Abstract
Fasciola hepatica is a trematode that causes zoonosis mainly in cattle and sheep and occasionally in humans. Fascioliasis has been reported in Korea; however, determining F. hepatica infection in snails has not been done recently. Thus, using PCR, we evaluated the prevalence of F. hepatica infection in snails at 4 large water-dropwort fields. Among 349 examined snails, F. hepatica-specific internal transcribed space 1 (ITS-1) and/or ITS-2 markers were detected in 12 snails and confirmed using sequence analysis. Morphologically, 213 of 349 collected snails were dextral shelled, which is the same aperture as the lymnaeid snail, the vectorial host for F. hepatica. Among the 12 F. hepatica-infected snails, 6 were known first intermediate hosts in Korea (Lymnaea viridis and L. ollula) and the remaining 6 (Lymnaea sp.) were potentially a new first intermediate host in Korea. It has been shown that the overall prevalence of the snails contaminated with F. hepatica in water-dropwort fields was 3.4%; however, the prevalence varied among the fields. This is the first study to estimate the prevalence of F. hepatica infection using the vectorial capacity of the snails in Korea.
Keywords: Fasciola hepatica, snail, prevalence, Korea, ITS-1, ITS-2, PCR
INTRODUCTION
Fascioliasis is a zoonosis caused by the trematode Fasciola hepatica, prevalent in cattle, and emerging as a cause of disease in humans. F. hepatica is distributed mainly in Europe, the Americas, and Oceania [1]. The life cycle of F. hepatica involves lymnaeid freshwater snails as the first intermediate host and depends on the development and survival of larval stages both in the snail intermediate host and in the environment. Humans are infected mainly by ingesting raw aquatic vegetables such as watercress and water dropwort that are contaminated with the metacercariae. Infections are also induced by consuming the raw liver of an infected animal and drinking water containing viable metacercariae of F. hepatica [1]. WHO added fascioliasis and other foodborne trematodiases to the list of important helminthiases significantly impacting human development, and knowing the infection status of F. hepatica in the natural environment is important [2].
Transmission dynamics of the fluke life stages is an important issue both for implementing proper fascioliasis control programs and risk assessment [3]. The use of tracer animals is valuable for investigating transmission to the definitive hosts, while microscopic investigation of snails for F. hepatica larvae is useful in the intermediate host. However, the use of tracer calves or sheep is expensive and the microscopic examination of snails has a low diagnostic sensitivity [4]. Therefore, the development of molecular biology provided a range of tools offering much higher specificity and sensitivity than conventional methods [4,5,6]. In Korea, cattle were heavily infected with Fasciola species before 2000 with a prevalence of up to 38.2% [7,8,9]. Additionally, there were several cases of human infections with F. hepatica [10,11]. However, recent studies on trace animals and the prevalence of fascioliasis are lacking. Moreover, little information is available in the literature on the larval forms of F. hepatica found in natural snails. Therefore, to evaluate the infection status of F. hepatica in the first intermediate snail host in Korea, we collected snails from large water-dropwort fields, monitored their contamination with F. hepatica, and evaluated the vectorial capacity of F. hepatica-infected snails.
MATERIALS AND METHODS
Preparation of F. hepatica genomic DNA
Adult F. hepatica specimens were obtained from one of the authors, Prof. Sung-Jong Hong (Chung-Ang University, Seoul, Korea), and the fluke was stored at -70℃. For DNA isolation, adult F. hepatica worms were cut into 20 mg tissue samples using a sterile scalpel. Genomic DNA was isolated from adult F. hepatica worms using a G-DEX™ genomic DNA extraction kit (iNtRON Biotechnology, Seoul, Korea) according to the manufacturer's instructions.
