Abstract
Many xenobiotics are considered reproductive toxins because of their ability to interact with the nuclear estrogen receptors (ERα and ERβ). However, there is evidence that these xenobiotics can regulate gene expression in the reproductive targets by mechanisms that do not involve these ERs. To examine this further, we compared the effects of estrogenic (o,p′-DDT [1-(o-chlorophenyl)-1-(p-chlorophenyl)2,2,2-trichloroethane] and Kepone, chlordecone) and nonestrogenic (p,p′-DDD [1,1-dichloro-2,2-bis(p-chlorophenyl)ethane], a metabolite of p,p′-DDT) xenobiotics with those of 17β-estradiol (E2) and 4-hydroxyestradiol-17β (4-OH-E2), a catechol metabolite of E2, on uterine expression of lactoferrin (LF) and progesterone receptor (PR). These genes are estrogen responsive in the mouse uterus. Normally, LF is expressed in the uterine epithelium, whereas PR is expressed in both the epithelium and stroma in response to estrogenic stimulation. Ovariectomized mice were injected with xenobiotics (7.5 mg/kg), E2 (10 μg/kg), 4-OH-E2 (10 μg/kg), or the vehicle (oil, 0.1 ml/mouse), and uterine tissues were processed for Northern blot and in situ hybridization. The pure antiestrogen ICI-182780 (ICI; 1 or 20 mg/kg) was used to interfere with estrogenic responses that were associated with the ERs. The results of Northern and in situ hybridization demonstrated increased uterine levels of PR and LF messenger RNAs (mRNAs) by all of these xenobiotics, but quantitatively the responses were much lower than those induced by E2 or 4-OH-E2. The results further showed that the E2-inducible epithelial LF mRNA accumulation was markedly abrogated by pretreatment with ICI (20 mg/kg). In contrast, this treatment retained the epithelial expression of PR mRNA, but down-regulated the stromal expression. In contrast, ICI had negligible effects on LF and PR mRNA responses to 4-OH-E2, indicating that this catechol estrogen exerted its effects primarily via a mechanism(s) other than the ERs. The heightened accumulation of LF mRNA in the epithelium in response to Kepone and o,p′-DDT was also severely compromised by pretreatment with ICI, but this antiestrogen had little effect on responses to p,p′-DDD. Similar to E2, Kepone increased the expression of PR mRNA in both uterine epithelium and stroma. However, pretreatment with ICI decreased stromal cell expression, whereas epithelial cell expression remained unaltered or increased. These responses were not noted in mice treated with o,p′-DDT or p,p′-DDD. Collectively, the results demonstrate that catechol estrogens or xenobiotics can alter uterine expression of estrogen-responsive genes by mechanisms that are not totally mediated by the classical nuclear ERs, and these alterations are cell type specific. We conclude that an interaction of a compound with the nuclear ERα and/or ERβ is not an absolute requirement for producing specific estrogen-like effects in the reproductive target tissues.
Our environment contains a large variety of compounds, both natural and synthetic, that can mimic natural estrogens in binding to the nuclear transcription factor now designated estrogen receptor-α (ERα) (1–4). Whether these xenobiotics interact with recently identified ERβ is not yet clearly established, although recent studies show that a few of these compounds possess an even higher affinity for this receptor (5). It is possible that environmental estrogens (xenoestrogens) could exert their effects by binding to homodimers of ERβ or heterodimers of ERα and ERβ. This is consistent with the finding that antiestrogens, 4-hydroxytamoxifen and ICI-182780 [ICI; 7α-(9 – 4,4,5,5,5-pentafluoropenylsulfinyl)nonyl-estra-1,3,4(10-triene-3,17β-diol)], bind ERβ as well as heterodimers of ERα and ERβ (6). Interactions of xenoestrogens with ER have been repeatedly offered as the basis for their reproductive toxicity (7, 8). However, the requirement for large concentrations (micro-molar) of xenoestrogens to induce any estrogenic phenotypes and their low affinity for ERα have challenged the idea that prevailing xenoestrogens in the environment are significant health hazards (9). This argument assumes that the major effects of xenoestrogens depend upon their interaction with the classical ER.
The present investigation examined whether xenoestrogens produce target-specific effects via gene expression that may or may not involve classical ERs. We selected two natural estrogens, 17β-estradiol (E2) and its catechol metabolite 4-hydroxyestradiol-17β (4-OH-E2), as well as three polychlorinated hydrocarbons. Two of the latter are insecticides, chlordecone (Kepone) and p,p′-DDD (Rhothane; [1,1-di-chloro-2,2-bis-(p-chlorophenyl)ethane]), and the third is o,p′-DDT [1-(o-chlorophenyl)-1-(p-chlorophenyl)2,2,2-trichloroethane], the estrogenic isomer of the insecticide p,p′-DDT (7, 10, 11). Kepone and o,p′-DDT have relatively weak affinity for ERα and exhibit estrogenic effects in vivo (7, 10–13). Although p,p′-DDD does not bind to rodent ERα or produce estrogenic effects in vivo, it has weak affinity for recombinant human ERα (14). To examine differential effects of these xenoestrogens and natural estrogens in uterine functions, the expression of two estrogen-responsive genes, lactoferrin (LF) and progesterone receptor (PR), in the adult ovariectomized mouse uterus was studied. Although they are established as estrogen-responsive genes, their expression can be influenced by transcription factors other than ER (15–17). The contribution of classical ERs in these responses was examined using the pure antiestrogen ICI (18). All of the compounds increased the expression of LF and PR, but only the effects of E2 on LF messenger RNA (mRNA) were markedly attenuated by pretreatment with ICI. Alteration in cell-specific expression of PR mRNA after exposure to this antiestrogen suggests that E2, 4-OH-E2, and the xenobiotics have distinct and overlapping effects in the uterus. Thus, an additional factor(s) other than the trans-activation effects of the classical ERs must be considered when evaluating the effects of environmental toxins on the reproductive tract.
