Sir,
Pseudomonas aeruginosa accounts for approximately 10 per cent of all nosocomial infections and is associated with widespread antibiotic resistance1,2,3. Inappropriate selection of antibiotics leads to increased mortality and, hence, management of this infection needs to be tailored according to local antibiogram patterns4,5. Cefepime is one of the few antibiotics reported to have consistent anti-pseudomonal activity, though reports on cefepime resistance are rising6,7,8. We, therefore, analyzed the susceptibility pattern of P. aeruginosa to cefepime and compared the same with other anti-pseudomonal antibiotics.
This prospective study was performed in the department of Microbiology, Himalayan Institute of Medical Sciences, Dehradun, Uttrakhand, India during March 2010 to February 2012. The study protocol was approved by the institutional ethics committee. All consecutive patients with positive P. aeruginosa culture were included in the study. The calculation of sample size was based on presumptive prevalence of 40-50 per cent resistance among the recovered P. aeruginosa isolates to third-generation cephalosporins9,10,11. The optimum sample size calculated was between 600 and 617. Antimicrobial sensitivity, with commercial discs Himedia, India was tested against piperacilin, piperacillin-tazobactam, ceftazidime, cefepime, ciprofloxacin, gentamicin, amikacin and imepenem by Kirby-Bauer method, following the recommendations of Clinical and Laboratory Standards Institute (CLSI)12. ATCC 25873 strain of P. aeruginosa was used as control and interpretation of antimicrobial sensitivity was done according to interpretive criteria published in CLSI guidelines.
Proportions of sensitive isolates recovered from out-patients and in-patients were compared using Chi-square test. Sensitivity to cefepime vis-a-vis other antibiotics was compared using Chi-square test and Fisher's exact test, wherever appropriate. SPSS version 17.0 (Chicago: SPSS Inc. USA) was used and P<0.05 was considered significant.
A total of 618 consecutive isolates of P. aeruginosa, recovered from as many patients, were included. The median (5th - 95th percentile) age of the patients was 68 (range 45-88) years. The isolates were recovered from a variety of specimens, including pus (n= 249), urine (n=123), endotracheal secretions (n=84), throat swab (n=12), sputum (n=31), trans-tracheal secretions (n=49), bronchoalveolar lavage fluid (n=27), double lumen jugular catheter (n=24) and eye discharge (n=19). Approximately two-thirds of the isolates were recovered from in-patients (n= 408; 66%).
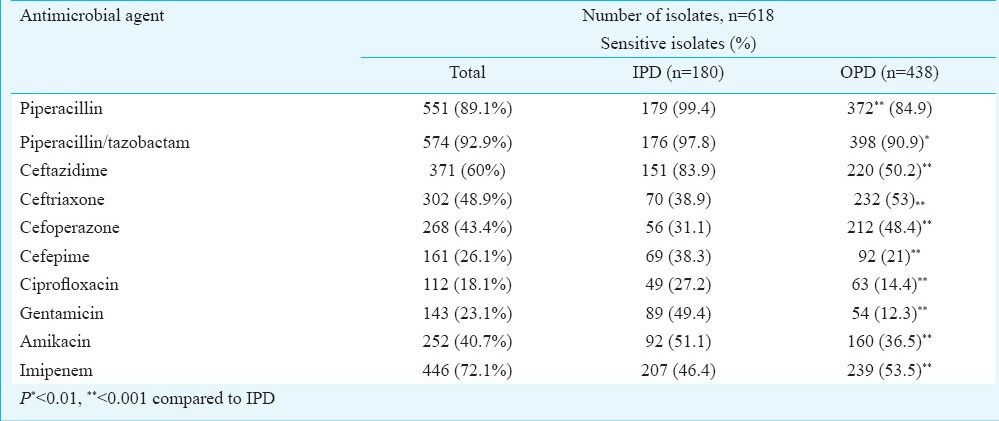
We observed sensitivity to piperacillin-tazobactam in >90 per cent isolates (n= 574, 92.9%), followed by piperacillin (n=551, 89.1%) and imipenem (n=446, 72.1%). Moderate sensitivity, ranging from 40.7 to 60 per cent, was observed for amikacin and the third-generation cephalosporins. However, cefepime demonstrated poorer anti-pseudomonal activity compared to each of the third-generation cephalosporins (Table I). Considering the isolates from out-patients and in-patients to be representatives of community-acquired and nosocomial isolates respectively, we observed that a significantly higher proportion of nosocomial isolates were resistant to ceftriaxone (48.93%) and cefoperazone (43.4%). Resistance was significantly higher (P<0.001) among community-acquired isolates for majority of the antibiotics (Table I).
