Summary
The immune system is designed to discriminate between self and tumor tissue. Through genetic recombination, there is fundamentally no limit to the number of tumor antigens that immune cells can recognize. Yet, tumors use a variety of immunosuppressive mechanisms to evade immunity. Insight into how the immune system interacts with tumors is expanding rapidly and has accelerated the translation of immunotherapies into medical breakthroughs. Herein, we appraise the state of the art in immunotherapy with a focus on strategies that exploit the patient’s immune system to kill cancer. We review various forms of immune-based therapies, which have shown significant promise in patients with hematological malignancies, including (i) conventional monoclonal therapies like rituximab, (ii) engineered monoclonal antibodies called bispecific T cell engagers (BiTEs), (iii) monoclonal antibodies and pharmaceutical drugs that block inhibitory T-cell pathways (i.e. PD-1, CTLA-4 and IDO), and (iv) adoptive cell transfer (ACT) therapy with T cells engineered to express chimeric antigen receptors (CARs) or T-cell receptors (TCRs). We also assess the idea of using these therapies in combination and conclude by suggesting multi-prong approaches to improve treatment outcomes and curative responses in patients.
Keywords: allogeneic hematopoietic stem cell transplantation; monoclonal antibodies; bispecific T-cell engagers; immune checkpoint modulators; adoptive T-cell transfer therapy, gene transfer
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a powerful approach for treating hematologic malignancies (1, 2). This approach involves infusing patients with blood-forming stem cells from a genetically similar but not identical (i.e. allogeneic) donor, with the goal of eradicating the patient’s malignancy while re-establishing hematopoiesis. Alloreactive T cells play a key role in de-bulking the patients’ leukemia (3, 4). However, these cells also target nonmalignant tissues and cause the potentially fatal complication of graft-versus-host disease (GVHD) (5, 6). To circumvent GVHD in patients, immunotherapists have invested considerable time and effort into finding alternative approaches to allo-HSCT that bolsters the patients’ (i.e. autologous) T cells to kill tumors and mediate curative responses.
In the context of autologous cell therapy, recent advances in gene transfer technology demonstrate that the patient’s T cells can be engineered with a chimeric antigen receptor (CAR) to recognize tumor antigen CD19 expressed on malignant cells (7-9). With the adoptive T-cell transfer (ACT) approach, these cells are expanded to large numbers and then infused into the patient. Such therapies have unlocked treatment outcomes of unprecedented efficacy (10-12). One example of the potency of this approach was reported in a landmark clinical trial by a research team at the University of Pennsylvania (13). In this trial, two children with refractory leukemia were treated with autologous anti-CD19 CAR T cells. Both children experienced a complete remission of their disease, and, remarkably, one child remains tumor free more than two years later.
Results from this clinical trial were striking. The lead investigator of this trial, Carl June, described the captured the importance of this finding in a film where he states: ‘It was like the calm after the storm: the clouds went away and she [Emma Whitehead] woke up and there was no leukemia. When that child survived, it was of course, an amazing event (film Fire with Fire directed by Ross Kauffman: http://vimeo.com/54668275).’ Investigators at the National Cancer Institute and Memorial Sloan
Kettering Cancer Center have reported similarly ‘amazing [therapeutic] events’ in their adult and pediatric patients treated with anti-CD19 CAR T cells (8, 9). Consequently, this approach has quickly gaining momentum. Several academic centers across the world have opened clinical trials to treat leukemia and lymphoma patients with CAR T cells (14, https://clinicaltrials.gov).
In addition to the above-mentioned cellular therapies, a number of other exciting new immunotherapeutic approaches has emerged as promising in recent clinical trials. Physicians have reported robust tumor regression after patients were treated with a very low dose of bispecific antibodies that link the cancer to endogenous effector T cells (15). Also, patients with advanced melanoma experienced noteworthy antitumor responses after treatment with a combination of two antibodies that block co-inhibitory molecules PD-1 (nivolumab) and CTLA-4 (ipilimumab) expressed on tumor-reactive T cells (16, 17). It will be critical to determine if these therapies will also improve treatment outcomes in patients with leukemia and lymphoma.
Given the promise of treatments that ignite the immune system against cancer, enthusiasm for the field has expanded dramatically. Academic centers and pharmaceutical companies across the nation have begun to invest billions in immunotherapy. Moreover, Science Magazine recognized cancer immunotherapy as the breakthrough of the year in 2013 (18). There is no question that the immune system can be exploited to destroy cancer and can yield durable responses in patients. The questions that remain are why are some immunotherapies still unable to help everyone, and what are the best approaches moving forward to treat hematologic malignancies?
Herein, we appraise the state of the art in immunotherapy with a focus on approaches that exploit the patient’s immune system to kill hematologic malignancies. We review various forms of immune-based therapies that have shown significant promise in patients: (i) conventional monoclonal therapies like rituximab, (ii) engineered monoclonal antibodies called bispecific T-cell engagers (BiTEs), (iii) monoclonal antibodies and pharmaceutical drugs that block inhibitory T-cell pathways (i.e. PD-1, CTLA-4, and IDO). We also briefly discuss the recent clinical findings with adoptive immunotherapy with T cells engineered to express chimeric antigen receptors (CARs) or T-cell receptors (TCRs). Finally, we assess the idea of using these therapies in combination and conclude by suggesting multi-prong approaches to improve treatment outcomes and curative responses in patients.
Conventional tumor antigen-specific monoclonal therapies
While results of ACT therapy with genetically re-directed CAR or TCR T cells have been encouraging, its broad utility in the treatment of hematologic malignancies is restricted by the difficulty of generating individual cellular products for each patient (19). As this technology continues to advance and academic centers and industry partners continue to invest in this approach, it is likely that this platform will expand to treat a greater population of patients (20). Conversely, monoclonal antibodies are easy to generate and can be readily exploited to treat patients with leukemia, lymphoma, and other forms of hematological malignancies.
As leukemia cells express surface antigens not expressed on normal tissue, monoclonal antibodies (mAbs) that specifically recognize tumor antigens have been widely investigated (21). The concept of using mAbs to target tumors was first proposed by Paul Ehrlich over a century ago (22). There are a number of advantageous to using this therapy to treat patients: mAbs are easy to produce as secreted proteins in mammalian cell culture, they are off-the-shelf reagents with high protein stability, and they can treat a wide range of patients with hematologic cancers (23). Most importantly, monoclonal antibodies, such as rituximab, alemtuzumab, and trastuzumab, have been widely used in patients and are reported to mediate antitumor responses in the clinic (24).
Monoclonal antibodies are exquisitely specific against their target antigen. Kohler and Milstein (25) published a proficient way of produce mAbs from hybridomas in 1975, raising hope for the development of novel antibodies to treat patients with cancer. Optimization of this platform was needed before therapeutic immunoglobulin G (IgG) molecules could be generated, and thus the first antitumor chimeric mAb against the protein CD20 called rituximab (trade names Rituxan, MabThera and Zytux) was not approved by the U.S. Food and Drug Administration (FDA) until 1997 (26). Approval of rituximab was motivated by results from a clinical trial lead by Ronald Levy and co-workers (27) in patients having B-cell non-Hodgkin’s lymphoma (B-NHL). In this historic trial, clinical remissions were observed in 17 patients (3 complete remissions and 14 partial remissions), yielding an impressive objective response rate of nearly 50%. A large number of clinical trials have repeated that finding, demonstrating that rituximab is an effective mAb treatment against a number of hematological malignancies, including large B-cell lymphoma, follicular lymphoma, and mantle cell lymphoma (28). In rare forms of lymphoma, where only a few randomized trials have been conducted, rituximab is a feasible treatment of marginal zone lymphomas, Waldenströms’ disease, Hairy cell lymphoma, human immunodeficiency virus (HIV)-associated lymphomas, and chronic lymphocytic lymphoma. For more insight the role of rituximab in hematologic malignancies, please refer to the previously published reviews (28-31).
Manipulating antibody structure for therapeutic gain
Several monoclonal antibody therapies have been approved for the treatment of hematological cancers. Although these therapies are well tolerated and can spur tumor regression, most are not able to cure the patient (26). Preclinical studies and human clinical trials have revealed many limitations of this approach: (i) redundant pathways leading to the survival of malignant cells, (ii) influences of the immunosuppressive tumor milleu and regulatory elements [e.g. myeloid derived suppressor cells (MDSCs)], (iii) suboptimal interaction with effector natural killer (NK), CD4+, and CD8+ T cells, and (iv) competition with circulating IgG molecules (32).
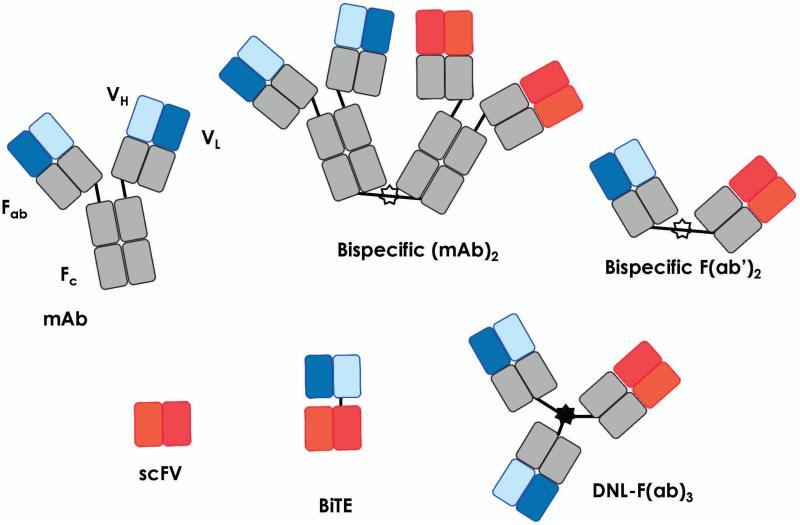
Genetics and chemical modifications to antibodies have enhanced their clinical use. The Bevan group (33) first reported in 1985 that hybrid antibodies (i.e. bispecific antibodies) could be synthesized to target cancer antigens for attack by T cells. Nearly 30 years later, a large array of novel antibodies have been created that harness T-cell-mediated antitumor immunity. Molecular engineers have generated antibody fragments that recapitulate the full binding activity of the original molecule. A functionally active small fragment—also called the single chain variable fragments (scFv)—is comprised of the heavy and light variable domains connected via a short peptide linker (consisting of about 5 amino acid residues) (34). scFv molecules have revolutionized the field and served as a platform to create a plethora of distinct bispecific antibodies. The evolution of antibody constructs is displayed in Fig. 1 and includes not only the structure of the conventional mAb but also several other chemical and synthetic constructs inspired by conventional mAbs over the years [such as bispecific (mAb)2, bispecific F(ab’)2, scFv, DNL-F(ab)3, and bispecific T-cell engagers (BiTEs)]. Greater details of these constructs and additional formats have been reviewed (26, 35, 36).
