Abstract
Di-n-Butyl (DBP) and Di-(2-EthylHexyl) (DEHP) phthalates can leach from daily-use products resulting in environmental exposure. In male rodents, phthalate exposure results in reproductive effects. To evaluate effects on the immature primate testis, testis fragments from 6-month-old rhesus macaques were grafted subcutaneously to immune-deficient mice, which were exposed to 0, 10, or 500 mg/kg of DBP or DEHP for 14 weeks or 28 weeks (DBP only). DBP exposure reduced the expression of key steroidogenic genes, indicating that Leydig cell function was compromised. Exposure to 500 mg/kg impaired tubule formation and germ cell differentiation and reduced numbers of spermatogonia. Exposure to 10 mg/kg did not affect development, but reduced Sertoli cell number and resulted in increased expression of inhibin B. Exposure to DEHP for 14 week also affected steroidogenic genes expression. Therefore, long-term exposure to phthalate esters affected development and function of the primate testis in a time and dosage dependent manner.
Keywords: phthalates, testis, testis development, steroidogenesis, spermatogenesis, non-human primates
1. Introduction
Phthalate esters, or phthalates, are present in a wide variety of products, from personal care products to medical devices, as they provide flexibility and other desirable characteristics. Di-n-Butyl phthalate (DBP) and Di-(2-EthylHexyl) phthalate (DEHP) are two of the most commonly used phthalates (http://www.epa.gov/teach/chem_summ/phthalates_summary.pdf). As these compounds are not covalently bound to the products to which they are added, they leach into the environment, resulting in human exposure. Currently, there is growing concern regarding the teratogenic, carcinogenic, and endocrine disrupting properties of phthalates. Because phthalates have been described as anti-androgenic compounds, males are considered a particularly susceptible population (David, 2003; Fisher, 2004; Frederiksen et al., 2007; Knez, 2013; Lyche et al., 2009; Ventrice et al., 2013). While the measured exposure to phthalates in the general population has been considered below tolerable levels of intake, children undergoing medical interventions may be exposed to significantly higher quantities of phthalates through equipment and medical devices (Fischer et al., 2013; Lyche et al., 2009; Wittassek and Angerer, 2008). The dose of phthalate exposure in these children has been estimated up to 10 – 20 mg/kg/day (Loff et al., 2000). Moreover, as the mechanisms of actions and effects of some groups of phthalates are similar enough, they may be considered additive (Gray et al., 2000; Rider et al., 2010).
Numerous studies performed in rats have shown that testosterone secretion and testis development are susceptible to disruption by phthalate exposure. For example, rats exposed to DBP and DEHP during the prenatal period show developmental abnormalities that are typical of the Testicular Dysgenesis Syndrome: cryptorchidism and alterations of the reproductive tract. These abnormalities have been associated with reductions in testosterone secretion and expression of steroidogenic enzymes (Barlow et al., 2003; Chen et al., 2013; Foster, 2005; Foster, 2006; Lehmann et al., 2004; Mylchreest et al., 1998). The postnatal period of development has been considered to be susceptible as well; prepubertal rats exposed to DEHP exhibit reduced testosterone secretion (Akingbemi et al., 2001), increased testicular apoptosis, and loss of the seminiferous epithelium (Park et al., 2002). Studies performed in mice have shown that some species differences exist in regards to sensitivity to phthalates. Mice exposed prenatally to a single dose of 500 mg/kg of phthalates exhibit germ cell abnormalities, but testosterone production is not affected (Gaido et al., 2007; Heger et al., 2012). However, oral administration of 500 mg/kg DBP to male mice from 4 to 14 days of age resulted in lower serum testosterone (Moody et al., 2013). This indicates the sensitivity of testis to phthalates effects is likely developmental stage dependent.
Studies in humans have been fewer and often contradictory. Epidemiological studies found a negative association between the anogenital distance in newborn boys and the concentration of phthalate metabolites in their mother’s urine (Swan et al., 2005). However, others have failed to find similar associations (Huang et al., 2009). In adults, several studies indicated a possible association between phthalate exposure and increase in sperm abnormalities (Duty et al., 2004; Duty et al., 2003a; Duty et al., 2003b; Hauser et al., 2006; Hauser et al., 2007; Rozati et al., 2002) and lower plasma testosterone concentration (Pan et al., 2006). However, another study did not show any association between phthalate metabolites and reproductive biomarkers (Jonsson et al., 2005). Thus, due to the limitations of epidemiological studies, there is yet no definite answer to whether phthalate exposure in humans imposes a significant health risk (Fischer et al., 2013; Johnson et al., 2012; Kamrin, 2009; Lyche et al., 2009).
Experimental studies in non-human primates have also not led to conclusive results. In juvenile marmosets, exposure to DEHP at a daily dose as high as 2500 mg/kg for 13 or 65 weeks did not elicit any effect on the hormonal profiles nor the testicular histology (Kurata et al., 1998; Tomonari et al., 2006). Also, no effects were detected in the testicular histology of young adult cynomolgus monkeys exposed to 500 mg/kg/day of DEHP for 14 days (Pugh et al., 2000). However, administration of a single dose of 500 mg/kg MBP (DBP metabolite) to newborn marmosets suppressed blood testosterone levels 5 hr later. Moreover, continuous administration of MBP for 14 days did not affect testosterone levels, but it resulted in an increase of Leydig cell volume, indicating compensatory hypertrophy of Leydig cells in response to compromised steroidogenesis (Hallmark et al., 2007). Ethical and logistical considerations have not allowed more extensive studies on phthalate effects in primates.
Testis tissue xenografting has emerged as a feasible strategy to study testis development and spermatogenesis from large animals in mice (Honaramooz et al., 2002). In this approach, testis fragments transplanted ectopically from immature males into immunodeficient adult castrated mice are able to survive, establish hormonal signaling with the rodent host, and undergo full development (for review see (Rodriguez-Sosa and Dobrinski, 2009)). Previously, we have shown that testis tissue from 6-month-old rhesus macaques attains full maturity at ~7 months after grafting in mice, as long as exogenous gonadotropins are administered to recipient mice (Rathi et al., 2008). Here, we used testis tissue xenografting to evaluate whether the developing primate testis is susceptible to the disrupting effects of phthalates.
2. Materials and Methods
2.1 Donor Tissue, Recipient Mice, and Ectopic Xenografting
Testes were obtained from 6-month-old rhesus macaques and 6- to 8-week-old SCID mice (Taconic, Germantown, NY, USA) were used as recipients. Testes were cut into fragments of ~1 mm3 (~1 mg each), kept in ice-cold DMEM (Life Technologies, Burlington, ON, Canada), and transplanted under the dorsal skin of castrated recipient mice as described previously (Rathi et al., 2008). Eight fragments of donor testes were placed under the back skin of each mouse, using 12 mice per donor. A total of 12 and 8 donors were used for the DBP and DEHP studies, respectively. Fragments of donor tissue that were not transplanted were fixed in 4% PFA or Bouin’s solution and processed as described below. All animal procedures were in accordance with the Animal Care and Use Committees of the University of Pennsylvania and the University of Calgary.
2.2 Treatment of Recipient Mice
For each donor, recipients were randomly divided into three groups. One week after surgery, recipients in each group started to receive 0, 10, or 500 mg/kg of DBP or DEHP by daily oral gavage using corn oil (all from Sigma, Oakville, ON, Canada) as vehicle. For the DBP study, recipient mice from 7 and 5 donors were treated for 14 and 28 weeks, respectively. In the DEHP study, all recipients were treated for 14 weeks. From week 4 after surgery, recipients received 10 IU of human chorionic gonadotropin (hCG, EMD Chemicals, Gibbstown, NJ, USA) subcutaneously twice per week to stimulate xenograft development and support complete spermatogenesis (Rathi et al., 2008). Recipients were weighed every other day and an average weight was determined for each group.
2.3 Sacrifice of Recipient Mice and Collection of Xenografts
At the end of treatment (14 weeks or 28 weeks), mice were anaesthetized and bled by cardiac puncture. Serum was collected and kept frozen until testosterone analyses. Mouse body weight, liver weight, and seminal vesicle weight were measured. Xenografts were collected, weighed, snap frozen on dry ice for testosterone analysis, placed to RNA Later Stabilization solution (Qiagen, Toronto, ON, Canada) and used for RNA isolation, or fixed as mentioned above. Fixed xenografts were processed and embedded in paraffin.
2.4 Evaluation of Xenografts and Stereological Analysis
Three sections per xenograft, separated at least 50 μm from each other, were considered for all analyses. Sections from xenografts fixed in Bouin’s solution were stained with H&E and examined at 20× magnification. All cords and tubules present along the longitudinal and transverse axes of each section were scored for the most advanced type of germ cell present (51 – 518 tubules / xenograft, average = 208.6 tubules/xenograft). Also, these H&E-stained sections were used for morphometrical evaluations as described previously (Avelar et al., 2010; Rodriguez-Sosa et al., 2012). Briefly, the volume densities of the testis components in xenografts were determined by light microscopy using a 441-intersection grid placed in the ocular of the microscope. In each group, 15 fields (400× magnification, 6615 grid points) were randomly chosen and scored per treatment group from each donor. In total, 105 section fields per treatment group were scored for 14 weeks and 75 section fields per treatment group were scored for 28 weeks.
