Infection with Babesia microti can cause severe illness, particularly among asplenic individuals. Blacklegged and Western blacklegged ticks (Ixodes scapularis and Ixodes pacificus, respectively) are the vector through which B microti is transmitted. The distribution of these ticks in Canada has increased over the past several years. In this article, the authors present the first case of babesiosis in Canada that was not diagnosed following travel to an area in which the disease is endemic.
Keywords: Babesia microti, Babesiosis, Blacklegged ticks, Canada, Emerging infection, Local acquisition
Abstract
A child with a complicated medical history that included asplenia acquired an infection with Babesia microti in the summer of 2013 and had not travelled outside of Manitoba. Although the clinical findings were subtle, astute laboratory work helped to reach a preliminary identification of Babesia species, while reference laboratory testing confirmed the diagnosis. Blacklegged ticks (Ixodes scapularis) are known to transmit Borrelia burgdorferi and Anaplasma phagocytophilum in the province; however, the present case represents the first known instance of tick-borne B microti, both in Manitoba and in Canada. The expanding territory of the blacklegged tick increases the relevance of this emerging infection. Clinicians, laboratory medical practitioners and public health officials should be aware of B microti as a potential locally acquired infection in Canada.
Abstract
Un enfant ayant des antécédents médicaux complexes, qui incluaient une asplénie, a contracté une infection à Babesia microti pendant l’été 2013, sans avoir quitté le Manitoba. Même si les résultats cliniques étaient discrets, un travail de laboratoire astucieux a contribué à l’identification préliminaire d’une espèce de Babesia. Le test du laboratoire de référence a confirmé le diagnostic. On sait que les tiques occidentales à pattes noires (Ixodes scapularis) transmettent le Borrelia burgdorferi et l’Anaplasma phagocytophilum dans la province. Le présent cas est toutefois la première occurrence connue de B microti à tique, tant au Manitoba qu’au Canada. L’expansion du territoire de la tique occidentale à pattes noires renforce la pertinence de cette infection émergente. Les cliniciens, les praticiens de laboratoires médicaux et les directeurs de la santé publique devraient savoir que le B microti peut être transmis localement au Canada.
CASE PRESENTATION
A seven-year-old boy presented to the emergency department at the Winnipeg Children’s Hospital (Winnipeg, Manitoba) on August 7, 2013, with a five-day history of fever (up to 39.5°C) and a headache. He also complained of mild anorexia and malaise. He experienced no other meningeal or respiratory tract symptoms and there was no nausea, vomiting or diarrhea. His urine output was maintained, although urine was darker than normal. He did not complain of arthralgias, arthritis or myalgias. No rash, jaundice or icterus had been noted by his parents. His medical history consisted of multiple congenital anomalies related to a midline defect syndrome that had not been formally diagnosed. These consisted of hydrocephalus treated with a ventriculoperitoneal shunt; panhypopituitarism; partially corrected tetrology of Fallot and dextrocardia; and asplenia secondary to mid-gut malrotation, which was surgically corrected at two weeks of age. The patient had travelled with his relatives to the southeast corner of Manitoba to stay at a cabin four weeks before the onset of symptoms. He did not recall specific tick bites but had numerous mosquito bites during the 48 h he was there. He did not report any other animal exposures. The patient had received blood transfusions for his surgeries during his first month of life, but not after.
Screening blood tests, including electrolyte, urea and creatinine levels, were all within normal limits. His white blood cell count, hemoglobin and platelet levels were also normal. A manual slide review was performed due to abnormalities consistent with his asplenia, and a parasite believed to represent Plasmodium falciparum was noted. Blood smears were produced using the remaining blood sample. Numerous ring-form trophozoite parasites were observed within erythrocytes, and a lack of pigment and occasional tetrads/Maltese cross formations were noted (Figure 1). Based on these findings, and a lack of a significant travel history, identification was deemed to be consistent with Babesia species.
Figure 1).