Fig. 1. Bispecific antibodies.
Bispecific antibodies were generated by genetic or chemical cross-linking of monoclonal antibodies (mAbs) or by F(ab’)2 fragments (upper row). Antibody engineering permitted the generation of small bispecific antibodies comprising the variable heavy (VH) and light (VL) domains of the original mAbs (lower row). Single-chain fragment variable (scFv); single-chain bi-specific tandem variable domain (BiTEs) and dock-and-lock trivalent Fab (DNL-(F(ab)3)). Modified figure from Stamova et al. (35).
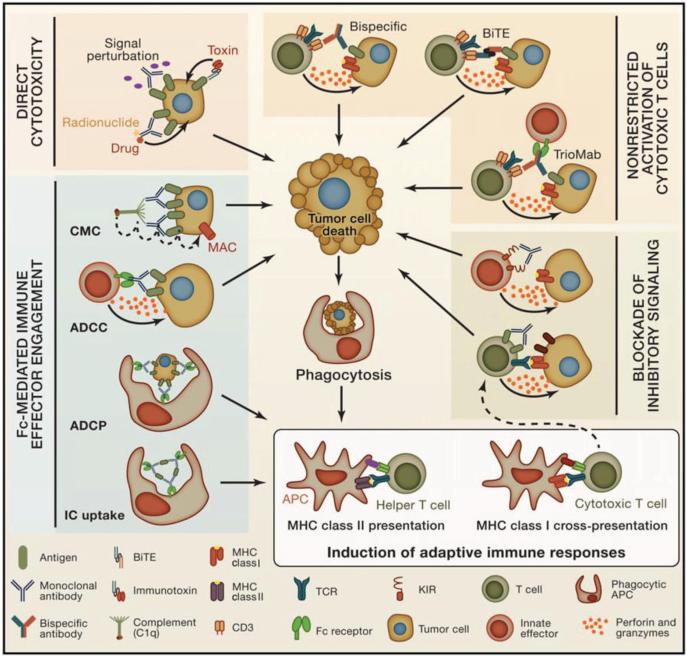
Mechanisms of tumor regression differ between conventional unconjugated monoclonal antibodies and novel bispecific antibodies. Depending on how mAbs are constructed, tumor cell death is executed via various modes of action. The mechanisms of action are schematized in Fig. 2 and include several possibilities: direct cytotoxicity on the tumor, Fc-mediated immune effector engagement, non-restricted activation of cytotoxic lymphocytes, or blockade of inhibitor signals on antitumor T cells (37, 38). While some novel mAbs have not been translated into the clinic, triomabs and BiTES have been used in a number of trials and have been reported to mediate potent antitumor responses in patients with hematologic malignancies (39).
Fig. 2. Mechanisms of action of antibody-based immunotherapy in cancer.
Mechanisms of antitumor antibody therapies are distinct and based on the antibody structure and function. Displayed are various strategies for antibody mediated tumor death. Upper left: direct cytotoxicity, in which mAbs induce cytotoxicity directly to the tumors by trumping signaling pathways or in which immune-conjugates kill targeted cells. Lower left: FcReceptor-mediated immune effector engagement, in which the Fc portion of mAbs engage immune effector functions, including soluble CMC (through the membrane attack complex MAC) as well as NK cells, macrophages, and dendritic cells, through FcRs, allowing for ADCC, ADCP, and IC uptake. Upper right: Non-restricted activation of cytotoxic T cells, in which tumor-infiltrating CTLs can be activated against tumor cells—independent of T cell receptor (TCR) specificity—by engaging co-receptors on the T cells and tumor antigens. Lower right: blockade of inhibitory signaling, in which cytotoxic lymphocytes, including NK cells and CTLs, express inhibitory receptors for various ligands (such as PD-L1) that may be expressed by tumor cells. Antagonistic antibodies that target these inhibitory receptors can block ligand-receptor interactions so that targeted cytotoxicity can ensue (such as the FDA approved CTLA-4 and PD-1 blocking antibodies). These four strategies enhance tumor cell death, which can promote phagocytosis of tumor cell antigens, and induction of adaptive immune responses (bottom right) in two ways: MHC class I cross-presentation and priming of cytotoxic T cells and MHC class II presentation and priming of helper T cells. These adaptive immune responses can enhance antitumor immunity. Figure reprinted from Cell, Volume 128, pages 1081-1084, Weiner LM, Murray JC, Shuptrine CW, 'Antibody-based immunotherapy of cancer'. Copyright 2012, with permission from Elsevier (38).
Bispecific T-cell engaging antibodies
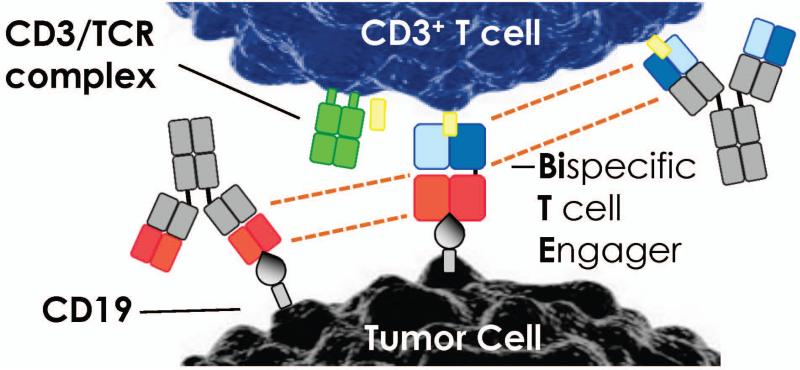
Clinical trials suggest that BiTEs are promising for treating patients with hematologic malignancies (40). These artificial antibodies transiently crosslink T cells with tumors (41). BiTEs are comprised of minimal binding domains of two distinct antibodies via a short peptide (42)(Fig. 3). Even when peptide linked, these two antibodies retain their capacity to bind their respective antigens.. BiTEs derived from antibodies differ in both size and specificity for the antigen recognized on the lymphocyte and on the tumor. Most researchers use antibodies against the ε chain of the CD3 complex to target host T cells (33, 43-46), as all lymphocytes express CD3 and its engagement activates them. A short flexible linker fuses the two single chain fragments in the BiTEs, which likely permit their rotation. This design may be important for mediating T-cell activation, as it permits the tight interaction with target epitopes on two different cells and ensures proximity of the T cells to the tumor (Fig. 3).
Fig. 3. Construction of a BiTE.
The variable domains of two monoclonal antibodies recognizing either tumor or T cell are genetically linked, as indicated by dotted lines. A single polypeptide chain is produced in which two single-chain antibodies are flexibly linked (BiTE). This small molecule tethers the tumor cells to the T cells by the tumor-associated antigen (TAA-in this cased CD19 expressed on hematologic malignancies) to the CD3 antigen on T cells.
BiTEs have many properties that make them clinically appealing. They redirect cancer cell death via T cells at sub-picomolar concentrations, activate host T cells, and drive a T cell’s ability to serially kill tumor cells in vivo (47). This chain of events leads to the sustained activation, proliferation, and cytotoxicity of T cells as long as target cells are available. Additional investigation revealed that BiTEs possess an enhanced efficacy against cancer cells at low T-cell numbers without requisite costimulation (48). This is an attractive feature of BiTEs, as T cells, particularly CAR T cells (49), require costimulation to optimally function and clear tumors. Endogenous T cells can be at a resting state and still kill tumors in the presence of BiTEs, underscoring the finding that T-cell pre-activation regimens are not required to ignite BiTE-mediated antitumor immunity. However, BiTEs are target cell-dependent and mediated by polyclonal lymphocytes; therefore, they do not drive off-target immune response to self. In fact, of significant clinical importance, no T-cell-mediated autoimmune disorders have been observed with BiTE therapies in preclinical models or in the clinical setting (15, 50, 51).
Clinical experience with BiTEs for treating hematologic malignancies
Conventional monoclonal antibodies do not directly recruit T cells to the tumor. In contrast, synthetic BiTEs, due to their engineered structure, incite the homing of cytotoxic lymphocytes to the malignancy. The first BiTE in clinical trials, called blinatumomab (or MT103), transiently tethers resting T cells to tumors via a bispecific CD3/CD19 construct (15). This construct targets CD3+ T cells to CD19-expressing cells, which include both hematologic malignancies and normal B cells. In preclinical work, sub-microgram concentrations of this drug were adequate to prevent of growth aggressive leukemia in a xenograft mouse model (51). Moreover, studies in chimpanzees showed that frequent treatment (every 2 h) with a low dose (0.1 μg/kg) of this molecule were well tolerated and completely ablated normal peripheral B cells (52). Collectively, these preclinical data were encouraging, as they suggested that blinatumomab might not only be safe but also effective in treating patients with CD19-positive malignancies.