Materials and Methods
Chemicals
E2, 4-OH-E2, and p,p′-DDD were purchased from Sigma Chemical Co. (St. Louis, MO). Kepone [chlordecone; 1,1a,3,3a,4,5,5a,5b,c-decachlorooctoahydro-1,3,4-metheno-2H-cyclobuta(cd)pentalen-2-one] and o,p′-DDT were purchased from Radian Corp. (Austin, TX) and were at least 99% pure. ICI was a gift from Zeneca Pharmaceuticals (Cheshire, UK). All of the test compounds were dissolved in absolute ethanol and diluted to the desired concentrations in sesame oil.
Animals and injection schedules
Adult CD-1 mice (Charles River Laboratories, Raleigh, NC) were maintained in the animal care facilities of the University of Kansas Medical Center in accordance with NIH standards for care and use of experimental animals. Virgin female mice (7–9 weeks of age) were ovariectomized and rested for 10 days before receiving any treatments. They received a single sc injection (0.1 ml/mouse) of oil (control), Kepone (3.25, 7.5, 15, or 30 mg/kg), o,p′-DDT (3.25, 7.5, 15, or 30 mg/kg), p,p′-DDD (3.25, 7.5, 15, or 30 mg/kg), E2 (10 μg/kg), and 4-OH-E2 (10 μg/kg), or ICI (1 or 20 mg/kg) or were given ICI 30 min before the injection of the xenobiotics or natural estrogens. Mice were killed at 1, 2, 4, 6, 12, and 24 h. Mice injected with oil were killed 6 or 24 h after the injection and served as controls.
Northern blot hybridization
Total RNA was extracted from tissues by a modified method as previously described (19, 20). Total RNA (6.0 μg) was denatured, separated by formaldehyde/agarose gel electrophoresis, transferred to nylon membranes, and UV cross-linked. Blots were prehybridized and hybridized as described previously (20, 21). Briefly, hybridization was carried out for 20 h at 68 C in 3 × SET (1 × SET = 150 mM NaCl, 5 mM EDTA, and 10 mM Tris-HCl, pH 8.0), 0.1% SDS, 20 mM phosphate buffer (pH 7.2), 250 μg/ml transfer RNA, 10% dextran sulfate, and 1 × 106 counts/min of 32P-labeled antisense complementary RNA (cRNA) probe/ml hybridization buffer. Hybridization was performed in sequence with probes for mouse PR, LF, and ribosomal protein L7 (rpL7). Stripping of hybridized probe before subsequent rehybridization was achieved by boiling blots for 5 min in 0.5 × SET and 0.1% SDS (21). Transcripts were detected by autoradiography. Quantitation of radioactivity to hybridized bands was obtained using a radioimage analysis system (Ambis Systems, San Diego, CA).
In situ hybridization
In situ hybridization was performed as described previously (20, 22). Briefly, each uterine horn was excised, cut into halves, and flash frozen in freon. Frozen sections (10 μm) were mounted onto poly-L-lysine-coated slides and stored at −70 C until used. After removal from −70 C, the slides with the uterine sections were placed on a slide warmer (37 C) for 2 min and then fixed in 4% paraformaldehyde in PBS for 15 min at 4 C. After prehybridization, uterine sections were hybridized to 35S-labeled antisense PR and LF cRNA probes for 4 h at 45 C. As negative controls, uterine sections were hybridized with the 35S-labeled sense PR and LF cRNA probes. After hybridization and washing, the slides were incubated with ribonuclease A (RNase A; 20 μg/ml) at 37 C for 20 min. RNase A-resistant hybrids were detected by autoradiography using Kodak NTB-2 liquid emulsion (Eastman Kodak, Rochester, NY). The slides were poststained with hematoxylin and eosin.
Hybridization probes
The subcloning and vectors for mouse LF and rpL7 have been described (22, 23). A 499-bp complementary DNA fragment for mouse PR in the DNA- and ligand-binding domains, was derived by RT-PCR using mouse day 4 pregnant uterine RNA sample. The fragment was then subcloned in pCR-Script (SK)+ vector, and the sequencing was performed using the dideoxy method to confirm the nucleotide sequence for the mouse PR. For Northern blot hybridization, antisense 32P-labeled cRNA probes were generated using SP6 polymerase. For in situ hybridization, sense or antisense 35S-labeled cRNA probes were generated. Probes had specific activities of about 2 × 109 dpm/μg.
Results
Effects of natural estrogens and xenobiotics on the temporal expression of LF and PR genes in the mouse uterus
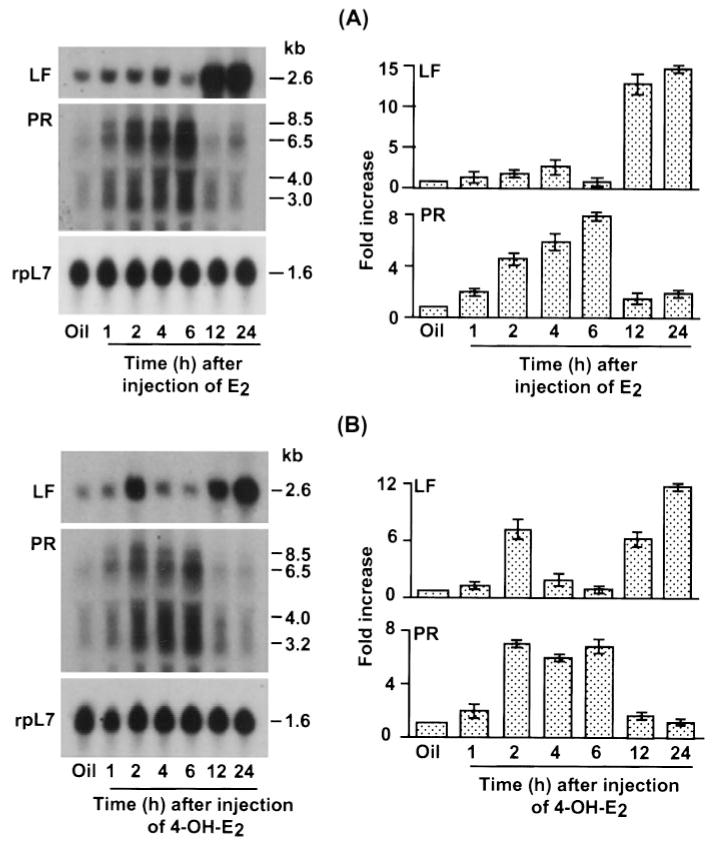
Ovariectomized mice were injected with natural estrogens (E2 or 4-OH-E2) or xenobiotics (Kepone, o,p′-DDT, or p,p′-DDD) to determine their effects on uterine accumulation of LF and PR mRNAs. Total RNA samples obtained from whole uteri from several mice were analyzed by Northern blot hybridization at various times after treatment (Fig. 1, A and B). As shown previously (24), the induction of uterine LF mRNA in response to E2 was bimodal, with an early increase (4-fold) at 4 h followed by further increases at 12 and 24 h (15-fold; Fig. 1A). The induction by 4-OH-E2 on uterine LF mRNA levels was also biphasic (Fig. 1B); however, the first induction was noted at 2 h (7-fold) followed by further increases at 12 and 24 h (12-fold). With respect to PR mRNA, both E2 and 4-OH-E2 showed a similar patter of induction; the peak (7–8 fold) induction was seen at 6 h.