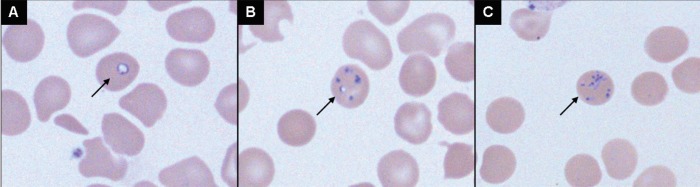
Babesia species in a thin blood smear stained with Giemsa (oil, original magnification ×1000). Vacuolated intraerythrocytic form (A), multiple forms within erythrocyte (B) and tetrads (C) are indicated
Twenty-four hours after initial evaluation, the patient was notified to return to the emergency department and the Pediatric Infectious Diseases Service was consulted. At this point, the patient was asymptomatic and the parasitemia level was determined to be 1%. He was diagnosed with mild babesiosis and prescribed a six-week course of atovaquone and azithromycin. Serology testing for Borrelia burgdorferi was ordered and found to be negative. Follow-up bloodwork was performed one week into his treatment course. At that time, the patient continued to have headache and intermittent, nonspecific abdominal pain. A mild anemia and slightly increased transaminase levels and bilirubin were noted. A blood specimen was collected and sent to the National Microbiology Laboratory for confirmation of Babesia microti infection, and to rule out infection with B burgdorferi and/or Anaplasma phagocytophilum. While polymerase chain reaction (PCR) was negative for the latter two organisms, real-time PCR was performed using primers that target the chaperonin-containing t-complex eta (CCTη) (1) and subsequently confirmed using a second real-time PCR assay targeting the 18S ribosomal RNA gene (in-house/Applied Biosystems, USA). To generate sequence data, nested PCR was performed using primers for the 18S ribosomal RNA gene (2) and the sequence of this product was compared with those in GenBank (National Center for Biotechnology Information, www.ncbi.nlm.nih.gov/genbank/), confirming the diagnosis. The patient was medically reassessed every two weeks for the six weeks of his therapy. The medication was well tolerated, with only mild gastrointestinal intolerance. Aside from the previously mentioned mild laboratory abnormalities, the patient responded well to treatment. On final follow-up visit at six weeks, the patient had fully recovered to baseline activity and health.
DISCUSSION
Babesiosis is a rare intraerthrocytic parasitic disease in Canada, caused primarily by B microti in North America and Babesia divergens in Europe, though cases of babesiosis have also been attributed to Babesia duncani on the Pacific Coast of the United States. It is primarily transmitted to humans through the bites of nymphal or adult female black-legged and Western blacklegged ticks (3), Ixodes scapularis and pacificus, respectively. These tick species also serve as the vector for B burgdorferi and A phagocytophilum (4). A diverse variety of mammal species can be infected and serve as reservoirs for B microti (5), although in most localities white-footed mice, Peromyscus leucopus, appear to be the most important. States in close proximity to Manitoba have reported numerous cases of babesiosis (6–8). For example, Minnesota reported 41 cases of confirmed or probable babesiosis in 2012 (6) while Wisconsin had 46 in 2011 (7). North Dakota reported a single case of babesiosis in 2011 (8). A population of blacklegged ticks, I scapularis, infected with the agents of Lyme disease, B burgdorferi, and anaplasmosis, A phagocytophilum, has been known to be established at the location where our patient had stayed, since 2006 (4). Since 2010, blacklegged ticks infected with B microti have been collected from six different localities in Manitoba where populations of these ticks have recently become established. The overall prevalence of B microti detected in blacklegged ticks collected from the established populations in Manitoba was 1.8% (six of 326 tested); however, at sites where B microti was detected, the prevalence was as high as 10%. B microti has also been detected in the tissues from field-collected rodents at two of these localities. Comparison of sequence data from the B microti DNA from the ticks and rodents, as well as from the reported case, revealed at least 98% homology with those in GenBank, indicating that the strains of B microti in Manitoba are similar to those reported in the United States. The detection of B microti in blacklegged ticks in multiple years and from the tissues of resident rodents suggests that this pathogen has become endemic in some localities in the province. In addition, all blacklegged ticks submitted through the ongoing passive tick surveillance program in Canada (9) have been screened for B microti infection since late 2012 and infected ‘bird-borne’ ticks have been observed in Ontario (n=1), although the prevalence of infection is very low. These surveillance data support the interpretation that the risk of human exposure to B microti-infected blacklegged ticks is currently low across much of central and eastern Canada; however, this pathogen is likely becoming endemic in some of many recently established blacklegged tick populations in Canada (10) including those in Manitoba.
Excellent descriptions of key clinical features and laboratory investigations for babesiosis have been summarized elsewhere (3,11,12).