In 2008, Bargou and colleagues (15) reported the first results of a Phase I clinical trial indicating that doses of blinatumomab as low as 5 μg/m2/day eliminate malignant cells in the blood of patients with relapsed B-NHL. All seven patients in the study who received a continuous infusion of BiTEs at 6 μg/m2/day had objective tumor regression, including five partial responses and two complete responses with remarkable clearance of tumor from multiple organs. In all responding patients, the majority of tumor shrinkage occurred within the first month of treatment, and the longest duration of curative responses was 13 months. Three more patients experienced tumor regression greater than 6 months. Due to the small size of this BiTE (60 kDa), it has a half-life in the blood of only several hours. Thus, constant infusion by a portable mini-pump is needed for treating the patient. Even with multiple rounds of treatment, the effective dose was five orders of magnitude lower than reported effective doses of the CD19-specific standard-of-care, rituximab (375 mg/m2, intravenously, every 8 weeks, with a maximum of 12 doses) (53). While no dose-limiting cytokine release syndrome was observed in patients, adverse events included confusion, tremor, focal neurologic deficits, and seizures (these manifestations were all fully reversible). As mentioned previously, no autoimmune manifestations were observed in these patients, but the treatment depleted healthy CD19-expressing normal B cells.
The therapeutic utility of blinatumomab was also reported in a Phase II clinical trial in patients with B-precursor acute lymphoblastic leukemia having minimal residual disease (MRD) in their bone marrow (50). The results from this trial indicated that the T-cell engager was able to kill rare disseminated tumor cells, which could only be detected by quantitative polymerase chain reaction (PCR) assays in relapse patients. Among 20 adult patients, blinatumomab mediated potent responses in 81% of patients, as they were MRD negative with a relapse-fee survival rate of 61% after a median follow-up of 33 months. Moreover, this therapy is highly efficacious even in situations with larger tumor burden, as evidenced by preliminary data showing that 26 or 36 adults with relapse/refractory acute lymphoblastic leukemia (ALL) achieved complete remission with regained hematological recovery. Importantly, this work also suggests that BiTEs are non-cross resistant to commonly used forms of chemotherapy and could be effective in otherwise treatment refractory patients with aggressive forms of leukemia and lymphomas. Collectively, this was an amazing finding, since MDR+ ALL patients have few treatment options and more than half die within 2 years.
Given the excitement of the results from the blinatumomab trial, it is not surprising that other BiTEs have emerged in the pipeline. Another BiTE, MT110, was tested in patients is in 2009. The construct targets host lymphocytes to epithelial cell adhesion molecule (EpCAM) on a wide array of solid tumors in patients with lung, gastric, and colorectal cancers (54). Phase I study results show disease stabilization in 7 of 19 patients following infusion of low doses of MT110. Evaluation of the therapy at higher doses is ongoing. Recent preclinical work suggests that this BiTE might also be effective in treating other aggressive forms of cancer, as MT110 was found to eliminate primary human pancreatic cancer stem cells and hepatocellular carcinoma cells in humanized xenograft models (55, 56).
New BiTE constructs have been engineered to target several tumor antigens. These targets include CD19, EpCAM, Her2/neu, PSMA, EGFRvIII, CEA, EphA2, MCSP, folate receptor, and CD33. For more insight into these exciting novel constructs, please refer to the previously published reports (57-64).
The first BiTE for the treatment of patients with acute myeloid leukemia (AML) is the CD33/CD3 construct called AMG 330 (65). Myeloid marker CD33 is an attractive target, as it is expressed on greater than 90% of AML cells (66) and preclinical studies reveal that AMG 330 destroys human AML cells in the presence of T cells from patients. Of clinical relevance, epigenetic modifier drugs (such as azacitidine) were found to increase CD33 on some AML cells and, in turn, augmented AMG 330-induced cytotoxicity. These findings demonstrate that AMG 330 has potent cytolytic activity, which may be further enhanced with clinically available therapeutics (67, 68). It will be exciting to determine if AMG 330 improves treatment outcomes in AML patients.
What are the mechanisms by which BiTEs mediate tumor immunity?
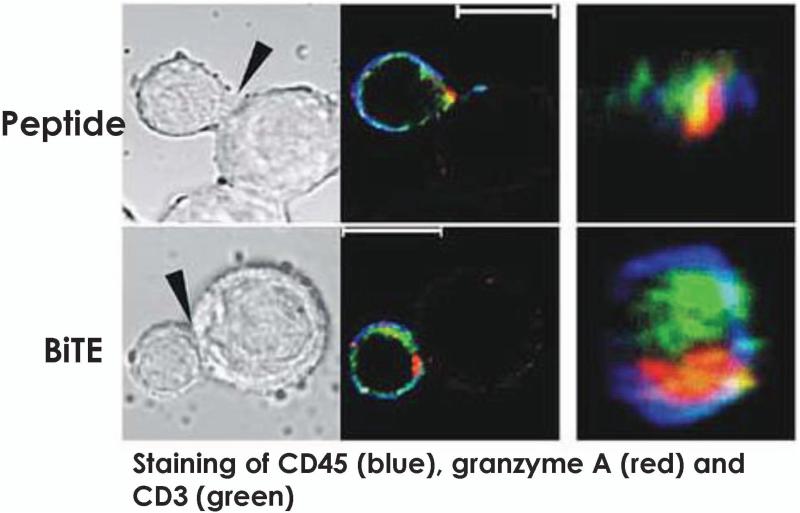
One reason BiTEs mediate robust T-cell activation is that they induce immunological synapses between lymphocytes and malignant cells that are nearly identical in composition and size from native cytolytic synapses (69). Offner and colleagues (69) used confocal microscopy (Fig. 4) to visualize the potency of BiTEs in triggering immunological synapses in lymphocytes. Following antigen-specific BiTE-mediated activation, apoptosis occurs via perforin-mediated membrane disruption of the cancer by the T cells (70). Perforin punches holes in the membrane of cancer cells, enabling the entry of the granzymes that trigger tumor death by igniting caspase pathways. Along with their elevated cytotoxicity, T cells activated by BiTEs secrete a large array of cytokines, including IL-2, TNF-α, and IFN-γ, which in turn, further bolster their antitumor effector function. Additional investigation reveals that the BiTE activated T cells express activation markers CD69 and CD25 on their surface (71), which may augment their persistence in vivo.
Fig. 4. Confocal imaging of T cell to target cell via BiTEs.
Staining of CD45 (blue), granzyme A (red) and CD3 (green). Results on synapse formation are depicted in this picture. The upper image depict synapses induced by erbB2 peptide while the lower rows show synapses formed in the presence of BiTE. The left columns show the cell conjugates in the differential interference contrast mode; the middle columns show confocal images of immunofluorescence staining of the same cell conjugates. The white bars represent 10 μm. The right columns show the synapse interface. Image reproduced from Molecular Immunology, Volume 43, pages 763-771, Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA, 'Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells,' Copyright 2006, with permission from Elsevier (69).
BiTEs initiate lytic synapses in the presence of tumor antigen. Robust T-cell responses are possible even against tumors that do not express MHC class I. This is important given that tumors avoid immune detection via this mechanism (72). BiTEs have also been shown to revive dysfunctional T cells, which could be exploited to improve the effectiveness of antigen-exhausted tumor-infiltrating lymphocytes (TILs) in cellular therapy approaches (73). Furthermore, video-assisted microscopy showed that BiTEs sustain repeated rounds of serial lysis of the tumor cells, which may explain their effectiveness in patients (74).
As mentioned earlier, BiTEs activate lymphocyte independent of costimulation (48). This is surprising given that T cells need TCR engagement (signal 1) and costimulation (signal 2) to undergo optimal activation, function, and expansion (75). However, there exist at least two reasons why BiTEs thrive independent of signal 2. One theory is that malignant cells may express sufficient amounts of B7 and ICOS ligand on their surface, thus permitting co-signaling of the T cell via the CD28 costimulatory family (76). The other theory is that memory T cells, which do not require costimulation during recall responses, mediate the majority of tumor killing by BiTEs (77). Thus, CD28 ligation may only amplify TCR signaling rather than exciting a unique array of signaling pathways. Thus, if the signaling threshold for activation in different memory T-cell populations varies, triggering the TCR-CD3 pathway via BiTEs might be enough to drive activation without cosignaling.
BiTE therapy not only activates effector T cells via CD3 signaling but also Tregs, which may impair treatment efficacy (78). However, it is also possible that Tregs convert into cytotoxic lymphocytes via cross-linkage with tumor via the BiTE format. A report by Choi et al. (79) supports this idea, as they surprisingly found that Treg redirection via BiTEs regressed glioblastoma multiforme in mice. These BiTEs targeted a mutated form of epidermal growth factor receptor (EGRFvIII) and redirected Tregs via CD3 signaling to kill glioblastoma via the granzyme-perforin pathway. Although this finding in mice is interesting, the mechanisms by which BiTEs potentiate the antitumor activity of human Tregs in patients remains incompletely elucidated. For that matter, it would be worth understanding how BiTEs also shape the generation of other immune cells in the body, as altering the biology of T cells will impact the balance and function of MDSCs and NK cells. In humans, if BiTEs increase the suppressive properties of Treg cells, it would be logical to ablate them. Indeed, in melanoma mouse models, CTLA-4 blockade was found to augment effector CD8+ T cells by depleting Tregs via Fcγ-macrophage mediated events (80). Consequently, if Treg cells impair tumor immunity in patients treated with BiTE therapies, it will be worthwhile to use CTLA-4 blocking antibody imilumomab to license BiTE-activated T cells to lyse tumor to a greater extent.