Fig. 1.
Temporal effects of E2 or 4-OHE2 on uterine expression of LF and PR mRNAs in ovariectomized mice. Adult ovariectomized mice were given a single injection of E2 (A, 10 μg/kg) or 4-OH-E2 (B, 10 μg/kg) and killed at the times indicated. Uteri from mice injected with oil and killed 24 h later served as a control. Total uterine RNA (6 μg) pooled from three or four mice was used for each group. Autoradiographic exposures were 2 h for both LF and rpL7 and 6 h for PR. These experiments were repeated twice with independent RNA samples, and the average value with the range of responses from two experiments are shown in the bar plots. Fold increases were calculated with respect to oil, and the increases were normalized with rpL7 expression (a housekeeping gene).
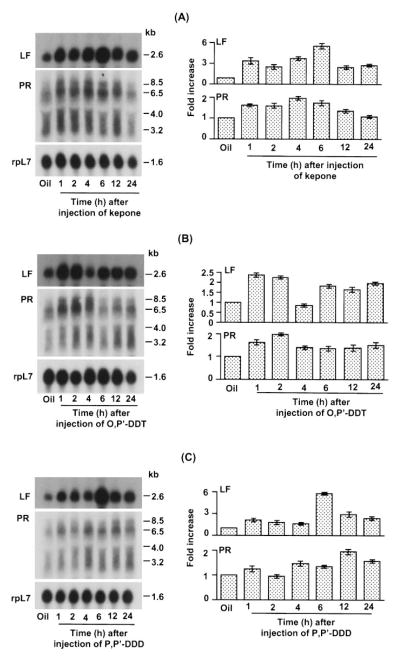
To examine the temporal effects of xenobiotics on the expression of uterine LF and PR mRNAs, we first examined the dose-response (3.25, 7.5, 15, or 30 mg/kg) effects of Kepone, o,p′-DDT, or p,p′-DDD at 24 h. These studies showed modest increases at 3.75 mg/kg, but maximal responses at 7.5 mg/kg with respect to both the LF and PR genes. The higher doses of Kepone (15 or 30 mg/kg) failed to sustain the levels of LF mRNA, whereas o,p′-DDT- or p,p′-DDD at these doses maintained the levels. With respect to PR mRNA levels, the degree of induction by these xenobiotics remained unchanged even at higher doses (15 or 30 mg/kg). The results indicated that the uterine induction of LF and PR mRNA levels by these xenobiotics was dose dependent, and a dose of 7.5 mg/kg showed a consistent inductive response for all of the xenobiotics examined. Thus, in subsequent studies, the temporal changes in uterine expression of LF and PR mRNAs in response to xenobiotics were examined at this dose (Fig. 2). Unlike the natural estrogens, this dose of either Kepone or o,p′-DDT does not produce a uterotropic response even if given daily for 3 days, and p,p′-DDD, has not been shown to be uterotropic at the doses examined (7). As shown in Fig. 2, uterine LF mRNA levels increased rapidly after exposure to all of these xenobiotics. With Kepone and p,p′-DDD, the maximal response (6-fold) was noted at 6 h, followed by a decline at 12 and 24 h. The induction of LF mRNA levels caused by o,p′-DDT was less than that produced by Kepone or p,p′-DDD and was biphasic.
Fig. 2.
Temporal effects of Kepone, o,p′-DDT, and p,p′-DDD on uterine expression of LF and PR mRNAs in ovariectomized mice. Adult ovariectomized mice were given a single injection of Kepone (A, 7.5 mg/kg), o,p′-DDT (B, 7.5 mg/kg), and p,p′-DDD (C, 7.5 mg/kg) and killed at the times indicated. Uteri of animals exposed to oil for 24 h served as the control group. Total uterine RNA (6 μg) pooled from four or five mice was used for each group. Autoradiographic exposures were 2 h for rpL7, 3 h for LF, and 12 h for PR. These experiments were repeated twice with independent RNA samples, and the average value with the range of responses from two experiments are shown in the bar plots. Fold increases were calculated with respect to oil, and the increases were normalized with rpL7 mRNA expression.
The multiple transcripts for PR mRNA [8.5, 6.5, 4.0, and 3.2 kilobases (kb)] have been previously reported (25). In general, xenobiotics showed induction of all transcripts. However, under a few experimental conditions, a differential expression of the highest (8.5 kb) and the lowest (3.2 kb) transcripts was noted. However, the significance of this finding is not clear. A recent report suggests that the alteration in the levels of A and B forms of PR in transgenic mice leads to functional abnormalities in the mammary gland (26).
PR mRNA levels increased rapidly within 2–4 h after the injection of Kepone or o,p′-DDT, although the maximal response (2-fold) occurred somewhat earlier with the latter compound. In the case of the nonestrogenic p,p′-DDD, the response was similar in magnitude to that obtained with Kepone or o,p′-DDT, but the peak level was reached at 12 h. In general, the degree of responses induced by these xenobiotics was much less than that observed with natural estrogens.