Survey areas and snail collection
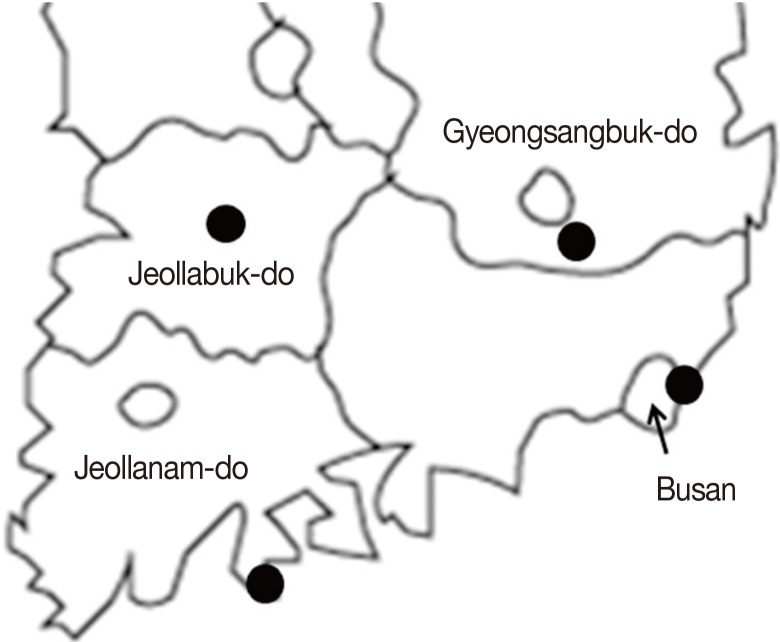
Freshwater snails were collected from 4 large water-dropwort fields in Cheongdo (Gyeongsangbuk-do), Jeonju (Jeollabuk-do), Suncheon (Jeollanam-do), and Gijang (Busan) from September 2013 to June 2014 (Fig. 1). Collected snails were placed in 50-ml plastic containers and transferred within 6 hr to the laboratory. They were rinsed with tap water and identified according to their shell morphology [12]. Then, a half was used to isolate DNA, and the remainder was preserved in a -70℃ freezer.
Fig. 1.
Locations of sites at which freshwater snails were collected. Dot (•); water-dropwort fields for snail collection.
Molecular detection of F. hepatica in snails using PCR
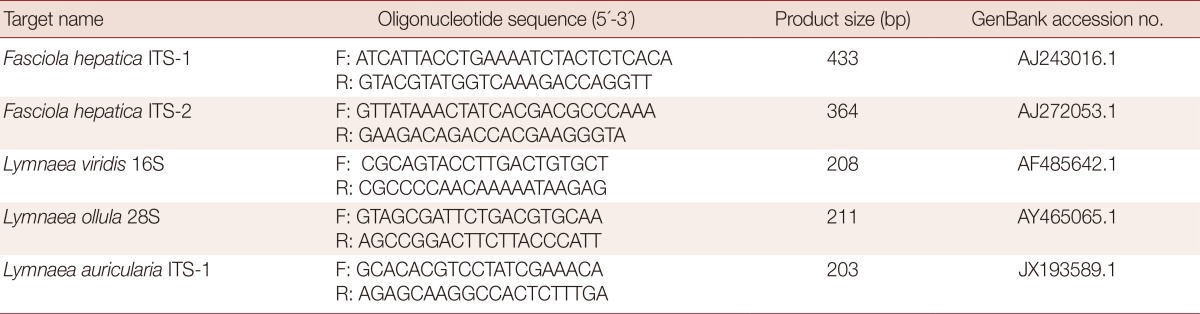
The presence of internal transcribed spacer 1 (ITS-1) and ITS-2 genes of F. hepatica in each snail was determined using PCR amplification. Briefly, genomic DNA was extracted from the whole snail body using G-DEX™ genomic DNA extraction kit according to the manufacturer's instructions. The primers used for PCR amplification are listed in Table 1. The PCR mixture for the PCR amplification contained 5 µl genomic DNA, 3 µl each of forward and reverse primers, 4 µl dNTP, 5 µl 10× Ex Taq buffer, 0.25 µl Ex Taq polymerase, and 29.8 µl DDW. PCR assays were performed with ITS-1 and ITS-2 primers and an initial denaturation step of 94℃ for 30 sec, followed by 30 cycles of denaturation at 98℃ for 10 sec, annealing at 60℃ for 30 sec and extension at 72℃ for 30 sec, followed by 1 cycle at 72℃ for 10 min and a final hold at 4℃.