Immune checkpoint blockers for the treatment of hematologic malignancies
Attenuating co-inhibitory molecules for the treatment of cancer has garnered attention as an immunotherapy strategy due to recent clinical success (81-84). An interaction between a T cell and an antigen-presenting cell (APC) through the TCR-antigen/MHC complex results in both costimulatory and co-inhibitory signals occurring simultaneously. The balance between these signals ultimately affects the overall activation and function of T cells. Costimulation typically involves the interaction of B7 with CD28 and is negatively impacted by the presence of the co-inhibitory molecule CTLA-4 on the surface of T cells (85, 86). Allison and colleagues (87) were the first to report that blocking CTLA-4 induces a potent antitumor immune response in mice with melanoma. This group has published a series of elegant papers on the mechanisms underlying the effectiveness of CTLA-4 inhibitors (88, 89). This body of work pioneered clinical investigations that eventually resulted in a phase III trial that blocked CTLA-4 using an anti-CTLA-4 mAb, where it was shown to improve the overall survival in patients with metastatic melanoma compared to patients receiving a tumor vaccine (84). CTLA-4 blockade extended the patient’s life, on average, by 4 months. Immune-related adverse events (IRAEs) have been observed in patients after CTLA-4 blockade (90); the most common IRAEs are rash, uveitis, colitis and hepatitis (91, 92). Interestingly, IRAEs appear to be associated with positive outcomes such as tumor regression and prolonged time to relapse (93). This trial paved the way for ipilimumab (an anti-CTLA-4 antibody therapy) to be approved by the FDA in March 2011 as an anti-melanoma therapy (94). Today, investigators are exploring the role of ipilimumab in patients with hematologic malignancies, as discussed immediately below.
An open and accruing Phase 1/1b clinical trial is now testing whether ipilimumab might rescue the immune response in patients with hematologic malignancies that have relapsed following allogeneic hematopoietic stem cell transplants (http://clinicaltrials.gov/show/NCT01822509). The motivation behind this trial is based on a promising pilot study in 29 patients with hematologic malignancies who relapsed after A-HSC transplantation. These patients were treated with a single, low dose of ipilimumab (0.1-3 mg/kg), which did not drive significant GVHD and was well tolerated in these patients (95). Importantly, three patients with lymphoid malignancies experienced an objective tumor response. Moving forward, the primary objectives of the open Phase 1/1b trial are to determine the maximum tolerated dose and to characterize toxicity in patients with a wide array of hematologic malignancies. As the therapeutic dose of ipilimumab is 10 mg/kg for patients with melanoma, which is 100-fold more drug than what was used in the pilot study, this new open trial will be important for shedding light on the optimal therapeutic yet safe dose that can be used in these patients.
The dynamic duo: CTLA-4 plus PD-1 blockers
T cells express a number of other co-inhibitory molecules on their cell surface besides CTLA-4, including PD-1, LAG-3, BTLA-4, CD160, and TIM-3 (96-100). Like CTLA-4 blockade, PD-1 inhibition was found to mediate clinical activity in a variety of cancers, showing durable responses in a proportion of patients who failed other therapies (81-84). With regards to toxicity, the overall incidence of adverse events with probable immune etiology was not much different in the anti-PD-1 study than the ipilimumab studies but that the toxicity did appear less severe in the majority of patients (101). In September 2014, the FDA approved a new PD-1 inhibitor, pembrolizumab (Keytruda®, Merck). This approval is timely, given recent reports that sought to address the question of whether blocking both inhibitors would be synergistic in patients. The results of two distinct phase 1 clinical trials were therapeutically impressive (16, 82). Both reports revealed that the combination of PD-1 and CTLA-4 blocking antibodies augmented treatment outcomes in melanoma patients (16, 82). Importantly, these patients did not experience an escalation of toxic effects often associated with improved treatment outcome. The results of these two trials were not only complementary but also quite auspicious. In the trial by Hamid et al. (82), a blocking PD-1 monoclonal antibody was given to patients who relapsed after monotherapy with ipilimumab (CTLA-4 antibody). Durable responses were as potent as those observed in patients who had not received ipilimumab therapy. These findings are notable, as they underscore the concept that tumor progression after anti–CTLA-4 therapy reduces the possibility of a positive antitumor response by anti–PD-1 therapy. Wolchok and colleagues (16) also tested concomitant administration of these two blockers in patients. At the maximum tolerated dose, more than half of the melanoma patients experienced objective tumor responses. Moreover, a remarkable shrinkage of 80% of the total tumor was observed in these responding patients. Unexpectedly, regardless of how PD-1 and CTLA-4 antibodies were given, no higher levels of toxicity were observed in those patients compared to patients that were only treated with individual drugs.
PD-1 and CTLA-4 negatively regulate T-cell activation. When ligated, they induce signaling pathways that trump lymphocyte activation (102). Thus, these inhibitors are often termed immune checkpoint blockers, as they permit T-cell activation in the tumor microenvironment (103). It is essential to understand the mechanism by which these antibodies operate and to identify the immune cells regulated by these promising therapeutic antibodies. Tumor-specific T cells can express CTLA-4 and PD-1, and antigen-presenting cells express the ligands that engage them (CD80, CD86, CD273 and CD274) (104). Moreover, the PD-1 and CTLA-4 signaling cascades use distinct ways to block the activation of T cells and thus blocking that signal profoundly potentiates T cell-mediated expansion and antitumor activity (105). There are a number of mechanisms that might explain how combining these drugs are working together. One way that CTLA-4 and PD-1 blockers might synergize is by concomitantly engaging the same cell, tipping the scale for T-cell costimulation and enhancing T-cell-mediated regression of tumors. Alternatively, it is possible that PD-1 and CTLA-4 antibodies modulate the function and expansion of distinct CD4+ helper or CD8+ memory subsets that work together to eradicate tumors. For instances, PD-1 antibody blockade has been reported to revive the function of exhausted CD8+ T cells (106). On the other hand, CTLA-4 antibodies may deplete suppressive Tregs that impair the antitumor activity of CD8+ T cells (107, 108). Simpson and coworkers (80) found that blocking the CTLA-4 pathway reduced the activity of Treg cells. If so, then restoring exhausted CD8+ T cells via PD-1 blockade while reducing Treg activity via CTLA-4 antibody should exponentially bolster the tumor immunity. Understanding the underlying mechanisms by which these antibodies cooperate to mediate antitumor immunity may shed light on how these treatments synergize to promote robust antitumor immunity.
Results from these combination checkpoint blockade studies have fueled enthusiasm for its use in other diseases. A clinical trial with this combination approach is open and recruiting patients to treat various types of hematological malignancies (http://clinicaltrials.gov/show/NCT01592370). The success of this combination therapy between CTLA-4/PD-1 inhibitors has also sparked the interest of investigators to create the ‘optimal recipes’ of stimulators and inhibitors to destroy tumor: such ‘ingredients’ include molecularly targeted therapies (JAK/STAT inhibitors and BRAF inhibitors), chemotherapeutics (oral drugs or radiation strategies), or other immune-modulating therapies (LAG-3 inhibitors, ICOS agonists, CD40 agonists, vaccines, cytokines, indolamine 2–3 dioxygenase inhibitors or genetically redirected T cells). Decoding the optimal recipe for therapeutic success is a major goal of the field.
IDO for the treatment of hematological malignancies
Another therapeutic approach involving the attenuation of immunosuppressive effects is centered around the compelling work pioneered by Mellor and Munn, who found that inhibiting indoleamine 2,3-dioxygenase (IDO) breaks T-cell tolerance to self/tumor tissue. Interestingly, this field of research was inspired by the investigators’ basic findings that trypotophan catabolism (via IDO) prevents allogeneic fetal rejection (109). Thus, it is not a surprise that IDO has a protective and immunosuppressive effect on the immune system. Additional reports revealed that IDO is an intracellular enzyme that is highly expressed in myeloid cells and some tumor cells, where it catalyzes the degradation of the essential amino acid tryptophan (110). Since this discovery, from nearly 20 years ago, many studies have linked IDO overexpression to the stage of progression in hematological malignancies and in various types of solid tumors (such as ovarian carcinoma, hepatocellular carcinoma, invasive cervical carcinoma, non–small cell lung carcinoma, colon carcinoma, and endometrial carcinoma) (111).
IDO dampens immunity to danger signals, as IDO expression is correlated with an increase in IFN-γ, IFN-α, CpG ODNs, and 4-1BB (112-115). Importantly, tumor-draining lymph nodes contain IDO-expressing DCs that polarize naive T cells to a regulatory phenotype resulting in a tolerizing rather than immunizing milieu (116-118). Tryptophan starvation reduced cell growth—not only in monocyte-derived DCs and T cells—but also by tumor cells. Therefore, tumor cells are able to balance the ability to thwart immune attack with its own metabolic status (119).