This represents the first confirmed case of tick-borne B microti infection acquired within Canada. Imported (13) and transfusion-acquired (14) B microti infections have been previously described. Locally acquired human babesiosis in Canada has important implications for clinical, laboratory and transfusion medicine as well as public health. Babesiosis remains a rare infection in Canada. Clinically, it should be considered in individuals at highest risk, including those at the extremes of age (<1 year of age and >50 years of age), the immunocompromised (congenital, HIV-infected, organ/blood and marrow transplant or immunosuppressive/chemotherapeutic medications), and in asplenic individuals in particular. Persistent fever without diagnosis, flu-like illness during spring and summer months (outside of influenza season), and an appropriate travel history, history of exposure to blacklegged ticks and history of blood transfusion in the preceding six months are key features that should prompt clinicians to consider babesiosis. Laboratory practitioners should suspect B microti when pleomorphic and predominantly intraerythrocytic parasites with hyperparasitized erythrocytes are noted. It is further distinguished from P falciparum by the lack of pigment or gametocytes. Intraerthrocytic or Maltese cross tetrads are highly suggestive of Babesia species, particular when other clinical information is inconsistent with P falciparum. Microscopic diagnosis of B microti should be supplemented with PCR-based confirmation by reference laboratories capable of full characterization. Clinicians are encouraged to provide appropriate travel and blacklegged tick exposure history on laboratory requisitions to assist the laboratory in prioritization of appropriate testing. B microti seroprevalence studies and screening of I scapularis populations in Canada are critical tools to identify emerging areas of endemicity and keep practitioners informed of the risks patients may face. Currently, babesiosis is not a nationally notifiable disease but it is reportable in Manitoba. Since January 2011, babesiosis has been a nationally reportable disease in the United States. In our opinion, given its emerging nature and potential to infect the Canadian blood supply, babesiosis is a good candidate to be made nationally reportable in Canada.
SUMMARY
The present report is the first published case of a patient with tick-borne babesiosis acquired within Canada. Fortunately, infection with B microti is readily detected by routine microscopy supplemented by appropriate clinical history, and can be confirmed by PCR. Although our patient fared well with standard treatment of atovaquone and azithromycin, it is important to consider this infection, particularly in individuals at higher risk for severe disease, because babesiosis can be fatal. Human infection remains a rare event but is likely to increase as the range of blacklegged ticks expands in central and eastern Canada. It is prudent to add babesiosis to the nationally notifiable disease list in Canada to further track this emerging infection.
REFERENCES
- 1.Nakajima R, Tsuji M, Oda K, et al. Babesia microti-group parasites compared phylogenetically by complete sequencing of the CCTeta gene in 36 isolates. J Vet Med Sci. 2009;71:55–68. doi: 10.1292/jvms.71.55. [DOI] [PubMed] [Google Scholar]
- 2.Persing DH, Mathiesen D, Marshall WF, et al. Detection of Babesia microti by polymerase chain reaction. J Clin Micro. 1992;30:2097–103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366:2397–407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 4.Ogden NH, Lindsay LR, Morshed M, Sockett PN, Artsob H. The emergence of Lyme disease in Canada. CMAJ. 2009;180:1221–4. doi: 10.1503/cmaj.080148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hersh MH, Tibbetts M, Strauss M, Ostfeld RS, Keesing F. Reservoir competence of wildlife host species for Babesia microti. Emerg Infect Dis. 2012;18:1951–7. doi: 10.3201/eid1812.111392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minnesota Department of Health. Babesiosis Statistics. < www.health.state.mn.us/divs/idepc/diseases/babesiosis/statistics.html> (Accessed September 5, 2013)
- 7.Wisconsin Department of Health Services. Babesiosis. < www.dhs.wisconsin.gov/communicable/tickborne/Babesiosis.htm> (Accessed September 5, 2013)
- 8.North Dakota Department of Health Tickborne Diseases. Babesiosis. < www.ndhealth.gov/disease/tickborne/Babesiosis/Babesiosis.htm> (Accessed September 5, 2013)
- 9.Ogden NH, Trudel L, Artsob H, et al. Ixodes scapularis ticks collected by passive surveillance in Canada: Analysis of geographic distribution and infection with Lyme borreliosis agent Borrelia burgdorferi. J Med Entomol. 2006;43:600–9. doi: 10.1603/0022-2585(2006)43[600:ISTCBP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Ogden NH, Lindsay LR, Leighton PA. Predicting the rate of invasion of the agent of Lyme disease Borrelia burgdorferi. J App Ecol. 2013;50:510–8. [Google Scholar]
- 11.Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am. 2008;22:469–88. doi: 10.1016/j.idc.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 13.Kunimoto D, Krause K, Morrison D. First case of (imported) babesiosis diagnosed in Canada. Can J Infect Dis. 1998;9:387–9. doi: 10.1155/1998/564190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kain KC, Jassoum SB, Fong IW, Hannach B. Transfusion-transmitted babesiosis in Ontario: First reported case in Canada. CMAJ. 2001;164:1721–3. [PMC free article] [PubMed] [Google Scholar]


