Abstract
The longitudinal arch (LA) of the human foot compresses and recoils in response to being cyclically loaded. This has typically been considered a passive process, however, it has recently been shown that the plantar intrinsic foot muscles have the capacity to actively assist in controlling LA motion. Here we tested the hypothesis that intrinsic foot muscles, abductor hallucis (AH), flexor digitorum brevis (FDB) and quadratus plantae (QP), actively lengthen and shorten during the stance phase of gait in response to loading of the foot. Nine participants walked at 1.25 m s−1 and ran at 2.78 and 3.89 m s−1 on a force-instrumented treadmill while foot and ankle kinematics were recorded according to a multisegment foot model. Muscle–tendon unit (MTU) lengths, determined from the foot kinematics, and intramuscular electromyography (EMG) signals were recorded from AH, FDB and QP. Peak EMG amplitude was determined during the stance phase for each participant at each gait velocity. All muscles underwent a process of slow active lengthening during LA compression, followed by a rapid shortening as the arch recoiled during the propulsive phase. Changes in MTU length and peak EMG increased significantly with increasing gait velocity for all muscles. This is the first in vivo evidence that the plantar intrinsic foot muscles function in parallel to the plantar aponeurosis, actively regulating the stiffness of the foot in response to the magnitude of forces encountered during locomotion. These muscles may therefore contribute to power absorption and generation at the foot, limit strain on the plantar aponeurosis and facilitate efficient foot ground force transmission.
Keywords: foot biomechanics, arch stiffness, electromyography, running, locomotion
1. Introduction
The human foot is a unique structure characterized by the presence of a pronounced longitudinal arch (LA) that provides considerable stiffness to enable forward propulsion, while also retaining sufficient flexibility to adapt and conform to alterations in surface and loading demand [1,2]. When encumbered with load, the LA lengthens and lowers, subsequently recoiling as the load is removed [3,4]. This compression–recoil process has been termed the ‘foot spring’ mechanism and allows mechanical energy to be stored and subsequently released during each foot contact, which may improve the metabolic efficiency of gait [3]. The contribution of the passive ligamentous structures to this mechanism have been well established [3,4], however, to date very little attention has been paid the potential contributions of the contractile tissues of the LA in this mechanism.
The plantar intrinsic foot muscles are a group of muscles that contain both origin and insertion within the foot. The three largest of these muscles, abductor hallucis (AH), flexor digitorum brevis (FDB) and quadratus plantae (QP) have muscle–tendon units (MTUs) that span the length of the LA and follow similar anatomical pathways to the medial and central slips of the plantar aponeurosis [5–7]. Recent work from our own laboratory has shown that similar to the plantar aponeurosis, these muscles stretch in response to controlled LA compression, with muscle activation increasing in response to the magnitude of encumbering load [8]. Furthermore, we have shown that additional activation of these muscles counteracts LA compression under load and subsequently increases the stiffness of the LA [8].
During human locomotion, the muscles and tendons of the lower limb perform positive and negative work on the body [9]. Active MTU lengthening is achieved through the application of an external load to forcibly extend muscles as they actively generate tension. This muscle action acts to absorb mechanical energy (power). Conversely, active MTU shortening (or contractions) generates mechanical power [9–12]. Early electromyographic (EMG) measurements from the intrinsic foot muscles suggest that these muscles are active during the stance phase of gait [13], however, it is unclear whether this activation occurs relative to lengthening or shortening of the MTUs.
Previous experiments from our laboratory have shown that the MTUs of AH, FDB and QP activate in response to being forcibly lengthened owing to LA compression during loading of the foot [8]. During locomotion, it is likely that these MTUs will also activate in response to LA compression that occurs during early stance phase. On the basis of our previous data [8], activation of the intrinsic foot muscles would stiffen the LA during early stance while also contributing to absorption of power within the stretched MTUs, effectively reducing the total load encumbered by the passive ligamentous structures. Deactivation of these muscles during late stance, during which time the MTUs presumably shorten, may also contribute to power being delivered through muscle contraction or elastic recoil of the elastic structures within the MTUs. Given that we have previously found that the magnitude of activation of these muscles is dependent on the load encountered [8,14], we expect to see an increase in the activation with speed of locomotion. An active contribution of the plantar intrinsic foot muscles could potentially enhance the capacity of the foot to adapt to the variations in external load as they are encountered, allowing efficient force transmission between the foot and the ground during tasks such as walking and running, when the magnitude of forces encountered are constantly changing. This may also reduce the total load, and hence strain, experienced by the passive ligamentous structures of the foot (plantar aponeurosis).
As such, we tested the hypothesis that the MTUs of AH, FDB and QP undergo an active stretch and shortening process in response to LA deformation during the stance phase of gait, and therefore are capable of contributing positive and negative power at the foot. Furthermore, we hypothesized that the magnitude of MTU deformation and muscle activation would increase with the increasing loads that are encountered when gait velocity is increased during walking and running.
2. Material and methods
2.1. Participants
Nine healthy male subjects (mean ± standard deviation for age 32 ± 5 years; height: 181 ± 8 cm; mass: 81 ± 11 kg) with no history of lower limb injury in the previous six months or known neurological impairment volunteered to participate in the study. Written informed consent was obtained from each subject.
2.2. Experimental procedures
Subjects performed walking trials at 1.25 m s−1, as well as running trials at 2.78 and 3.89 m s−1 on a force-instrumented treadmill (AMTI, force-sensing tandem treadmill, Watertown, MA, USA). To ensure familiarity with the treadmill and each gait velocity, subjects were allowed 1 min to adapt and familiarize themselves to each speed, prior to the commencement of data capture. Kinetic, kinematic and EMG data were collected simultaneously during all walking and running trials, with approximately 15–20 strides (toe-off to ipsilateral toe-off) being recorded at each gait velocity for subsequent data analysis.