Breaking tolerance by inhibiting the immunosuppressive effects of IDO has become an appealing therapy, as studies show that patients with poor survival have IDO overexpressing hematological malignancies (120). Specifically, IDO expression has been reported in AML, ATLL and CLL patients (121-123). 1-methyl-D,L-tryptophan (1-MT) is the most widely examined IDO inhibitory drug and has been shown to synergize with the effects of cyclophosphamide in a murine breast cancer model (124). Several IDO inhibitors are being studied but are only capable of reducing the immunosuppressive effects of IDO (125), suggesting that a more potent candidate molecule may be required to mediate durable antitumor immunity. Despite this, numerous active clinical studies are underway, most of which are targeting solid tumors. An IDO1 inhibitor is now being tested in a phase II clinical study for myelodysplastic syndrome (http://clinicaltrials.gov/show/NCT01822691). Additionally, IDO-specific CD8+ T cells have been reported to boost T-cell immunity against viral and tumor-associated antigens by directly killing IDO-expressing Treg cells ex vivo. This immunological event had a profound effect on the balance of IL-17 producing Th17 and Treg cells (126). These findings have inspired a phase I clinical trial in which an IDO-specific peptide vaccine was given to patients with non-small cell lung carcinoma. Based on recent findings that co-inhibitory CTLA-4 blocker increase IDO expression in tumor-bearing hosts and that its concomitant inhibition potentiates T-cell-mediated tumor immunity in mice with melanoma (127), it is possible that combining IDO with CTLA-4 and/or PD-1 blocking therapies will bolster the antitumor activity of T cells in patients with leukemia, lymphomas, or other types of hematological malignancies. However, the design and execution of a clinical trial focused on this therapeutic ménage à trois of CTLA-4/ PD-L1/IDO inhibition represents a reasonable and timely approach (128), given the availability of all three reagents for use in the clinical setting.
Adoptive T-cell transfer therapy for hematologic malignancies
One of the most promising strategies for treating patients with leukemia in recent years involves the use of genetically re-directed autologous lymphocytes for ACT therapy (129-131). The ACT approach entails ex vivo selection and expansion of tumor-infiltrating lymphocytes for reinfusion into the tumor-bearing hosts. Engineering T cells for ACT therapy allows for the treatment of a broader range of malignancies and a wider patient population. Patients with hematological malignancies have benefitted wildly from this treatment. As mentioned earlier, techniques of gene therapy offer new possibilities to transduce autologous polyclonal T cells with CAR or TCR specificity for relevant tumor antigens, such as CD19, CD20, CD33, and many more. There exist numerous advantageous with the use of gene transfer technology to modify T cells including the ability to select distinct T-cell subsets for infusion that have been identified as promising in clinically relevant models of cancer. Indeed, type 1 and type 17 cells (helper CD4+ as well as cytotoxic CD8+ T cells) have been reported to mediate potent tumor immunity in murine and humanized models of solid tumors (such as melanoma and mesothelioma) (132-138). Moreover, CD8+ and CD4+ T cells with stem cell-like properties or with a central memory phenotype can be redirected and, based on clinically relevant murine and humanized tumor models, may mediate tumor immunity to a far greater extent than effector memory lymphocytes (139-142). Another advantage of this approach is that unlike monoclonal antibodies or BiTEs, cells are a personalized ‘living therapy’ capable of expanding and persisting for the life of the patient.
Allogeneic stem cell transplantation for hematologic malignancies
Allo-HSCT, as mentioned earlier, is a powerful approach for treating hematologic malignancies (1, 2). Alloreactive donor T cells play a key role in killing the patients’ leukemia; however, they also target normal epithelial tissues. This therapy mediates both desired (graft-versus-leukemia) and adverse (graft-versus-host disease) effects in patients (3-5). These patients are also at high risk for viral illness, particularly from reactivation of chronic viruses such as cytomegalovirus (CMV), Epstein-Barr virus (EBV), and human herpesviruses (143). Although pharmacologic treatments for these viruses are available, they often have limited efficacy, must be administered repeatedly, and have side effects. For these reasons, several transplant centers have produced donor-derived virus-specific T cells that can be administered as a donor lymphocyte infusion (DLI) (144, 145). Some centers have developed ‘third-party’ T-cell banks derived from a panel of donors selected to span the most common HLA alleles (146). The group at Baylor first initiated the use of T-cell lines specific for 3-5 viruses simultaneously and has infused these cells to patients (147-149). They found that the incidence and severity of GVHD was tolerable in all these studies. Collectively, these forms of cell therapy are the most clinically advanced, with publication of phase II multicenter trials (146).
There has recently been an increased interest in using autologous T cells that have been engineered with CARs to treat hematologic malignancies. The desire for using the patient’s own T cells to kill cancer is inspired by significant preclinical and clinical work by the Restifo (150) and Rosenberg (151) groups at the National Cancer Institute, who have reported long ago the power of ACT transfer with both naturally arising or genetically redirect autologous T cells (with TCRs and CARs) in patients with metastatic melanoma. This strategy mediates robust and long lasting immunity in some patients, and in contrast to patients treated with third party donor cells, avoids the risk of GVHD. A full discussion of these important studies is beyond the scope of this review but can be found elsewhere (130, 152).
Autologous T cells and chimeric antigen receptors
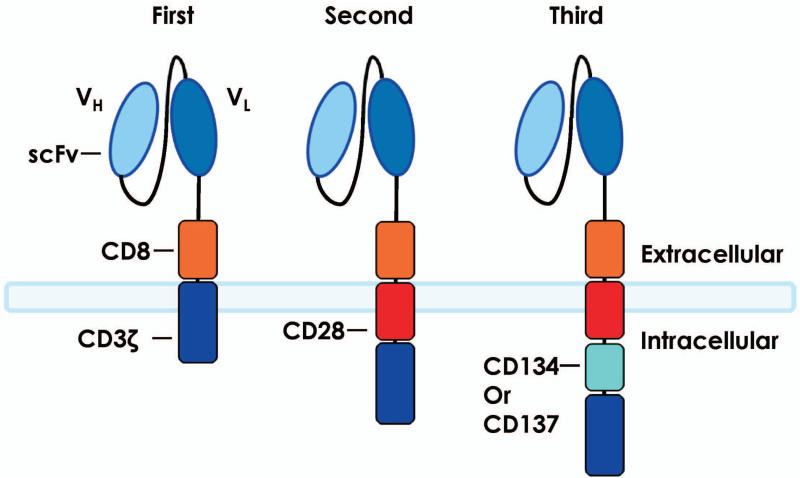
Genetically modifying T cells to express synthetic CARs are an attractive strategy for treating patients with advanced malignancies (150, 151, 153-155). Zelig Eshhar and co-workers (156) pioneered the first generation CAR T cells in 1989. As shown in Fig. 5, these CARs usually consisted of the single-chain variable fragment of an antibody specific for tumor antigen linked to the transmembrane and extracellular signaling domains via CD3ζ or FcRγ. Their objective with CARs was to generate alternative ways beyond TCR/MHC-1 signaling by which T cells can engage antigens expressed by target cells. Thus, in contrast to TCRs, CARs are cytotoxic to antigen in an MHC-unrestricted fashion and secreted IL-2 in an antigen-specific manner (156). This work formed the foundation to build next generation CAR T cells. CARs are a particularly attractive approach, as they resist classic mechanisms of tumor immune-evasion by MHC downregulation and/or the failure to process and present peptide antigens (129).
Fig. 5. T cells redirected to recognize and kill tumors.
Gene transfer can be used to engineer T cells to express CARs that target antigens in an MHC-independent manner. CARs are fusion proteins composed of an extracellular portion that is usually derived from an antibody and intracellular signaling modules derived from T cell signaling proteins. First-generation CARs contain CD3ζ, whereas second-generation CARs possess a costimulatory endodomain (e.g. CD28 or 4-1BB) fused to CD3ζ. Third-generation CARs consist of two costimulatory endodomains linked to CD3ζ. Abbreviations: CAR, chimeric antigen receptor; MHC, major histocompatibility complex; scFv, single-chain variable fragment; TCR, T-cell receptor.
While first generation CAR T cells are cytotoxic in vitro, they are unable to become fully activated and drive memory responses to the tumor in vivo. Thus, in retrospect, it is not surprising that the clinical efficacy of first generation CARs was modest in patients with various types of cancer, including lymphoma, ovarian cancer, and renal carcinoma (157-159). When one considers that tumors often express nominal levels of costimulatory ligands on their surface, it also makes sense that engagement of first generation CARs leads to T-cell anergy and the failure to persist (99). Thus, investigators synthesized second generation CARs that incorporated costimulation into the Fc-CD3ζ construct. While most work with second generation CARs involved those that express the classic costimulatory molecule CD28 (depicted in Fig. 4), investigators have expanded their toolkit to induce other types of costimulatory molecules into CAR constructs, such as OX40, 41BBL, or ICOS (136, 160).
Second-generation CAR T cells resulted in potent expansion and persistence of anti-tumor T cells both in vitro and in vivo (161). Third generation CARs include a combination of multiple costimulatory ectodomains, such as incorporating both CD28 and 41BBL into the Fc-CD3ζ construct (162, 163). Fig. 5 summarizes the relative history and construction of first, second, and third generation CARs. The possibilities for developing various types of CAR T cells is limited only by the imagination, as they could be constructed in a number of ways to be more effective in vivo, including with the ability to secrete cytokines, monoclonal antibodies, checkpoint inhibitors, and even BiTES or to express receptors that improve their ability to consume cytokines (CD25, CD127, etc.) or traffic to tumors (CCR7, CCR6, etc.).
CARs in clinical trials for hematological malignancies
The most promising results from gene therapy with CARs have been with CD19-based targeting of B-cell malignancies. CD19 is a B-lineage surface antigen that is expressed by certain lymphoid cancers as well as normal mature B cells. The Surgery Branch at the NIH (8) reported that patients with a B-cell malignancy successfully treated using an anti-CD19 CAR expressing CD3ζ and CD28 downstream of antigen recognition. This patient received two infusions of CAR T cells and has an ongoing response 4 years later. Additional patients were treated with this protocol at the NIH. Six of the eight individuals with B-cell lymphoma or chronic lymphocytic leukemia experienced clinical responses. One patient in this cohort had a complete response and at the time of publication (164), three partial responses were ongoing with 7-18 months follow-up, and the complete response was ongoing with 15 months of follow-up. While patients experienced prolonged B-cell depletion, the more difficult acute toxicities to manage were related to elevated serum cytokine levels and included hypotension, fevers, fatigue, and renal failure. Importantly, this group has taken measures in reducing these toxicities by discontinuing adjuvant IL-2 and reducing the cell dose.
