Abstract
Until 2012, a total of 48 cases of diphyllobothriasis had been reported in Korea, all of which were morphologically identified as Diphyllobothrium latum. However, some of these specimens were analyzed by nucleotide sequencing of the mitochondrial cox1 gene, which showed that all were D. nihonkaiense, not D. latum. After that, 3 further cases of diphyllobothriasis were confirmed as D. nihonkaiense. In the present study, 3 new cases of D. nihonkaiense were detected from 2011 through 2013. The hosts were infected through consumption of salmonid fishes, such as the trout or salmon, and 2 of them experienced severe diarrhea prior to proglottid passage. All of the tapeworms were confirmed to be D. nihonkaiense by genetic identification. This proved again that most diphyllobothriasis in Korea have been caused by D. nihonkaiense.
Keywords: Diphyllobothrium nihonkaiense, salmon, trout, sequencing
INTRODUCTION
The genus Diphyllobothrium (Cobbold, 1858) has long been recognized as a human intestinal parasite. Among its 18 species, Diphyllobothium latum (Linnaeus, 1758) is the most important, and this species is usually associated with human diphyllobothriasis, especially in north temperate and subarctic regions of Eurasia and North America [1]. The infection sources of this species are predatory fish, for example, perch, pike, and burbot in Europe, and pikeperch or walleye in North America [2]. A total of 48 cases of human diphyllobothriasis had been reported in Korea until 2012, and all of them were morphologically identified as D. latum [3].
However, it was suggested that many records on larval diphylllobothriids from salmonid fish (e.g., salmon, trout, and whitefish) actually belonged to other species [4]. In addition, it was proved that Pacific salmons such as cherry, pink, and chum harbor D. nihonkaiense, but not D. latum [5]. The Korean cases were infected mostly through consumption of raw salmonid fish such as salmon or trout [3,6], suggesting that they had possibly been infected with species other than D. latum. In fact, several nucleotide sequencing studies along with morphological identification revealed that previously identified Diphyllobothrium specimens in Korea were D. nihonkaiense [7]. After then, 3 further cases of diphyllobothriasis were confirmed as D. nihonkaiense [8,9]. It is suggested that all Korean diphyllobothriasis cases were caused by D. nihonkaiense, and that D. latum may not exist in Korea. Herein, we report another 3 cases of D. nihonkaiense infection, the possible transmission of which was consumption of salmonid fish.
CASE RECORD
Case 1
A 38-year-old man, living in Cheonan-si, Chungcheongnam-do, visited the Department of Internal Medicine, Dankook University Hospital in April 2011, complaining of the periodical passage of tapeworm segments beginning in August 2010, the last 60 cm in length each time. He was otherwise healthy and without any apparent helminth infection symptoms. He self-treated with albendazole twice, but 3 further discharges of segments occurred after the treatments. He recalled that he had consumed raw trout in Cheonan-si restaurants more than 10 times in May 2010 with friends, 3 months before the tapeworm discharges. Under the diagnosis of diphyllobothriasis, he was treated with praziquantel at a single dose of 10 mg/kg and purged with magnesium sulfate at a dose of 40 mg/kg, expelling a complete worm in the first diarrheic stool (Fig. 1A). All the proglottids were wider than long and rosette-shaped uteri were observed in gravid proglottids. After acetocarmine stain, it was shown that genital pore was located anteriorly to the uterine pore, and the wing-shaped ovary is posterior to the uterus (Fig. 1B, C). The specimen was easily identified as Diphyllobothrium sp. After DNA extraction from the proglottids, PCR was performed in a GeneAmp PCR System 9700 (Applied Biosystems, Langen, Germany). The primers used were Dl/n-COIF1 and Dl/n-COIR1, and the PCR conditions were 94℃ for 3 min, 35 cycles of 94℃ for 1 min, 48℃ for 1 min, 72℃ for 1 min, and final extension at 72℃ for 10 min. By nucleotide sequencing of the mitochondrial cox1 gene, the specimen was finally confirmed to be D. nihonkaiense.
