Abstract
The existence of a complete and functional renin-angiotensin system along the nephron is widely recognized. However, its precise role in blood pressure control and, by extension, hypertension is still uncertain. While most investigators agree that overexpressing RAS components along the nephron results in hypertension, two important issues remain: whether the local RAS works as a separate entity or represents an extension of the systemic RAS and whether locally generated angiotensin II has specific renal effects on blood pressure that are distinct from systemic angiotensin II. This review addresses these issues while emphasizing the unique role of local angiotensin II in the response of the kidney to hypertensive stimuli and the induction of hypertension.
Keywords: ACE, hypertension, kidney, RAS
Introduction
The human kidney filters approximately 180 l of plasma every day, the equivalent to 60 times the whole plasma volume of a normal individual. Said another way, by the time you finish reading this review (in about 1 h), your kidneys will have filtered the entirety of your plasma at least twice. Most of the filtered volume is reclaimed because of the powerful sodium and water retention capacity of the renal tubules and the actions of the octapeptide angiotensin II. Thus, it is not surprising that angiotensin II can be produced locally within the kidneys because of the activity of a complete renin-angiotensin system (RAS) expressed along the nephron. In fact, the renal RAS can play an important role in disease. This review will discuss the evidence showing that the renal RAS is pathologically activated by renal injury and inflammation and that this activation represents a powerful mechanism to generate hypertension.
Expression of RAS Components Along the Nephron
Large amounts of angiotensinogen accumulate in the proximal tubular epithelium. Because this angiotensinogen is excreted into the tubular lumen and in the urine, many investigators believe that it can be cleaved by tubular renin and ACE to form angiotensin II [1–3]. Support for this concept is provided by multiple experiments showing that transgenic mice overexpressing human, rat or mouse angiotensinogen in proximal tubular epithelium develop hypertension and renal injury with no apparent alterations of the systemic RAS [4–6]. The source of proximal tubule angiotensinogen is still a point of contention. Recently, Matsusaka et al. demonstrated that liver angiotensinogen is the main source of angiotensinogen under basal conditions, at least in mice [7•]. However, their observation does not explain the increases of angiotensinogen mRNA in kidneys from angiotensin II infused animals [8, 9]. As it stands today, it appears that the angiotensinogen in segments 1 and 2 of the proximal tubule is of systemic origin, while the angiotensinogen in segment 3 is produced locally. Regardless of the source, an increased urinary angiotensinogen excretion correlates with increases in renal angiotensin II content [10]. The former observation serves as the basis for clinical studies exploring urinary angiotensinogen excretion in humans. Multiple studies show that patients with hypertension and several forms of renal disease display increased urinary angiotensinogen when compared to normal subjects [11•].
Tubular renin has at least three sources: systemic (i.e., juxtaglomerular) renin can be filtered. Renin can also be produced, although in small quantities, by proximal tubule cells and [12]; renin is also abundantly expressed in the distal nephron, mainly by connecting tubules and collecting ducts [1]. Multiple studies by Prieto-Carrasquero demonstrate that distal renin expression is augmented in several forms of experimental hypertension, even in conditions when there is substantial plasma renin suppression [13]. Recently, it was shown that overexpressing collecting duct renin causes hypertension in mice [14]. Angiotensin-converting enzyme (ACE) is expressed in multiple cell types within the kidney [15]. However, by far the site of highest expression is the brush border of the proximal tubule. ACE expression has also been detected in the distal nephron, although whether the source of distal ACE activity is local production or absorbed proximal tubule ACE is not clear. What is important is that ACE activity is detectable throughout the nephron and in the urine [16]. Finally, AT1 receptors are ubiquitously expressed along the nephron, although particularly high concentrations are found in the thick ascending limb [17].