2.3. Data acquisition
2.3.1. Kinematic and kinetic measurements
Three-dimensional (3D) motion capture of the foot and shank, and ground reaction force data were collected during each walking and running trial. Fourteen retro-reflective markers (diameter 9.0 mm) were placed on the skin of the right foot and ankle according to a multisegment foot model developed to describe rear-, mid- and forefoot motion [15]. Two additional markers were applied to the skin over the second and fourth toes, at the level of the middle phalanx, in order to track the movement of the lesser toes.
Kinematic data were captured at 200 Hz using an eight camera 3D optoelectronic motion capture system (Qualysis, Gothenburg, Sweden) while ground reaction force and EMG data were synchronously captured at 2000 Hz through an analogue to digital converter. Kinematic, force and EMG data were collected simultaneously and synchronized using the Qualysis Track Management software from the same company.
2.3.2. Electromyography
Identification of the AH, FDB and QP muscles was conducted using real-time B-mode ultrasound imaging (10 MHz linear array, Ultrasonix RP, USA) in the right foot of each subject. Subsequently, bipolar fine-wire electrodes (0.051 mm stainless steel, Teflon coated, Chalgren, USA) with a detection length of 2 mm and inter-electrode distance of 2 mm were inserted using delivery needles (0.5 × 50 mm) into the muscle tissue of AH, FDB and QP under ultrasound guidance, in accordance with previously described methods [14]. The size of the active area and separation between sites was chosen to give the best chance of recording representative activity from each muscle, while reducing the possibility of crosstalk from nearby muscles. Once the wires were positioned appropriately in each muscle the delivery needles were removed and the muscle was imaged once more to determine that the electrode sensitive ends of the wires remained within the muscle tissue. Sterile techniques were used for the insertion of all wires.
All EMG signals were amplified 1000 times and recorded with a bandwidth of 30–1000 Hz (MA300, Motion Labs, LA, USA). In order to prevent movement artefacts, the fine-wire electrodes, connectors, cabling and pre-amplifiers were secured with cohesive bandage around the distal part of the shank. A surface ground electrode (Ag–AgCl electrode, 24 mm diameter; Tyco Healthcare Group) was secured to the skin overlying the fibula head.
Prior to data collection, the participant was asked to perform foot manoeuvres known to activate each muscle separately. When predicted EMG patterns could be detected, it was concluded that the electrodes were in the correct position. If not, the electrodes were withdrawn approximately 1 mm until appropriate activation patterns could be detected and possible crosstalk excluded. A Velcro strap was secured around the participant's waist, which enabled the EMG amplifier box to be secured to the subject without interfering with their gait. A lightweight optical cable connected the amplifier box to the analogue to digital converter.
2.4. Data analysis
Kinetic, kinematic and EMG data files were exported to Visual3D (C-motion Inc., Germantown, MD, USA) for analysis. A vertical ground reaction force threshold was set to define each toe-off as occurring when vertical ground reaction force fell below 50 N, while foot contact was defined as occurring when vertical force subsequently rose above 50 N. Swing phase was defined as the period from right toe-off to right foot contact, while stance phase was defined as occurring between right foot contact and right toe-off. One gait cycle was considered as right toe-off to the subsequent ipsilateral toe-off.
Force plate data recorded during each experimental trial were digitally filtered with a 20 Hz low pass, fourth order Butterworth filter. Subsequently, the vertical component of the ground reaction force was calculated for each gait velocity and normalized to bodyweight for each participant.
Marker trajectories were digitally filtered with a 6 Hz low pass, fourth order Butterworth filter. Assumed rigid segments were created according to a previously described multisegment foot model [15] including the shank, foot, calcaneus, mid-foot and metatarsals. Joint rotations were calculated in accordance with International Society of Biomechanics recommendations (y-up, z-lateral, x-anterior) with rotation about the z-axis—sagittal plane motion, rotation about the x-axis—frontal plane motion and rotation about the y-axis—transverse plane motion [16]. The LA angle was defined as rotation of the metatarsals relative to the calcaneus, about the z-axis, with metatarsal extension being positive and flexion negative [15]. Thus, an increase in LA angle is indicative of a reduction in LA height (figure 1). For each gait velocity, LA compression was calculated by subtracting the LA angle at foot contact in the 1.25 m s−1 condition from the maximal LA angle recorded during each stance phase. Mean peak LA compression was calculated for each gait velocity by averaging the LA compression occurring over a minimum of 10 gait cycles.
Figure 1.
Compression and recoil of the longitudinal arch (LA). The LA angle is defined as the sagittal plane rotation of the metatarsals relative to the calcaneus. An increase in LA angle indicates compression of the LA which is calculated by subtracting LA angle at foot contact from peak LA angle, which generally occurred at mid-stance. Group mean LA angles are presented at foot contact (a), peak LA angle (b) and toe-off (c) when running at 3.89 m s−1 with data indicating that the LA compresses and recoils during stance phase. (Online version in colour.)