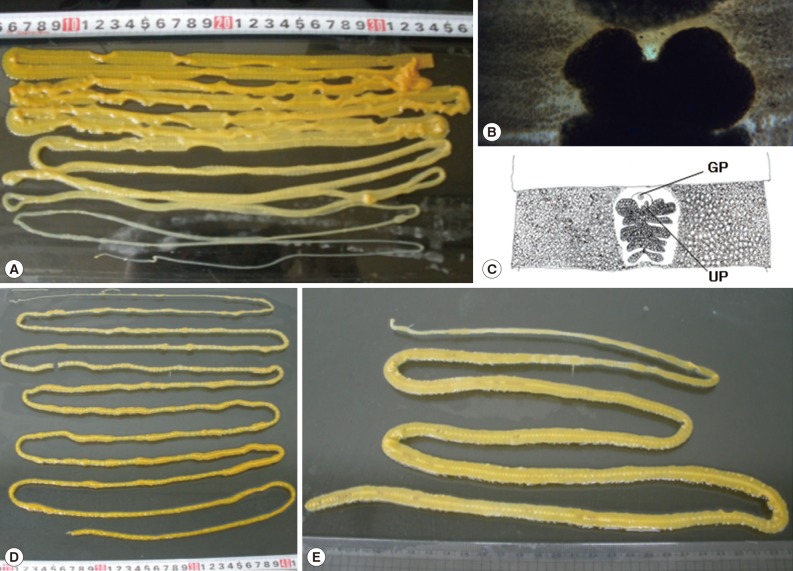
Fig. 1.
Gross findings of adult Diphyllobothrium nihonkaiense worms from case 1 (A), case 2 (D), and case 3 (E). The worm of case 1 was discharged after praziquantel treatment and purgation with magnesium sulfate, and the rest worms were spontaneously discharged from the patients during defecation. (B) A gravid proglottid of case 1, stained by acetocarmine. (C) The graphical view of (B). GP, genital pore; UP, uterine pore.
Case 2
A 50-year old man noticed, upon defecation, a tape-like substance hanging from the anus in February 2013. With great care, he pulled out what proved to be worm segments; 1 hr later, he discovered another worm segment under the same circumstances as the first. The 2 worm segments, of approximately 6 m in total length (Fig. 1D), were observed to wriggle on the floor. He had been healthy without gastrointestinal troubles, and with no worm-segment discharge. However, 4 weeks before the segment discharge, he developed severe diarrhea of a frequency of 10-20 incidences per day, which lasted for 2 weeks. He reported a dislike of all raw fish except for trout, which he had occasionally consumed. His most recent such experience was at about 1 year previously. The segments were identified, on the basis of the lack of a scolex, as those of Diphyllobothrium. By nucleotide sequencing, the specimen showed 93.0% (587/631 bp) similarity with D. latum (GenBank no. AB269325) and 99.5% (628/631 bp) similarity with D. nihonkaiense (GenBank no. AB268585). The specimen was finally diagnosed as D. nihonkaiense (Fig. 2). He was treated with praziquantel, and thereafter showed no signs of this parasitic infection.
Fig. 2.
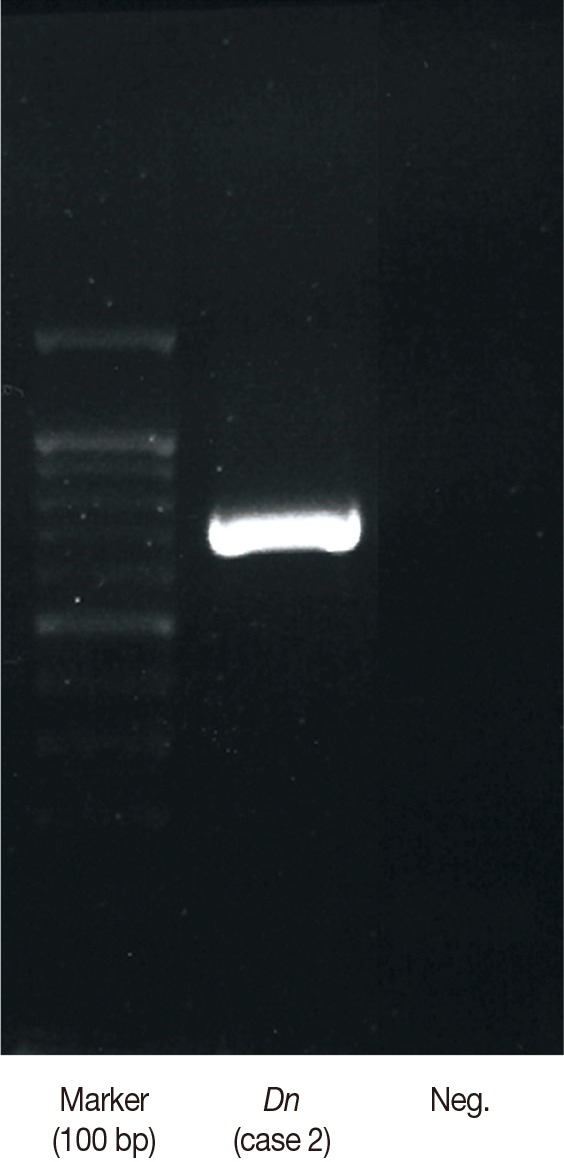
Representative data (from case 2) of nucleotide sequencing of mitochondrial DNA partial cox1 in case 2. Lane 1, size marker (100 bp); lane 2, the present sample; lane 3, negative control.