The Renal RAS and the Response to Local Parenchymal Injury
Experiments in Goldblatt and angiotensin II-infused animals showed that renal angiotensin II levels are much higher than those in the systemic circulation and subject to local independent regulation [18]. This raised the question of whether there was local angiotensin II production during hypertension, even in high plasma angiotensin II states. Unequivocal evidence in favor of this hypothesis was provided by experiments in rats infused with Val5-angiotensin II, an isoform of angiotensin II separable from endogenous angiotensin II (Ile5-angiotensin II) by high-performance liquid chromatography [19]. These studies demonstrated that chronic Val5-angiotensin II (exogenous angiotensin II) infusion induced renal Ile5-angiotensin II (endogenous angiotensin II) synthesis. In another study, the increase in renal angiotensin II content normally observed in angiotensin II-infused mice was significantly reduced by concomitant treatment with an ACE inhibitor [20]. Thus, several lines of evidence indicate that local synthesis is an important contributor to the local pool of angiotensin II in hypertensive states.
Why does the kidney respond to increases in plasma angiotensin II with local angiotensin II production?
While the idea of a feed-forward mechanism is not the prevailing view of the RAS, the renal expression of several of its components, namely tubular angiotensinogen, ACE, tubular renin, and the AT1 receptor, is either augmented or sustained during several forms of experimental and human hypertension [21]. For instance, renal RAS upregulation is well documented in the hypertension induced by angiotensin II infusion and also by nitric oxide synthesis inhibition [22, 23]. One hypothesis to explain this is that renal RAS activation is an “alarm” response and not a physiological process. For instance, in vitro studies demonstrate that the proximal tubule angiotensinogen gene is activated by several proinflammatory cytokines, including IL-6 and IFN-γ [24–27]. These cytokines can act independently or synergistically with other factors, including angiotensin II, high glucose, and reactive oxygen species to upregulate angiotensinogen expression and secretion into the bathing media. Although angiotensinogen is the best understood example, reports indicate that, at least in rodents, ACE and tubular renin expression are also upregulated in many conditions associated with renal parenchymal injury. Another suggestion is that inflammatory cells express RAS components and therefore, in conditions of renal inflammation, can become a separate source of local angiotensin II [28, 29].
The Specific Effects of Renal Angiotensin II
Experiments in the 1970s and 1980s showed that direct application of angiotensin I or RAS blockers (ACE inhibitors or AT1 receptor blockers) into the renal artery led to acute changes in the glomerular filtration rate (GFR) and sodium excretion [30–33]. The importance of this pioneering work was summarized elsewhere [34•]. Here we focus on experiments in gene-targeted mice as an important strategy to analyze the exact effects of renal angiotensin II. For instance, our laboratory exploited the well-established fact that ACE is the main source angiotensin II in renal tissues [35]. Specifically, we analyzed the renal response of ACE gene-targeted mice to hypertensive stimuli. One experiment was performed in mice termed ACE 9/9 in which ACE expression is strictly limited to renal tubular epithelium [18]. When exposed to chronic angiotensin I infusion, ACE 9/9 mice showed increased levels of renal angiotensin II and urinary angiotensin II excretion. More significantly, these mice developed hypertension similar in magnitude to that observed in equally treated wild-type littermates. This experiment showed that renal tubular epithelial ACE has the unique ability to increase the local concentration and urinary excretion of angiotensin II and to induce hypertension even in the total absence of ACE and angiotensin II in extra-renal tissues [18].
To verify this hypothesis, separate experiments studied ACE 3/3 and ACE 10/10 mice. In the ACE 3/3 mouse, ACE expression is restricted mostly to hepatocytes [36]. ACE 10/10 mice are an inbred line that expresses ACE exclusively in myelomonocytic cells [37]; these animals have essentially no renal ACE. Both mouse strains have normal circulating levels of ACE. They also have normal blood pressure and normal baseline renal morphology and function. Remarkably, the absence of renal ACE in the ACE 10/10 and ACE 3/3 mice significantly reduced the hypertension in response to angiotensin II infusion (a high serum angiotensin II model) or to reduced nitric oxide production (a low serum angiotensin II model) [38••, 39••].
