Abstract
Background & objectives:
The National AIDS Control Organization (NACO) of India has been providing free ARV (antiretroviral) drugs since 2004. By 2012, 486,173 patients had received treatment through the antiretroviral therapy (ART) centres. The objective of this observational study was to assess the factors determining survival of patients on ART under routine programme conditions in an ART centre in north India five years after its inception.
Methods:
Treatment naive HIV positive patients who were enrolled in the ART centre between May 2009 and May 2010 and started on ART as per the Revised NACO guidelines 2009, were included in the study and outcome was assessed after two years of follow up.
Results:
A total of 1689 patients were included in the analysis, of whom 272 (16.10%) expired, 205 (12.13%) were lost to follow up (LFU), 526 (31.14%) were transferred out to other facilities and 686 (40.63%) were alive at the end of two years. Majority (92%) of the deaths occurred in the first six months of therapy. Age >30 yr, male gender, poor functional status, haemoglobin level <11 g/dl, body weight <45 kg and CD4 count <100/μl at baseline had significantly higher relative hazard of death. Most LFU also occurred in the first six months and these patients had significantly low CD4 count, weight, haemoglobin level and higher number of patients in Stages III and IV as compared to those who survived.
Interpretation & conclusions:
The study findings revealed poor survival in the first six months of therapy especially in those with severe immunosuppression. This emphasizes the need for early enrolment into the programme. The high LFU occurring early after initiation of therapy suggests the urgent need to build an efficient patient retrieval system in the programme.
Keywords: Antiretroviral therapy, CD4, HIV/AIDS, survival analysis
Antiretroviral therapy (ART) is now available for 6.65 million people in low- and middle-income countries, accounting for 47 per cent of the 14.2 million people eligible to receive it1. The clinical benefit of ART for AIDS patients in terms of mortality reduction and improved quality of life is well known. The efficacy of ART, as reflected by virological and immunological responses, is similar among patients treated in high-income as well as in resource-limited countries2,3. However, high early mortality after starting ART has been observed in the resource poor setting2. The impact of ART programmes in low-income countries is, therefore, unlikely to be related to questions of drug efficacy, but rather to health system issues and programme effectiveness4.
Several studies have been done to know the predictors of mortality of those accessing ART services. Early survival outcome of ART scale-up services showed advanced clinical stage, anaemia, low body weight, and lack of co-trimoxazole prophylaxis to be independent predictors of mortality in Ethiopia5. In Cameroon CD4 count, haemoglobin, BMI, sex and clinical stage at enrolment predicted increased risk of mortality6. Anaemia, thrombocytopenia and severe malnutrition were found to be important predictors in Tanzania7.
Of the 4.8 million people living with HIV in Asia, nearly half (49%) are in India. It has an estimated 2.4 million (CI: 1.93-3.04) people living with HIV/AIDS (PLHA); with children (<15 yr) accounting 3.5 per cent of all infections, women 39 and 83 per cent in the age group 15-49 yr8. Initiation of free ART services was started in the country in April 2004 by the National AIDS Control Programme (NACO). As on March 2014, there were 425 ART centres functioning throughout the country providing free ARV (antiretroviral) drugs to 7.68 lakh patients9.
Despite this large number of patients accessing ART through National Programme, there are only a few studies to document the outcome of ART in terms of survival and its determinants. Early in the programme a centre from the north showed a mortality of 12 per cent at two years10. A retrospective cohort analysis from an ART centre showed mortality rate at one year to be 7.66 deaths/100 patient-years with > 50 per cent of the deaths occurring during the first three months of ART initiation11.
The ART centre at Banaras Hindu University (BHU), Varanasi, Uttar Pradesh, India, established in 2005, is a large ART centre catering to approximately 16,000 patients with a drainage area covering eastern part of Uttar Pradesh, Bihar and Jharkhand. The objective of this observational study was to assess the factors determining survival of patients on ART under routine programme conditions five years after its inception.
Material & Methods
This study was done between May 2011 to May 2012 in the NACO funded ART centre of Banaras Hindu University, Varanasi, India. The study was approved by the Ethics Committee of the Institute of Medical Sciences, BHU.