Table I.
Susceptibility pattern of P. aeruginosa isolates to anti-pseudomonal antibiotics from OPD (Out patient department) and IPD (Inpatient department)
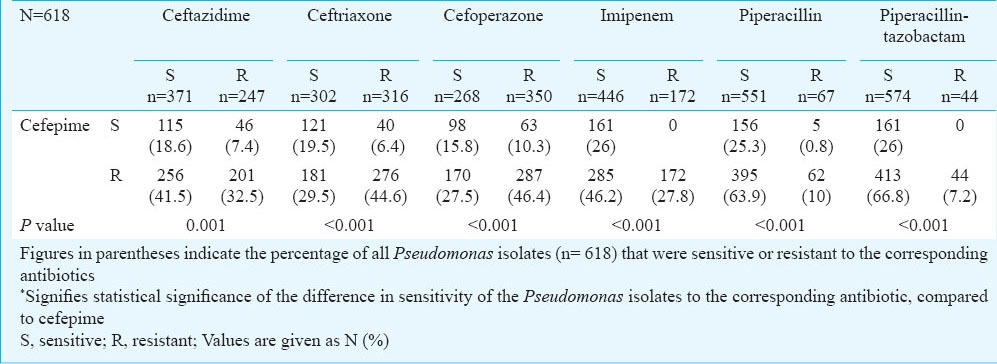
A significantly higher proportion of isolates was resistant to cefepime and sensitive to other antibiotics. Piperacillin-tazobactam, piperacillin and imipenem were most effective against cefepime-resistant isolates (Table II). Resistance to all cephalosporins was seen in 74 (11.9%) isolates, of which 44 (59.5%), 61 (82.4%) and 58 (78.3%) were sensitive to piperacillin, piperacillin-tazobactam and imipenem, respectively, thereby further validating the anti-pseudomonal efficacy of piperacillin-tazobactam.
Table II.
Comparison of the anti-pseudomonal activity of cefepime vis-à-vis other antibiotics
An alarming level of resistance was observed to almost all anti-pseudomonal antibiotics with the community-acquired P. aeruginosa isolates demonstrating higher resistance compared to hospital isolates. We had earlier reported antibiotic resistance in a relatively higher proportion of P. aeruginosa isolates recovered from this region9,13. In this study a higher percentage of antibiotic resistance was seen among community-acquired isolates, though the mechanism underlying this phenomenon is unclear. The reason could be an indiscriminate use of antibiotics in the local community.
The considerably higher resistance to cefepime, compared than to the older third-generation cephalosporins was also unexpected. Compared to third-generation cephalosporins, the fourth-generation cephalosporins are poor inducers of, and relatively resistant to type I chromosomally-encoded and some plasmid-mediated β-lactamases and extended-spectrum β-lactamases14. Consequently these are traditionally described to have an extended antibacterial spectrum and have been recommended for empirical treatment of serious infections in hospitalized patients, in whom Gram-positive microorganisms, Enterobacteriaceae, and Pseudomonas are potential pathogens7,8. Furthermore, cefepime has been reported to have comparable activity to ceftazidime for P. aeruginosa14. Cefepime is also recommended as the drug of choice in febrile neutropenic patients in whom Pseudomonas is a predominant pathogen15. Similar studies need to be done in other parts of the country to assess the actual situation. Further studies should be conducted to elucidate the predominant mechanisms of resistance to β-lactam antibiotics among such isolates. These mechanisms might hint at novel therapeutic targets for the better management of infections due to multi-drug resistant strains of Pseudomonas.