To better understand the effects of renal angiotensin II on kidney function, we measured sodium and urine output of wild-type and mice lacking renal ACE (ACE 10/10 mice) during angiotensin II infusion [38••]. This approach clamped circulating angiotensin II to similar elevated levels. Angiotensin II infusion caused a transient reduction of sodium and urine output in wild-type mice that returned to pre-infusion levels after 48 h. These changes were substantially blunted in the ACE 10/10 mice. After 3 days of infusion, sodium and urine outputs were similar in both groups. However, in wild-type mice, sodium balance was attained at the expense of hypertension, consistent with a major shift in the pressure-natriuresis relationship. Importantly, the absence of hypertension in ACE 10/10 mice implies that the lack of kidney ACE prevents angiotensin II infusion from shifting the pressure-natriuresis relationship, a major mechanism in establishing hypertension according to Guyton’s kidney-fluids hypothesis [39••].
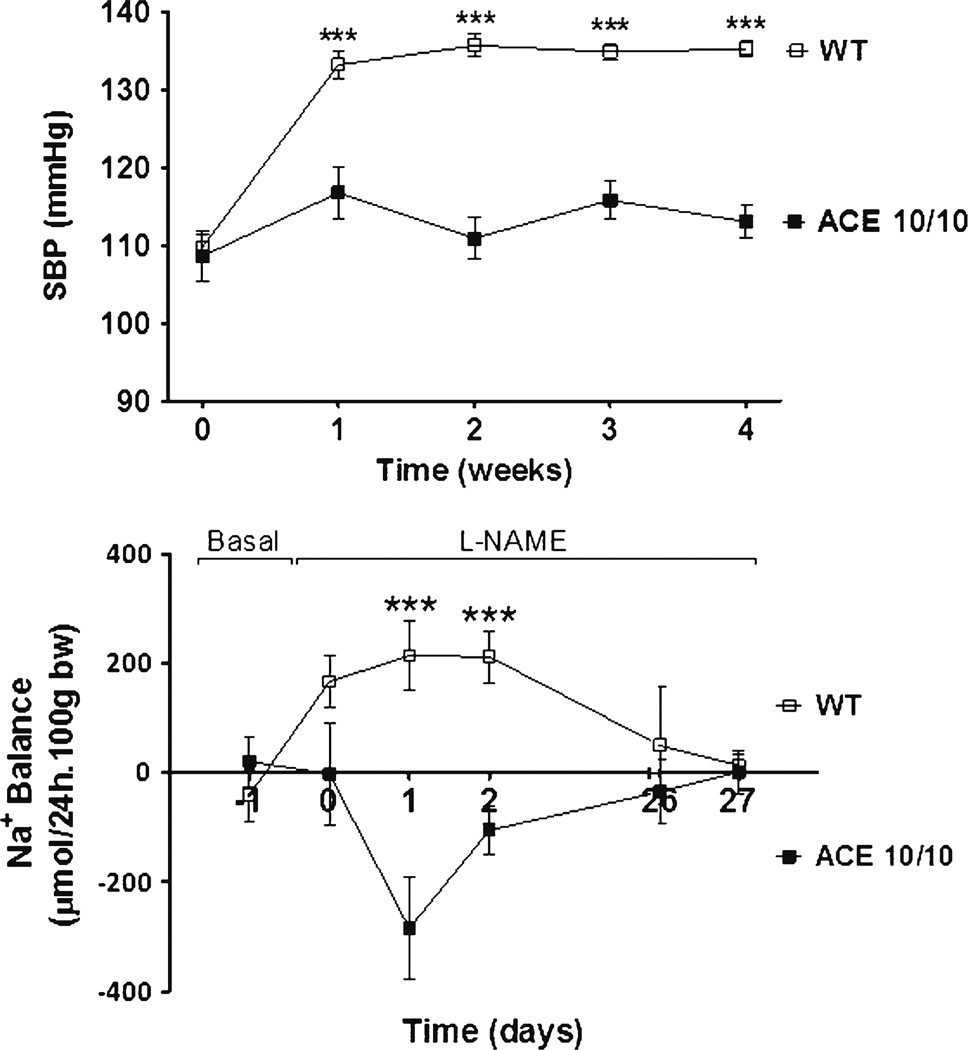
Similar observations were made during hypertension induced by nitric oxide synthase inhibition with L-NAME (L-NAME induced hypertension) [40]. In this model, the protection against the hypertension by the lack of renal ACE was even more pronounced; while wild-type mice became hypertensive, the blood pressure of ACE 10/10 mice remained essentially unchanged (Fig. 1). Sodium balance studies reveal that in L-NAME treated wild-type mice it was restored at the expense of hypertension. In sharp contrast, ACE 10/10 mice showed an enhanced natriuretic response that allowed them to maintain normal blood pressure values (Fig. 1). As a whole, these observations support a very novel concept, namely that shifting of the renal pressure–natriuresis relationship toward hypertension ultimately depends on renal ACE and the angiotensin II produced locally in the kidney, and not on systemic effects of angiotensin II.