2.5 Immunohistochemistry
Tissue sections from xenografts fixed in Bouin’s solution were immunostained for UCH-L1, AR and PCNA as previously described (Rathi et al., 2008; Rodriguez-Sosa et al., 2012). Antigen retrieval was performed by boiling sections in citrate buffer (pH = 6.0) for 10 min in a microwave operated at high power. Blocking was performed with PBS containing 5% normal donkey serum and all antibodies were used at 1:400 dilution with overnight incubation. Primary antibodies used were: rabbit polyclonal anti-PGP9.5 (cat # 7863-0504, lot 131109, AbD Serotec, Raleigh, NC, USA) for UCH-L1, rabbit polyclonal anti-Androgen Receptor (cat # ab74272, lot # GR99534, Abcam, Cambridge, MA, USA), mouse anti-PCNA (cat # M087901-2, lot # 00083603, DakoCytomation, Burlington, ON, Canada). Secondary antibodies were used at concentration of 2.5 μg/ml for 45 min. For AR and UCH-L1, donkey anti-rabbit antibodies conjugated to peroxidase (cat # 711-035-152, Jackson Immunoresearch, West Grove, PA, USA) were used. Staining was developed by exposure to Vector Nova Red (Vector Labs, Burlington, ON, Canada) according to manufacturer instructions. Sections were dehydrated and mounted in Permount (Fisher Scientific, Ottawa, ON, Canada). For double immunofluorescence of AR and PCNA, secondary antibodies were donkey anti-rabbit antibody conjugated to alexaflour-488 and donkey anti-mouse conjugated to alexaflour-555 (both from Life Technologies, Burlington, ON, Canada). Sections were mounted in Vectashield with DAPI (Vector Labs, Burlington, ON, Canada). For all staining procedures, sections incubated in buffer without primary antibody as well as rabbit and mouse IgG isotype controls were used as negative controls. In three sections per xenograft, seminiferous cords or tubules were randomly chosen as above and scored. When the percentage of tubules with UCH-L1+ germ cells was evaluated, on average 2500-3500 tubule cross sections were scored per donor for each treatment group at both time points. To obtain the number of UCH-L1 positive cells per tubule cross-sections, we counted cells in 140-316 tubule cross-sections per treatment group at each time points.
2.6 Apoptosis
Sections from xenografts fixed in 4% PFA were used for TUNEL assay, which was performed using the ApopTag Plus Fluorescein Detection Kit (Millipore, Temecula, CA, USA) according to manufacturer instructions. Sections incubated in buffer without terminal deoxynucleotidyl tranferase (TdT) were used as negative control. Seminiferous cords and tubules were randomly chosen as above and scored for the presence of apoptotic structures (nuclei or nuclear fragments). Between 600 and 1000 tubule cross sections per treatment group were scored at each time point.
2.7 Reverse transcription and real-time PCR
Total RNA was isolated from xenografts using RNeasy Mini Kit (Qiagen, Toronto, ON, Canada). For reverse transcription, 2 μg of total RNA was used in a final volume of 25 μl reaction containing 0.5 μg of Oligo d(T)12-18, RT buffer (1x), 10 mM dithiothreitol, 0.5 mM of dNTP, 5U of RNase-inhibitor, and 10U of SuperScript II Reverse transcriptase (Life Technologies, Burlington, ON, Canada). Reverse transcription was carried out at 42°C for 1 hour. Quantitative RT-PCR amplification was performed using SsoFast Eva Green SYBR Green Master Mix (Bio-Rad, Mississauga, ON, Canada) in 7500 Fast Real Time PCR System (Applied Biosystems, Life Technologies, Burlington, ON, Canada). The GAPDH gene was amplified as an internal control for each Real-Time PCR. Primer sequences, length of amplified products, and annealing temperatures were as outlined in the Supplemental Table 1. All amplified products were verified by High Resolution Melting Curve analysis, and relative levels of gene expression were analyzed by delta-dCt method using 7500 Software (Applied Biosystems, Life Technologies, Burlington, ON, Canada).
2.8 Testosterone Analyses
Serum testosterone was determined by heterologous radioimmunoassay, similar to previous reports (Stabenfeldt et al. 1979). Serum samples were extracted with diethyl ether and incubated overnight with diluted antisera (S-250, Dr. G. Niswender, 1:130,000) and tracer (1,2,6,7-3H-testosterone). Free tracer was separated using dextran-coated charcoal and the bound fraction counted after addition of scintillation cocktail. The standard curve ranged from 2.5-200 pg/tube. All samples were assayed in a single assay and the intra-assay co-efficient of variation was <10% (Stabenfeldt et al. 1979).
To determine tissue testosterone, whole xenografts were sonicated, mixed, and extracted overnight at 4°C in a MeOH volume proportional to fresh tissue weight. A 100 μl aliquot of supernatant (approximately 2 mg of tissue-equivalent) was spiked with 20 μl of internal standard in MeOH, and diluted to 1 ml in water for solid phase extraction (SPE) followed by liquid chromatography – tandem mass spectrometry (APCI) method of Koren et al, 2012 with two modifications. The calibration curve was prepared in water, rather than stripped serum, and the deuterated internal standard contained only testosterone, rather than a mix of steroids. Only one sample fell below of the calibration range (0.01 ng/ml).
2.9 Statistical Analyses
Parameters involving percentages (mouse mortality and xenograft recovery) were compared by Chi-square test using the FREQ procedures of the SAS 9.1.2 program (Cary, NC, USA), while all other parameters involving means (mouse and xenograft weight, seminal vesicle weight, etc.), presented ± SEM, were compared by analysis of variance and Tukey tests using the GLM procedure of the same statistical software.
3. Results
3.1 DBP Exposure
3.1.1 General parameters of recipient mice and testis xenografts
DBP exposure did not affect the mortality of recipient mice at any collection time (P>0.05; data not shown). The body weight of mice in the 500 mg/kg group was lower at 14 weeks (P<0.05), but became comparable to the 0 and 10 mg/kg groups at 28 weeks (P>0.05; data not shown). The percentage of liver weight in relation to body weight was higher at 14 weeks in the 500 mg/kg group (P<0.05), but it was not different at 28 weeks (P>0.05; data not shown). Thus, while DBP affected the body and liver weight, this was a transient effect and only occurred at the higher dose of exposure. DBP exposure did not affect recovery of xenografts (P>0.05; data not shown). In total, 81% (449/554) and 88% (329/376) of xenografts were collected at 14 and 28 weeks, respectively. The xenograft weight was not different at 14 weeks, but at 28 weeks it was lower in the 500 mg/kg group (P<0.05; data not shown).
3.1.2 Leydig Cell Function
Testosterone levels in blood serum of recipient mice and xenograft tissue were examined. However, high variation across groups and monkey donors precluded the direct analysis of the endocrine activity of the testis xenografts. As an alternative approach, we measured the seminal vesicle weight. Seminal vesicles are highly androgen dependent and in castrated mice they undergo complete atrophy (Tsuji et al., 1998). Therefore, the seminal vesicle weight has been successfully used in numerous studies to indirectly evaluate the androgens levels produced by testis xenografts (reviewed in (Rodriguez-Sosa and Dobrinski, 2009)). At both 14 and 28 weeks, the seminal vesicle weight was lower in the 500 mg/kg group (P<0.05, Fig. 1a), indicating reduced androgen production by testis xenografts of this group.
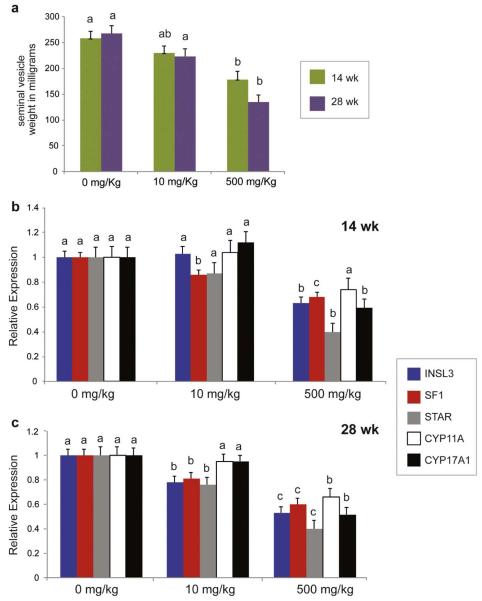
Figure 1.
Endocrine function of testis xenografts from mice treated with different doses of DBP. a) Seminal vesicle weight of recipient mice at time of sacrifice. b) Relative expression of Leydig cell markers at 14 weeks and c) 28 weeks. In each panel, different letters between bars of the same color indicate statistical difference (P<0.05).