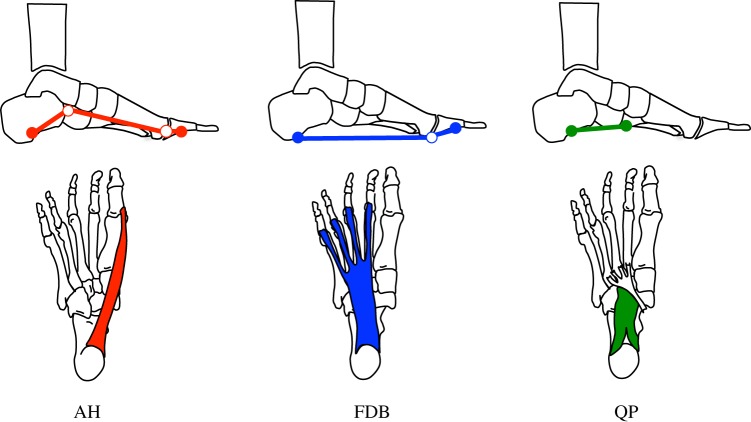
MTU lengths for the AH, FDB and QP muscles were determined based on a geometrical model according to the multisegment kinematic data by defining virtual markers corresponding to the origin, tether and insertion points for each individual muscle in accordance with previous cadaveric descriptions [5–7]. The points were expressed as fixed locations on the bony segment to which they were attached, allowing estimation of changes in MTU length according to the motion of the rigid foot segments. MTU length was defined as the straight line distance from the origin to the insertion, via any tether points. Tether points were created at the distal end of the metatarsal segments for AH and FDB, representing the point where each MTU wraps around the metatarsophalangeal (MTP) joints (figure 2). Additionally, a second tether point was created for the AH MTU, representing the fascial encapsulation of this muscle that occurs posterior to the navicular bone, extending from the deltoid ligament [17]. This encapsulation serves as a pulley, changing the anatomical pathway of AH. Within this geometric model, any length changes observed for the AH and FDB MTUs will be due to a combination of rotations about the LA and MTP joints, while QP MTU length changes will be due to rotation about the LA only (figure 2). Peak MTU strain was calculated during stance phase at each gait velocity by dividing the change in MTU length (peak MTU length minus MTU length at foot contact) during stance phase by the MTU length at foot contact.
Figure 2.
Depiction of the muscle–tendon unit (MTU) pathways (top row) and anatomical pathways (bottom) for abductor hallucis (AH, red), flexor digitorum brevis (FDB, blue) and quadratus plantae (QP, green). Filled circles indicate origin and insertion points for each MTU, while open circles indicate tether points. The MTU length changes for AH and FDB will be due to a combination of rotations occurring about the longitudinal arch (LA) and metatarsophalangeal (MTP) joints, while QP MTU length changes will occur owing to changes in the LA angle. (Online version in colour.)
Raw EMG signals were visually inspected in order to identify data that may have been contaminated by movement artefact, which was defined as an abnormal spike in the signal associated with foot contact. In the event that movement artefact was identified in the EMG signal, data from that particular stride was excluded from the analysis. Following removal of any DC offset from each EMG signal, root mean square (RMS) signal amplitude was calculated using a moving window of 50 ms. Subsequently for each muscle, peak EMG RMS amplitude was selected during the stance phase for each stride cycle, allowing comparisons in magnitude of activation occurring at each gait velocity. EMG data for each muscle was normalized to the peak RMS amplitude recorded across all gait velocities for each muscle.
For each individual, the kinetic, kinematic and EMG data from each gait cycle were time normalized to 100 points and a minimum of 10 gait cycles were averaged from a single velocity to form an individual mean for each variable, at each gait velocity. This process allows for comparison of data across gait cycles at varying velocities.
2.5. Statistics
A one-way repeated measures analysis of variance (ANOVA) was used to describe the effects of gait velocity on mean maximum vertical ground reaction force, LA compression, peak MTU strain and peak stance phase EMG RMS amplitude for each muscle. Post hoc multiple comparison tests including Sidak corrections were performed between each gait velocity (1.25 versus 2.78 versus 3.89 m s−1). Statistical differences were established at p ≤ 0.05. Results are presented as mean difference ± standard error of the mean unless otherwise stated.
3. Results
A representative example of raw kinetic, kinematic and EMG data from a representative individual running at 3.87 m s−1 is presented in figure 3. The data show a high degree of similarity between the five sequential strides. The prominent peaks in the vertical ground reaction force indicate stance phase, which is approximately divided equally into deceleration and propulsion phases as shown by the horizontal ground reaction force. The change in LA angle for this subject at this running velocity was cyclic and highly reproducible. A process of LA compression and recoil is shown by the rapid increases in LA angle occurring during early stance, followed by a rapid decrease in LA angle occurring in late stance, associated with propulsion. While small variations in muscle activity were observed between the three intrinsic foot muscles, for the most part their activity was similar with significant periods of activity during stance and silence during swing, except for AH.
Figure 3.
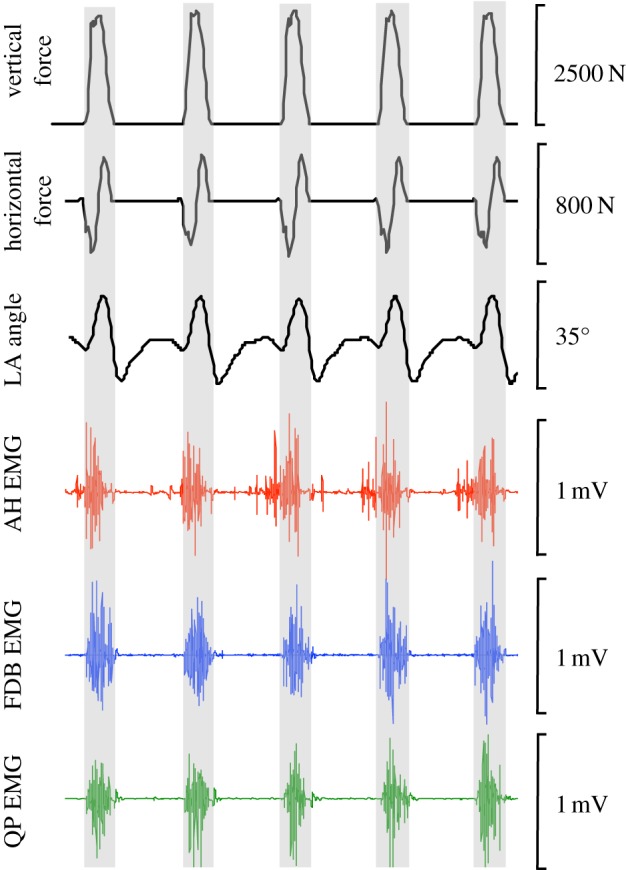
Raw data collected from a representative participant while running at 3.87 m s−1. Vertical and horizontal forces are calculated from the force-instrumented treadmill. Longitudinal arch (LA) angle is calculated based on multisegment foot kinematics and intramuscular electromyography (EMG) recordings are collected from the abductor hallucis (AH) (top), flexor digitorum brevis (FDB) (middle) and quadratus plantae (QP) (bottom). Shaded areas indicate stance phase. (Online version in colour.)