Case 3
In April 2013, a 36-year old man discovered a tapeworm segment in his stool. He had been suffering from mild diarrhea, which, 1 week prior to an interview, became severe. He had long enjoyed raw fish, especially salmon, often as salmon sushi. He recalled that he had eaten a significant amount of raw salmon at a wedding reception 5 months previously. According to the morphological characteristics of the uterus, the segment was judged to be Diphyllobothrium sp. (Fig. 1D), and by genetic identification, it was confirmed to be D. nihonkaiense.
DISCUSSION
Although a total of 48 Korean cases of D. latum were reported from 1971 through 2012 [3], there had been serious confusion as to their species identification. On the basis of the taxonomic differences discovered between Finnish D. latum and that in Japan, the latter were renamed as D. nihonkaiense [10]. Further, plerocercoid larvae detected in trout (Oncorhynchus masou) blocks kept in the freezer of a patient, who had expelled D. nihonkaiense in diarrhea, were molecularly identified as those of D. nihonkaiense [11]. D. nihonkaiense infection also has been reported in North America, for example the case of a Czech tourist infected through consumption of raw Pacific sockeye salmon (Oncorhynchus nerka). Also in Europe, specifically of a patient living in Switzerland who had acquired D. nihonkaiense infection after eating imported Pacific salmon [12,13].
In 2009, some of the Korean specimens that had already been diagnosed as D. latum were re-analyzed, this time by nucleotide sequencing of the mitochondrial cox1 gene in combination with morphological identification, and shown to be D. nihonkaiense [5]. Since the present 3 infection cases were the results of the consumption of raw trout or salmon, it is reasonable to guess that the relevant tapeworms were also D. nihonkaiense. However, the presence of other Diphyllobothrium spp. such as D. latum cannot be discounted [11]. Molecular speciation should be conducted on as many Diphyllobothrium specimens as possible.
A number of epidemiological surveys on second intermediate hosts of Diphyllobothrium sp. have been undertaken. For example, 4-10% of Lake Geneva perch fillets harbored the plerocercoid of D. latum, and Pacific salmons were proved to be an infection source of D. nihonkaiense [12,13]. In Japan, the chum salmon (Oncorhynchus keta) detection rate for D. nihonkaiense plerocercoids was found to be 51.1%, suggesting the most important source of diphyllobothriasis [14]. Although the salmon and trout have been strongly implicated as important diphyllobothriasis infection sources, no plerocercoid larvae have yet been discovered in those species in Korea. Surveys on Diphyllobothrium sp. larvae infection among salmonid fish are urgently required.
Most reported Diphyllobothrium infections have been asymptomatic; diarrhea, abdominal pain, or discomfort occurs in only about 1 in 5 infections [4]. With respect to the present 3 cases, 2 patients experienced severe diarrhea prior to the proglottid discharge. However, it was uncertain whether their condition was tapeworm-caused. Acute peristalsis, alternatively, effects detachment of the scolex from the intestinal villi, and eventually leads to their expulsion through defecation. Recently, a gastrointestinal contrast medium, Gastrografin, an amidotrizoic acid, has been utilized in D. latum extirpation. Gastrografin has some advantages over anthelminthics, including its availability in hospitals, and the lack of known serious side effects [15]. Since colonoscopic removal incurs risk of tapeworm breakage, Gastrografin is a potential treatment alternative to praziquantel [16].