Effects of the antiestrogen ICI on the expression of uterine LF and PR mRNAs induced by natural estrogens or xenobiotics
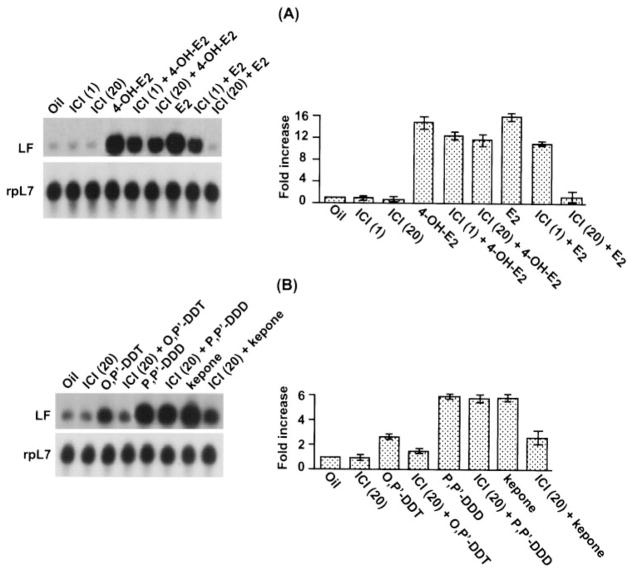
To determine whether the effects of natural estrogens or xenobiotics on the induction of LF and PR mRNAs in the mouse uterus was mediated by ERs, ovariectomized mice were injected with either 1 or 20 mg/kg ICI 30 min before the injections of the natural estrogens (10 μg/kg) or xenobiotics (7.5 mg/kg). The uterine RNA samples were analyzed by Northern blot hybridization (Fig. 3). Although the induction levels (12- to 15-fold) of LF mRNA in response to E2 or 4-OH-E2 were same, the effects of pretreatment with ICI on the responses to these natural estrogens were quite different (Fig. 3A). The treatment with 1 mg/kg ICI, a dose 100-fold higher than that of the estrogens, had little effect on the responses to E2 or 4-OH-E2. However, increasing the dose of ICI to 20 mg/kg almost completely inhibited the response to E2, but had only a small inhibitory effect on the response to 4-OH-E2, suggesting that much of the response to this catechol estrogen was not mediated by the estrogen receptors, either ERα or ERβ. Neither dose of antiestrogen alone had any effect compared with levels in control animals treated with oil (Fig. 3A). These results are consistent with our recent findings in mice lacking ERα (ERKO) (27). The effects of antiestrogen pretreatment on the responses induced by xenobiotics are shown in Fig. 3B. A dose of 20 mg/kg ICI given 30 min before o,p′-DDT or Kepone prevented much of the increase in LF mRNA induced by these xenobiotics (Fig. 3B). In contrast, the antiestrogen had no effect on the response to p,p′-DDD, indicating that this response was not mediated by uterine ER.
Fig. 3.
Effects of ICI on uterine expression of LF in response to estrogens and xenobiotics in ovariectomized mice. Adult ovariectomized mice were given a single injection of oil, E2 (10 μg/kg), 4-OH-E2 (10 μg/kg), Kepone (7.5 mg/kg), o,p′-DDT (7.5 mg/kg), p,p′-DDD (7.5 mg/kg), or ICI (1 or 20 mg/kg) or were given ICI 30 min before the injection of each of the other compounds. Mice were killed 6 h after injection of the xenobiotics and/or ICI (B) and 24 h after the injection of estrogens and/or ICI (A). Total uterine RNA (6 μg) pooled from three or four mice was used for each group. These experiments were repeated twice with independent RNA samples, and the average value with the range of responses from two experiments are shown in the bar plots. Fold increases were calculated with respect to oil, and the increases were normalized with rpL7 mRNA expression.
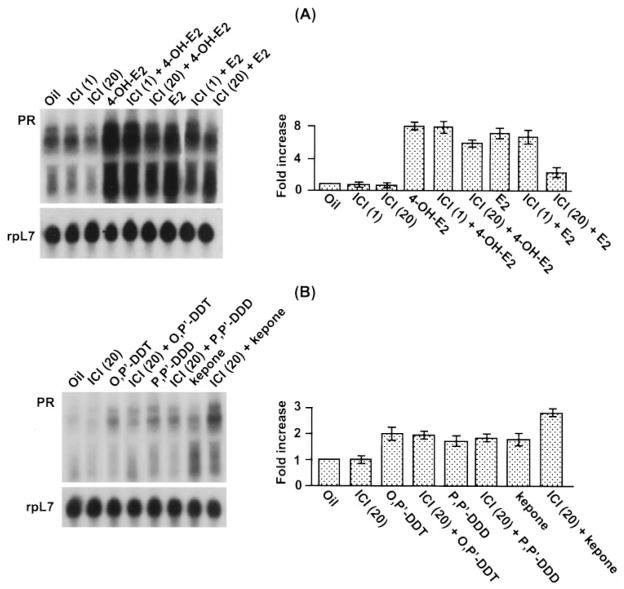
The effects of ICI at a dose of 1 mg/kg had no effects on the increased levels of PR mRNA 2 h after the injection of E2 or 4-OH-E2 (Fig. 4A). Increasing the dose of ICI (20 mg/kg) had a pronounced inhibitory effect on the responses to E2, but a minimal effect on the responses to 4-OH-E2, again supporting the view that much of the response to this catechol estrogen was not mediated by the ERs. The uterine responses to xenobiotics on uterine PR mRNA accumulation were much less than those seen with the estrogens (Fig. 4B). ICI at a dose of 20 mg/kg had little, if any, inhibitory effect on the response to o,p′-DDT or p,p′-DDD. In contrast, the response to Kepone on PR mRNA levels increased after pretreatment with the antiestrogen (Fig. 4B). Again, these results are consistent with the view that certain steroid-responsive uterine genes activated by xenobiotics are not solely mediated by the ERs.