Likewise, the translational research program at the University of Pennsylvania reported dramatic tumor regression and cytokine-related toxicities following administration of anti-CD19 CARs in three patients with CLL. In this trial, the anti-CD19 CAR expressed CD3ζ and 4-1BB in the extracellular domains, whereas the NCI used CD28 instead of 4-1BB. They University of Pennsylvania group (7, 12) found that all patients experienced objective tumor responses. Elevated serum cytokine levels and a febrile syndrome associated with dyspnea or hypotension was noted in two patients. One patient developed fevers, constitutional symptoms, and cardiac dysfunction requiring corticosteroid administration. Similar to the NIH study, all toxicities resolved over time.
In one study of adult ALL by the group at the Memorial Sloan-Kettering Cancer Center (9), two patients with bone marrow blasts and two with MRD were treated with infusion of T cells transduced with an anti-CD19 CAR; all experienced MRD− status and went on to allo-HSCT. Elevated cytokine levels and cytokine-related toxicities were noted consistently, and two patients developed relative hypotension and mental status changes requiring high-dose corticosteroids. Two pediatric patients with ALL were also treated with CD19-CAR therapy at Children’s Hospital of Pennsylvania (13). Remarkably, complete disease remission occurred in both patients with aggressive refractory disease. One response remains ongoing today, nearly two years later. However, the other lasted only 2 months and was associated with recurrence of CD19-negative disease. Both patients experienced B-cell aplasia and cytokine release syndrome; one required cytokine blockade with etanercept (anti-TNF-α) and tocilizumab (anti-IL-6) to dampen the cytokine-related toxicities. To date, the University of Pennsylvania group has found that the curative response rates are 90% in the first 30 ALL patients treated with CAR T cells that incorporate CD3ζ and 41BB downstream of CD19 recognition (June C, et al., NEJM in press, Carl June, personal communication).
The NIH group (165) also found recently that chemotherapy-refractory diffuse large B-cell lymphoma (DLBCL) and indolent B-cell malignancies can be treated with autologous anti-CD19 CARs. Of the 15 patients treated, eight achieved complete remissions, four achieved partial remissions, one had stable disease, and two were not evaluable for response. CRs were obtained by four of seven evaluable patients with chemotherapy-refractory DLBCL; three of these four CRs are ongoing, with durations ranging from 9-22 months. Acute toxicities including fever, hypotension, delirium, and other neurologic toxicities occurred in some patients after infusion of anti-CD19 CAR T cells. These toxicities resolved within 3 weeks. One patient died suddenly as a result of an unknown cause 16 days after cell infusion.
From the early clinical studies at the University of Pennsylvania, NIH and Memorial Sloan-Kettering Cancer Center, it is clear that T-cell therapy with anti-CD19 CARs appears to have robust clinical activity against a variety of malignancies. Treatment is associated with transient but frequently severe adverse events related to elevated serum cytokine levels. However, given the morbidity and mortality of alternative treatments, including allogeneic HSCT and of refractory lymphoma/leukemia, these transient toxicities may be justified, particularly in the setting of more aggressive cancers.
In contrast to the aforementioned monoclonal antibodies and immune checkpoint blockers, cellular therapies are not yet approved by the FDA. Consequently, these therapies are available in few locations around the world. A hurdle of cell therapy is their expense, and that the treatments require specialized facilities and highly trained researchers and clinicians. Yet, improvements in translating these therapies into the clinic have emerged with advances in isolating T cells from the patients and effective ways to expand these T cells with artificial antigen presenting cells and culture techniques. Similar to work with the now widely used A-HSCT approach, it is possible that blood banks could generate tumor-specific T cells for clinical use or that T cells could be mass-produced in a central facility. Finally, state-of-the-art technology could be used to ablate MHC molecules on allogeneic T cells (which could reduced their immunogenicity against self-tissue) and then be redirect with CARs to recognize and kill tumors in a wide variety of patients. Given the potency of this approach, it will be paramount to find ways to broaden the use of cell therapies for all patients. A comprehensive review of CAR therapies for hematologic malignancies can be found in this issue of Immunological Reviews.
Future combination immunotherapies to bolster tumor regression
Monoclonal antibodies, immune checkpoint blockers, BiTEs, and CAR T cells have all shown promise in the clinic. While some patients experience curative responses with these approaches, others do not respond or relapse after initial treatment response. This observation begs the question: why are some patients able to benefit from immunotherapy while others are not? The answer might stem from the fact that tumors are immunosuppressive and find ways to evade detection by the immune system (103).
Emerging data suggest that combining distinct therapies (CTLA-4 plus PD-1 inhibitors) (16) might improve treatment outcome and the number of patients that respond to therapy, which is not a new idea. For example, transfer of ex vivo expanded lymphocytes post preconditioning with an intense lymphodepleting preparative regimen is one of the most effective combination therapies for patients with advanced malignancies (166, 167). When antitumor T cells are infused after lymphodepletion, more than 70% of patients refractory to other treatments experience objective responses. The mechanisms underlying the effectiveness of lymphodepletion have been determined, at least in part, and have provided insight into choosing and combining distinct therapies that might synergize together to improve immunotherapy (150). The mechanisms underlying the effectiveness of lymphodepletion include (i) activation of the innate immune system, (ii) removal of endogenous cells that act as cytokine sinks, and (iii) depletion of suppressor cells (i.e. Treg cells and MDSCs) (168). As discussed below, alternative reagents that mimic these mechanisms could be used in combination to improve immunotherapy in patients with hematological malignancies.
The innate immune system of the patient might be activated with safe adjuvants approved for clinical use, including CpG ODN 7909 (169). Moreover, the CD40 costimulatory molecule on innate immune cells might enhance immunotherapy through engaging CD40L on T cells. A recombinant human CD40L, which has been used in clinical trials for patients with pancreatic cancer, could be used to activate the innate immune system (170). Given that CTLA-4 blockers induce ICOS (171), which further improves the function and survival of antitumor Th17 cells (135), administering an ICOS agonist might also enhance treatment outcome in patients.
Numerous approaches to eliminate human Treg cells as well as bulk lymphocytes that function as cytokines sinks have been performed in the clinic with varying degrees of success. For example. suppressor cells have been depleted with ONTAK, HuMax-CD4 and RFT5 (172-174). Regulatory B cells can also dampen an immune responses and thus their removal with rituximab might enhance cell therapy (175, 176). To bolster the infused T cells, homeostatic cytokines IL-7, IL-15, or IL-21 and their respective complexes could be administered systemically to support the tumor-reactive lymphocytes, as these cytokines are of low basal level in cancer patients (171, 177, 178). Approaches aimed at neutralizing specific immunoregulatory mechanisms or the addition of reagents targeting immune checkpoints to BiTEs or CAR T-cell therapy approaches may overcome immune suppression and enhance T-cell-mediated tumor regression.
Conclusions
New curative treatments are needed for patients with hematological malignancies. The discovery of pathways in the immune system that promote antitumor immunity has spurred a new era of cancer immunotherapy. There have been a number of advancements in immunotherapies that treat these patients, including synthetic antibodies, immune checkpoint blockers, and CAR T cells. An improved understanding of how the immune system regulation and the translation of these discoveries into effective treatments have dramatically prolonged the survival in patients with hematological malignancies. Yet, there is still considerable work to be done, as not all patients respond to immunotherapy. Mounting evidence shows that combinatorial immunotherapies can dramatically enhance treatment outcomes in patients and even promote long-term curative responses. This work has shed light on designing next generation immunotherapies for patients with advanced malignancies.