3.1. Vertical force, LA compression and MTU strain
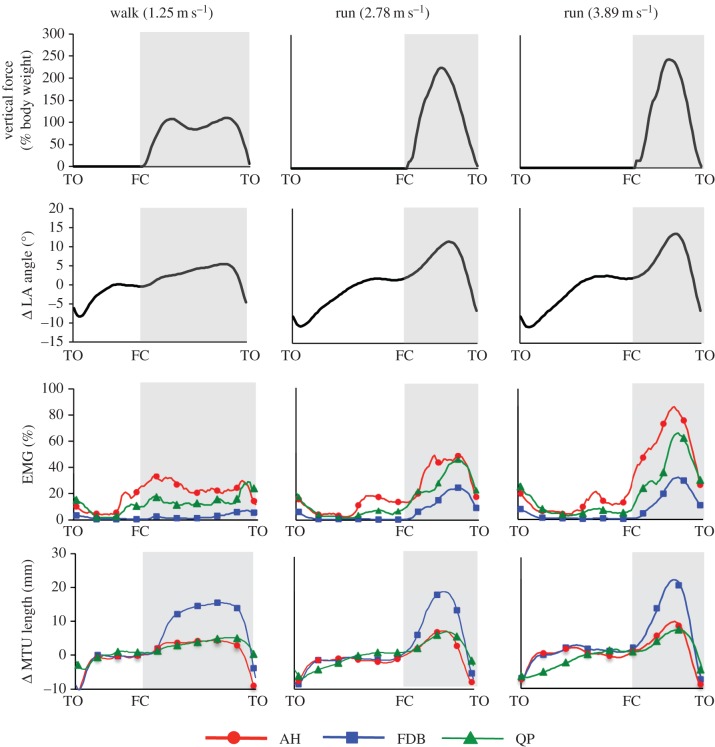
During early to mid-stance LA compression occurred (figure 1) and the MTUs of AH, FDB and QP lengthened as vertical force increased. From mid- to late stance, as vertical force decreased, the LA recoiled and the MTUs of AH, FDB and QP rapidly shortened (figure 3). It was observed that vertical force, LA compression and peak MTU strain all increased significantly with increasing gait velocity (all p ≤ 0.05, figure 4).
Figure 4.
Group mean ensembles ± standard error of the mean for vertical ground reaction force, longitudinal arch (LA) angle (degrees, °), electromyography (EMG) root mean square (RMS) signal amplitude and changes (Δ) in muscle–tendon unit (MTU) length for abductor hallucis (AH, red circles), flexor digitorum brevis (FDB, blue squares) and quadratus plantae (QP, green triangles). Group mean ensembles are defined from toe-off (TO) to ipsilateral toe-off for the right foot. Data recorded during walking at 1.25 m s−1 and running at 2.78 and 3.89 m s−1. For each muscle EMG data are normalized to the maximal amplitude recorded for all trials. Change in MTU length and LA angle is calculated by offsetting the MTU length and LA angle at heel contact in the 1.25 m s−1 condition, respectively. Vertical ground reaction force (GRF) data are normalized to body mass. FC, foot contact. (Online version in colour.)
3.2. Muscle activation
All muscles displayed EMG patterns that were similar in nature, highlighted by substantial bursts of activation during stance and periods of relative inactivity during the early swing phase (figure 3). For all muscles, stance phase activation increased with increasing gait velocity and the associated increase in ground reaction force.
The AH activation pattern consisted of two discrete bursts, with the initial burst occurring during late swing phase, prior to foot contact (figures 3 and 4). The second more substantial burst of AH activity occurred during stance for both walking and running. Peak activation generally coincided with peak vertical ground reaction force with deactivation occurring during late stance (propulsion phase), as the AH underwent shortening (cf. figures 3 and 4). Peak AH activation during stance increased significantly with increasing gait velocity (p ≤ 0.05) as did AH total EMG activity over the stride cycle (p ≤ 0.05, figure 5).
Figure 5.