Table 1.
Primers used for detection of F. hepatica and identification of snail species collected from water-dropwort fields in Korea
Adult F. hepatica was used as a positive control for detection of specific ITS-1 and ITS-2 genes. Amplifications were generated using a TaKaRa PCR Thermal Cycler (Takara Bio Inc., Otus, Japan). Agarose gel electrophoresis (1.5%) with ethidium bromide staining was used to visualize the ITS-1 and ITS-2 PCR products.
Snail identification using morphological and molecular methods
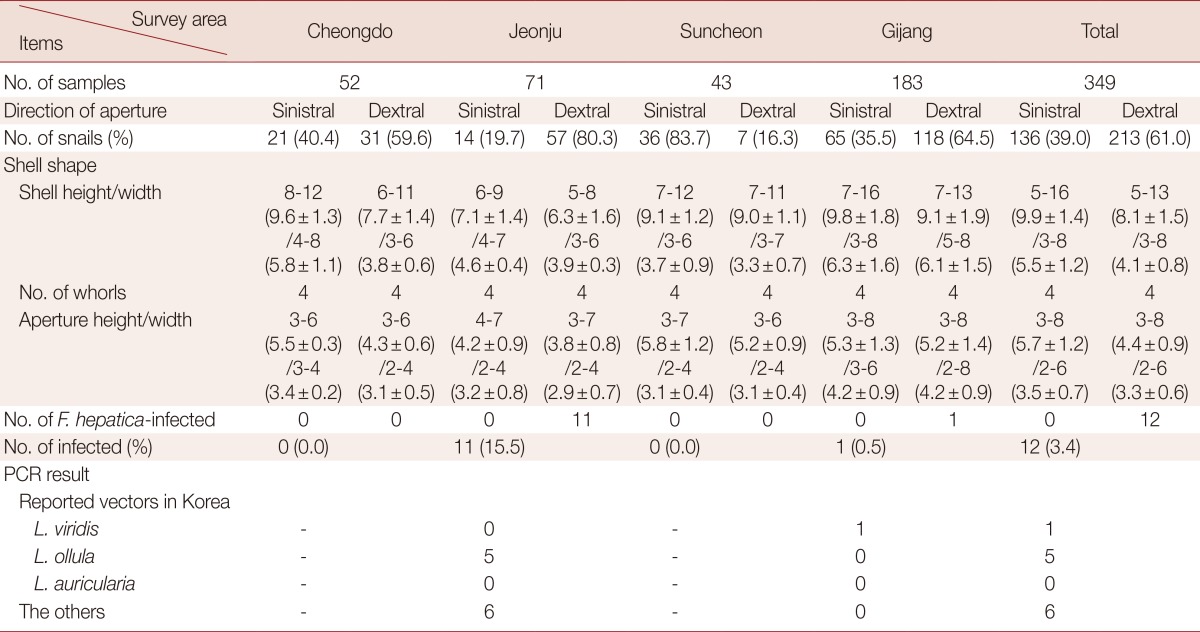
Lymnaeid freshwater snails can act as the first intermediate host of F. hepatica [1,12]. Collected snails were sorted according to morphological and molecular characteristics. First, collected snails were divided into 2 groups according to the direction of shell aperture such as sinistral (left-handed aperture) and dextral (right-handed aperture), and then the following criteria were recorded for each snail: shell size, number of whorls, and size of aperture.