All newly diagnosed treatment naive HIV positive patients above 18 years, who were enrolled in the ART centre between May 2009 and May 2010 and started on ART as per Revised NACO guidelines 200912 were included in the study. The follow up was done till May 2012. Patients were started on ART after baseline investigations, if they were in clinical stage I or II with CD4 count <250/μl, or in clinical stage III and CD4 count <350/μl or stage IV irrespective of CD4 count. Zidovudine (AZT) + lamivudine (3TC) + nevirapine (NVP) was the preferred regimen if haemoglobin was >9g/dl, and stavudine (d4T) + lamivudine +nevirapine was given to those with haemoglobin <9 g/dl. Efavirenz (EFV) was substituted for nevirapine in those taking antitubercular drugs and those with nevirapine toxicity.
Patients were followed at 15 days intervals after initiation of ARV and then monthly as per NACO guidelines for a duration of two years. During each visit, patients were counselled for adherence and evaluated for drug toxicity, clinical improvement and opportunistic infections. Patients’ weight, clinical stage, functional status, drug toxicity, adherence to ART medication, presence of opportunistic infection, regimen initiated, any change in therapy were documented in their white card provided by the NACO. At the end of the month, patients were labelled as ‘on treatment’ if they picked up their drugs, ‘missed’ if they did not pick up drugs for the month, dead if they expired and ‘transferred out’ if they were transferred out to another ART centre. Those patients who did not come for three consecutive months were labelled as lost to follow up (LFU) at the end of fourth month. CD4 count was repeated and documented six monthly. All missed patients were called up telephonically by the counsellor or outreach workers in the centre. A list of missed and LFU patients who could not be traced by the centre was given to the community care centre (CCC). Mortality data were taken from hospital records for those who died in the hospital. Those patients who died at home or other hospitals, information was collected from relatives who came to the ART centre or from the records of outreach workers.
Statistical analysis: The data were extracted from the white card analyzed by using SPSS version 16.0 Chicago, USA. All patients were coded as non-surviving or surviving or lost to follow up. The data were presented as mean ± standard deviation for continuous variables and frequency with their respective percentages for categorical variables. Patient characteristics were described in terms of median with their inter quartile range (IQR) for skewed continuous data.
The main outcome variable was death and the time of its occurrence during the 2-year follow up. The data were treated as censored when either patient was lost to follow up or transferred out to other ART centres while calculating survival function. However, if a patient reappeared after discontinuing drug, they were not considered as censored at the time of discontinuation. A sensitivity analysis called ‘the worst-case scenario’ was also done in which all LFUs were classified as ‘deceased’ immediately after their last contact with the centre. The Kaplan-Meier model was used to estimate the survival probability after ART initiation, and survival curves were compared by Log-Rank test; confidence intervals of survival probabilities were also provided. The predictor variables used in the analysis were age, sex, body weight, WHO clinical stage, functional status, CD4 count, haemoglobin, ART drugs and presence of tuberculosis on the day ART was initiated. Variables that were significant (P<0.05) in the bivariate analysis were subsequently considered for multivariate analysis (Cox proportional hazard model) to assess the relationship between these variables and mortality.
Results
A total of 1913 adult HIV positive patients were enrolled during the study period in ART centre, of whom 224 (218 receiving ART medication from outside and 6 for incomplete record) were excluded from the analysis. A total of 1689 patients were included in the analysis, of whom 272 (16.10%) expired, 205 (12.13%) were lost to follow up, 526 (31.14%) were transferred out to other facilities and 686 (40.63%) were alive at the end of two years. A very small number of patients (12, 0.7%) missed their drugs for 1-2 months but re-entered the study and were alive at the end of follow up.
Baseline characteristics of patients are given in Tables I and II. Of the 272 deaths in the 2-year follow up period, most occurred within six months. The probability of survival at 3 months was 87.5 per cent (95% CI: 85.8-88.9), at 6 months was 84.1 per cent (95% CI: 82.2-85.8), at 12 months 82.7 per cent (95% CI: 80.7-84.6) and at 24 months 82.3 per cent (95% CI: 80.3-84.2). The probability of survival decreased till six months only and beyond that it was same throughout the study period (Fig. 1).
Table I.