Fig. 1.
The absence of renal ACE derived-angiotensin II formation prevents the hypertension and sodium retention induced by systemic nitric oxide synthesis inhibition. Wild-type and mice lacking renal ACE (ACE 10/10) were given L-NAME, a nitric oxide synthesis inhibitor, in the drinking water (5 mg/10 ml). Values represent mean±SEM; N=6–10, ***P<0.001 versus wild-type mice. Data were published elsewhere [38••]
In theory, the renal sodium dysregulation facilitated by the renal ACE/angiotensin II pathway can be induced by changes in glomerular filtration rate (GFR) and/or sodium reabsorption. With this in mind, the GFR response to L-NAME was studied in conscious, unrestrained wild-type and ACE 10/10 mice via a transcutaneous method [41]. Whereas wild-type mice responded to L-NAME with acute reductions in GFR, the GFR of equally treated ACE 10/10 mice remained unchanged. The explanation for this observation is not clear, but it suggests that the renal ACE/angiotensin II pathway is also important in modulating renal hemodynamic responses to hypertensive stimuli.
The effects of the renal ACE/angiotensin II pathway on sodium transport have also been explored. An extensive expression analysis included the Na+/H+ exchanger (NHE3), loop of Henle Na+/K+/2Cl− co-transporter 2 (NKCC2), distal tubule NaCl co-transporter (NCC), epithelial sodium channel (ENaC), anion exchangers pendrin and NDBCE, and Na+/K+ ATPase, among others. In both the L-NAME hypertension and angiotensin II infusion models, the presence of kidney ACE was associated with “increased sodium transport.” The most striking differences between wild-type and mutant mice were observed in response to angiotensin II infusion, where the presence of kidney ACE facilitated substantial increases in abundance, phosphorylation, and/or processing of NKCC2, NCC, ENaC, and pendrin in the loop of Henle and the distal nephron. In the specific case of NKCC2 and NCC, the two transporters with the most significant changes, increased abundance and/or phosphorylation were consistent with increased in vivo transporter activation (as measured by the response to specific blockers). Remarkably, the described changes were either totally absent or substantially attenuated in equivalently treated ACE 10/10 mice. Hence, while it is well established that angiotensin II regulates NKCC2, NCC, ENaC, and pendrin, a major conclusion from these studies is the dominant effect of locally generated angiotensin II in regulating sodium transport along the nephron. Thus, the evidence suggests that the renal ACE/angiotensin II pathway serves as a master switch for sodium transport along the nephron.
Conclusions
Emerging evidence supports an important and obligatory role of angiotensin II generated within the kidney in hypertension. Experiments in gene-targeted mice reveal the importance of the renal ACE/angiotensin II pathway in eliciting sodium retention through its positive modulatory effects on renal filtration and sodium reabsorptive mechanisms. Further, these effects are largely independent of the plasma angiotensin II status. This is because the protective effects of a lack of renal ACE were observed even during the high plasma angiotensin II levels caused by angiotensin II infusion. Finally, we posit that a better understanding of the physiologic effects of local renal ACE/angiotensin II will uncover important mechanistic knowledge about the origins of hypertension.