Insulin-like factor 3 (INSL3) has been described as a marker of Leydig cell functionality as it closely mirrors the secretion of testosterone by these cells (Heng et al., 2012; Ivell and Anand-Ivell, 2011; Ivell et al., 2013). Consistent with the seminal vesicle weight, the relative expression of INSL3 was significantly lower in xenografts from the 500 mg/kg group than in xenografts from the 0 mg/kg or 10 mg/kg group at 14 weeks (P<0.05, Fig. 1b). Notably, at 28 weeks, INSL3 expression was not only reduced in the 500 mg/kg group, but also in the 10 mg/kg group (P<0.05, Fig. 1c). We also measured the expression of the Steroidogenic Factor-1 (SF1), which is an orphan member of the nuclear hormone receptor superfamily of transcription factors responsible, at least in part, for the development and differentiation of steroidogenic tissues (Val et al., 2003) and the tissue-specific expression of genes involved in steroid hormone biosynthesis (Parker and Schimmer, 1997). At both collection times, the relative expression of SF1 was lower in xenografts of both 10 and 500 mg/kg groups, and affected in a dose-dependent manner (P<0.05, Fig. 1b, c).
Effects on INSL3 and SF1 were accompanied by alterations in the expression of key steroidogenic enzymes. Steroidogenic acute regulatory protein (StAR) is involved in cholesterol transfer into the mitochondria, which is the rate-limiting step in the production of steroid hormones (Christenson and Strauss, 2001). The relative expression of StAR was affected by DBP in a dose and time dependent manner; at 14 weeks the expression level was reduced only in the 500 mg/kg group (P<0.05), but at 28 weeks StAR expression was decreased in both 10 and 500 mg/kg groups (P<0.05) (Fig. 1b, c). Cytochrome P450 (CYP) Cholesterol side-chain cleavage enzyme (CYP11A) and 17α-hydroxylase/17,20-lyase (CYP17A1) are members of the Cytochrome 450 superfamily of monooxygenases and are involved in key steps of the steroidogenic pathway in Leydig cells (Payne and Hales, 2004). The relative expression of both genes was reduced in the 500 mg/kg group at 28 weeks (P<0.05); while at 14 weeks only CYP17A1 was reduced. (Fig. 1b, c). Thus, the steroidogenic pathway was affected by exposure of testicular tissue to DBP in a time and dose-dependent manner.
As expression of some genes indirectly related to Leydig cell steroidogenesis have been reported to be affected by phthalate exposure in rodents, we also examined the expression level of the following genes: the peroxisome-proliferator activated receptors (PPARα, δ, and γ) (Gazouli et al., 2002), translocator protein (TSPO) (Gazouli et al., 2002), 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) (Ma et al., 2011), and the aldosterone receptors 1 and 2, (NR3C1 and 2) (Martinez-Arguelles et al., 2009). However, there was no effect of DBP exposure on the expression of these genes in xenografts of primate testis tissue (P>0.05; data not shown).
3.1.3 Seminiferous tubule development and germ cell differentiation
In comparison to the donor tissue at the time of grafting, at 14 weeks the development of seminiferous tubules was evident by the larger diameter of the seminiferous cords, but no apparent effect of treatment was observed at this time point (Fig. 2a – d). Stereological analyses did not show differences in the density of the tubular and intertubular compartments of testis xenografts (Suppl. Table 2). However, at 28 weeks while tubule formation and full germ cell differentiation was observed in some xenografts (2 out of 5 donors) of the 0 or 10 mg/kg group, neither lumina nor haploid germ cells were observed in xenografts of the 500 mg/kg group. In the latter, only seminiferous cords with presence of spermatocytes were observed (Fig. 2e – h).
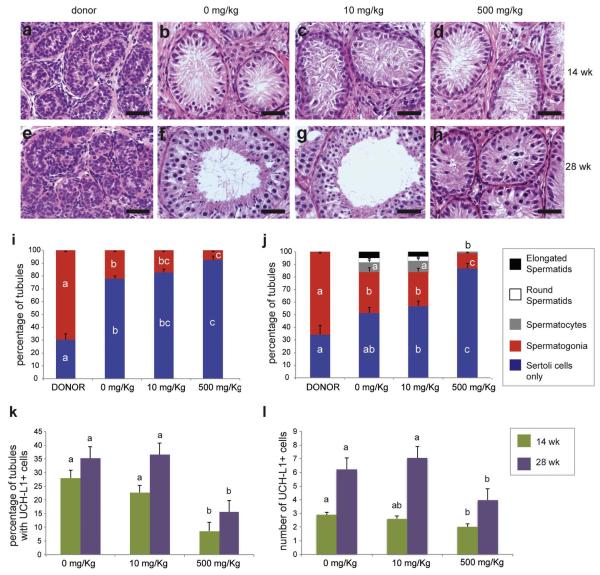
Figure 2.
Development and germ cell differentiation of testis xenografts from mice treated with different doses of DBP. a-h) Histology of donor tissue at the time of grafting (a & e), and that of testis xenografts from mice treated with 0, 10, and 500 mg/kg of DBP for 14 weeks (b-d) and 28 weeks (f-h); i) and j) Percentage of seminiferous tubules with the most advanced type of germ cell present at 14 (i) and 28 (j) weeks; k) Percentage of seminiferous tubules with the presence of spermatogonia (UCH-L1-positive); l) Number of UCH-L1-positive spermatogonia per tubule cross-section. Different letters between bars of the same color indicate statistical difference (P<0.05). Scale bars = 50 μm.
Seminiferous cords or tubules were classified according to the most advanced type of germ cell present. At both 14 and 28 weeks the number of tubules with spermatogonia was lower in xenografts from mice treated with 500 mg/kg than in those from mice treated with 0 or 10 mg/kg of DBP (P<0.05; Fig 2i and j). Moreover, the number of tubules with spermatocytes was also reduced in xenografts from mice treated with 500 mg/kg of DBP for 28 weeks (P<0.05; Fig 2j). The number of tubules containing haploid cells in xenografts was not different between xenografts from mice treated with 0 and 10 mg/kg of DBP for 28 weeks (P>0.05; Fig 2j). Thus, while tubule formation and germ cell differentiation was evidently affected in xenografts from mice treated with 500 mg/kg, this was not observed in xenografts from mice that received 10 mg/kg of DBP.
The number of spermatogonia was evaluated by using ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) as a marker. Overall, the percentage of tubules with UCH-L1+ cells and the number of UCH-L1+ cells per tubule cross-section increased over time (from 14 weeks to 28 weeks) within each group (Fig. 2k and l). However, at both time points, the percentage of tubules with UCH-L1+ cells and the number of UCH-L1+ cells per tubule cross-section were significantly lower in the 500 mg/kg group (P<0.05).
3.1.4 Sertoli cell function
Anti-Mullerian hormone (also known as Mullerian inhibiting substance or MIS) is predominantly expressed by immature Sertoli cells, and this expression decreases as the testis matures (Sharpe et al., 2003). In xenografts, the relative expression of MIS evaluated by quantitative RT-PCR was not affected by treatment (P>0.05; data not shown). We also evaluated the expression of androgen receptor (AR), which increases as Sertoli cells mature (Sharpe et al., 2003). Immuno-histochemical analysis on tubule cross-sections from xenografts revealed strong expression of AR protein in Sertoli cells from all groups (Fig. 3a), indicating that DBP did not affect Sertoli cell maturation.
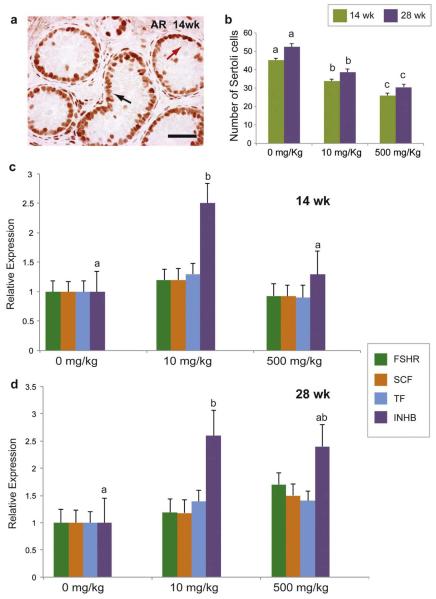
Figure 3.
Sertoli cell maturation and function in testis xenografts from mice treated with different doses of DBP. a) Immunohistochemistry for AR in testis xenografts treated with 0 mg/kg of DBP for 14 weeks. Black arrow shows AR-positive Sertoli cells while red arrow shows AR-negative germ cells; b) Number of Sertoli cells (AR-positive) per tubule cross-section; c) Relative expression of several genes associated with the function of Sertoli cells in xenografts at 14 (c) and 28 weeks (d). Different letters between bars of the same color indicate statistical difference (P<0.05). Scale bar = 50 μm.