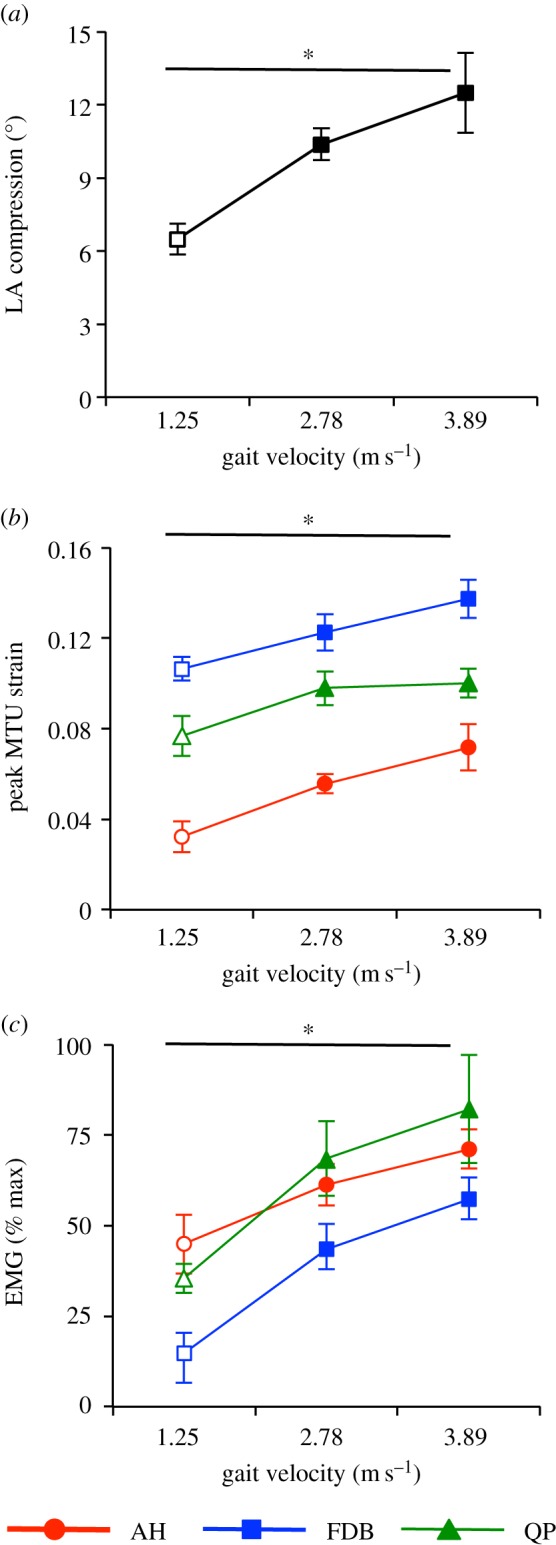
Group mean data for longitudinal arch (LA) compression (a), peak muscle–tendon unit (MTU) strain (b) and electromyography (EMG) root mean square (RMS) amplitude (c) during stance for abductor hallucis (AH, red circles), flexor digitorum brevis (FDB, blue squares) and quadratus plantae (QP, green triangles). LA compression is calculated by subtracting the LA angle at heel strike in the 1.25 m s−1 condition from the peak angle occurring during stance, at each gait velocity. EMG RMS values are normalized to the maximal amplitude recorded during all trials. * Denotes significant difference, with all values increasing with increasing gait velocity (all p ≤ 0.05). (Online version in colour.)
The FDB displayed a burst of activity commencing at foot contact and continuing throughout stance during running and to a lesser extent during walking. Peak activation occurred at mid- to late stance (figure 4). Deactivation occurred during the later part of stance usually associated with the propulsion phase (cf. figure 3). FDB activity during stance significantly increased with increasing gait velocity (p ≤ 0.05, figure 5).
The QP displayed a small increase in activation during late swing that continued into early stance, followed by a second larger burst of activity in mid-stance during running and late stance during walking (figures 3 and 4). Peak stance phase activity increased with gait velocity (p ≤ 0.05, figure 5).
4. Discussion
This study provides unique insight into the neuromechanical function of the plantar intrinsic foot muscles during walking and running at different velocities. These novel findings provide the first in vivo evidence that the plantar intrinsic foot muscles actively lengthen during early stance, absorbing mechanical power and stiffening the arch in response to increasing ground reaction force. Subsequently, in late stance as ground reaction force subsides, shortening of the MTUs allow mechanical power to be returned, presumably aiding forward progression during propulsion. We suggest that this mechanism to actively adapt the stiffness of the foot in response to the magnitude of load encountered may enhance foot ground force transmission and also reduce strain experienced by passive ligamentous structures of the foot.
The foot is the conduit for force transmission between the body and the ground during locomotion. The presence of a pronounced LA gives the foot the capacity to compress and conform in response to load, whilst retaining sufficient stiffness to enable forward propulsion [1,18]. The ligamentous plantar aponeurosis is known to stretch during early stance, providing some resistance to LA compression, while in late stance the windlass mechanism increases LA stiffness in preparation for propulsion [2,19]. While the plantar aponeurosis has been considered the primary contributor to LA stiffness, this passive structure is limited in its ability to respond and adapt to the loading variations that are commonly encountered during locomotion. Additionally, the suggestion that the regulation of foot stiffness is entirely passive does not completely account for the highly adaptable nature of the LA [20], which is known to display increased stiffness when encumbered with higher loads in the absence of increased plantar aponeurosis tension [21]. Recently, we have shown that the plantar intrinsic foot muscles also possess the capacity to stiffen the LA [8]. When considering this knowledge in the light of the current findings that plantar intrinsic foot muscle activation increases with increasing gait velocity, we suggest that these muscles are recruited in order to stiffen the LA, countering the LA compression that occurs owing to higher ground reaction forces. The ability of the plantar intrinsic foot muscles to provide force-dependent alterations in LA stiffness may facilitate effective foot ground force transmission, enabling higher ground reaction forces to be transmitted over a shorter period of time, as required at higher gait velocities [22].