Baseline characteristics of the cases (N=1689)
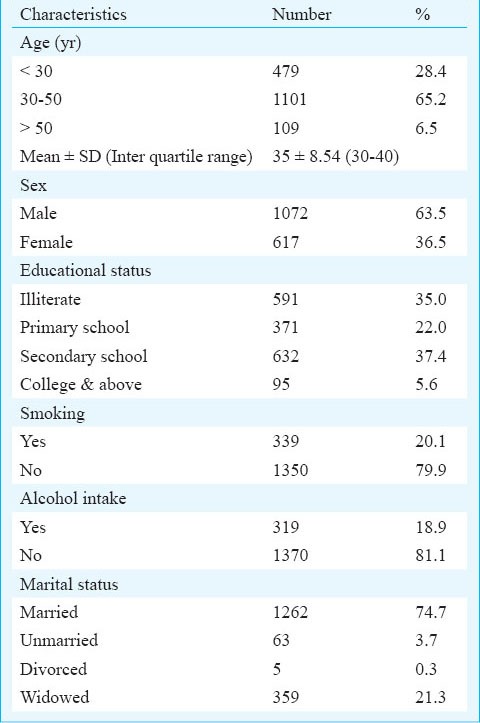
Table II.
Clinical characteristics of the cases at initiation of ART at baseline (N=1689)
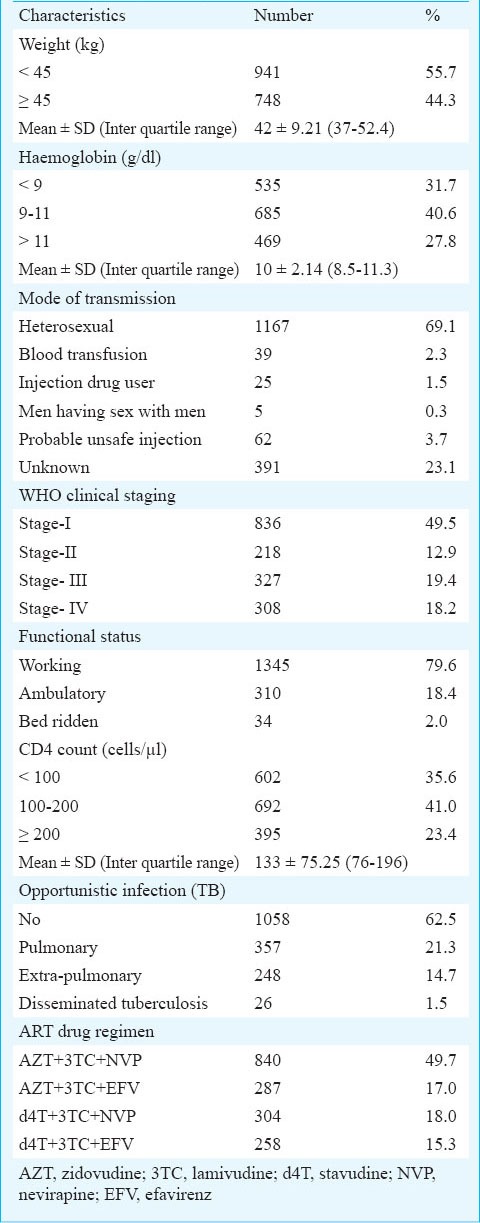
Fig. 1.
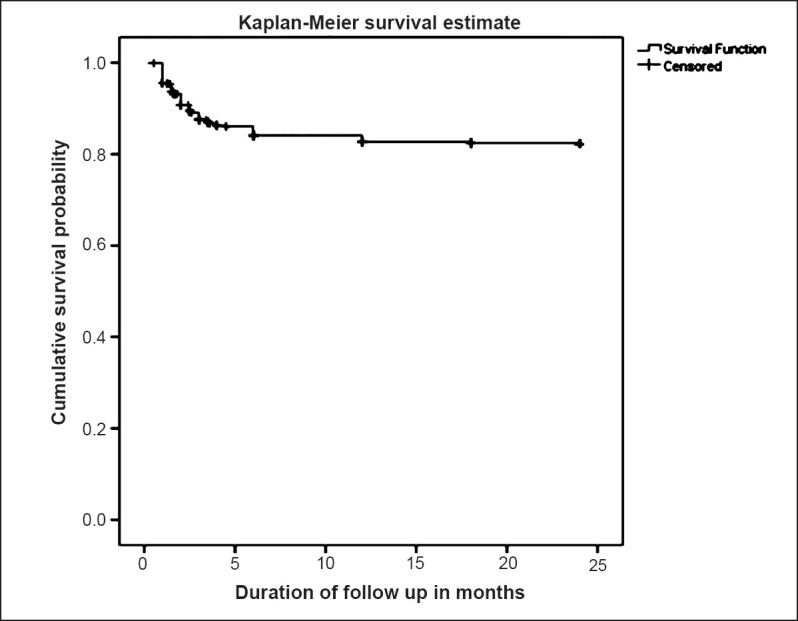
Survival probability of patients of ART in months.