As AR was detected in all Sertoli cells, we used this marker to quantify Sertoli cells. At both 14 and 28 weeks, DBP exposure caused a reduction in the number of Sertoli cells per tubule cross-section in a dose dependent manner (P<0.05; Fig. 3b).We then evaluated the relative expression of several genes associated with Sertoli cell functionality. FSH is a positive regulator of Sertoli cell proliferation and function (O’Shaughnessy et al., 2010), and its receptor (FSHR) has been shown to be affected by phthalate exposure in mice (Pocar et al., 2012). We did not detect any effect of DBP on the expression of FSHR in primate testis xenografts (P>0.05; Fig. 3c and d). We also measured the expression of Stem cell factor (SCF) and Transferrin (TF). SCF induces germ cell differentiation (Rossi et al., 2000), while Transferrin (TF) is responsible for providing iron to germ cells (Sylvester and Griswold, 1994). Neither SCF nor TF expression were affected by DBP exposure (P>0.05; Fig. 3c and d). However, treatment resulted in alterations in relative expression level of inhibin B (INHB) as revealed by qRT-PCR (Fig. 3c and d). INHB is the predominant form of inhibin in the testis and is responsible for regulating FSH secretion via a negative feedback at the pituitary (Meachem et al., 2001; O’Connor and De Kretser, 2004). Exposure of DBP at the dose of 10 mg/kg resulted in a notable increase in INHB expression at both 14 and 28 weeks (P>0.05). In the 500 mg/kg group, INHB expression was not different from that of the 0 mg/kg group at either time point (P>0.05).
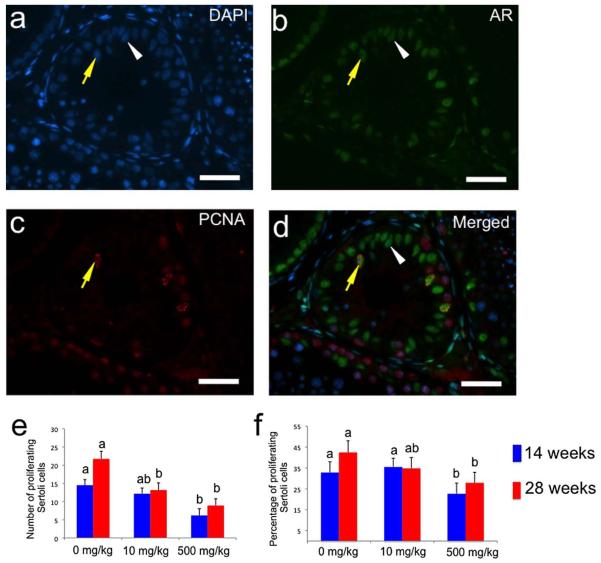
Proliferating cell nuclear antigen (PCNA) was used as a marker for proliferating cells (Leonardi et al., 1992) with AR to detect differences in the proliferation of Sertoli cells (Fig. 4a – d). At 14 weeks, the number of proliferating Sertoli cells (PCNA+ AR+) per tubule cross-section was significantly lower in xenografts from the 500 mg/kg group (P<0.05; Fig. 4e). At 28 weeks, a significant difference in the number of these cells was detected in both the 10 mg/kg and 500 mg/kg groups (P<0.05). Having detected differences in the total number of Sertoli cells (AR+) cells, this number was used to calculate the percentage of proliferating Sertoli cells ((PCNA+AR+)/AR+) in each specimen. The percentage of proliferating Sertoli cells was lower in the 500 mg/kg group at both collection times (P<0.05, Fig. 4f). Thus, DBP affected the number of Sertoli cells and this was associated with lower cell proliferation.
Figure 4.
Sertoli cell proliferation in testis xenografts collected from mice treated with different doses of DBP. a – d) Fluorescence micrographs of a tissue section from a xenograft (0 mg/kg group) collected at 28 weeks in which double immunohistochemistry for AR and PCNA was performed. Yellow arrow points to a proliferating Sertoli cell (AR+ PCNA+), while white arrowhead points to a non-proliferating Sertoli cell (AR+ PCNA-). a) Nuclear counterstaining with 4′,6-diamidino-2-phenylindole (DAPI), b) staining with anti-AR antibody, c) staining with anti-PCNA antibody, and d) merged image. e) Number of proliferating Sertoli cells (AR+ PCNA+ cells) per seminiferous cord and tubule cross-section. f) Percentage of proliferating Sertoli cells (No. of AR+ PCNA+ cells per cross section × 100 / No. of AR+ cells per cross section). In a – d bars = 50 μm. In e – f, different letters between bars of the same color indicate statistical difference (P<0.05).
3.1.5 Apoptosis in the seminiferous epithelium
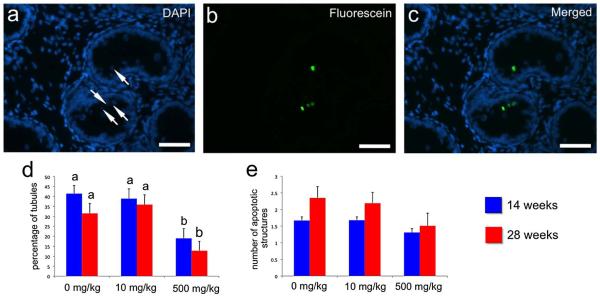
We then focused on cell death, evaluating apoptosis by TUNEL assay (Fig. 5a – c). Treatment reduced the number of tubules with apoptotic cells or bodies. At both collection points the percentage of tubules with apoptotic structures (positive nuclei or nuclear fragments) was lower in xenografts from mice treated with 500 mg/kg (P<0.05; Fig. 5d). However, the number of these structures per tubule cross-section was not affected (P>0.05; Fig. 5e).
Figure 5.
Apoptosis in the seminiferous tubules of testis xenografts from mice treated with different doses of DBP. a – c) Fluorescence micrographs of a tissue section from a xenograft (0 mg/kg group) collected at 14 weeks in which TUNEL assay was performed. a) DAPI nuclear counterstaining, b) staining with anti-Digoxigenin-fluorescein antibody, c) merged image. Arrows in a) points to apoptotic structures (apoptotic nuclei or nuclear fragments) clearly visible in b) and c), bars = 50 μm. d) Percentage of tubules with presence of apoptotic structures. e) Number of apoptotic structures per seminiferous cord or tubule cross-section. In d) and e), different letters between bars of the same color indicate statistical difference (P<0.05).
3.2 DEHP exposure
Recipient mice with infant monkey testis tissue xenografts were treated with 0, 10, or 500 mg/kg DEHP for 14 weeks. DEHP exposure did not affect the mortality and body weight of recipient mice (P>0.05; data not shown). However, the liver weight was increased in the 500 mg/kg group (P<0.05; data not shown). In total, 80 % (71/96) of xenografts were collected. There were no effects of treatment on the xenograft recovery and xenograft weight (P>0.05; data not shown).
Similar to the DBP experiment, testosterone concentrations in both blood serum of recipient mice and xenograft tissue were highly variable, precluding detection of any possible effects of DEHP treatment. Evaluation of the seminal vesicle weight did not detect any effect of treatment (P>0.05; Suppl. Fig. 1a and b). However, the relative expression of INSL3 and StAR were significantly reduced in xenografts of the 500 mg/kg group (P<0.05; Suppl. Fig. 1c). Unexpectedly, the relative level of CYP11A or CYP17A1 was higher in the 10 mg/kg group compared to the 0 and 500 mg/kg groups (P<0.05; Suppl. Fig. 1c), and the relative expression of SF1 was not affected (P>0.05; Suppl. Fig. 1c).
DEHP exposure did not affect seminiferous tubule development, germ cell differentiation, and the density of testicular compartments in testis xenografts (P>0.05, Suppl. Fig. 1d and Table 2). Moreover, relative expression of MIS, number of tubules with UCH-L1+ cells, and number of UCH-L1+ cells per tubule cross section were not affected by DEHP exposure (P>0.05; Suppl. Fig. 1 e and f).
4. Discussion
In this study we investigated the effect of commonly used phthalate plasticizers, DBP and DEHP, on the development of primate testis tissue, using a previously validated testis tissue xenografting model (Honaramooz et al., 2002; Rathi et al., 2008; Rodriguez-Sosa et al., 2012). In the testis xenografting model, recipient mice provide the extragonadal regulation and support for testicular tissue that undergoes full development similar to testes in situ (Honaramooz et al., 2002; Rodriguez-Sosa et al., 2012; Zeng et al., 2007). Recipient mice also provide the metabolic products that result from phthalate ingestion. As phthalate metabolism is qualitatively similar between mouse and humans (Kluwe, 1982), testis tissue xenografting offers a unique opportunity to study phthalate effects on the developing primate testis. This approach allowed us to demonstrate that phthalates impair the development of male gonadal tissue in primates in a time and dose dependent manner.
The xenografting model used in the current study used immune-deficient nude mice. It was reported recently that phthalate exposure is associated with increased inflammation and oxidative stress and phthalates activate macrophages in different tissues (Murphy et al., 2014; Campioli et al., 2014; Ferguson et al., 2014). Therefore, using immune-deficient recipient mice may have underestimated the effects of phthalates.
In the current study, two exposure doses were used: 500 mg/kg was considered as a ‘positive control’ and used to evaluate presence of any effect. The dose of 10 mg/kg was more relevant to the environmental exposure for children undergoing medical interventions and for adult blood transfusion patients (Fischer et al., 2013; Loff et al., 2000; Lyche et al., 2009; Schettler, 2006; Wittassek and Angerer, 2008).