Compression and recoil of the LA in response to load during stance allows mechanical energy to be both absorbed and returned during each foot contact and may provide metabolic energy savings [3,23]. This process has traditionally been considered passive in nature, with energy being stored and released via elastic stretch and recoil of the plantar aponeurosis [3,4]. However, if activation of the plantar intrinsic foot muscles provides the capacity to actively absorb and generate mechanical power at the foot during locomotion, then this may change our interpretation of the mechanical function of the foot. Stiffening the LA will essentially reduce compression, and reducing the strain experienced by the plantar aponeurosis and other ligamentous structures that would otherwise stretch further in the absence of muscular intervention. While this may provide some protection to the plantar aponeurosis and other structures, it also reduces the amount of energy storage and return from these structures. However, as the intrinsic foot muscles have relatively short muscle fibres (approx. 23 mm) relative to the MTU length (approx. 116 mm), the elastic structures of these muscles (tendon and aponeurosis) are well suited for storing the energy that is absorbed by the muscle during early stance and returning it to generate power during deactivation of the muscle in the shortening phase (push-off) [23]. The extent to which these muscles might be able to store and return the energy as well as tuning the stiffness of the foot is yet to be explored.
It is important to note that within the current experimental design we were unable to separate the individual contribution of the plantar aponeurosis and plantar intrinsic foot muscles to the foot spring mechanism, as these two structures act in parallel to regulate LA stiffness during locomotion. Based on the EMG profiles of these muscles during walking, it is apparent that at lower gait velocities the majority of energy absorption and return may occur in the passive plantar aponeurosis with some contribution from the active intrinsic foot muscles. However, as gait velocity increases and the magnitude of force required to be transmitted between the foot and ground also increases, it is likely that the contribution from the intrinsic foot muscles increases substantially, as noted by the significant increase in muscle activation. Caravaggi et al. [21] have previously reported that the compression of the arch during fast walking is significantly less than that which would be expected based on the passive stiffness of the arch reported by Ker et al. [3]. We suggest that this is due to the role of the intrinsic foot muscles in increasing the stiffness of the arch with increased force demand. This force-dependent contribution from the intrinsic foot muscles may serve to stiffen the foot at higher velocities, allowing ankle plantar flexion torque to be transmitted to the ground rapidly, while also serving to modulate the amount of energy that is stored within the elastic element of the MTU. Further research exploring the relative contribution of these structures to the energetics of locomotion may provide valuable insight to human locomotor function.
The role of the foot in generating or absorbing power at the level of the body centre of mass also deserves consideration. Recently, Zelik & Kuo [24] compared measures of total joint work from the ankle, knee and hip against work performed on the centre of mass during constant velocity locomotion with the aim of quantifying the magnitude of mechanical energy dissipation performed by soft tissue. They reported a substantial disparity in total joint work when compared with the total work performed on the centre of mass, with most of this disparity being dissipatory in nature and occurring during the first half of stance. Because Zelik & Kuo [24] assumed a rigid foot segment in their inverse dynamics analysis, they attributed the differences in joint work and centre of mass work to the inability of rigid body inverse dynamics to measure dissipative work performed by the soft tissues. They concluded that it is likely that passive soft tissues do play an important role in mechanical energy dissipation [3,25,26]. However, when considering their findings in the light of the findings from our current study, it is possible that some of this difference may also be due to the role of both the passive and active components contributing to foot stiffness and subsequently contributing to both negative and positive power during stance.
We have provided a detailed description of the activation patterns of AH, FDB and QP recorded from a range of walking and running velocities. An early intramuscular EMG study by Mann & Inman [13] reported that some plantar intrinsic foot muscles are activated as a functional group during late stance in order to stabilize the forefoot during propulsion. Results from the current study provide evidence that while these muscles may have similar mechanical functions, specific differences in activation patterns exist. For example, AH, a muscle that is known to be a slowly discharging, fatigue-resistant muscle [14] displayed a substantial amount of late swing and early stance activation, which may indicate that this muscle provides preparatory stiffening of the LA prior to foot contact, as well as mechanical energy absorption during early stance. Recruitment of FDB and QP occurred largely at foot contact, with peak activation occurring in mid-stance and continuing into the propulsive phase, giving FDB a primary function of generating power during propulsion. Despite specific differences in activation patterns between the three muscles, it is apparent that regardless of specific function, activation is regulated in response to the magnitude of vertical force and subsequent LA compression encountered by the foot.
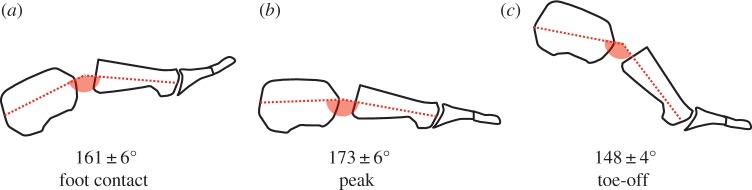
This study has focused on the behaviour of the AH, FDB and QP MTUs during locomotion. It needs to be acknowledged that in addition to LA kinematics, rotation of the MTP joints may also influence length changes of the MTUs of AH and FDB. Extension of the MTP joints that occurs in late stance as the heel rises from the ground would presumably have a lengthening effect on the MTU as it wraps around the joint. However, based on our data this lengthening effect is minimal (figure 6a) with this probably being due to the relatively small moment arm of the FDB and AH MTUs across the MTP joint when compared with their moment arm across the joints of the LA (figure 6b,c). Thus, length changes of the MTUs are closely aligned to the kinematics of the LA, as is reflected by the data in this study.
Figure 6.
