Figure 1.
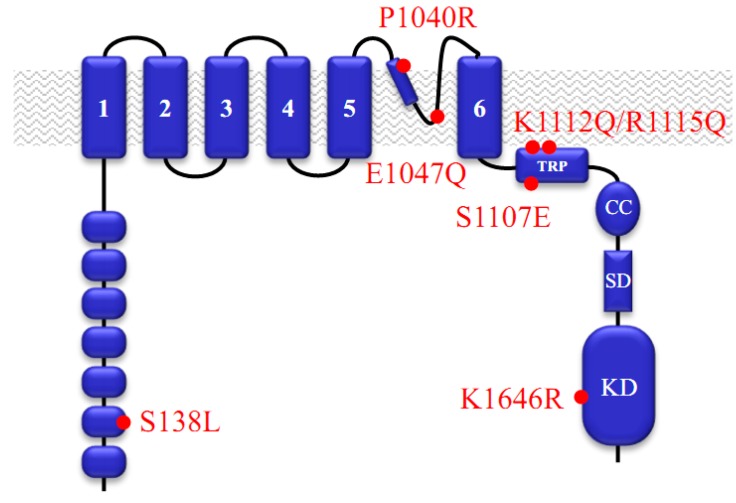
Domain topology of the murine kinase-coupled channel Transient receptor potential cation channel subfamily M member 7 (TRPM7).
The plasma membrane channel segment of TRPM7 comprises six transmembrane helices (1–6). A short stretch between the 5 and 6 helices contains a predicted pore forming loop and pore helix [16]. A large cytosolic N-terminus of TRPM7 contains a set of domains that are highly conserved among the TRPM gene family and resemble ankyrin repeats as revealed by 3D modeling [11]. A C-terminus of TRPM7 contains a highly conserved transient receptor potential (TRP) domain, a coiled-coil (CC) domain, a kinase substrate domain (SD) and a kinase domain (KD). Red dots indicate the location of point mutations that were highly instrumental in probing of TRPM7 functions. Mutations S138L and P1040R correspond to TRPM6 missense mutations causing an inherited disorder in humans known as hypomagnesemia and secondary hypocalcemia (HSH) [9,17]. S138L disrupts assembly of TRPM7 channel complexes [9], whereas P1040R results in a dominant-negative channel subunit [17]. E1047 is a negatively charged residue located in a ‘selectivity’ filter of the TRPM7 channel pore and E1047Q mutation results in an active channel permeable to monovalent cations and impermeable to divalent ions like Ca2+ and Mg2+ [16,18] recapitulating the characteristic feature of the taste-signaling TRPM5 channel [19,20]. The S1107E mutation produces a constitutively active channel insensitive to intracellular Mg2+ and PIP2 [21]. Positively charged residues K1112 and R1115 were suggested to be required for PIP2 dependent gating of TRPM7 and, consequently, the K1112Q/R1115Q double mutation ablates TRPM7 currents [22]. K1646 is a highly conserved residue located in the catalytic site of the kinase domain [4] and the K1646R mutation is sufficient to block the kinase function of TRPM7 (‘kinase-dead’ mutation) [23,24].