Acknowledgement
The authors are supported by NIH grants DK083785 (AMcD), R01HL110353 (KEB), and R00DK083455 (RAGV), and an AHA Beginning Grant-in-Aid 13BGIA14680069 (XZS).
Xiao Z. Shen has received a grant from the American Heart Association.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Jorge F. Giani, Tea Janjulia, Brian Taylor, Ellen Bernstein, Kandarp Shah, Alicia A. McDonough, Kenneth E. Bernstein, and Romer A. Gonzalez-Villalobos declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Jorge F. Giani, Departments of Biomedical Sciences and Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Tea Janjulia, Departments of Biomedical Sciences and Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Brian Taylor, Departments of Biomedical Sciences and Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Ellen A. Bernstein, Departments of Biomedical Sciences and Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Kandarp Shah, Departments of Biomedical Sciences and Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Xiao Z. Shen, Departments of Biomedical Sciences and Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Alicia A. McDonough, Department of Cell and Neurobiology, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
Kenneth E. Bernstein, Departments of Biomedical Sciences and Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Romer A. Gonzalez-Villalobos, Departments of Biomedical Sciences and Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA Pfizer, DSRD CoE, 274 Eastern Point Road, MS 8274-1245, Groton, CT 06340, USA, romer.gonzalezvillalobos@pfizer.com.
Reference
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34(6):1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 2.Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins I and II in anesthetized rats. J Am Soc Nephrol. 1994;5(4):1153–1158. doi: 10.1681/ASN.V541153. [DOI] [PubMed] [Google Scholar]
- 3.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37(5):1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am J Physiol Renal Physiol. 2004;286(5):F965–F971. doi: 10.1152/ajprenal.00402.2003. [DOI] [PubMed] [Google Scholar]
- 5.Sachetelli S, Liu Q, Zhang SL, Liu F, Hsieh TJ, Brezniceanu ML, et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69(6):1016–1023. doi: 10.1038/sj.ki.5000210. [DOI] [PubMed] [Google Scholar]
- 6.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, et al. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293(3):F938–F945. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, et al. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012 doi: 10.1681/ASN.2011121159.. This manuscripts demonstrates that liver angiotensinogen is the main source for angiotensin peptides, under basal conditions, in mice
- 8.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12(3):431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, et al. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal physiol. 2008;295(3):F772–F779. doi: 10.1152/ajprenal.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61(2):579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Navar LG. Translational studies on augmentation of intratubular renin-angiotensin system in hypertension. Kidney inter, Suppl. 2013;3(4):321–325. doi: 10.1038/kisup.2013.67.. This manuscript summarizes recent advances in urinary angiotensinogen excretion as a marker for renal human RAS activation.
- 12.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, et al. Renin expression in renal proximal tubule. J Clin Invest. 1993;91(3):774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prieto MC, Gonzalez AA, Navar LG. Evolving concepts on regulation and function of renin in distal nephron. PflugersArchiv: Eur J Physiol. 2013;465(1):121–132. doi: 10.1007/s00424-012-1151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramkumar N, Ying J, Stuart D, Kohan DE. Overexpression of renin in the collecting duct causes elevated blood pressure. Am J Hypertens. 2013;26(8):965–972. doi: 10.1093/ajh/hpt071. [DOI] [PubMed] [Google Scholar]
- 15.Alhenc-Gelas F, Baussant T, Hubert C, Soubrier F, Corvol P. The angiotensin converting enzyme in the kidney. J Hypertens Suppl : Off J Int Soc Hypertens. 1989;7(7):S9–S13. doi: 10.1097/00004872-198909007-00003. discussion S4. [DOI] [PubMed] [Google Scholar]