Acknowledgements
We thank the scientific and clinical team and the patients at the Medical University of South Carolina for help and guidance in the development of new cancer immunotherapies. This work was supported in part by NIH grant 5R01CA175061,KL2 South Carolina Clinical and Translational Research grant UL1TR000062, ACS-IRG grant 016623-004, MUSC Start-up funds to Chrystal M. Paulos and Jeane B. Kempner Foundation grant and ACS Post-doctoral fellowship (122704-PF-13-084-01-LIB) grant support for Michelle H. Nelson. This work was also supported in part by NIH grant 5P01CA154778.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annual review of immunology. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh A, Holland AM, van den Brink MR. Genetically engineered donor T cells to optimize graft-versus-tumor effects across MHC barriers. Immunological reviews. 2014;257:226–236. doi: 10.1111/imr.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolb HJ, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004;103:767–776. doi: 10.1182/blood-2003-02-0342. [DOI] [PubMed] [Google Scholar]
- 4.Kolb HJ, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 5.Gooley TA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. The New England journal of medicine. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corradini P, Farina L. Allogeneic transplantation for lymphoma: long-term outcome. Current opinion in hematology. 2010;17:522–530. doi: 10.1097/MOH.0b013e32833e5b41. [DOI] [PubMed] [Google Scholar]
- 7.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science translational medicine. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Current opinion in immunology. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nature reviews Clinical oncology. 2013;10:267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bargou R, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 16.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riley JL. Combination checkpoint blockade--taking melanoma immunotherapy to the next level. The New England journal of medicine. 2013;369:187–189. doi: 10.1056/NEJMe1305484. [DOI] [PubMed] [Google Scholar]
- 18.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 19.Cheadle EJ, Gornall H, Baldan V, Hanson V, Hawkins RE, Gilham DE. CAR T cells: driving the road from the laboratory to the clinic. Immunological reviews. 2014;257:91–106. doi: 10.1111/imr.12126. [DOI] [PubMed] [Google Scholar]
- 20.Kohn DB, et al. CARs on track in the clinic. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:432–438. doi: 10.1038/mt.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritz J, Schlossman SF. Utilization of monoclonal antibodies in the treatment of leukemia and lymphoma. Blood. 1982;59:1–11. [PubMed] [Google Scholar]
- 22.Ehrlich P. Collected studies on immunity. J Wiley & Sons; New York: 1906. [Google Scholar]
- 23.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature reviews Immunology. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abou-Jawde R, Choueiri T, Alemany C, Mekhail T. An overview of targeted treatments in cancer. Clinical therapeutics. 2003;25:2121–2137. doi: 10.1016/s0149-2918(03)80209-6. [DOI] [PubMed] [Google Scholar]
- 25.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 26.Chames P, Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? mAbs. 2009;1:539–547. doi: 10.4161/mabs.1.6.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maloney DG, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- 28.Hauptrock B, Hess G. Rituximab in the treatment of non-Hodgkin's lymphoma. Biologics : targets & therapy. 2008;2:619–633. doi: 10.2147/btt.s3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bello C, Sotomayor EM. Monoclonal antibodies for B-cell lymphomas: rituximab and beyond. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2007:233–242. doi: 10.1182/asheducation-2007.1.233. [DOI] [PubMed] [Google Scholar]
- 30.Hamburg MA, Collins FS. The path to personalized medicine. The New England journal of medicine. 2010;363:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 31.von Mehren M, Adams GP, Weiner LM. Monoclonal antibody therapy for cancer. Annual review of medicine. 2003;54:343–369. doi: 10.1146/annurev.med.54.101601.152442. [DOI] [PubMed] [Google Scholar]
- 32.Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. British journal of pharmacology. 2009;157:220–233. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staerz UD, Kanagawa O, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature. 1985;314:628–631. doi: 10.1038/314628a0. [DOI] [PubMed] [Google Scholar]
- 34.Holliger P, Prospero T, Winter G. "Diabodies": small bivalent and bispecific antibody fragments. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamova S, Koristka S, Keil J, Arndt C, Feldmann A, Michalk I, Bartsch, Bippes CC, Schmitz M, Cartellieri, Bachmann M. Cancer Immunotherapy by Retargeting of Immune Effector Cells via Recombinant Bispecific Antibody Constructs. Antibodies. 2012;1:172–198. [Google Scholar]
- 36.Muller D, Kontermann RE. Bispecific antibodies for cancer immunotherapy: Current perspectives. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2010;24:89–98. doi: 10.2165/11530960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Jiang XR, et al. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nature reviews Drug discovery. 2011;10:101–111. doi: 10.1038/nrd3365. [DOI] [PubMed] [Google Scholar]
- 38.Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell. 2012;148:1081–1084. doi: 10.1016/j.cell.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wickramasinghe D. Tumor and T cell engagement by BiTE. Discovery medicine. 2013;16:149–152. [PubMed] [Google Scholar]
- 40.Choi BD, Cai M, Bigner DD, Mehta AI, Kuan CT, Sampson JH. Bispecific antibodies engage T cells for antitumor immunotherapy. Expert opinion on biological therapy. 2011;11:843–853. doi: 10.1517/14712598.2011.572874. [DOI] [PubMed] [Google Scholar]
- 41.Kontermann RE. Dual targeting strategies with bispecific antibodies. mAbs. 2012;4:182–197. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bird RE, Walker BW. Single chain antibody variable regions. Trends in biotechnology. 1991;9:132–137. doi: 10.1016/0167-7799(91)90044-i. [DOI] [PubMed] [Google Scholar]
- 43.Titus JA, Garrido MA, Hecht TT, Winkler DF, Wunderlich JR, Segal DM. Human T cells targeted with anti-T3 cross-linked to antitumor antibody prevent tumor growth in nude mice. Journal of immunology. 1987;138:4018–4022. [PubMed] [Google Scholar]
- 44.Weiner GJ, Hillstrom JR. Bispecific anti-idiotype/anti-CD3 antibody therapy of murine B cell lymphoma. Journal of immunology. 1991;147:4035–4044. [PubMed] [Google Scholar]
- 45.Renner C, et al. Cure of xenografted human tumors by bispecific monoclonal antibodies and human T cells. Science. 1994;264:833–835. doi: 10.1126/science.8171337. [DOI] [PubMed] [Google Scholar]
- 46.Kipriyanov SM, Moldenhauer G, Strauss G, Little M. Bispecific CD3 × CD19 diabody for T cell-mediated lysis of malignant human B cells. International journal of cancer Journal international du cancer. 1998;77:763–772. doi: 10.1002/(sici)1097-0215(19980831)77:5<763::aid-ijc16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 47.Mack M, Riethmuller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7021–7025. doi: 10.1073/pnas.92.15.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dreier T, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. International journal of cancer Journal international du cancer. 2002;100:690–697. doi: 10.1002/ijc.10557. [DOI] [PubMed] [Google Scholar]
- 49.Gilham DE, Debets R, Pule M, Hawkins RE, Abken H. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends in molecular medicine. 2012;18:377–384. doi: 10.1016/j.molmed.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Handgretinger R, Zugmaier G, Henze G, Kreyenberg H, Lang P, von Stackelberg A. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia. 2011;25:181–184. doi: 10.1038/leu.2010.239. [DOI] [PubMed] [Google Scholar]
- 51.Dreier T, et al. T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19−/CD3- bispecific single-chain antibody construct. Journal of immunology. 2003;170:4397–4402. doi: 10.4049/jimmunol.170.8.4397. [DOI] [PubMed] [Google Scholar]
- 52.Schlereth B, et al. T-cell activation and B-cell depletion in chimpanzees treated with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Cancer immunology, immunotherapy : CII. 2006;55:503–514. doi: 10.1007/s00262-005-0001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hainsworth JD, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:1746–1751. doi: 10.1200/JCO.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 54.Fiedler WM, Wolf M, Kebenko M, Goebeler M-E, Ritter B, Quaas A, Vieser E, Hijazi Y, Patzak I, Friedrich M, Kufer P, Frankel S, Seggewiss-Bernhardt R, Kaubitzsch S. J Clin Oncol. 2012;30 suppl; abstr 2504. [Google Scholar]
- 55.Cioffi M, Dorado J, Baeuerle PA, Heeschen C. EpCAM/CD3-Bispecific T-cell engaging antibody MT110 eliminates primary human pancreatic cancer stem cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:465–474. doi: 10.1158/1078-0432.CCR-11-1270. [DOI] [PubMed] [Google Scholar]
- 56.Zhang P, et al. An EpCAM/CD3 bispecific antibody efficiently eliminates hepatocellular carcinoma cells with limited galectin-1 expression. Cancer immunology, immunotherapy : CII. 2014;63:121–132. doi: 10.1007/s00262-013-1497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friedrich M, et al. Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-Bispecific BiTE antibody cross-reactive with non-human primate antigens. Molecular cancer therapeutics. 2012;11:2664–2673. doi: 10.1158/1535-7163.MCT-12-0042. [DOI] [PubMed] [Google Scholar]
- 58.Lutterbuese R, et al. T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12605–12610. doi: 10.1073/pnas.1000976107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amann M, et al. Antitumor activity of an EpCAM/CD3-bispecific BiTE antibody during long-term treatment of mice in the absence of T-cell anergy and sustained cytokine release. Journal of immunotherapy. 2009;32:452–464. doi: 10.1097/CJI.0b013e3181a1c097. [DOI] [PubMed] [Google Scholar]
- 60.Lutterbuese R, et al. Potent control of tumor growth by CEA/CD3-bispecific single-chain antibody constructs that are not competitively inhibited by soluble CEA. Journal of immunotherapy. 2009;32:341–352. doi: 10.1097/CJI.0b013e31819b7c70. [DOI] [PubMed] [Google Scholar]
- 61.Kularatne SA, et al. Recruiting cytotoxic T cells to folate-receptor-positive cancer cells. Angewandte Chemie. 2013;52:12101–12104. doi: 10.1002/anie.201306866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi BD, et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:270–275. doi: 10.1073/pnas.1219817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hammond SA, et al. Selective targeting and potent control of tumor growth using an EphA2/CD3-Bispecific single-chain antibody construct. Cancer research. 2007;67:3927–3935. doi: 10.1158/0008-5472.CAN-06-2760. [DOI] [PubMed] [Google Scholar]
- 64.Torisu-Itakura H, et al. Redirected lysis of human melanoma cells by a MCSP/CD3-bispecific BiTE antibody that engages patient-derived T cells. Journal of immunotherapy. 2011;34:597–605. doi: 10.1097/CJI.0b013e3182307fd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walter RB. Biting back: BiTE antibodies as a promising therapy for acute myeloid leukemia. Expert review of hematology. 2014;7:317–319. doi: 10.1586/17474086.2014.896190. [DOI] [PubMed] [Google Scholar]
- 66.De Propris MS, et al. High CD33 expression levels in acute myeloid leukemia cells carrying the nucleophosmin (NPM1) mutation. Haematologica. 2011;96:1548–1551. doi: 10.3324/haematol.2011.043786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rowe JM, Lowenberg B. Gemtuzumab ozogamicin in acute myeloid leukemia: a remarkable saga about an active drug. Blood. 2013;121:4838–4841. doi: 10.1182/blood-2013-03-490482. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez HF, et al. Autologous transplantation gives encouraging results for young adults with favorable-risk acute myeloid leukemia, but is not improved with gemtuzumab ozogamicin. Blood. 2011;117:5306–5313. doi: 10.1182/blood-2010-09-309229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Molecular immunology. 2006;43:763–771. doi: 10.1016/j.molimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 70.Haas C, et al. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology. 2009;214:441–453. doi: 10.1016/j.imbio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 71.Mack M, Gruber R, Schmidt S, Riethmuller G, Kufer P. Biologic properties of a bispecific single-chain antibody directed against 17-1A (EpCAM) and CD3: tumor cell-dependent T cell stimulation and cytotoxic activity. Journal of immunology. 1997;158:3965–3970. [PubMed] [Google Scholar]
- 72.Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 73.Miescher S, Whiteside TL, Carrel S, von Fliedner V. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. Journal of immunology. 1986;136:1899–1907. [PubMed] [Google Scholar]
- 74.Hoffmann P, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19−/CD3-bispecific single-chain antibody construct. International journal of cancer Journal international du cancer. 2005;115:98–104. doi: 10.1002/ijc.20908. [DOI] [PubMed] [Google Scholar]