The rate of occurrence of death during the follow up period was 14/100 person-years. In the worst-case scenario, considering all those lost to follow up as deceased immediately after the last date of contact, mortality rate was estimated at 27 per 100 person-years at risk.
The Kaplan Meir survival function (Table III) indicated significantly higher survival of patients with baseline characteristics of age below 30 yr, females (88%; 95% CI: 86-91), weight ≥ 45 kg (86%; 95% CI: 83-88), clinical stage 1 and 2 (86%; 95% CI: 83-88), functional status working (85%; 95% CI: 83-87), and haemoglobin level >11g/dl. CD4 count showed significantly poor survival when it was below 100 cells/μl3. Patients who had tuberculosis at baseline had lower survival (79%) as compared to those who did not (84%). A total of 357 patients had pulmonary tuberculosis, 248 had extra-pulmonary tuberculosis and 26 patients had disseminated tuberculosis. Among patients with extra-pulmonary tuberculosis, 133 had abdominal tuberculosis, 50 had pleural effusion, 34 had tubercular meningitis, 27 had lymph node tuberculosis and four had bone tuberculosis.
Table III.
Probability of survival beyond two years (Kaplan Meier method)
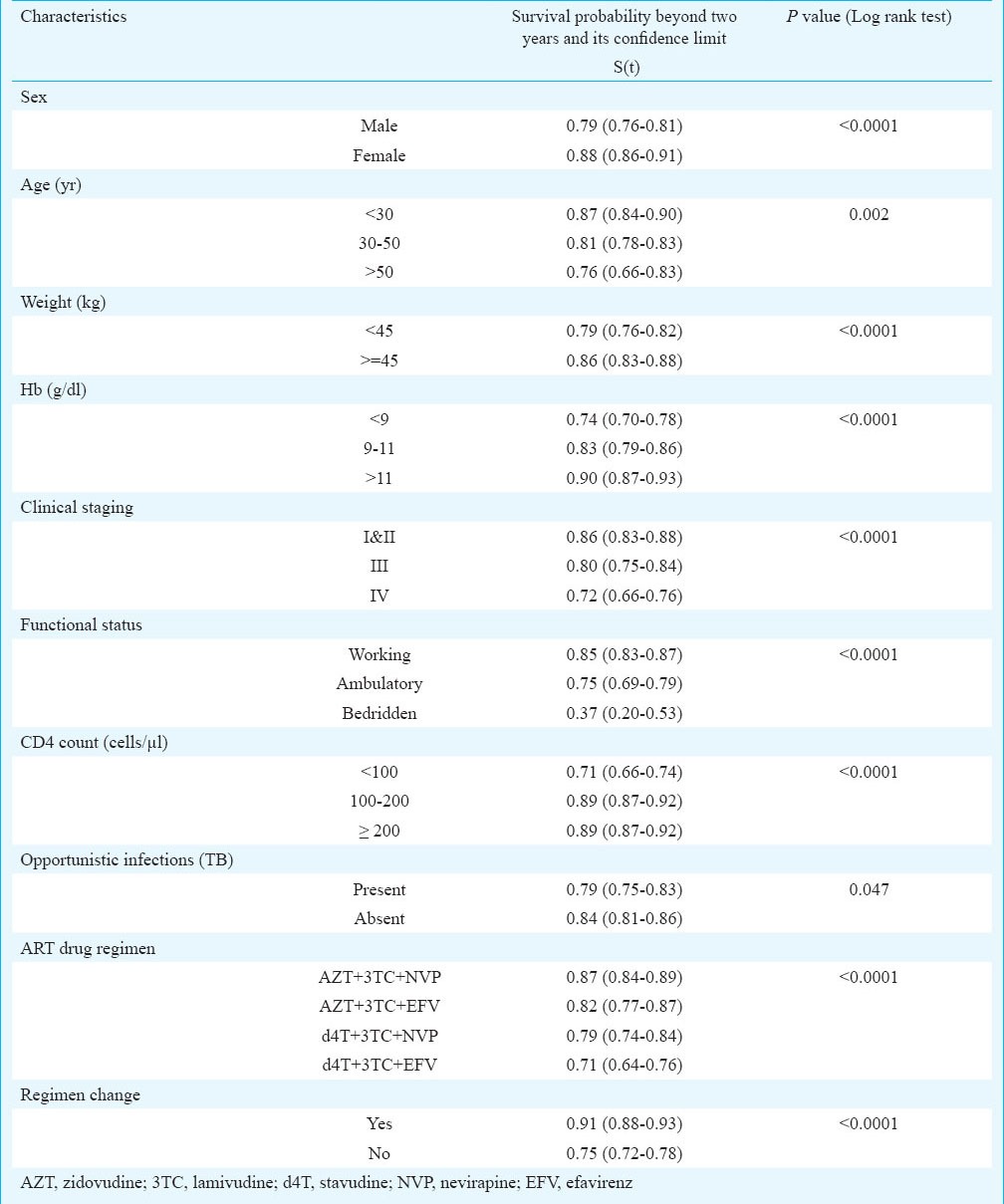
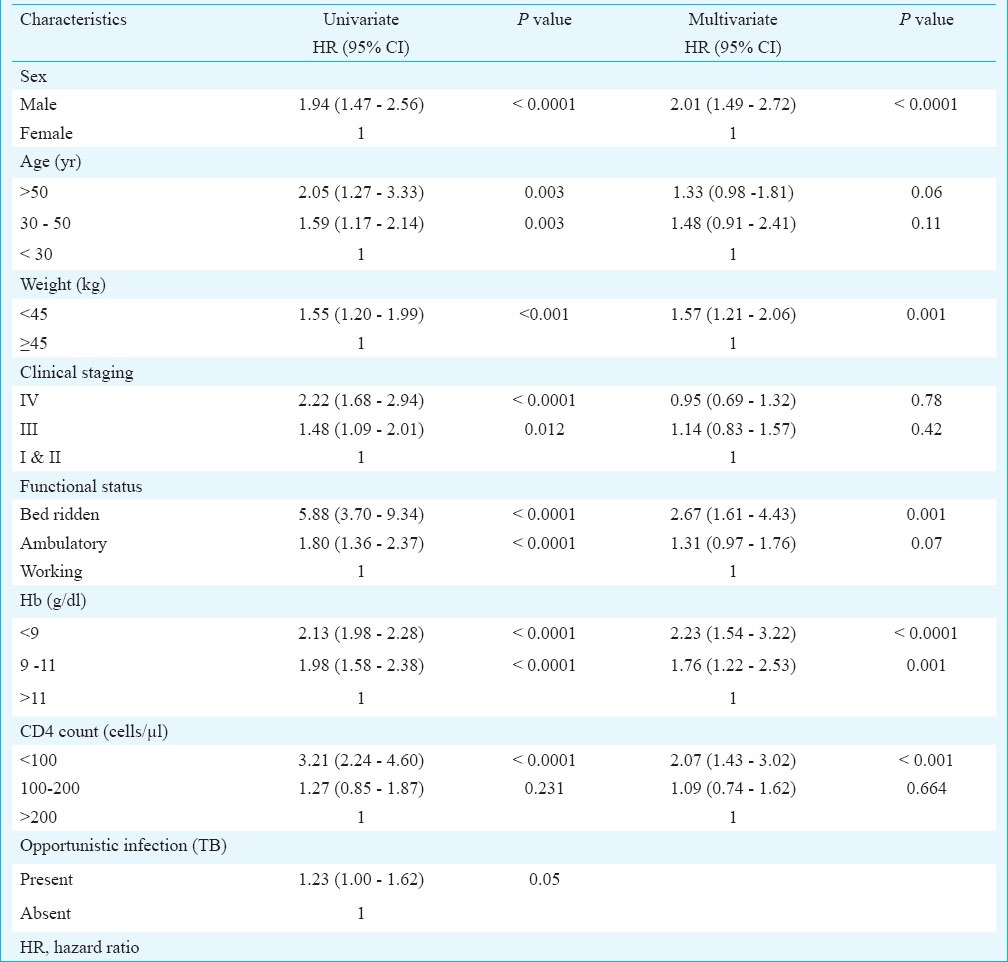
Multivariate analysis was done on all those predictors found significantly associated with survival in univariate analysis. All characteristics except clinical stage, ART drug regimen and presence of tuberculosis were found to significantly affect survival of the patients (Fig. 2). The hazard of death was 2.01 (CI: 1.49-2.72) times higher in males