Next, the collected snail species were identified using a molecular method. We prepared specific primers for detection of Lymnaea viridis, L. ollula (=L. pervia, Austropeplea ollula), and L. auricularia (=Radix auricularia; Table 1), which were reported as the first intermediate host of F. hepatica in Korea [8,13,14]. PCR amplification of the specific gene fragments of L. viridis, L. ollula, and L. auricularia was performed using 5 µl of genomic DNA from each sample, 5 µl of 10× Ex Taq buffer, 4 µl of a dNTP mixture, 3 µl each of forward and reverse primers, 0.25 µl of TaKaRa Ex Taq DNA Polymerase, and 29.8 µl of DDW using a TaKaRa PCR Thermal Cycler. All PCR assays were performed using 1 cycle at 94℃ for 30 sec and 30 cycles at 98℃ for 10 sec, 60℃ for 30 sec and 72℃ for 30 sec, followed by 1 cycle at 72℃ for 7 min and a final hold at 4℃. Agarose gel electrophoresis (1.5%) with ethidium bromide staining was used to visualize the PCR products.
Sequencing and analysis of the F. heptica ITS-2 region
To confirm that the PCR products from F. hepatica-infected snails were not a result of unspecific amplification, sequencing of a single band was performed. Briefly, after electrophoretic separation, the ITS-2 PCR products were clearly delineated and sequenced directly by SolGent (Daejeon, Korea). The complete ITS-2 sequences of F. hepatica from 6 positive field samples (snails act as the first intermediate host) were compared with those isolated from Spain (GenBank accession no. AJ27205.1) using the Clone Manager software (Sci-Ed Software, Cary, North Carolina, USA).
Statistical analyses
The data are presented as means±SD. Statistical significance was determined using ANOVA (StatView; Abacus Concepts Inc., Berkeley, California, USA). All experiments were performed at least twice; P-values <0.05 were considered to indicate statistically significant differences.
RESULTS
F. hepatica infection in snails
The PCR amplification of the positive control, i.e., adult 3 DNA, showed a characteristic pattern of bands at 433 and 346 bp for ITS-1 and ITS-2, respectively. These results were consistent in all subsequent reactions. The sequences obtained from the PCR products were identical to the target sequence (GenBank nos. AJ243016.1 and AJ272053.1).
PCR was performed on 349 snails to determine F. hepatica infection. As shown in Fig. 2, PCR amplification using ITS-1 and ITS-2 specific primers produced 433-bp and 346-bp fragments, respectively. Among the 349 snails collected from the 4 fields, ITS-1 or ITS-2 bands of F. hepatica were detected in 12 snails. Thus, the overall prevalence of F. hepatica infection in snails was 3.4%, ranging from 0.0% to 15.5% depending on the water-dropwort field (Table 2).
Fig. 2.
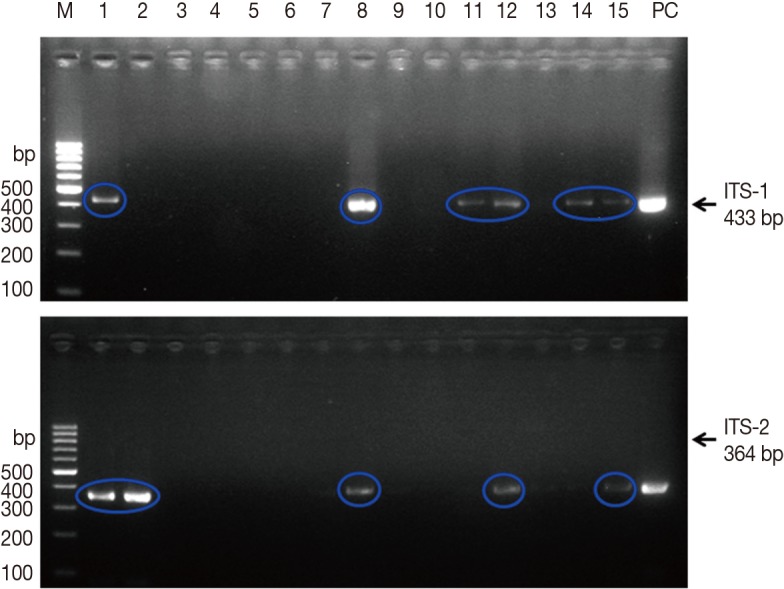
Agarose gel electrophoresis of PCR products containing internal transcribed spacer 1 (ITS-1) and ITS-2 markers of Fasciola hepatica. M, 100 bp marker; lanes 1-15, field snail samples; PC, positive control (F. hepatica worm). Upper panel, 433 bp for ITS-1; lower panel, 364 bp for ITS-2.