Fig. 4.
Effects of ICI on uterine expression of PR mRNA in response to estrogens or xenobiotics in ovariectomized mice. Adult ovariectomized mice were given a single injection of oil, E2 (10 μg/kg), 4-OH-E2 (10 μg/kg), Kepone (7.5 mg/kg), o,p′-DDT (7.5 mg/kg), p,p′-DDD (7.5 mg/kg), or ICI (20 mg/kg) or were given ICI 30 min before the injection of the other compounds. Mice were killed 6 h after the injections of estrogens and/or ICI (A) or 2 h after xenobiotics and/or ICI (B), respectively. Total uterine RNA (6 μg) pooled from four or five mice was used for each group. The experiments were repeated twice with independent RNA samples, and the average value with the range of responses from two experiments are shown in the bar plots. Fold increases were calculated with respect to oil, and the increases were normalized with rpL7 mRNA expression.
Effects of natural estrogens and xenobiotics on cell-specific expression of LF mRNA in the ovariectomized mouse uterus
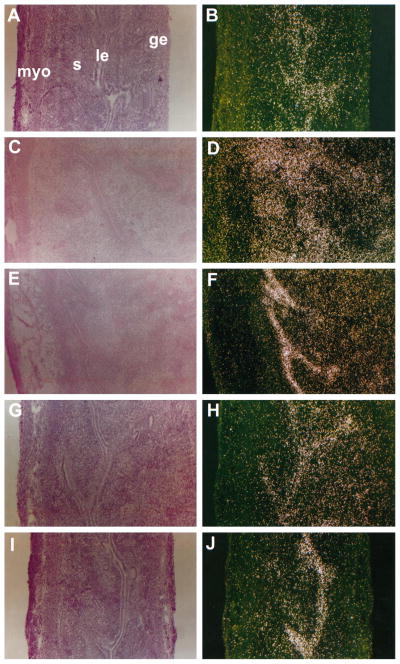
To determine whether natural estrogens or xenobiotics influence uterine expression of LF in a cell-type specific manner and whether the effects are ER mediated, in situ hybridization was performed. As expected, the accumulation of LF mRNA was not detected in any uterine cell types after injections of oil or ICI in ovariectomized mice (Fig. 5, A and B). However, either E2 or 4-OH-E2 increased the levels of LF mRNA in the luminal and glandular epithelial cells (Fig. 5, C and E). The response induced by E2 was totally abrogated by pretreatment with ICI at 20 mg/kg (Fig. 5D). In contrast, the response induced by 4-OH-E2 in epithelial cells was not altered by pretreatment with this dose of ICI (Fig. 5F). These results are consistent with those of the Northern blot hybridization experiments (Fig. 3A).
Fig. 5.
In situ hybridization of LF mRNA in the ovariectomized mouse uterus after exposure to oil, antiestrogen (ICI), estrogens (E2 and 4-OH-E2), or xenobiotics (Kepone, o,p′-DDT, and p,p′-DDD), or antiestrogen plus these compounds. Adult ovariectomized mice were injected with: A, oil; B, ICI; C, E2; D, E2 plus ICI; E, 4-OH-E2; F, 4-OH-E2 plus ICI; G, Kepone; H, Kepone plus ICI; I, o,p′-DDT; J, o,p′-DDT plus ICI; K, p,p′-DDD; and L, p,p′-DDD plus ICI. Mice injected with the natural estrogens and/or ICI were killed 24 h after injections, whereas those given xenobiotics and/or ICI were killed after 6 h. Results with control mice injected with oil or ICI are shown at 24 h. Hybridization signals 6 h after oil or ICI injections were similar to those obtained at 24 h (data not shown). Paraformaldehyde-fixed frozen longitudinal sections (10 μm) were mounted onto glass slides, prehybridized, and hybridized with 35S-labeled LF sense or antisense cRNA probes. RNase A-resistant hybrids were detected after 3–5 days of autoradiography. Darkfield photomicrographs of uterine sections are shown at ×100. No positive signals were observed when sections were hybridized with the sense probe (data not shown). le, Luminal epithelium; ge, glandular epithelium; s, stroma; myo, myometrium. These experiments were repeated twice with three mice in each group, and similar results were obtained.
The cell-type specific localization of LF mRNA in uteri of mice treated with Kepone or o,p′-DDT was similar to that found with the natural estrogens, and much of the activity was inhibited by pretreatment with ICI (Fig. 5, G–J); ICI appeared to be more effective against the action of o,p′-DDT than against Kepone. In contrast, this antiestrogen had little effect on the luminal epithelial expression of LF mRNA by p,p′-DDD (Fig. 5, K and L). No positive signals were observed when uterine sections were hybridized with the sense probe (data not shown).