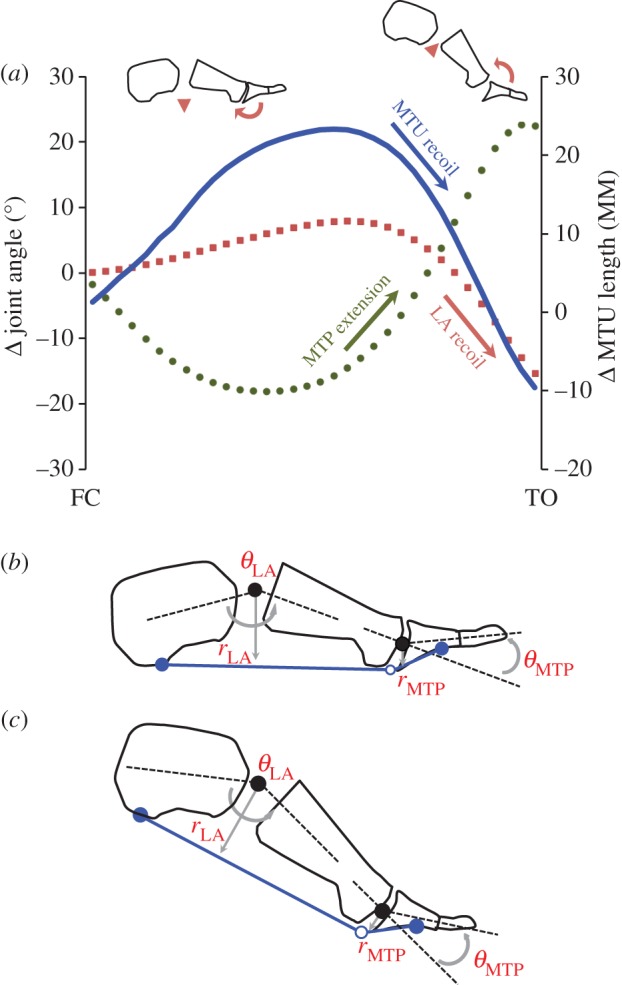
(a) Changes in FDB muscle–tendon unit (MTU) length (blue line), metatarsophalangeal (MTP) joint flexion/extension (green circles) and longitudinal arch (LA) angle (red squares) during stance phase of running at 2.78 m s−1. Data shows that MTU length recoils rapidly during late stance in parallel to LA recoil. This recoil happens despite the opposing influence of MTP joint extension occurring at the same time that should presumably lengthen the MTU. (b,c) The large moment arm of FDB across the LA, compared with its relatively small moment arm across the MTP joints, thus providing a biomechanical rationale for why MTP extension has minimal effect on overall length changes of the MTU. (Online version in colour.)
There are some methodological limitations within the current experimental design that need to be acknowledged. The use of skin-mounted markers to determine changes in foot segment motion may underestimate some of the motion of the mid-foot [27,28] and, therefore, may also impact on our modelling of MTU lengths. However, we are confident that the general movement directions measured are consistent with what actually occurred during each gait trial and therefore the patterns of MTU lengthening and shortening should be representative of what has occurred. The use of intramuscular fine-wire electrodes had the potential to influence running biomechanics of some participants, owing to discomfort from the fine wires. In order to address this issue, all participants were asked to acknowledge any pain or discomfort arising from the electrodes. None of our participants experienced pain or discomfort during the experimental task, thus we are confident that this was not the case.
In summary, the plantar intrinsic foot muscles are activated in order to provide dynamic support of the LA during locomotion. These muscles undergo active lengthening and shortening during stance, with muscle activation and stretch increasing in response to increasing vertical load. Thus, these muscles have the capacity to contribute to power absorption in early to mid-stance and power return and generation in late stance. The AH, FDB and QP muscles displayed distinct patterns of activation that may be related to differences in function, however, activation of all muscles appears to be regulated in response to the magnitude of loading forces encountered.
Ethics statement
The study protocol was approved by the institutional human research ethics committee and conducted in accordance with the Declaration of Helsinki.
References
- 1.Vereecke EE, Aerts P. 2008. The mechanics of the gibbon foot and its potential for elastic energy storage during bipedalism. J. Exp. Biol. 211, 3661–3670. ( 10.1242/jeb.018754) [DOI] [PubMed] [Google Scholar]
- 2.Hicks J. 1954. The mechanics of the foot. II. The plantar aponeurosis and the arch. J. Anat. 88, 25. [PMC free article] [PubMed] [Google Scholar]
- 3.Ker RF, Bennett MB, Bibby SR, Kester RC, Alexander RM. 1987. The spring in the arch of the human foot. Nature 325, 147–149. ( 10.1038/325147a0) [DOI] [PubMed] [Google Scholar]
- 4.Erdemir A, Hamel AJ, Fauth AR, Piazza SJ, Sharkey NA. 2004. Dynamic loading of the plantar aponeurosis in walking. J. Bone Joint Surg. Am 86-A, 546–552. [DOI] [PubMed] [Google Scholar]
- 5.Kura H, Luo ZP, Kitaoka HB, An KN. 1997. Quantitative analysis of the intrinsic muscles of the foot. Anat. Rec. 249, 143–151. () [DOI] [PubMed] [Google Scholar]
- 6.Ledoux WR, Hirsch BE, Church T, Caunin M. 2001. Pennation angles of the intrinsic muscles of the foot. J. Biomech. 34, 399–403. ( 10.1016/S0021-9290(00)00194-9) [DOI] [PubMed] [Google Scholar]
- 7.Tosovic D, Ghebremedhin E, Glen C, Gorelick M, Brown JM. 2012. The architecture and contraction time of intrinsic foot muscles. J. Electromyogr. Kinesiol. 22, 930–938. ( 10.1016/j.jelekin.2012.05.002) [DOI] [PubMed] [Google Scholar]
- 8.Kelly LA, Cresswell AG, Racinais S, Whiteley R, Lichtwark G. 2014. Intrinsic foot muscles have the capacity to control deformation of the longitudinal arch. J. R. Soc. Interface 11, 20131188 ( 10.1098/rsif.2013.1188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavagna GA, Kaneko M. 1977. Mechanical work and efficiency in level walking and running. J. Physiol. 268, 467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter DA. 1983. Energy generation and absorption at the ankle and knee during fast, natural, and slow cadences. Clin. Orthop. Relat. Res. 175, 147–154. [PubMed] [Google Scholar]