Table 2.
Morphological and molecular characteristics of the collected snails
Snail identification and vectorial capacity
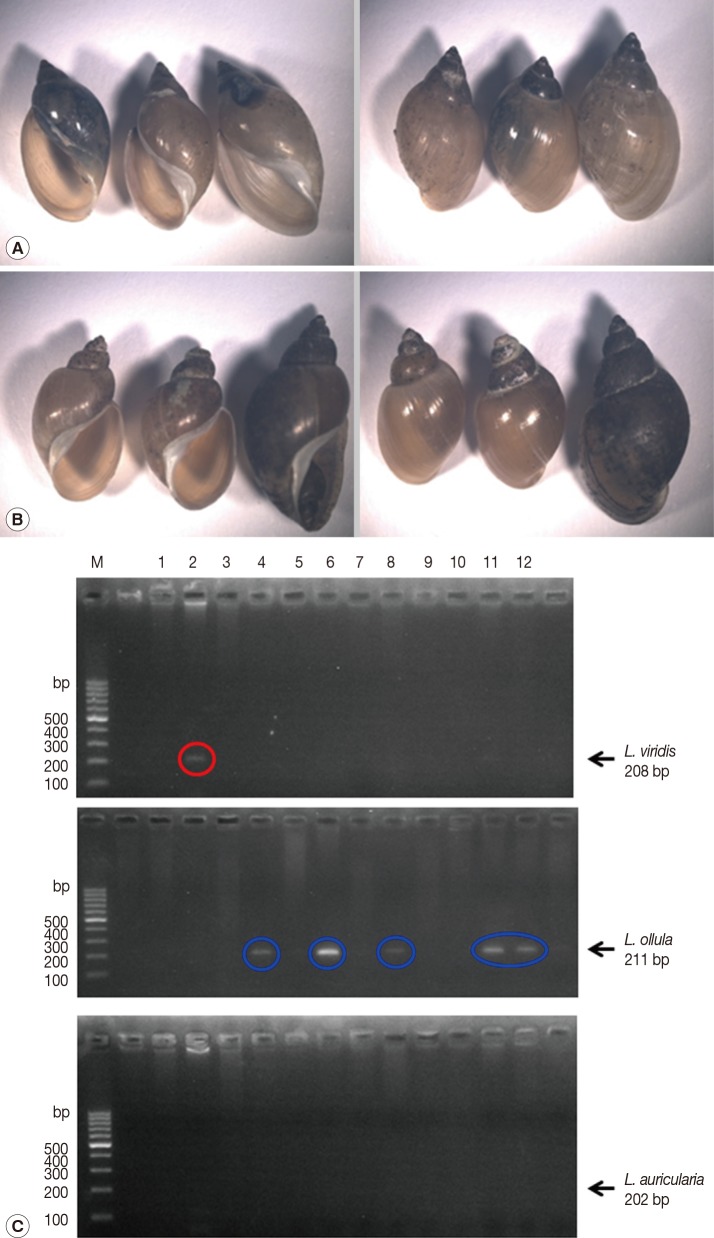
A total of 349 snails were classified based on morphological and molecular characteristics. According to the direction of their aperture, snails were divided into 2 groups, sinistral and dextral. Lymnaea snails are dextral [12]. Among the 349 snails, 136 were sinistral (Fig. 3A; Table 2), and 213 were dextral (Fig. 3B; Table 2). The morphological characteristics of snails with a sinistral shell were as follows: the shells were elongated and their mean height and width were 9.9±1.4 and 5.5±1.2 mm, respectively. Each snail had 4 whorls, and the mean aperture height and width were 5.7±1.2 and 3.5±0.7 mm, respectively (Fig. 3A; Table 2). The morphological features of dextral snails (Lymnaea spp.) were as follows: the height and width were 8.1±1.5 and 4.1±0.8 mm, respectively, which were smaller than those of sinistral snails (P>0.05). The snails had 4 rounded whorls, and the mean aperture height and width were 4.4±0.9 and 3.3±0.6 mm, respectively. The aperture was consistently oval without angles (Fig. 3B; Table 2).