Effects of natural estrogens and xenobiotics on cell-specific expression of PR mRNA in the mouse uterus
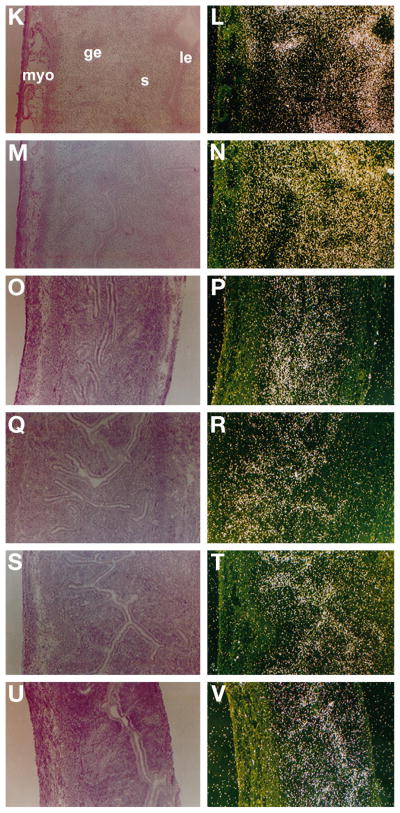
The cell-type specific effects of the natural estrogens or xenobiotics on uterine PR mRNA accumulation were examined by in situ hybridization. In mice treated with oil, autoradiographic signals for PR mRNA were detected mainly in the uterine luminal epithelium (Fig. 6, A and B), and an injection of ICI (20 mg/kg) maintained the low levels of PR mRNA in the same cell types (data not shown). The expression of PR mRNA significantly increased in both epithelial and stromal cells after an injection of E2 (Fig. 6, C and D). The pretreatment with ICI greatly reduced the E2-induced up-regulation of PR mRNA levels in stromal cells, but this treatment distinctly maintained the up-regulated PR mRNA levels in the luminal epithelial cells (Fig. 6, E and F). This change in the pattern of cellular expression of PR mRNA was duplicated, albeit with a lower intensity of signals, in the uteri of mice treated with Kepone and/or ICI (Fig. 6, G–J). Thus, the pretreatment with ICI significantly attenuated the Kepone-induced accumulation of PR mRNA in stromal cells, but the luminal epithelial accumulation remained unaffected. With respect to 4-OH-E2, PR mRNA accumulation was markedly up-regulated in the epithelial and subepithelial stromal cells. In contrast to E2 or Kepone, the pretreatment with ICI failed to show any reduction of PR mRNA accumulation by 4-OH-E2 in either cell type, although this treatment modestly increased the signals of PR mRNA throughout the stroma (Fig. 6, M and N). o,p′-DDT and p,p′-DDD showed a localization pattern similar to that of 4-OH-E2, but with a reduced signal intensity; ICI had very little effect on this expression (Fig. 6, O–V).
Fig. 6.
In situ hybridization of PR mRNA in the ovariectomized mouse uterus after exposure to oil, antiestrogen (ICI), estrogens (E2 and 4-OH-E2), or xenobiotics (Kepone, o,p′-DDT, and p,p′-DDD), or the antiestrogen plus the estrogens or xenobiotics. Adult ovariectomized mice were injected with: oil (A and B), E2 (C and D), E2 plus ICI (E and F), Kepone (G and H), Kepone plus ICI (I and J), 4-OH-E2 (K and L), 4-OH-E2 plus ICI (M and N), o,p′-DDT (O and P), o,p′-DDT plus ICI (Q and R), p,p′-DDD (S and T), and p,p′-DDD plus ICI (U and V). Mice were killed 6 h after the injections of natural estrogens and/or ICI and 2 h after the injections of xenobiotics and/or ICI. Results obtained 6 h after injection of oil are shown. ICI alone did not produce any difference in signals compared with those obtained after oil injection (data not shown). Paraformaldehydefixed frozen longitudinal sections (10 μm) were mounted onto glass slides, prehybridized, and hybridized with 35S-labeled PR sense or antisense cRNA probes. RNase A-resistant hybrids were detected after 5–10 days of autoradiography. Bright- and darkfield photomicrographs of uterine sections are shown at ×100. No positive signals were observed when sections were hybridized with the sense probe (data not shown). le, Luminal epithelium; ge, glandular epithelium; s, stroma; myo, myometrium. These experiments were repeated twice with three mice per group, and similar results were obtained.
Discussion
The highlights of the present investigation are that the uterine responses to primary estrogen, catechol estrogen, or xenobiotics are differentially regulated and dependent upon the cell types involved. Another important finding is that classical nuclear ERs are not the sole mediators of these responses. These findings are important with regard to the reproductive toxicity attributed to a large variety of polychlorinated hydrocarbons present in the environment (2, 3, 7, 8). Until recently, the basis for this toxicity was thought to be mediated by their interactions with the nuclear ER of the target tissues in producing estrogenic effects. The finding that some persistent metabolites of DDT could bind to the androgen receptor at lower concentrations than they do with the ER initiated interest in the possibility that other transcription factors are involved in the observed effects of environmental toxins (28). This is further supported by in vitro studies using MCF-10A breast epithelial cells that lack ER (29). This study showed that p,p′-DDT, which lacks in vivo estrogenicity and does not bind ER, stimulated growth factor receptor tyrosine kinase activity and cell proliferation. In contrast, the estrogenic analog of DDT (o,p′-DDT) was ineffective, indicating structural specificity for the response.
Kepone, o,p′-DDT, or p,p′-DDT has similar low affinity (IC50, ~100 μM) for the androgen receptor, and thus, interaction with this receptor is not considered a factor for any possible reproductive toxicity of these compounds (28). In contrast, although the affinities of Kepone and o,p′-DDT for the ER (IC50, ~4 μM) are considerably lower than that of E2 (IC50, ~0.002 μM), it is much higher than that of p,p′-DDD (IC50, >1000 μM) (28). When given in large doses, o,p′-DDT or Kepone mimics the action of E2 in several in vivo responses (7, 8, 10, 13). This requirement for large doses of xenoestrogens to produce estrogenic effects suggests that mechanisms other than those based on DNA-ER interactions are involved in reproductive toxicity. Models for examining such a view include examination of gene expression in animals that lack functional ERs produced by gene targeting (ER knock-out, ERKO) (25, 27) or antagonism of ER action by a potent antiestrogen (27, 30, 31).
LF and PR are well known estrogen-responsive genes in the mouse uterus (15, 16, 17, 24). However, there is evidence that these genes can be up-regulated by many other factors. For example, although the LF gene is inducible in the uterus and mammary gland by epidermal growth factor (32) and PRL (15, 33), respectively, the PR gene is known to be induced by dopamine, cAMP, and growth factors (17, 34, 35). In the present investigation, the increased accumulation of their mRNAs in the mouse uterus after exposure to E2 or 4-OH-E2 was anticipated. In contrast, increased expression after exposure to o,p′-DDT or Kepone at a relatively low dose (7.5 mg/kg) was unexpected, as little or no uterotropic effects are produced by either of these compounds at this dose. Further, the effects of p,p′-DDD, which is not estrogenic at any dose and does not bind to the rodent uterine ER, was even more surprising. Thus, these xenobiotics influence estrogen-responsive uterine genes at doses that are considered inconsequential when examining for typical phenotypic estrogenic effects.