A significant effect observed in rats after exposure comparable to the higher dose was a reduction of testosterone secretion (Akingbemi et al., 2001; Foster, 2005; Foster, 2006; Lehmann et al., 2004; Mylchreest et al. 1998). This was associated with a reduction in the expression of steroidogenic enzymes (Barlow et al., 2003; Chen et al., 2013; Lehmann et al., 2004; Wang et al., 2007). While we could not detect effects on testosterone concentrations in either serum or tissue, a significant reduction in seminal vesicle weight was observed in the 500 mg/kg group at 28 weeks, providing indirect evidence of alterations in androgen secretion by Leydig cells.
Our results showed that DBP exposure affected the expression of key genes involved in the steroidogenic pathway of Leydig cells in a dosage dependent manner and there was a cumulative effect over time. This is in line with previous studies reporting reduced expression of steroidogenic enzymes after phthalate exposure (Barlow et al., 2003; Chen et al., 2013; Lehmann et al., 2004; Wang et al., 2007). Interestingly, expression of most of these genes was affected at 14 weeks, suggesting that steroidogenesis may have been affected at this time point, even if not to the extent that it affected seminal vesicle weight. Accordingly, seminiferous tubule development and germ cell differentiation were notably affected at 28 weeks, but not at 14 weeks.
In the current study, Sertoli cell maturation (as evaluated by MIS and AR expression) was not affected by phthalate exposure, but the number of Sertoli cells was significantly reduced for both exposure groups and both collection points in a time and dose-dependent manner. This is an important observation as Sertoli cells play a key role in spermatogenesis by providing important environmental, nutritional, and regulatory support to germ cells (Skinner, 1991). Mature Sertoli cells under androgen stimulation secrete the fluid that induce lumen (and hence tubule) formation and factors that support germ cell differentiation (Jegou et al., 1983; Zirkin, 1993). Sertoli cells are considered to be one of the primary targets of phthalates, particularly in exposure during the postnatal period. Effects on Sertoli cells include vacuolization, lost integrity of vimentin filaments and alterations of tight junctions, resulting in germ cell sloughing and germ cell loss (reviewed by (Mazaud-Guittot, 2011). We did not observe abnormalities in the general histological attributes of seminiferous epithelium (i.e. vacuolization, germ cell sloughing). However, the numbers of Sertoli cells and spermatogonia were reduced by phthalate exposure. In addition, the relative expression of INHB was significantly higher in the 10 mg/kg group. Similarly, an elevated inhibin level was also reported in a study where male mice were fed with 500 mg/kg DBP from day 4 to day 14 of age (Moody et al., 2013). INHB regulates FSH secretion via a negative feedback on the pituitary (Meachem et al., 2001; O’Connor and De Kretser, 2004). As FSH is the main positive regulator of Sertoli cell proliferation and function, this result suggests that FSH stimulation may have been compromised, which in turn may also contribute to Sertoli cell and germ cell defects. As FSH levels were not measured in our study, a link between the reduced numbers of Sertoli cells and circulating level of FSH could not be established.
The numbers of spermatogonia and the percentage of tubules with spermatogonia were reduced in the 500 mg/kg group, but not in the 10 mg/kg group. Lower numbers in the 500 mg/kg group may have been due to the effects of phthalates on colonization and proliferation of SSCs. Phthalates have been shown to affect SSC proliferation in vitro (Lucas et al., 2012). Moreover, the transplantation assays showed that phthalate exposure also affects colonization of seminiferous epithelium niches by SSCs (Doyle et al., 2013). Another factor contributing to the lower number of germ cells in the 500 mg/kg group might be the reduced number of Sertoli cells.
In rats, phthalates reduce proliferation of fetal Sertoli cells (Hutchison et al., 2008; Scott et al., 2008) and this effect has been associated with the reduction of testosterone as Sertoli cell proliferation during the fetal period is positively regulated by testosterone (Scott et al., 2007). We were not able to detect significant differences in testosterone levels due to high variability among samples. We therefore analyzed the proliferation of Sertoli cells to help explain the lower numbers of these cells that results from phthalate exposure. At both collections times, percentage of proliferating Sertoli cells was lower in the 500 mg/kg group, indicating that lower proliferation contributed to the lower Sertoli cell number in this group. However, there was no difference in the percentage of proliferating Sertoli cells in the 10 mg/kg group. Sertoli cell proliferation may have been affected earlier during xenograft development but not been evident at xenograft collection. The fact that Sertoli cell number did not seem to notably change after 14 weeks suggests that this is possible.
To help explain lower Sertoli and germ cell numbers, we also evaluated cell death in the seminiferous epithelium by the TUNEL assay. In rats, DBP exposure was shown to affect mostly proliferation rather than apoptosis of testicular somatic cells (Boekelheide et al., 2009). Unexpectedly, we found a lower number of tubules with apoptotic structures (apoptotic cells or bodies) in the 500 mg/kg group. It is interesting to note that in rodents, exposure to high levels of phthalates (>250 mg /kg) induced a quick increase of the apoptotic rate, and in a few days this rate decreased and reached low numbers (Park et al., 2002). Thus, it is possible that in the chronic exposure to 500 mg/kg of DBP, the apoptotic rate significantly increased early in treatment and then gradually reached low levels afterwards. It is also possible that the reduced number of apoptotic structures was a consequence of the lower number of Sertoli and germ cells in 500 mg/kg group.
Overall, DBP treatment resulted in detrimental effects on developing testis tissue that intensified with time and dose of exposure. In a parallel experiment mice were treated with DEHP for 14 weeks. However, unlike the DBP experiment, we only detected alterations in the relative expression of Leydig cell markers. While DBP and DEHP exposures were not compared directly, results from both experiments suggest that DEHP exposure resulted in more subtle effects. This is different from data generated in rodents that suggest that potency of DEHP is higher than that of DBP (Foster, 2005). However, species differences may be responsible for this effect. In sub-fertile couples a relationship was found between male low semen quality and their exposure to DBP, but not to DEHP (Hauser et al., 2006; Hauser et al., 2007). Additional studies are required to evaluate the potency of different types of phthalates and the specific mechanisms behind their effects in primate testis tissue.
While our study was in progress, two reports were published describing the use of testis tissue xenografting to evaluate the effects of short-term exposure to phthalates on the endocrine function of testis tissue from human fetuses. In the first report, fetal testis tissue from rats, mice, and humans was grafted under the kidney capsule of adult immunodeficient rats that were exposed for 1 – 3 days to 100 – 500 mg/kg of DBP. Unlike in rat testis xenogratfs, no endocrine effects were observed in mouse and human tissue (Heger et al., 2012). Another study involved the treatment of recipient mice with 500 mg/kg of DBP (or its monobutyl metabolite) for up to 21 days. Similarly, while effects were found in rat testis xenografts, no alterations were observed in the steroidogenic function of human testis tissue (Mitchell et al., 2012). This has led to the believe that the human and mouse fetal Leydig cells are insensitive to phthalate exposure (Johnson et al., 2012). While it is uncertain whether longer periods of exposure will lead to disrupting effects on the endocrine function of the human fetal testis, our results show that postnatal primate Leydig cells are susceptible to disruption by chronic exposure to phthalates. A different susceptibility between fetal and postnatal Leydig cells may likely be responsible for this discrepancy of results; while two in vitro studies did not show any effect of phthalate exposure on testosterone production in fetal human testis tissue (Hallmark et al., 2007; Lambrot et al., 2009), another study showed an inhibition of steroidogenesis in adult human testis explants induced by phthalates (Desdoits-Lethimonier et al., 2012). Moreover, the reduced testosterone concentration in men associated with higher exposure to phthalates (Pan et al., 2006) supports the hypothesis that postnatal Leydig cells are sensitive to phthalates.
In conclusion, the present study demonstrates that the development of the primate testis is susceptible to disruption by phthalates widely used in everyday life. This effect may include a decrease in expression of steroidogenic enzymes, and reduced number of testicular somatic and germ cells. While most of the effects were observed at the high exposure level that is orders of magnitude higher than environmental exposure, notably, some of these effects result from levels of exposure that are relevant in humans. Furthermore, this report shows the efficiency of the testis tissue xenografting model in toxicological studies involving phthalates and similar compounds in primates and non-rodent species. Finally, this work provides an important insight into the potential effect of exposure to endocrine disruptors during postnatal testis development in primates and their potential detrimental effects on male fertility.
Supplementary Material
Supplemental Figure 1. Effects of treatment of recipient mice with 0, 10 or 500 mg/kg DEHP for 14 weeks. a) Seminal vesicle weight of recipients; b) Testosterone in blood serum; c) Relative expression of genes associated with Leydig cell function; d) Percentage of seminiferous tubules with the most advanced type of germ cell present; e) Percentage of tubules with germ cells (UCH-L1 positive); f) Number of germ cells (UCH-L1 positive) per tubule cross-section. Different letters between bars of the same color indicate statistical difference (P<0.05).
Supplemental Figure 2 (for review only): Immunohistochemistry control staining. (A-C) 6 month old monkey donor tissue with rabbit and mouse IgG isotype control (A) merged DAPI, 555 and 488, (B) DAPI alone and (C) 555 and 488 merged channels. (D-E) monkey testis tissue xenograft collected at 28 weeks with rabbit and mouse IgG isotype control (D) merged DAPI, 555 and 488, (E) DAPI alone and (F) 555 and 488 merged channels. (G) 6 month old monkey donor tissue with rabbit IgG isotype control visualized with VECTOR NovaRED substrate kit and (H) monkey testis tissue xenograft collected at 28 weeks with rabbit IgG isotype control visualized with VECTOR NovaRED substrate kit. Scale bar = 50μm.