Fig. 3.
Identification of snails collected from 4 large water-dropwort fields in Korea. (A) Sinistral (left-handed aperture) snails. (B) Dextral (right-handed aperture) snails. (C) Agarose gel electrophoresis of PCR products for detection of L. viridis, L. ollula, and L. auricularia from field snail samples. M, 100 bp marker; lanes 1-12, field snail samples.
To evaluate the vectorial activity of F. hepatica-infected snails, we performed PCR to identify the snail species of 12 F. hepatica-infected ones. Among the 12 infected snails, 1 was identified as L. viridis and 5 as L. ollula; however, the species of the remaining 6 could not be identified.
Confirmation of F. hepatica-infected snails using sequencing and analysis
We sequenced the ITS-2 PCR products amplified from the F. hepatica-infected snails to confirm whether the PCR products were real F. hepatica or not. As shown in Fig. 4, the nucleotide sequences of the PCR products from the 6 F. hepatica-infected samples obtained from 2 fields were 100% identical to the ITS-2 sequences of F. hepatica-infected samples obtained from Spain (GenBank accession no. AJ272053.1). Thus, this banding pattern was considered a positive result specific for F. hepatica DNA.
Fig. 4.
F. hepatica ITS-2 nucleotide sequences of 6 positive samples obtained from PCR products compared with a GenBank sequence (accession no. AJ272053.1). Base homologies are indicated by a dot (∙); base changes are shown in orange. The total length of the F. hepatica ITS-2 region is 468 bp.
DISCUSSION
The water dropwort is one of the major second intermediate hosts of human F. hepatica infection in Korea. To determine the potential infectivity with F. hepatica, we collected snails from 4 large water-dropwort fields in Korea. The snails were examined using PCR targeting the ITS-1 and ITS-2 regions of F. hepatica. Of the 349 snails, 12 were infected with F. hepatica (overall prevalence, 3.4%). However, the prevalence varied from 0.0% to 15.5% depending on the collection field. Among 12 F. hepatica-infected snails, 6 were identified as L. viridis (1 snail) and L. ollula (5 snails), and the other 6 could not be identified. The unidentified Lymnaea sp. snails may be a new first intermediate host in Korea.
F. hepatica is a major foodborne trematode, and its infection is a good example of an emerging/reemerging parasitic disease as a consequence of diverse phenomena related to both environmental change and man-made modifications [1,2]. In Korea, F. hepatica infection had been one of the endemic diseases affecting cattle during the 1980s [7,9]. Of 524 dairy cattle surveyed, 200 were infected with Fasciola spp. (38.2%) [7]. From 1987 to 1990, the prevalence of F. hepatica in 6,011 cows raised at 387 farms was 27.7% [9]. Therefore, the Korean government implemented F. hepatica control programs during the 1980s [7,9]. Currently, no information exists regarding the prevalence of F. hepatica infection in Korea; however, human F. hepatica infection cases have been reported [10,11]. Traditionally, F. hepatica infection in cattle was evaluated using a microscope; however, this method is time-consuming and laborious. Thus, various molecular approaches have been used to identify Fasciola species [4,5,6,10,15]. ITS of nuclear ribosomal DNA has been used widely due to its highly repetitive and variable regions flanked by more conserved regions [16]. Characterization of Fasciola samples from different host animals was performed by sequencing the first and second ITS regions of ribosomal DNA in Vietnam, Belgium, Luxembourg, Sweden, and Korea [3,17,18]. In particular, ITS-2 sequences of nuclear ribosomal DNA were used to identify and determine the genetic variation of F. hepatica [1,15,17,18].