than females, 1.33 (CI: 0.98-1.81) and 1.48 (CI: 0.91-2.41) times higher in patients of age 30-50 yr and >50 yr, respectively as compared to patients of age < 30 yr, 1.57 (CI: 1.21-2.05) times higher in those weighing less than 45 kg than those weighing more than 45 kg, 1.76 (CI: 1.22-2.53) and 2.23 (CI: 1.54-3.22) times higher in patients with haemoglobin level 9-11 and <9 g/dl, respectively when compared with those of haemoglobin level ≥11 g/dl. The risk of death among cases with CD4 count <100/μl was 2.07 (CI: 1.43-3.20) times higher compared to patients with CD4 count ≥200/μl; while the risk was almost similar in those with CD4 count 100-200 μl (Table IV).
Fig. 2.
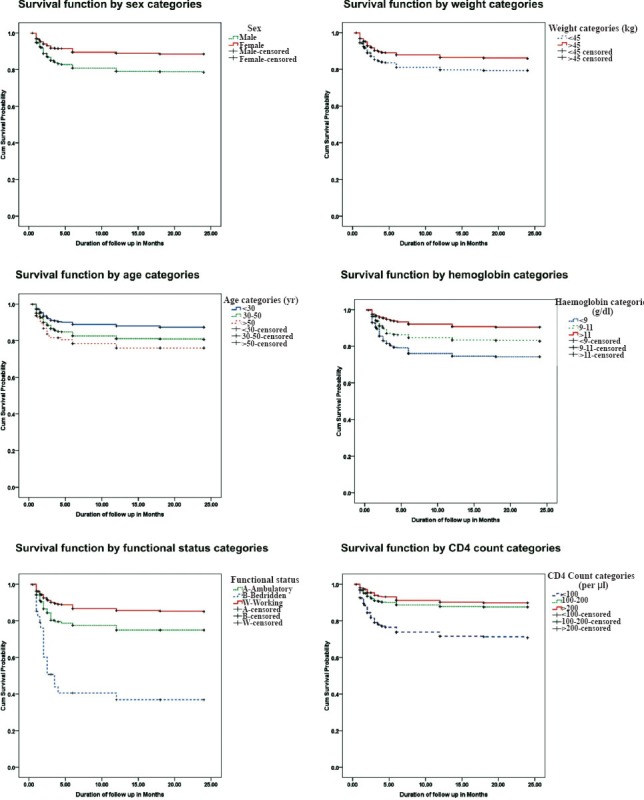
Survival function by sex, weight, age, haemoglobin, functional status and CD4 count category.
Table IV.