Supplemental Table 1. Primers used for quantitative Real-Time PCR
Supplemental Table 2. Volume density (%, Mean ± SEM) of the different compartments of testis xenografts collected from mice exposed to several doses of DBP or DEHP. In each study and within each column, there was no statistical difference between rows (P>0.05).
Highlights.
Phthalate esters can leach from plastic products leading to environmental exposure.
In fetal rats phthalate exposure causes abnormal development of the male reproductive tract.
Development of immature primate testis exposed to phthalates was studied after xenografting to mouse hosts.
Long-term exposure to phthalates reduced steroidogenic gene expression, Sertoli cell numbers and germ cell differentiation in a dosage and time dependent manner.
The developing primate testis is sensitive to phthalates at levels relevant to human exposure.
Acknowledgements
This study was supported by NIH/NIEHS (1 R21 ES014856-01A2). We thank Swapna Mohan for her assistance with animal handling. We also acknowledge the kind help of the staff at the Health Sciences Animal Research Facility of the University of Calgary.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akingbemi BT, Youker RT, Sottas CM, Ge R, Katz E, Klinefelter GR, Zirkin BR, Hardy MP. Modulation of rat Leydig cell steroidogenic function by di(2-ethylhexyl)phthalate. Biol Reprod. 2001;65:1252–9. doi: 10.1095/biolreprod65.4.1252. [DOI] [PubMed] [Google Scholar]
- Avelar GF, Oliveira CF, Soares JM, Silva IJ, Dobrinski I, Hess RA, França LR. Postnatal somatic cell proliferation and seminiferous tubule maturation in pigs: a non-random event. Theriogenology. 2010;74(1):11–23. doi: 10.1016/j.theriogenology.2009.12.014. doi:10.1016/j.theriogenology.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow NJ, Phillips SL, Wallace DG, Sar M, Gaido KW, Foster PM. Quantitative changes in gene expression in fetal rat testes following exposure to di(n-butyl) phthalate. Toxicol Sci. 2003;73:431–41. doi: 10.1093/toxsci/kfg087. doi:10.1093/toxsci/kfg087. [DOI] [PubMed] [Google Scholar]
- Boekelheide K, Kleymenova E, Liu K, Swanson C, Gaido KW. Dose-dependent effects on cell proliferation, seminiferous tubules, and male germ cells in the fetal rat testis following exposure to di(n-butyl) phthalate. Microsc Res Tech. 2009;72:629–38. doi: 10.1002/jemt.20684. doi:10.1002/jemt.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioli E, Martinez-Arguelles DB, Papadopoulos V. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate promotes local adipose and systemic inflammation in adult male offspring. Nutr Diabetes. 2014;4:e115. doi: 10.1038/nutd.2014.13. doi: 10.1038/nutd.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhou QH, Leng L, Chen X, Sun ZR, Tang NJ. Effects of di(n-butyl) and monobutyl phthalate on steroidogenesis pathways in the murine Leydig tumor cell line MLTC-1. Environ Toxicol Pharmacol. 2013;36:332–8. doi: 10.1016/j.etap.2013.04.013. doi:10.1016/j.etap.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Christenson LK, Strauss JF., 3rd Steroidogenic acute regulatory protein: an update on its regulation and mechanism of action. Arch Med Res. 2001;32:576–86. doi: 10.1016/s0188-4409(01)00338-1. [DOI] [PubMed] [Google Scholar]
- David RM. Summary of mammalian toxicology and health effects of phthalate esters. In: Hutzinger O, editor. The Handbook of Environmental Chemistry. Vol. 3. Springer; Berlin: 2003. pp. 299–316. a. G., G. a. S., C.A. [Google Scholar]
- De Franca LR, Hess RA, Cooke PS, Russell LD. Neonatal hypothyroidism causes delayed Sertoli cell maturation in rats treated with propylthiouracil: evidence that the Sertoli cell controls testis growth. Anat Rec. 1995;242:57–69. doi: 10.1002/ar.1092420108. doi:10.1002/ar.1092420108. [DOI] [PubMed] [Google Scholar]
- Desdoits-Lethimonier C, Albert O, Le Bizec B, Perdu E, Zalko D, Courant F, Lesne L, Guille F, Dejucq-Rainsford N, Jegou B. Human testis steroidogenesis is inhibited by phthalates. Hum Reprod. 2012;27:1451–9. doi: 10.1093/humrep/des069. doi:10.1093/humrep/des069. [DOI] [PubMed] [Google Scholar]
- Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod. 2013;88:112. doi: 10.1095/biolreprod.112.106104. doi:10.1095/biolreprod.112.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, Brock JW, Ryan L, Chen Z, Overstreet J, Hauser R. The relationship between environmental exposure to phthalates and computer-aided sperm analysis motion parameters. J Androl. 2004;25:293–302. doi: 10.1002/j.1939-4640.2004.tb02790.x. [DOI] [PubMed] [Google Scholar]
- Duty SM, Singh NP, Silva MJ, Barr DB, Brock JW, Ryan L, Herrick RF, Christiani DC, Hauser R. The relationship between environmental exposures to phthalates and DNA damage in human sperm using the neutral comet assay. Environ Health Perspect. 2003a;111:1164–9. doi: 10.1289/ehp.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, Herrick RF, Christiani DC, Hauser R. Phthalate exposure and human semen parameters. Epidemiology. 2003b;14:269–77. [PubMed] [Google Scholar]
- Ferguson KK, Cantonwine DE, Rivera-González LO, Loch-Caruso R, Mukherjee B, Anzalota Del Toro LV, Jiménez-Vélez B, Calafat AM, Ye X, Alshawabkeh AN, Cordero JF, Meeker JD. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environ Sci Technol. 2014;48:7018–25. doi: 10.1021/es502076j. doi: 10.1021/es502076j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer CJ, Bickle Graz M, Muehlethaler V, Palmero D, Tolsa JF. Phthalates in the NICU: is it safe? J Paediatr Child Health. 2013;49:E413–9. doi: 10.1111/jpc.12244. doi:10.1111/jpc.12244. [DOI] [PubMed] [Google Scholar]
- Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–15. doi: 10.1530/rep.1.00025. doi:10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- Foster PM. Mode of action: impaired fetal leydig cell function--effects on male reproductive development produced by certain phthalate esters. Crit Rev Toxicol. 2005;35:713–9. doi: 10.1080/10408440591007395. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29:140–7. doi: 10.1111/j.1365-2605.2005.00563.x. discussion 181-5, doi:10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res. 2007;51:899–911. doi: 10.1002/mnfr.200600243. doi:10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Hensley JB, Liu D, Wallace DG, Borghoff S, Johnson KJ, Hall SJ, Boekelheide K. Fetal mouse phthalate exposure shows that Gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol Sci. 2007;97:491–503. doi: 10.1093/toxsci/kfm049. doi:10.1093/toxsci/kfm049. [DOI] [PubMed] [Google Scholar]
- Gazouli M, Yao ZX, Boujrad N, Corton JC, Culty M, Papadopoulos V. Effect of peroxisome proliferators on Leydig cell peripheral-type benzodiazepine receptor gene expression, hormone-stimulated cholesterol transport, and steroidogenesis: role of the peroxisome proliferator-activator receptor alpha. Endocrinology. 2002;143:2571–83. doi: 10.1210/endo.143.7.8895. doi:10.1210/endo.143.7.8895. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr., Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–65. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, Coutts S, Anderson RA, Greig I, Morris K, Sharpe RM. Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ Health Perspect. 2007;115:390–6. doi: 10.1289/ehp.9490. doi:10.1289/ehp.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–91. doi: 10.1097/01.ede.0000235996.89953.d7. doi:10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Singh NP, Silva MJ, Ryan L, Duty S, Calafat AM. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum Reprod. 2007;22:688–95. doi: 10.1093/humrep/del428. doi:10.1093/humrep/del428. [DOI] [PubMed] [Google Scholar]
- Heger NE, Hall SJ, Sandrof MA, McDonnell EV, Hensley JB, McDowell EN, Martin KA, Gaido KW, Johnson KJ, Boekelheide K. Human fetal testis xenografts are resistant to phthalate-induced endocrine disruption. Environ Health Perspect. 2012;120:1137–43. doi: 10.1289/ehp.1104711. doi:10.1289/ehp.1104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng K, Anand-Ivell R, Teerds K, Ivell R. The endocrine disruptors dibutyl phthalate (DBP) and diethylstilbestrol (DES) influence Leydig cell regeneration following ethane dimethane sulphonate treatment of adult male rats. Int J Androl. 2012;35:353–63. doi: 10.1111/j.1365-2605.2011.01231.x. doi:10.1111/j.1365-2605.2011.01231.x. [DOI] [PubMed] [Google Scholar]