The LF gene expression by E2 was remarkably abrogated by pretreatment with ICI (20 mg/kg), a pure antiestrogen, establishing the involvement of the ER. In contrast, ICI showed only modest effects on 4-OH-E2-induced expression of this gene. This is consistent with our previous observation in ERKO mice (27). Thus, the effects of 4-OH-E2 on uterine expression of the LF gene does not appear to be solely dependent on its interaction with the ER. This catechol estrogen has an affinity for ERα and ERβ that is about 10% that of E2 (5). The attenuation of uterine LF mRNA accumulation by ICI in response to o,p′-DDT or Kepone indicates that this response to a considerable extent was mediated via ER. In contrast, ICI’s inability to influence uterine LF mRNA accumulation in response to p,p′-DDD suggests that this xenobiotic affects the expression of this gene by a factor other than the nuclear ER.
The regulation of uterine accumulation of PR mRNA by the test compounds and their interactions with the nuclear ER appear more complex. The changes in PR mRNA were similar in mice exposed to either E2 or 4-OH-E2, and ICI showed minimal reduction in the levels of PR mRNA. There is a report (25) that ICI-164384, a structurally similar but less potent antiestrogen than ICI (18), at a dose of 1 mg/kg inhibited the effects of E2 (20 μg/kg) on PR mRNA expression in the mouse uterus. In the present study, we observed that ICI at 1 mg/kg was essentially without any effect on the PR mRNA response to E2 at 10 μg/kg. One explanation for these observed differences could be the timing of analysis. We examined the effects of pretreatment of ICI in response to E2 at 6 h, the time of maximal uterine accumulation of PR mRNA, rather than at 24 h (25). One of the most intriguing findings of the present investigation is that the pretreatment with ICI altered the cell type-specific accumulation of PR mRNA in response to E2 or Kepone. The retention of PR mRNA accumulation in epithelial cells and its attenuation in stromal cells under these experimental conditions suggest that PR mRNA expression in the uterus in response to different estrogenic compounds is dependent upon the cell types involved. Current explanations for estrogen action involve ligand binding to homo- or heterodimers of ERα or ERβ and their activation of genes containing a consensus estrogen response element in their promoter region (Ref. 36 and references therein). However, ER can mediate gene transcription via other enhancer elements, such as activating protein-1 (AP-1) (37). Recent in vitro studies show that antiestrogens, including ICI-164384, and E2 can bind ERα and alter gene expression via an AP-1 site (37). The antiestrogens can also alter gene expression via the AP-1 site when bound to ERβ, but E2 was inhibitory at this site when acting via ERβ. The effects of E2 or Kepone on expression of PR mRNA in stromal cells are probably mediated via the nuclear ERs, as this response was essentially abolished by pretreatment with ICI. If the effect of E2 or Kepone on epithelial cells was directed via an ER action, it should have also been eliminated by the antiestrogen. Furthermore, even an indirect up-regulation of PR mRNA in epithelial cells produced by paracrine effects originating from stromal cells (38) would be expected to be removed by ICI. Therefore, the results suggest that increased PR mRNA expression in epithelial cells by either E2 or Kepone was mediated not by ER but by some other activator(s). The accumulation of uterine PR mRNA also exhibited differential regulation in response to 4-OH-E2. Similar to E2 or Kepone, this catechol estrogen enhanced accumulation of PR mRNA in both epithelial and subepithelial stromal cells, but unlike E2 or Kepone, pretreatment with ICI did not down-regulate the accumulation in stromal cells in response 4-OH-E2; rather an enhanced response in stromal cells was noted, suggesting that the ER-mediated inhibitory effects of ICI had been removed. Clearly, the effects of 4-OH-E2 on PR gene expression, similar to those on the LF gene expression, are not dependent upon ER.
In summary, these studies further establish that environmental reproductive toxins and other estrogens can alter the functions of estrogen-responsive genes in reproductive tissues by mechanisms that are independent of classical ERs. Thus, the affinity of a compound for ERs (ERα or ERβ) should not determine its possible in vivo effects on reproductive functions. The alteration of the LF and PR genes by environmentally relevant doses of xenobiotics may have significant impact on uterine responses at the molecular level. Admittedly, the responses seen in the present study are acute effects, and their relevance to the consequences of chronic exposure, which would be expected for exposure to environmental toxins, remain to be determined.
Footnotes
This work was supported in part by grants from the NIEHS (ES-078140 to Sa.K.D.) and the NICHD (HD-12304 and HD-29968 to Su.K.D.) and core support from NICHD center grants (HD-02528 and HD-33994).