Hazard ratios of mortality according to baseline characteristics of HIV patients on ART
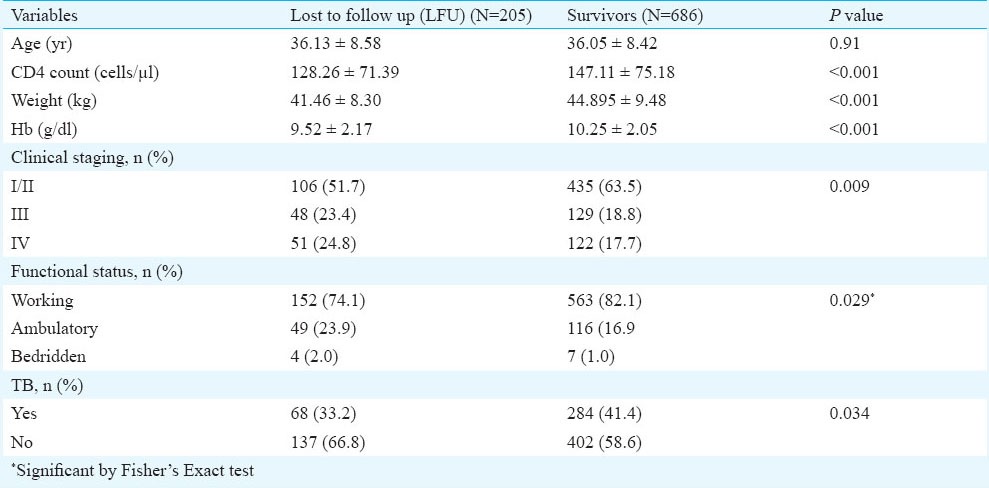
The median follow up time for lost to follow up cases was around five months indicating that most cases were lost in the first six months. On comparing the baseline characteristics of LFU cases it was observed that they had significantly low CD4 count, weight and haemoglobin level and higher number of patients in stages III and IV and poor functional status as compared to those who survived (Table V). Cause of death was ascertained only in 129 (47.4%) patients. Tuberculosis 71 (41.2%), diarrhoea 18 (13.9%) were the common causes of death.
Table V.
Comparison of lost to follow up and survivors
Discussion
Our study showed that the mortality in patients on ART was 14/100 person-years which was higher than a study from eastern India11 but similar to another study from western part of the country13. One reason for this variation in mortality rates could be the time of enrolment into the ART care. Critically ill HIV positive patients who are admitted for inpatient care are enrolled in many ART centres only if they survive, which may be a reason for the variation in mortality rates in different centres. However, a study on treatment outcomes of patients enrolled in 2004-2005 in the national programme showed a similar mortality (13%)14 as found in our study (16.1%) in which the patients were enrolled in the same programme five years later. In our study 31 per cent patients were transferred out to other ART centres as during the study period five new ART centres were started in the neighbouring districts.
In the first six month of therapy, 92 per cent of the deaths occurred, indicating a very high early mortality in our patients. Studies both from India as well as other resource poor countries have also shown high early mortality among patients accessing ART care2,11,14,15. Male sex, higher age, low weight, poor functional status, presence of anaemia and CD4 <100/μl at the time of enrolment were the main predictors of mortality in the present study.
Male sex was a predictor of mortality with a risk almost double that of female sex in our study as well as a study from Cameroon6. In this part of the country the disease is common in migrant population. It is usually the males who acquire the disease from regions of high prevalence and transmit it to their female partners. Females are usually tested after their spouses are symptomatic, leading to earlier diagnosis and treatment of females which could be a reason for their decreased risk of mortality. Older age at presentation also increased the risk of death in our study which has also been observed in other studies13,16,17.
Even after five years of the establishment of this ART centre 35 per cent of the patients enrolling into ART care had a CD4 count <100/μl, 37.6 per cent were in stage III/IV, 32 per cent had haemoglobin levels <9g/dl and 55 per cent had weight <45 kg suggesting late presentation. Thus, efforts are needed to bring patients under ART care early. This can be done by increasing awareness among public which is an important component of the National Programme. However, special attention should be given to educate the health care professionals especially in the private sector as approximately 75 per cent of the patients who are referred to the ART centre (unpublished data ART centre BHU) are from the private practitioners.
Although tuberculosis was associated with increased mortality in the univariate analysis, it did not show significant increase in the multivariate analysis. Among the patients who died, 41 per cent had tuberculosis and it was the most common cause of death.
One of the important findings was a high lost to follow up in our study which was also reported in other studies from India10,14. The baseline characteristics of LFU patients were a lower CD4 count, weight and haemoglobin levels and higher number of patients with poor functional status and stage III and IV condition as compared to survivors, suggesting severe immunosuppression in this subset. Most of these characteristics are also predictors of mortality. A meta-analysis of studies that determined the vital status of patients who were LFU after starting ART in low or middle-income countries showed that among African adults the combined mortality among LFUs was 46 per cent ranging from 12 to 87 per cent across studies18. This suggests that ART programmes with high rates of LFU may be underestimating mortality. Thus a major effort is needed not only to trace LFUs but to start a rigorous mechanism to trace patients early when they have missed their monthly dose. Active tracing with the help of District Level Network of PLHIV and Integrated counselling and testing centres (ICTC) counsellors in the district of patients residence would go a long way in improving patient retention in the programme. Regular updating of patients contact details including phone numbers needs to be done at the ART centre. A study to analyse the individual reasons leading to lost to follow up will greatly help the programme.