- 11.Ito A, Komi PV, Sjodin B, Bosco C, Karlsson J. 1983. Mechanical efficiency of positive work in running at different speeds. Med. Sci. Sports Exerc. 15, 299–308. ( 10.1249/00005768-198315040-00009) [DOI] [PubMed] [Google Scholar]
- 12.Donelan JM, Kram R, Kuo AD. 2002. Simultaneous positive and negative external mechanical work in human walking. J. Biomech. 35, 117–124. ( 10.1016/S0021-9290(01)00169-5) [DOI] [PubMed] [Google Scholar]
- 13.Mann R, Inman VT. 1964. Phasic activity of intrinsic muscles of the foot. J. Bone Joint Surg. Am. 46, 469–481. [PubMed] [Google Scholar]
- 14.Kelly LA, Kuitunen S, Racinais S, Cresswell AG. 2012. Recruitment of the plantar intrinsic foot muscles with increasing postural demand. JCLB 27, 46–51. [DOI] [PubMed] [Google Scholar]
- 15.Leardini A, Benedetti MG, Berti L, Bettinelli D, Nativo R, Giannini S. 2007. Rear-foot, mid-foot and fore-foot motion during the stance phase of gait. Gait Posture 25, 453–462. ( 10.1016/j.gaitpost.2006.05.017) [DOI] [PubMed] [Google Scholar]
- 16.Wu G, Cavanagh PR. 1995. ISB recommendations for standardization in the reporting of kinematic data. J. Biomech. 28, 1257–1261. ( 10.1016/0021-9290(95)00017-C) [DOI] [PubMed] [Google Scholar]
- 17.Wong YS. 2007. Influence of the abductor hallucis muscle on the medial arch of the foot: a kinematic and anatomical cadaver study. Foot Ankle Int. 28, 617–620. ( 10.3113/FAI.2007.0617) [DOI] [PubMed] [Google Scholar]
- 18.Donatelli R. 1985. Normal biomechanics of the foot and ankle. J. Orthop. Sports Phys. Ther. 7, 91 ( 10.2519/jospt.1985.7.3.91) [DOI] [PubMed] [Google Scholar]
- 19.Caravaggi P, Pataky T, Goulermas JY, Savage R, Crompton R. 2009. A dynamic model of the windlass mechanism of the foot: evidence for early stance phase preloading of the plantar aponeurosis. J. Exp. Biol. 212, 2491–2499. ( 10.1242/jeb.025767) [DOI] [PubMed] [Google Scholar]
- 20.Pataky TC, Caravaggi P, Savage R, Parker D, Goulermas JY, Sellers WI, Crompton RH. 2008. New insights into the plantar pressure correlates of walking speed using pedobarographic statistical parametric mapping (pSPM). J. Biomech. 41, 1987–1994. ( 10.1016/j.jbiomech.2008.03.034) [DOI] [PubMed] [Google Scholar]
- 21.Caravaggi P, Pataky T, Günther M, Savage R, Crompton R. 2010. Dynamics of longitudinal arch support in relation to walking speed: contribution of the plantar aponeurosis. J. Ana. 217, 254–261. ( 10.1111/j.1469-7580.2010.01261.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson J, Thorstensson A. 1989. Ground reaction forces at different speeds of human walking and running. Acta Physiol. Scand. 136, 217–227. ( 10.1111/j.1748-1716.1989.tb08655.x) [DOI] [PubMed] [Google Scholar]
- 23.Alexander RM. 1984. Elastic energy stores in running vertebrates. Am. Zool. 24, 85–94. [Google Scholar]
- 24.Zelik KE, Kuo AD. 2010. Human walking isn't all hard work: evidence of soft tissue contributions to energy dissipation and return. J. Exp. Biol. 213, 4257–4264. ( 10.1242/jeb.044297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gefen A, Megido-Ravid M, Itzchak Y. 2001. In vivo biomechanical behavior of the human heel pad during the stance phase of gait. J. Biomech. 34, 1661–1665. ( 10.1016/S0021-9290(01)00143-9) [DOI] [PubMed] [Google Scholar]
- 26.Pain MT, Challis JH. 2001. The role of the heel pad and shank soft tissue during impacts: a further resolution of a paradox. J. Biomech. 34, 327–333. ( 10.1016/S0021-9290(00)00199-8) [DOI] [PubMed] [Google Scholar]
- 27.Nester C, Jones RK, Liu A, Howard D, Lundberg A, Arndt A, Lundgren P, Stacoff A, Wolf P. 2014. Foot kinematics during walking measured using bone and surface mounted markers. J. Biomech. 40, 3412–3423. ( 10.1016/j.jbiomech.2007.05.019) [DOI] [PubMed] [Google Scholar]
- 28.Lundgren P, Nester C, Liu A, Arndt A, Jones R, Stacoff A, Wolf P, Lundberg A. 2008. Invasive in vivo measurement of rear-, mid- and forefoot motion during walking. Gait Posture 28, 93–100. ( 10.1016/j.gaitpost.2007.10.009) [DOI] [PubMed] [Google Scholar]