References
- 1.Clarkson TW. Environmental contaminants in the food chain. Am J Clin Nutr [Suppl] 1995;61:682S–686S. doi: 10.1093/ajcn/61.3.682S. [DOI] [PubMed] [Google Scholar]
- 2.Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995;103:582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colborn T, VomSaal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitten PL, Naftolin F. Dietary estrogens: a biologically active background for estrogen action. In: Hochberg RB, Naftolin F, editors. The New Biology of Steroid Hormones. Raven Press; New York: 1990. pp. 155–167. [Google Scholar]
- 5.Kuiper GGJM, Carlson B, Grandien K, Enmark E, Hâggblad J, Nilsson S, Gustafsson J-Å. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 6.Cowley SM, Hoare S, Mosselman S, Parker MG. Estrogen receptors α and β form heterodimers on DNA. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 7.Nelson JA, Struck RF, James R. Estrogenic activities of chlorinated hydrocarbons. J Toxicol Environ Health. 1978;4:325–339. doi: 10.1080/15287397809529664. [DOI] [PubMed] [Google Scholar]
- 8.Robison AK, Mukku VR, Stancel GM. Analysis and characterization of estrogenic xenobiotics and natural products. In: McLachlan JA, editor. Estrogens in the Environment. Elsevier; New York: 1985. pp. 107–115. [Google Scholar]
- 9.Safe SH. Environmental and dietary estrogen and human health: Is there a problem? Environ Health Perspect. 1995;103:346–351. doi: 10.1289/ehp.95103346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bitman J, Cecil HC. Estrogenic activity of DDT analogs and polychlorinated biphenyls. J Agr Food Chem. 1970;18:1008–1112. doi: 10.1021/jf60172a019. [DOI] [PubMed] [Google Scholar]
- 11.Bulger WH, Kupfer D. Estrogenic activity of pesticides and other xenobiotics on the uterus and male reproductive tract. In: Thomas JA, Korach KS, McLachlan JA, editors. Endocrine Toxicology. Raven Press; New York: 1985. pp. 1–34. [Google Scholar]
- 12.Hammond B, Katzenellenbogen BS, Krauthammer N, McConnell J. Estrogenic activity of the insecticide chlordecone (Kepone) and interaction with uterine estrogen receptor. Proc Natl Acad Sci USA. 1979;76:6641–6645. doi: 10.1073/pnas.76.12.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson DC, Sen M, Dey SK. Differential effects of dichlorodiphenyl-trichloroethane analogs, chlordecone, and 2,3,7,8-tetrachlorodebenzo-p-dioxin on establishment of pregnancy in the hypophysectomized rat. Proc Soc Exp Biol Med. 1992;199:42–48. doi: 10.3181/00379727-199-43326. [DOI] [PubMed] [Google Scholar]
- 14.Klotz DM, Beckman BS, Hill SM, McLachlan JA, Walters MR, Arnold SF. Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect. 1996;104:1084–1089. doi: 10.1289/ehp.961041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng CT, Pentecost BT, Chen YH, Newbold RR, Eddy EM, McLachlan JA. Lactoferrin gene expression in the mouse uterus and mammary gland. Endocrinology. 1989;124:992–999. doi: 10.1210/endo-124-2-992. [DOI] [PubMed] [Google Scholar]
- 16.McMaster MT, Teng CT, Dey SK, Andrews GK. Lactoferrin in the mouse uterus: analyses of the preimplantation period and regulation by ovarian steroids. Mol Endocrinol. 1992;5:101–111. doi: 10.1210/mend.6.1.1738363. [DOI] [PubMed] [Google Scholar]
- 17.Power RF, Mani SK, Codina J, Conneel OM, O’Malley BW. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991;254:1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- 18.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;15:3867–3874. [PubMed] [Google Scholar]
- 19.Han JH, Stratowa C, Rutter WJ. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987;26:1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- 20.Das SK, Wang X-N, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 21.Das SK, Chakraborty I, Paria BC, Wang X-N, Plowman GD, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol. 1995;9:691–705. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- 22.Lim H, Dey SK, Das SK. Differential expression of the erbB2 gene in the periimplantation mouse uterus: potential mediator of signaling by epidermal growth factor-like growth factors. Endocrinology. 1997;138:1328–1337. doi: 10.1210/endo.138.3.4991. [DOI] [PubMed] [Google Scholar]
- 23.Das SK, Tsukamura H, Paria BC, Andrews GK, Dey SK. Differential expression of epidermal growth factor receptor (EGF-R) gene and regulation of EGF-R bioactivity by progesterone and estrogen in the adult mouse uterus. Endocrinology. 1994;134:971–981. doi: 10.1210/endo.134.2.7507841. [DOI] [PubMed] [Google Scholar]
- 24.Wang X-N, Das SK, Damm D, Klagsbrun M, Abraham JA, Dey SK. Differential regulation of heparin-binding epidermal growth factor-like growth factor in the adult ovariectomized mouse uterus by progesterone and estrogen. Endocrinology. 1994;135:1264–1271. doi: 10.1210/endo.135.3.8070372. [DOI] [PubMed] [Google Scholar]
- 25.Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 26.Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci USA. 1998;95:696–701. doi: 10.1073/pnas.95.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das SK, Taylor JA, Korach KS, Paria BC, Dey SK, Lubahn DB. Estrogenic responses in estrogen receptor-α deficient mice reveal a novel estrogen signaling pathway. Proc Natl Acad Sci USA. 1997;94:12786–12791. doi: 10.1073/pnas.94.24.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 29.Shen K, Novak RF. DDT stimulates c-erbB2, c-met, and STATS tyrosine phosphorylation, Grb2-Sos association, MAPK phosphorylation, and proliferation of human breast epithelial cells. Biochem Biophys Res Commun. 1997;231:17–21. doi: 10.1006/bbrc.1996.6039. [DOI] [PubMed] [Google Scholar]
- 30.Das SK, Paria BC, Chakraborty I, Dey SK. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc Natl Acad Sci USA. 1995;92:4332–4336. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor β. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 32.Nelson KG, Takahashi T, Bossert NL, Walmer DK, McLachlan JA. Epidermal growth factor replaces estrogen in the stimulation of female genital-tract growth and differentiation. Proc Natl Acad Sci USA. 1991;88:21–25. doi: 10.1073/pnas.88.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green MR, Pastewka JV. Lactoferrin is a marker for prolactin response in mouse mammary explants. Endocrinology. 1978;103:1510–1513. doi: 10.1210/endo-103-4-1510. [DOI] [PubMed] [Google Scholar]
- 34.Aronica SM, Katzenellenbogen BS. Progesterone receptor regulation in uterine cells: stimulation by estrogen, cyclic adenosine 3′,5′-monophosphate, and insulin-like growth factor I and suppression by antiestrogens and protein kinase inhibitors. Endocrinology. 1991;128:2045–2052. doi: 10.1210/endo-128-4-2045. [DOI] [PubMed] [Google Scholar]
- 35.Sumida C, Pasqualini JR. Stimulation of progesterone receptors by phorbol ester and cyclic AMP in fetal uterine cells in culture. Mol Cell Endocrinol. 1990;69:207–215. doi: 10.1016/0303-7207(90)90014-y. [DOI] [PubMed] [Google Scholar]
- 36.Cowley SM, Hoare S, Mosselman S, Parker MG. Estrogen receptors α and β form heterodimers on DNA. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 37.Paech K, Webb P, Kuiper GGJM, Nilsson S, Gustafsson J-Å, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 38.Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]