- Hess RA, Cooke PS, Bunick D, Kirby JD. Adult testicular enlargement induced by neonatal hypothyroidism is accompanied by increased Sertoli and germ cell numbers. Endocrinology. 1993;132:2607–13. doi: 10.1210/endo.132.6.8504761. doi:10.1210/endo.132.6.8504761. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418:778–81. doi: 10.1038/nature00918. doi:10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Chou YY, Lin SJ, Lee CC. Association between prenatal exposure to phthalates and the health of newborns. Environ Int. 2009;35:14–20. doi: 10.1016/j.envint.2008.05.012. doi:10.1016/j.envint.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Hutchison GR, Scott HM, Walker M, McKinnell C, Ferrara D, Mahood IK, Sharpe RM. Sertoli cell development and function in an animal model of testicular dysgenesis syndrome. Biol Reprod. 2008;78:352–60. doi: 10.1095/biolreprod.107.064006. doi:10.1095/biolreprod.107.064006. [DOI] [PubMed] [Google Scholar]
- Ivell R, Anand-Ivell R. Biological role and clinical significance of insulin-like peptide 3. Curr Opin Endocrinol Diabetes Obes. 2011;18:210–6. doi: 10.1097/MED.0b013e3283453fe6. doi:10.1097/MED.0b013e3283453fe6. [DOI] [PubMed] [Google Scholar]
- Ivell R, Wade JD, Anand-Ivell R. INSL3 as a biomarker of Leydig cell functionality. Biol Reprod. 2013;88:147. doi: 10.1095/biolreprod.113.108969. doi:10.1095/biolreprod.113.108969. [DOI] [PubMed] [Google Scholar]
- Itman C, Wong C, Hunyadi B, Ernst M, Jans DA, Loveland KL. Smad3 dosage determines androgen responsiveness and sets the pace of postnatal testis development. Endocrinology. 2011;152:2076–2089. doi: 10.1210/en.2010-1453. doi:10.1210/en.2010-1453. [DOI] [PubMed] [Google Scholar]
- Jegou B, Le Gac F, Irby DC, de Kretser DM. Studies on seminiferous tubule fluid production in the adult rat: effect of hypophysectomy and treatment with FSH, LH and testosterone. Int J Androl. 1983;6:249–60. doi: 10.1111/j.1365-2605.1983.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Johnson KJ, Heger NE, Boekelheide K. Of mice and men (and rats): phthalate-induced fetal testis endocrine disruption is species-dependent. Toxicol Sci. 2012;129:235–48. doi: 10.1093/toxsci/kfs206. doi:10.1093/toxsci/kfs206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson BA, Richthoff J, Rylander L, Giwercman A, Hagmar L. Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology. 2005;16:487–93. doi: 10.1097/01.ede.0000164555.19041.01. [DOI] [PubMed] [Google Scholar]
- Kamrin MA. Phthalate risks, phthalate regulation, and public health: a review. J Toxicol Environ Health B Crit Rev. 2009;12:157–74. doi: 10.1080/10937400902729226. doi:10.1080/10937400902729226. [DOI] [PubMed] [Google Scholar]
- Kluwe WM. Overview of phthalate ester pharmacokinetics in mammalian species. Environ Health Perspect. 1982;45:3–9. doi: 10.1289/ehp.82453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knez J. Endocrine-disrupting chemicals and male reproductive health. Reprod Biomed Online. 2013;26:440–8. doi: 10.1016/j.rbmo.2013.02.005. doi:10.1016/j.rbmo.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Koren LZ, Ng ESM, Soma KK, Wynne-Edwards KE. Sample preparation and liquid chromatography-tandem mass spectrometry for multiple steroids in mammalian and avian serum. PLoS One. 2012;7(2):e32496. doi: 10.1371/journal.pone.0032496. doi: 10.1371/journal.pone.0032496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata Y, Kidachi F, Yokoyama M, Toyota N, Tsuchitani M, Katoh M. Subchronic toxicity of Di(2-ethylhexyl)phthalate in common marmosets: lack of hepatic peroxisome proliferation, testicular atrophy, or pancreatic acinar cell hyperplasia. Toxicol Sci. 1998;42:49–56. doi: 10.1006/toxs.1997.2414. doi:10.1006/toxs.1997.2414. [DOI] [PubMed] [Google Scholar]
- Lambrot R, Muczynski V, Lecureuil C, Angenard G, Coffigny H, Pairault C, Moison D, Frydman R, Habert R, Rouiller-Fabre V. Phthalates impair germ cell development in the human fetal testis in vitro without change in testosterone production. Environ Health Perspect. 2009;117:32–7. doi: 10.1289/ehp.11146. doi:10.1289/ehp.11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann KP, Phillips S, Sar M, Foster PM, Gaido KW. Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol Sci. 2004;81:60–8. doi: 10.1093/toxsci/kfh169. doi:10.1093/toxsci/kfh169. [DOI] [PubMed] [Google Scholar]
- Leonardi E, Girlando S, Serio G, Mauri FA, Perrone G, Scampini S, Dalla Palma P, Barbareschi M. PCNA and Ki67 expression in breast carcinoma: correlations with clinical and biological variables. J Clin Pathol. 1992;45:416–9. doi: 10.1136/jcp.45.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loff S, Kabs F, Witt K, Sartoris J, Mandl B, Niessen KH, Waag KL. Polyvinylchloride infusion lines expose infants to large amounts of toxic plasticizers. J Pediatr Surg. 2000;35:1775–81. doi: 10.1053/jpsu.2000.19249. doi:10.1053/jpsu.2000.19249. [DOI] [PubMed] [Google Scholar]
- Lucas BE, Fields C, Joshi N, Hofmann MC. Mono-(2-ethylhexyl)-phthalate (MEHP) affects ERK-dependent GDNF signalling in mouse stem-progenitor spermatogonia. Toxicology. 2012;299:10–9. doi: 10.1016/j.tox.2012.04.011. doi:10.1016/j.tox.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, Saunders M, Skaare JU. Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health B Crit Rev. 2009;12:225–49. doi: 10.1080/10937400903094091. doi:10.1080/10937400903094091. [DOI] [PubMed] [Google Scholar]
- Ma X, Lian QQ, Dong Q, Ge RS. Environmental inhibitors of 11β-hydroxysteroid dehydrogenase type 2. Toxicology. 2011;285:83–89. doi: 10.1016/j.tox.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, Culty M, Zirkin BR, Papadopoulos V. In utero exposure to di-(2-ethylhexyl) phthalate decreases mineralocorticoid receptor expression in the adult testis. Endocrinology. 2009;150:5575–85. doi: 10.1210/en.2009-0847. doi:10.1210/en.2009-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaud-Guittot S. Dissecting the phthalate-induced Sertoli cell injury: the fragile balance of proteases and their inhibitors. Biol Reprod. 2011;85:1091–3. doi: 10.1095/biolreprod.111.095976. doi:10.1095/biolreprod.111.095976. [DOI] [PubMed] [Google Scholar]
- Meachem SJ, Nieschlag E, Simoni M. Inhibin B in male reproduction: pathophysiology and clinical relevance. Eur J Endocrinol. 2001;145:561–71. doi: 10.1530/eje.0.1450561. [DOI] [PubMed] [Google Scholar]
- Merchant-Larios H, Moreno-Mendoza N. Onset of sex differentiation: dialog between genes and cells. Arch Med Res. 2001;32:553–8. doi: 10.1016/s0188-4409(01)00317-4. [DOI] [PubMed] [Google Scholar]
- Mitchell RT, Childs AJ, Anderson RA, van den Driesche S, Saunders PT, McKinnell C, Wallace WH, Kelnar CJ, Sharpe RM. Do phthalates affect steroidogenesis by the human fetal testis? Exposure of human fetal testis xenografts to di-n-butyl phthalate. J Clin Endocrinol Metab. 2012;97:E341–8. doi: 10.1210/jc.2011-2411. doi:10.1210/jc.2011-2411. [DOI] [PubMed] [Google Scholar]
- Moody S, Goh H, Bielanowicz A, Rippon P, Loveland KL, Itman C. Prepubertal mouse testis growth and maturation and androgen production are acutely sensitive to di-n-butyl phthalate. Endocrinology. 2013;154:3460–75. doi: 10.1210/en.2012-2227. doi: 10.1210/en.2012-2227. [DOI] [PubMed] [Google Scholar]
- Murphy CJ, Stermer AR, Richburg JH. Age- and species-dependent infiltration of macrophages into the testis of rats and mice exposed to mono-(2-Ethylhexyl) phthalate (MEHP) Biol Reprod. 2014;91:18. doi: 10.1095/biolreprod.113.115527. doi: 10.1095/biolreprod.113.115527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E, Cattley RC, Foster PM. Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: an antiandrogenic mechanism? Toxicol Sci. 1998;43:47–60. doi: 10.1006/toxs.1998.2436. doi:10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- O’Connor AE, De Kretser DM. Inhibins in normal male physiology. Semin Reprod Med. 2004;22:177–85. doi: 10.1055/s-2004-831893. doi:10.1055/s-2004-831893. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Monteiro A, Verhoeven G, De Gendt K, Abel MH. Effect of FSH on testicular morphology and spermatogenesis in gonadotrophin-deficient hypogonadal mice lacking androgen receptors. Reproduction. 2010;139:177–84. doi: 10.1530/REP-09-0377. doi:10.1530/REP-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JM. Cell Bioloy of testicular development in the fetus and neonate. In: Desjardins C, L.L E, editors. Cell and Molecular Biology of the Testis. Oxford University Press; New York, N.Y.: 1993. pp. 3–42. [Google Scholar]