In this study, we used the repetitive DNA sequences of ITS-1 and ITS-2 regions specific for F. hepatica to monitor the prevalence of F. hepatica infection. The overall prevalence of F. hepatica infection among 349 snails collected from water-dropwort fields was 3.4%. Also, we found 3 unpaired amplifications of ITS-1 and ITS-2 from F. hepatica-infected snails. The discrepancy may be caused by point mutation of ITS-1 or ITS-2 gene sequences of F. hepatica in each snail, because both ITS-1 and ITS-2 genes were detected at adult F. hepatica worm as a positive control. Interestingly, all F. hepatica-infected snails were found at dual cultivation fields such as rice and water-dropwort indicating that dual cultivation fields are easily contaminated with inappropriately fermented livestock manure for growing rice. This prevalence was lower than in previous reports in Korea [7,9] and current reports from Sweden, Switzerland, and Poland (13.0%, 7%, and 26.6%, respectively) [3,5,6].
F. hepatica is a snail-transmitted trematode. Approximately 20 species within the family Lymnaeidae play an essential role as the first intermediate host of F. hepatica worldwide [1]. In Europe, L. truncatula is considered the principal intermediate host in most environments [5,19], with infection levels ranging from 0% to 82% in Sweden [3]. The prevalence of natural F. hepatica infection in France was 1.1% in L. truncatula and 0.3% in L. glabra [19]. L. tomentosa and L. ollula are the major hosts in Australia and Japan, respectively, whereas L. cubensis and L. columella are the major hosts in the Americas [1]. In Korea, L. viridis is reportedly the dominant intermediate host for F. hepatica [13], and others include L. ollula [8] and L. auricularia [14]. These are distributed throughout the environment; the major habitats are rice paddies, followed by brooks, irrigation canals, and drains [8]. We also tried to identify the snail species collected from water-dropwort fields using morphological and molecular methods. Among 349 snails, 136 were sinistral (39.0%), and 213 were dextral (61.0%). Lymnaea is a dextral snail and the first intermediate host of F. hepatica infection. Beside these snails, there are many types of dextral snails such as Radix, Austropeplea, and Fossaria genera.
In this study, to determine the natural F. hepatica infection of snails in water-dropwort fields in Korea, a field study was performed. We found 12 F. hepatica-infected snails (3.4%). The probability of an individual snail being infected with F. hepatica varied significantly depending on the environmental status of cultivation fields and the types of snails found in the field. Identification of the potentially new Lymnaea sp. positive for F. hepatica is urgently needed.
ACKNOWLEDGMENT
The present research was performed by the support of Rural Development Administration, Republic of Korea (project no. PJ009879).
Footnotes
We have no conflict of interest related to this study.
