., Delling M., Penumaka A., Ramsey I.S., Manasian Y., et al. Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in fas-induced apoptosis. Dev. Cell. 2012;22:1149–1162. doi: 10.1016/j.devcel.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krapivinsky G., Krapivinsky L., Manasian Y., Clapham D.E. The TRPM7 chanzyme is cleaved to release a chromatin-modifying kinase. Cell. 2014;157:1061–1072. doi: 10.1016/j.cell.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monteilh-Zoller M.K., Hermosura M.C., Nadler M.J., Scharenberg A.M., Penner R., Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Demeuse P., Penner R., Fleig A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J. Gen. Physiol. 2006;127:421–434. doi: 10.1085/jgp.200509410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmitz C., Deason F., Perraud A.L. Molecular components of vertebrate mg2+-homeostasis regulation. Magnes. Res. 2007;20:6–18. [PubMed] [Google Scholar]
- 71.Runnels L.W., Yue L., Clapham D.E. The TRPM7 channel is inactivated by pip(2) hydrolysis. Nat. Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- 72.Kozak J.A., Matsushita M., Nairn A.C., Cahalan M.D. Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels. J. Gen. Physiol. 2005;126:499–514. doi: 10.1085/jgp.200509324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prakriya M., Lewis R.S. Separation and characterization of currents through store-operated crac channels and Mg2+-Inhibited Cation (MIC) channels. J. Gen. Physiol. 2002;119:487–507. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li M., Jiang J., Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kozak J.A., Kerschbaum H.H., Cahalan M.D. Distinct properties of crac and mic channels in RBL cells. J. Gen. Physiol. 2002;120:221–235. doi: 10.1085/jgp.20028601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen X., Numata T., Li M., Mori Y., Orser B.A., Jackson M.F., Xiong Z.G., MacDonald J.F. The modulation of TRPM7 currents by nafamostat mesilate depends directly upon extracellular concentrations of divalent cations. Mol. Brain. 2010;3:38. doi: 10.1186/1756-6606-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parnas M., Peters M., Dadon D., Lev S., Vertkin I., Slutsky I., Minke B. Carvacrol is a novel inhibitor of drosophila trpl and mammalian TRPM7 channels. Cell Calcium. 2009;45:300–309. doi: 10.1016/j.ceca.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen H.C., Xie J., Zhang Z., Su L.T., Yue L., Runnels L.W. Blockade of TRPM7 channel activity and cell death by inhibitors of 5-lipoxygenase. PLoS One. 2010;5:e11161. doi: 10.1371/journal.pone.0011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zierler S., Yao G., Zhang Z., Kuo W.C., Porzgen P., Penner R., Horgen F.D., Fleig A. Waixenicin a inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J. Biol. Chem. 2011;286:39328–39335. doi: 10.1074/jbc.M111.264341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chubanov V., y Schnitzler M.M., Meissner M., Schafer S., Abstiens K., Hofmann T., Gudermann T. Natural and synthetic modulators of SK (k(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br. J. Pharmacol. 2012;166:1357–1376. doi: 10.1111/j.1476-5381.2012.01855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qin X., Yue Z., Sun B., Yang W., Xie J., Ni E., Feng Y., Mahmood R., Zhang Y., Yue L. Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. Br. J. Pharmacol. 2013;168:1294–1312. doi: 10.1111/bph.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chokshi R., Fruasaha P., Kozak J.A. 2-aminoethyl diphenyl borinate (2-apb) inhibits TRPM7 channels through an intracellular acidification mechanism. Channels (Austin) 2012;6:362–369. doi: 10.4161/chan.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davis F.M., Azimi I., Faville R.A., Peters A.A., Jalink K., Putney J.W., Jr., Goodhill G.J., Thompson E.W., Roberts-Thomson S.J., Monteith G.R. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014;33:2307–2316. doi: 10.1038/onc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siddiqui T., Lively S., Ferreira R., Wong R., Schlichter L.C. Expression and contributions of TRPM7 and KCA2.3/SK3 channels to the increased migration and invasion of microglia in anti-inflammatory activation states. PLoS One. 2014;9:e106087. doi: 10.1371/journal.pone.0106087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schilling T., Miralles F., Eder C. TRPM7 channels regulate proliferation and polarisation of macrophages. J. Cell Sci. 2014 doi: 10.1242/jcs.151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim B.J., Nam J.H., Kwon Y.K., So I., Kim S.J. The role of waixenicin a as transient receptor potential melastatin 7 blocker. Basic Clin. Pharmacol. Toxicol. 2013;112:83–89. doi: 10.1111/j.1742-7843.2012.00929.x. [DOI] [PubMed] [Google Scholar]
- 87.Visser D., Langeslag M., Kedziora K.M., Klarenbeek J., Kamermans A., Horgen F.D., Fleig A., van Leeuwen F.N., Jalink K. TRPM7 triggers Ca2+ sparks and invadosome formation in neuroblastoma cells. Cell Calcium. 2013;54:404–415. doi: 10.1016/j.ceca.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nam J.H., Kim W.K., Kim B.J. Sphingosine and FTY720 modulate pacemaking activity in interstitial cells of cajal from mouse small intestine. Mol. Cells. 2013;36:235–244. doi: 10.1007/s10059-013-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sofuoglu M., Portoghese P.S., Takemori A.E. Differential antagonism of delta opioid agonists by naltrindole and its benzofuran analog (NTB) in mice: Evidence for delta opioid receptor subtypes. J. Pharmacol. Exp. Ther. 1991;257:676–680. [PubMed] [Google Scholar]