- Pan G, Hanaoka T, Yoshimura M, Zhang S, Wang P, Tsukino H, Inoue K, Nakazawa H, Tsugane S, Takahashi K. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ Health Perspect. 2006;114:1643–8. doi: 10.1289/ehp.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JD, Habeebu SS, Klaassen CD. Testicular toxicity of di-(2-ethylhexyl)phthalate in young Sprague-Dawley rats. Toxicology. 2002;171:105–15. doi: 10.1016/s0300-483x(01)00567-4. [DOI] [PubMed] [Google Scholar]
- Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997;18:361–77. doi: 10.1210/edrv.18.3.0301. doi:10.1210/edrv.18.3.0301. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–70. doi: 10.1210/er.2003-0030. doi:10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Pocar P, Fiandanese N, Secchi C, Berrini A, Fischer B, Schmidt JS, Schaedlich K, Borromeo V. Exposure to di(2-ethyl-hexyl) phthalate (DEHP) in utero and during lactation causes long-term pituitary-gonadal axis disruption in male and female mouse offspring. Endocrinology. 2012;153:937–48. doi: 10.1210/en.2011-1450. doi:10.1210/en.2011-1450. [DOI] [PubMed] [Google Scholar]
- Pugh G, Jr., Isenberg JS, Kamendulis LM, Ackley DC, Clare LJ, Brown R, Lington AW, Smith JH, Klaunig JE. Effects of di-isononyl phthalate, di-2-ethylhexyl phthalate, and clofibrate in cynomolgus monkeys. Toxicol Sci. 2000;56:181–8. doi: 10.1093/toxsci/56.1.181. [DOI] [PubMed] [Google Scholar]
- Rathi R, Zeng W, Megee S, Conley A, Meyers S, Dobrinski I. Maturation of testicular tissue from infant monkeys after xenografting into mice. Endocrinology. 2008;149:5288–96. doi: 10.1210/en.2008-0311. doi:10.1210/en.2008-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CV, Furr JR, Wilson VS, Gray LE., Jr. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int J Androl. 2010;33:443–62. doi: 10.1111/j.1365-2605.2009.01049.x. doi:10.1111/j.1365-2605.2009.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Sosa JR, Dobrinski I. Recent developments in testis tissue xenografting. Reproduction. 2009;138:187–94. doi: 10.1530/REP-09-0012. doi:10.1530/REP-09-0012. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sosa JR, Costa GM, Rathi R, Franca LR, Dobrinski I. Endocrine modulation of the recipient environment affects development of bovine testis tissue ectopically grafted in mice. Reproduction. 2012;144:37–51. doi: 10.1530/REP-12-0020. doi:10.1530/REP-12-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P, Sette C, Dolci S, Geremia R. Role of c-kit in mammalian spermatogenesis. J Endocrinol Invest. 2000;23:609–15. doi: 10.1007/BF03343784. [DOI] [PubMed] [Google Scholar]
- Rozati R, Reddy PP, Reddanna P, Mujtaba R. Role of environmental estrogens in the deterioration of male factor fertility. Fertil Steril. 2002;78:1187–94. doi: 10.1016/s0015-0282(02)04389-3. [DOI] [PubMed] [Google Scholar]
- Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29:134–9. doi: 10.1111/j.1365-2605.2005.00567.x. discussion 181-5, doi:10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- Scott HM, Hutchison GR, Jobling MS, McKinnell C, Drake AJ, Sharpe RM. Relationship between androgen action in the “male programming window,” fetal sertoli cell number, and adult testis size in the rat. Endocrinology. 2008;149:5280–7. doi: 10.1210/en.2008-0413. doi:10.1210/en.2008-0413. [DOI] [PubMed] [Google Scholar]
- Scott HM, Hutchison GR, Mahood IK, Hallmark N, Welsh M, De Gendt K, Verhoeven G, O’Shaughnessy P, Sharpe RM. Role of androgens in fetal testis development and dysgenesis. Endocrinology. 2007;148:2027–36. doi: 10.1210/en.2006-1622. doi:10.1210/en.2006-1622. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–84. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Skinner MK. Cell-cell interactions in the testis. Endocr Rev. 1991;12:45–77. doi: 10.1210/edrv-12-1-45. doi:10.1210/edrv-12-1-45. [DOI] [PubMed] [Google Scholar]
- Stabenfeldt GH, Hughes JP, Kennedy PC, Meagher DM, Neely DP. Clinical findings, pathological changes and endocrinological secretory patterns in mares with ovarian tumours. J Reprod Fertil Suppl. 1979:277–285. [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL, Study for Future Families Research, T. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–61. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester SR, Griswold MD. The testicular iron shuttle: a “nurse” function of the Sertoli cells. J Androl. 1994;15:381–5. [PubMed] [Google Scholar]
- Tomonari Y, Kurata Y, David RM, Gans G, Kawasuso T, Katoh M. Effect of di(2-ethylhexyl) phthalate (DEHP) on genital organs from juvenile common marmosets: I. Morphological and biochemical investigation in 65-week toxicity study. J Toxicol Environ Health A. 2006;69:1651–72. doi: 10.1080/15287390600630054. doi:10.1080/15287390600630054. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Terada N, Sugihara A, Tsujimura T, Donjacour AA, Cunha GR. Later onset of apoptosis in the bulbourethral glands after castration compared to that in the seminal vesicles. J Steroid Biochem Mol Biol. 1998;67:113–8. doi: 10.1016/s0960-0760(98)00079-x. [DOI] [PubMed] [Google Scholar]
- Val P, Lefrancois-Martinez AM, Veyssiere G, Martinez A. SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept. 2003;1:8. doi: 10.1186/1478-1336-1-8. doi:10.1186/1478-1336-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventrice P, Ventrice D, Russo E, De Sarro G. Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environ Toxicol Pharmacol. 2013;36:88–96. doi: 10.1016/j.etap.2013.03.014. doi:10.1016/j.etap.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Wang YB, Song L, Cui LB, Hong X, Zhang ZD, Wang XR. Monobutyl phthalate inhibits steroidogenesis by downregulating steroidogenic acute regulatory protein expression in mouse Leydig tumor cells (MLTC-1) J Toxicol Environ Health A. 2007;70:947–55. doi: 10.1080/15287390701290717. doi:10.1080/15287390701290717. [DOI] [PubMed] [Google Scholar]
- Wittassek M, Angerer J. Phthalates: metabolism and exposure. Int J Androl. 2008;31:131–8. doi: 10.1111/j.1365-2605.2007.00837.x. doi:10.1111/j.1365-2605.2007.00837.x. [DOI] [PubMed] [Google Scholar]
- Zeng W, Rathi R, Pan H, Dobrinski I. Comparison of global gene expression between porcine testis tissue xenografts and porcine testis in situ. Mol Reprod Dev. 2007;74:674–9. doi: 10.1002/mrd.20670. doi:10.1002/mrd.20670. [DOI] [PubMed] [Google Scholar]
- Zirkin BR. Regulation of spermatogenesis in the adult mammal: gonadotropins and androgens. In: C D, Ewing LL, editors. Cell and Molecular Biology of the Testis. Oxford University Press; New York, N.Y.: 1993. pp. 166–188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Effects of treatment of recipient mice with 0, 10 or 500 mg/kg DEHP for 14 weeks. a) Seminal vesicle weight of recipients; b) Testosterone in blood serum; c) Relative expression of genes associated with Leydig cell function; d) Percentage of seminiferous tubules with the most advanced type of germ cell present; e) Percentage of tubules with germ cells (UCH-L1 positive); f) Number of germ cells (UCH-L1 positive) per tubule cross-section. Different letters between bars of the same color indicate statistical difference (P<0.05).
Supplemental Figure 2 (for review only): Immunohistochemistry control staining. (A-C) 6 month old monkey donor tissue with rabbit and mouse IgG isotype control (A) merged DAPI, 555 and 488, (B) DAPI alone and (C) 555 and 488 merged channels. (D-E) monkey testis tissue xenograft collected at 28 weeks with rabbit and mouse IgG isotype control (D) merged DAPI, 555 and 488, (E) DAPI alone and (F) 555 and 488 merged channels. (G) 6 month old monkey donor tissue with rabbit IgG isotype control visualized with VECTOR NovaRED substrate kit and (H) monkey testis tissue xenograft collected at 28 weeks with rabbit IgG isotype control visualized with VECTOR NovaRED substrate kit. Scale bar = 50μm.
Supplemental Table 1. Primers used for quantitative Real-Time PCR
Supplemental Table 2. Volume density (%, Mean ± SEM) of the different compartments of testis xenografts collected from mice exposed to several doses of DBP or DEHP. In each study and within each column, there was no statistical difference between rows (P>0.05).