- 75.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 76.Martin-Orozco N, et al. Melanoma cells express ICOS ligand to promote the activation and expansion of T-regulatory cells. Cancer research. 2010;70:9581–9590. doi: 10.1158/0008-5472.CAN-10-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loffler A, et al. A recombinant bispecific single-chain antibody, CD19 × CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. [PubMed] [Google Scholar]
- 78.Koristka S, et al. Retargeting of human regulatory T cells by single-chain bispecific antibodies. Journal of immunology. 2012;188:1551–1558. doi: 10.4049/jimmunol.1101760. [DOI] [PubMed] [Google Scholar]
- 79.Choi BD, Gedeon PC, Sanchez-Perez L, Bigner DD, Sampson JH. Regulatory T cells are redirected to kill glioblastoma by an EGFRvIII-targeted bispecific antibody. Oncoimmunology. 2013;2:e26757. doi: 10.4161/onci.26757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simpson TR, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Azuma M, Cayabyab M, Buck D, Phillips JH, Lanier LL. CD28 interaction with B7 costimulates primary allogeneic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. The Journal of experimental medicine. 1992;175:353–360. doi: 10.1084/jem.175.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 87.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annual review of immunology. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 88.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Seminars in oncology. 2010;37:473–484. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wolchok JD, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Annals of the New York Academy of Sciences. 2013;1291:1–13. doi: 10.1111/nyas.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. The oncologist. 2007;12:864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 91.Beck KE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robinson MR, et al. Cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma: a new cause of uveitis. Journal of immunotherapy. 2004;27:478–479. doi: 10.1097/00002371-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 93.Attia P, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bashey A, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1581–1588. doi: 10.1182/blood-2008-07-168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ The Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsuzaki J, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fourcade J, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. The Journal of experimental medicine. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paulos CM, June CH. Putting the brakes on BTLA in T cell-mediated cancer immunotherapy. The Journal of clinical investigation. 2010;120:76–80. doi: 10.1172/JCI41811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunological reviews. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 101.Quezada SA, Peggs KS. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. British journal of cancer. 2013;108:1560–1565. doi: 10.1038/bjc.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nature reviews Immunology. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 105.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Molecular and cellular biology. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 107.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. The Journal of experimental medicine. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Antony PA, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. Journal of immunology. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Munn DH, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 110.Liu X, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 111.Uyttenhove C, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature medicine. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 112.Hayashi T, et al. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. The Journal of clinical investigation. 2004;114:270–279. doi: 10.1172/JCI21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seo SK, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nature medicine. 2004;10:1088–1094. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 114.Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. Journal of immunology. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 115.Shirey KA, Jung JY, Maeder GS, Carlin JM. Upregulation of IFN-gamma receptor expression by proinflammatory cytokines influences IDO activation in epithelial cells. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2006;26:53–62. doi: 10.1089/jir.2006.26.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. The Journal of clinical investigation. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Munn DH, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. The Journal of clinical investigation. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fallarino F, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. Journal of immunology. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 119.Aune TM, Pogue SL. Inhibition of tumor cell growth by interferon-gamma is mediated by two distinct mechanisms dependent upon oxygen tension: induction of tryptophan degradation and depletion of intracellular nicotinamide adenine dinucleotide. The Journal of clinical investigation. 1989;84:863–875. doi: 10.1172/JCI114247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chamuleau ME, et al. High INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica. 2008;93:1894–1898. doi: 10.3324/haematol.13113. [DOI] [PubMed] [Google Scholar]
- 121.Curti A, Trabanelli S, Salvestrini V, Baccarani M, Lemoli RM. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: focus on hematology. Blood. 2009;113:2394–2401. doi: 10.1182/blood-2008-07-144485. [DOI] [PubMed] [Google Scholar]
- 122.Hoshi M, et al. Changes in serum tryptophan catabolism as an indicator of disease activity in adult T-cell leukemia/lymphoma. Leukemia & lymphoma. 2009;50:1372–1374. doi: 10.1080/10428190903045393. [DOI] [PubMed] [Google Scholar]
- 123.Lindstrom V, et al. Indoleamine 2,3-dioxygenase activity and expression in patients with chronic lymphocytic leukemia. Clinical lymphoma, myeloma & leukemia. 2012;12:363–365. doi: 10.1016/j.clml.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 124.Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nature reviews Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 125.Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;111:2152–2154. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]
- 126.Sorensen RB, Hadrup SR, Svane IM, Hjortso MC, Thor Straten P, Andersen MH. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood. 2011;117:2200–2210. doi: 10.1182/blood-2010-06-288498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. The Journal of experimental medicine. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wainwright DA, Lesniak MS. Menage a trois: Sustained therapeutic anti-tumor immunity requires multiple partners in malignant glioma. Oncoimmunology. 2014;3:e28927. doi: 10.4161/onci.28927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vonderheide RH, June CH. Engineering T cells for cancer: our synthetic future. Immunological reviews. 2014;257:7–13. doi: 10.1111/imr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive immunotherapy for cancer or viruses. Annual review of immunology. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Corrigan-Curay J, et al. T-cell immunotherapy: looking forward. Molecular therapy : the journal of the American Society of Gene Therapy. 2014;22:1564–1574. doi: 10.1038/mt.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hinrichs CS, et al. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muranski P, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Frontiers in immunology. 2014;5:276. doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paulos CM, et al. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Science translational medicine. 2010;2:55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guedan S, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Muranski P, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rubinstein MP, et al. Ex vivo interleukin-12-priming during CD8(+) T cell activation dramatically improves adoptive T cell transfer antitumor efficacy in a lymphodepleted host. Journal of the American College of Surgeons. 2012;214:700–707. doi: 10.1016/j.jamcollsurg.2011.12.034. discussion 707-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gattinoni L, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nature medicine. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nature medicine. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Klebanoff CA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paulos CM, et al. Adoptive immunotherapy: good habits instilled at youth have long-term benefits. Immunologic research. 2008;42:182–196. doi: 10.1007/s12026-008-8070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin R, Liu Q. Diagnosis and treatment of viral diseases in recipients of allogeneic hematopoietic stem cell transplantation. Journal of hematology & oncology. 2013;6:94. doi: 10.1186/1756-8722-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Walter EA, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. The New England journal of medicine. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 145.Louis CU, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. Journal of immunotherapy. 2010;33:983–990. doi: 10.1097/CJI.0b013e3181f3cbf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Leen AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121:5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leen AM, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nature medicine. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 148.Hanley PJ, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114:1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leen AM, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature reviews Immunology. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nature reviews Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Focosi D, Amabile G, Di Ruscio A, Quaranta P, Tenen DG, Pistello M. Induced pluripotent stem cells in hematology: current and future applications. Blood cancer journal. 2014;4:e211. doi: 10.1038/bcj.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.June CH. Adoptive T cell therapy for cancer in the clinic. The Journal of clinical investigation. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Al-Khami AA, Mehrotra S, Nishimura MI. Adoptive immunotherapy of cancer: Gene transfer of T cell specificity. Self/nonself. 2011;2:80–84. doi: 10.4161/self.2.2.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vera JF, Brenner MK, Dotti G. Immunotherapy of human cancers using gene modified T lymphocytes. Current gene therapy. 2009;9:396–408. doi: 10.2174/156652309789753338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kershaw MH, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Till BG, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lamers CH, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:e20–22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 160.Hombach AA, Heiders J, Foppe M, Chmielewski M, Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology. 2012;1:458–466. doi: 10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nature biotechnology. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 162.Carpenito C, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Milone MC, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kochenderfer JN, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kochenderfer JN, et al. Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies Can Be Effectively Treated With Autologous T Cells Expressing an Anti-CD19 Chimeric Antigen Receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dudley ME, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Paulos CM, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. The Journal of clinical investigation. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Paulos CM, et al. Toll-like receptors in tumor immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5280–5289. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li AL, et al. Antileukemic effect of interleukin-7-transduced bone marrow stromal cells in mice following allogeneic T-cell-depleted bone marrow transplantation. Transplantation proceedings. 2005;37:2297–2299. doi: 10.1016/j.transproceed.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 172.Dang NH, et al. Phase II study of denileukin diftitox for relapsed/refractory B-Cell non-Hodgkin's lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:4095–4102. doi: 10.1200/JCO.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 173.Hagberg H, Pettersson M, Bjerner T, Enblad G. Treatment of a patient with a nodal peripheral T-cell lymphoma (angioimmunoblastic T-Cell lymphoma) with a human monoclonal antibody against the CD4 antigen (HuMax-CD4) Medical oncology. 2005;22:191–194. doi: 10.1385/MO:22:2:191. [DOI] [PubMed] [Google Scholar]
- 174.Powell DJ, Jr., Parker LL, Rosenberg SA. Large-scale depletion of CD25+ regulatory T cells from patient leukapheresis samples. Journal of immunotherapy. 2005;28:403–411. doi: 10.1097/01.cji.0000170363.22585.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Olkhanud PB, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer research. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McLaughlin P, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 177.Klebanoff CA, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. The Journal of experimental medicine. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]