References
- 1.WHO, unparalleled global progress in HIV response but sustained investment vital. [accessed on January 25, 2013]. Available from: http://www.who.int/mediacentre/news/releases/2011/hiv_20111130/en .
- 2.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 3.Ivers LC, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis. 2005;41:217–24. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 4.Lawn SD, Myer L, Wood R. Efficacy of antiretroviral therapy in resource poor settings: are outcomes comparable to those in the developed world? Clin Infect Dis. 2005;41:1683–4. doi: 10.1086/498030. [DOI] [PubMed] [Google Scholar]
- 5.Alemu AW, Sebastián MS. Determinants of survival in adult HIV patients on antiretroviral therapy in Oromiyaa, Ethiopia. Glob Health Action. 2010;3:5398. doi: 10.3402/gha.v3i0.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sieleunou I, Souleymanou M, Schonenberger AM, Menten J, Boelaert M. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far-North Province, Cameroon. Trop Med Int Health. 2009;14:36–43. doi: 10.1111/j.1365-3156.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- 7.Johannessen A, Naman E, Ngowi BJ, Sandvik L, Matee MI, Aglen HE, et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52. doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National AIDS Control Organization (NACO). Department of AIDS Control, Ministry of health and family welfare. Annual report 2009-2010. [accessed on January 27, 2013]. Available from: http://www.avertsociety.org/docs/NACO_AR_English.pdf .
- 9.National AIDS Control Organization (NACO). Department of AIDS Control, Ministry of Health and Family Welfare. Annual report 2013-14. [accessed on November 1, 2014]. Available from: http://www.naco.gov.in/upload/2014%20mslns/NACO_English%202013-14.pdf .
- 10.Sharma SK, Dhooria S, Prasad KT, George N, Ranjan S, Gupta D, et al. Outcomes of antiretroviral therapy in a northern Indian urban clinic. Bull World Health Organ. 2010;88:222–6. doi: 10.2471/BLT.09.068759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhowmik A, Bhandari S, De R, Guha SK. Predictors of mortality among HIV-infected patients initiating anti retroviral therapy at a tertiary care hospital in Eastern India. Asian Pac J Trop Med. 2012;5:986–90. doi: 10.1016/S1995-7645(12)60187-4. [DOI] [PubMed] [Google Scholar]
- 12.Department of AIDS Control, National AIDS Control Organization, Ministry of Health and Family Welfare. Care Support & Treatment: National AIDS Control Programme, Phase-III, India. [accessed on January 27, 2013]. Available from: http://www.naco.gov.in/upload/IEC%20Division/NACO%20monographs%20for%20Vienna/ART%20Monograph.pdf .
- 13.Ghate M, Deshpande S, Tripathy S, Godbole S, Nene M, Thakar M, et al. Mortality in HIV infected individuals in Pune, India. Indian J Med Res. 2011;133:414–20. [PMC free article] [PubMed] [Google Scholar]
- 14.Bachani D, Garg R, Rewari BB, Hegg L, Rajasekaran S, Deshpande A, et al. Two-year treatment outcomes of patients enrolled in India's national first-line antiretroviral therapy programme. Natl Med J India. 2010;23:7–12. [PubMed] [Google Scholar]
- 15.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and non death losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–6. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 16.Martin JN, Colford JM, Jr, Ngo L, Tager IB. Effect of older age on survival in human immunodeficiency virus (HIV) disease. Am J Epidemiol. 1995;142:1221–30. doi: 10.1093/oxfordjournals.aje.a117581. [DOI] [PubMed] [Google Scholar]
- 17.Chaisson RE, Keruly JC, Moore RD. Race, sex, drug use, and progression of human immunodeficiency virus disease. N Engl J Med. 1995;333:751–6. doi: 10.1056/NEJM199509213331202. [DOI] [PubMed] [Google Scholar]
- 18.Brinkhof MW, Pujades Rodriguez M, Egger M. Mortality of patients lost to follow up in antiretroviral treatment programmes in resource limited settings: systematic review and meta-analysis. PLoS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]