Acknowledgment
This study was supported by partial funding from Indian Council of Medical Research (ICMR), New Delhi.
References
- 1.Gaynes R, Edwards JR. Overview of nosocomial infections caused by Gram-negative bacilli. Clin Infect Dis. 2005;41:848–54. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 2.Fluit AC, Jones ME, Schmitz FJ, Acar J, Gupta R, Verhoef J. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY antimicrobial surveillance program, 1997 and 1998. Clin Infect Dis. 2000;30:454–60. doi: 10.1086/313710. [DOI] [PubMed] [Google Scholar]
- 3.Streit JM, Jones RN, Sader HS, Fritsche TR. Assessment of pathogen occurrences and resistance profiles among infected patients in the intensive care unit: report from the SENTRY Antimicrobial Surveillance Program (North America, 2001) Int J Antimicrob Agents. 2004;24:111–8. doi: 10.1016/j.ijantimicag.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother. 2005;49:1306–11. doi: 10.1128/AAC.49.4.1306-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidal F, Mensa J, Almela M, Martínez JA, Marco F, Casals C, et al. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch Intern Med. 1996;156:2121–6. [PubMed] [Google Scholar]
- 6.Sader HS, Fritsche TR, Jones RN. Potency and spectrum trends for cefepime tested against 65,746 clinical bacterial isolates collected in North American medical centers: results from the SENTRY Antimicrobial Surveillance Program (1998–2003) Diagn Microbiol Infect Dis. 2005;52:265–73. doi: 10.1016/j.diagmicrobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Croughs PD, Li B, Hoogkamp-Korstanje JA, Stobberingh E. Thirteen years of antibiotic susceptibility surveillance of Pseudomonas aeruginosa from intensive care units and urology services in the Netherlands. Eur J Clin Microbiol Infect Dis. 2013;32:283–8. doi: 10.1007/s10096-012-1741-4. [DOI] [PubMed] [Google Scholar]
- 8.Robinson CA, Kuhn RJ, Craigmyle J, Anstead MI, Kanga JE. Susceptibility of Pseudomonas aeruginosa to cefepime versus ceftazidime in patients with cystic fibrosis. Pharmacotherapy. 2001;21:1320–4. doi: 10.1592/phco.21.17.1320.34420. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar B, Biswas D, Prasad R, Sharma JP. A clinic-microbiological study on the importance of Pseudomonas in nosocomially infected ICU patients, with special reference to metallo beta1-lactamase production. Indian J Pathol Microbiol. 2006;49:44–8. [PubMed] [Google Scholar]
- 10.Sivanmaliappan TS, Sevanan M. Antimicrobial susceptibility patterns of Pseudomonas aeruginosa from diabetes patients with foot ulcers. Int J Microbiol 2011. 2011 doi: 10.1155/2011/605195. 605195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goswami NN, Trivedi HR, Goswami AP, Patel TK, Tripathi CB. Antibiotic sensitivity profile of bacterial pathogens in postoperative wound infections at a tertiary care hospital in Gujarat, India. J Pharmacol Pharmacother. 2011;2:158–64. doi: 10.4103/0976-500X.83279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wayne, PA: Clinical and Laboratory Standards Institute; 2007. CLSI. Performance standards for antimicrobial susceptibility testing; Seventeenth informational supplement. CLSI Document M100- S17. [Google Scholar]
- 13.Arya M, Prasad R, Arya PK, Gupta P, Biswas D. Antibiotic susceptibility of Pseudomonas aeruginosa isolates in Uttaranchal- a Hospital based study. Indian Med Gazette. 2003;137:4–6. [Google Scholar]
- 14.Petri WA. Antimicrobial agents: Penicillins, cephalosporins, and other β-lactam antibiotics. In: Hardman JG, Limbird LE, Goodman Gilman A, editors. Goodman & Gilman's the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill; 2001. pp. 1189–218. [Google Scholar]
- 15.Tamar F, Kasper DL. Approach to the acutely Ill infected febrile patient. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison's principles of internal medicine. 18th ed. I. New York: McGraw-Hill; 2008. pp. 1023–30. [Google Scholar]