References
- 1.Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255–1278. doi: 10.1016/j.ijpara.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Sripa B. Global burden of food-borne trematodiasis. Lancet Infect Dis. 2012;12:171–172. doi: 10.1016/S1473-3099(11)70321-8. [DOI] [PubMed] [Google Scholar]
- 3.Novobilský A, Engström A, Sollenberg S, Gustafsson K, Morrison DA, Höglund J. Transmission patterns of Fasciola hepatica to ruminants in Sweden. Vet Parasitol. 2014;203:276–286. doi: 10.1016/j.vetpar.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan RM, Dame JB, Reddy GR, Courtney CH. The prevalence of Fasciola hepatica in its snail intermediate host determined by DNA probe assay. Int J Parasitol. 1997;27:1585–1593. doi: 10.1016/s0020-7519(97)00139-2. [DOI] [PubMed] [Google Scholar]
- 5.Schweizer G, Meli ML, Torgerson PR, Lutz H, Deplazes P, Braun U. Prevalence of Fasciola hepatica in the intermediate host Lymnaea truncatula detected by real time TaqMan PCR in populations from 70 Swiss farms with cattle husbandry. Vet Parasitol. 2007;150:164–169. doi: 10.1016/j.vetpar.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Kozak M, Wedrychowicz H. The performance of a PCR assay for field studies on the prevalence of Fasciola hepatica infection in Galba truncatula intermediate host snails. Vet Parasitol. 2010;168:25–30. doi: 10.1016/j.vetpar.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Son BW, Bai GH, Jo JH, Park YS, Kim SJ. A survey on bovine fascioliasis in Gyeoggi-do: prevalence survey and comparative survey and comparative chemotherapeatical experiments with niclofolan and bithionol against Fasciola spp. and Paramphistomum spp. in dairy cattle. J Korean Vet Med Ass. 1977;13:161–164. [Google Scholar]
- 8.Jang DH, Youn HJ, Jun GS. Study on metacercarial productivity of Fasciola spp. in its intermediate host Austropeplea ollula (Lymnaea ollula) Korean J Vet Res. 1987;27:291–299. [Google Scholar]
- 9.Sohn BW, Kang GS, Han TH. Studies on the optimal time for therapy of Fasciola spp. infected cattle in central area of Korea. Korean J Vet Serv. 1992;15:1–6. [Google Scholar]
- 10.Kang BK, Jung BK, Lee YS, Hwang IK, Lim H, Cho J, Hwang JH, Chai JY. A case of Fasciola hepatica infection mimicking cholangiocarcinoma and ITS-1 sequencing of the worm. Korean J Parasitol. 2014;52:193–196. doi: 10.3347/kjp.2014.52.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YH, Kang KJ, Kwon JH. Four cases of hepatic fascioliasis mimicking cholangiocarcinoma. Korean J Hepatol. 2005;11:169–175. [PubMed] [Google Scholar]
- 12.Malek EA, Cheng TC. Medical and Economic Malacology. New York, USA: Academic Press; 1974. [Google Scholar]
- 13.Cho SH, Lee CG, Kim JH. Laboratory maintenance of field-collected Lymnaea viridis intermediate host for Fasciola hepatica. Korean J Vet Res. 1997;37:185–188. [Google Scholar]
- 14.Jung PL. Freshwater Mollusks in Korea. 1st ed. Seoul, Korea: Yeonhag-Sa; 2003. [Google Scholar]
- 15.Choe SE, Nguyen TT, Kang TG, Kweon CH, Kang SW. Genetic analysis of Fasciola isolates from cattle in Korea based on second internal transcribed spacer (ITS-2) sequence of nuclear ribosomal DNA. Parasitol Res. 2011;109:833–839. doi: 10.1007/s00436-011-2323-6. [DOI] [PubMed] [Google Scholar]
- 16.Morgan JA, Blair D. Nuclear rDNA ITS sequence variation in the trematode genus Echinostoma: an aid to establishing relationships within the 37-collar-spine group. Parasitology. 1995;111:609–615. doi: 10.1017/s003118200007709x. [DOI] [PubMed] [Google Scholar]
- 17.Dung BT, Doanh PN, The DT, Loan HT, Losson B, Caron Y. Morphological and molecular characterization of lymnaeid snails and their potential role in transmission of Fasciola spp. in Vietnam. Korean J Parasitol. 2013;51:657–662. doi: 10.3347/kjp.2013.51.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caron Y, Martens K, Lempereur L, Saegerman C, Losson B. New insight in lymnaeid snails (Mollusca, Gastropoda) as intermediate hosts of Fasciola hepatica (Trematoda, Digenea) in Belgium and Luxembourg. Parasit Vectors. 2014;7:66. doi: 10.1186/1756-3305-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rondelaud D, Vignoles P, Abrous M, Dreyfuss G. The definitive and intermediate hosts of Fasciola hepatica in the natural watercress beds in central France. Parasitol Res. 2001;87:475–478. doi: 10.1007/s004360100385. [DOI] [PubMed] [Google Scholar]