References
- 1.Bylund BG. Chapter 17. Diphyllobothrium latum. In: Akuffo H, editor. Parasites of the Colder Climates. London, UK and New York, USA: CRC Press; 2003. pp. 169–176. [Google Scholar]
- 2.Andersen KI, Gibson DI. A key to three species of larval Diphyllobothrium Cobbold, 1858 (Cestoda: Pseudophyllidea) occurring in European and North American freshwater fishes. Syst Parasitol. 1989;13:3–9. [Google Scholar]
- 3.Choi HJ, Lee J, Yang HJ. Four human cases of Diphyllobothrium latum infection. Korean J Parasitol. 2012;50:143–146. doi: 10.3347/kjp.2012.50.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scholz T, Garcia HH, Kuchta R, Wicht B. Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance. Clin Microbiol Rev. 2009;22:146–160. doi: 10.1128/CMR.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dick TA, Nelson PA, Choudhury A. Diphyllobothriasis: update on human cases, foci, patterns and sources of human infections and future considerations. Southeast Asian J Trop Med Public Health. 2001;32(suppl 2):59–76. [PubMed] [Google Scholar]
- 6.Lee EB, Song JH, Park NS, Kang BK, Lee HS, Han YJ, Kim HJ, Shin EH, Chai JY. A case of Diphyllobothrium latum infection with a brief review of diphyllobothriasis in the Republic of Korea. Korean J Parasitol. 2007;45:219–223. doi: 10.3347/kjp.2007.45.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon HK, Kim KH, Huh S, Chai JY, Min DY, Rim HJ, Eom KS. Morphologic and genetic identification of Diphyllobothrium nihonkaiense in Korea. Korean J Parasitol. 2009;47:369–375. doi: 10.3347/kjp.2009.47.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SH, Eom KS, Park MS, Kwon OK, Kim HS, Yoon JH. A case of Diphyllobothrium nihonkaiense infection as confirmed by mitochondrial COX1 gene sequence analysis. Korean J Parasitol. 2013;51:471–473. doi: 10.3347/kjp.2013.51.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song SM, Yang HW, Jung MK, Heo J, Cho CM, Goo YK, Hong Y, Chung DI. Two human cases of Diphyllobothrium nihonkaiense infection in Korea. Korean J Parasitol. 2014;52:197–199. doi: 10.3347/kjp.2014.52.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamane Y, Kamo H, Bylund G, Wikgren BJP. Diphyllobothrium nihonkaiense sp. nov (Cestoda: Diphyllobothriidae): revised identification of Japanese broad tapeworm. Shimane J Med Sci. 1986;10:29–48. [Google Scholar]
- 11.Ando K, Ishikura K, Nakakugi T, Shimono Y, Tamai T, Sugawa M, Limviroj W, Chinzei Y. Five cases of Diphyllobothrium nihonkaiense infection with discovery of plerocercoids from an infective source, Oncorhynchus masou ishikawae. J Parasitol. 2001;87:96–100. doi: 10.1645/0022-3395(2001)087[0096:FCODNI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Wicht B, Scholz T, Peduzzi R, Kuchta R. First record of human infection with the tapeworm Diphyllobothrium nihonkaiense in North America. Am J Trop Med Hyg. 2008;78:235–238. [PubMed] [Google Scholar]
- 13.Shimizu H, Kawakatsu H, Shimizu T, Yamada M, Tegoshi T, Uchikawa R, Arizono N. Diphyllobothriasis nihonkaiense: possibly acquired in Switzerland from imported Pacific salmon. Inter Med. 2008;47:1359–1362. doi: 10.2169/internalmedicine.47.1026. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki J, Murata R, Sadamasu K, Araki J. Detection and identification of Diphyllobothrium nihonkaiense plerocercoids from wild Pacific salmon (Oncorhynchus spp.) in Japan. J Helminthol. 2010;84:434–440. doi: 10.1017/S0022149X10000155. [DOI] [PubMed] [Google Scholar]
- 15.Ko SB. Observation of deworming process in intestinal Diphyllobothrium latum parasitism by gastrografin injection into jejunum through double-balloon enteroscope. Am J Gastroenterol. 2008;103:2149–2150. doi: 10.1111/j.1572-0241.2008.01982_11.x. [DOI] [PubMed] [Google Scholar]
- 16.Fujita M, Koga H, Iida M, Hirakawa K, Hoshika K, Haruma K, Okino T. The diagnostic yield of colonoscopy and the therapeutic value of intraduodenal amidotrizoic acid injection in intestinal Diphyllobothrium latum infection: report of a case. Am J Gastroenterol. 2002;97:2468–2470. doi: 10.1111/j.1572-0241.2002.06009.x. [DOI] [PubMed] [Google Scholar]