- 16.Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol. 1997;272(3 Pt 2):F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 17.Paxton WG, Runge M, Horaist C, Cohen C, Alexander RW, Bernstein KE. Immunohistochemical localization of rat angiotensin II AT1 receptor. Am J Physiol. 1993;264(6 Pt 2):F989–F995. doi: 10.1152/ajprenal.1993.264.6.F989. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Villalobos RA, Billet S, Kim C, Satou R, Fuchs S, Bernstein KE, et al. Intrarenal angiotensin-converting enzyme induces hypertension in response to angiotensin I infusion. J Am Soc Nephrol. 2011;22(3):449–459. doi: 10.1681/ASN.2010060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao W, Seth DM, Navar LG. Augmenation of endogenous angiotensin II levels in Val5-Ang II infused rats. JIM. 2008;56(1):344–492. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, et al. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010;298(1):F150–F157. doi: 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57(3):355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol. 2011;11(2):180–186. doi: 10.1016/j.coph.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graciano ML, Cavaglieri RDC, Delle H, Dominguez WV, Casarini DE, Malheiros DMAC, et al. Intrarenal renin-angiotensin system is upregulated in experimental model of progressive renal disease induced by chronic inhibition of nitric oxide synthesis. J Am Soc Nephrol. 2004;15(7):1805–1815. doi: 10.1097/01.asn.0000131528.00773.a9. [DOI] [PubMed] [Google Scholar]
- 24.Acres OW, Satou R, Navar LG, Kobori H. Contribution of a nuclear factor-kappaB binding site to human angiotensinogen promoter activity in renal proximal tubular cells. Hypertension. 2011;57(3):608–613. doi: 10.1161/HYPERTENSIONAHA.110.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Katsurada A, Navar LG, et al. Costimulation with angiotensin II and interleukin 6 augments angiotensinogen expression in cultured human renal proximal tubular cells. Am J Physiol Renal Physiol. 2008;295(1):F283–F289. doi: 10.1152/ajprenal.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Urushihara M, Acres OW, et al. IL-6 augments angiotensinogen in primary cultured renal proximal tubular cells. Mol Cell Endocrinol. 2009;311(1–2):24–31. doi: 10.1016/j.mce.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satou R, Miyata K, Gonzalez-Villalobos RA, Ingelfinger JR, Navar LG, Kobori H. Interferon-gamma biphasically regulates angiotensinogen expression via a JAK-STAT pathway and suppressor of cytokine signaling 1 (SOCS1) in renal proximal tubular cells. Faseb J. 2012;26(5):1821–1830. doi: 10.1096/fj.11-195198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Iturbe B, Franco M, Johnson RJ. Impaired pressure natriuresis is associated with interstitial inflammation in saltsensitive hypertension. Curr Opin Nephrol Hypertens. 2013;22(1):37–44. doi: 10.1097/MNH.0b013e32835b3d54. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Iturbe B, Johnson RJ. The role of renal microvascular disease and interstitial inflammation in salt-sensitive hypertension. Hypertens Res. 2010;33(10):975–980. doi: 10.1038/hr.2010.148. [DOI] [PubMed] [Google Scholar]
- 30.Fagard RH, Cowley AW, Jr, Navar LG, Langford HG, Guyton AC. Renal responses to slight elevations of renal arterial plasma angiotensin II concentration in dogs. Clin Exp Pharmacol & Physiol. 1976;3(6):531–538. doi: 10.1111/j.1440-1681.1976.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 31.Kimbrough HM, Jr, Vaughan ED, Jr, Carey RM, Ayers CR. Effect of intrarenal angiotensin II blockade on renal function in conscious dogs. Circ Res. 1977;40(2):174–178. doi: 10.1161/01.res.40.2.174. [DOI] [PubMed] [Google Scholar]
- 32.Navar LG, Rosivall L. Contribution of the renin-angiotensin system to the control of intrarenal hemodynamics. Kidney Int. 1984;25(6):857–868. doi: 10.1038/ki.1984.102. [DOI] [PubMed] [Google Scholar]
- 33.Rosivall L, Carmines PK, Navar LG. Effects of renal arterial angiotensin I infusion on glomerular dynamics in sodium replete dogs. Kidney Int. 1984;26(3):263–268. doi: 10.1038/ki.1984.168. [DOI] [PubMed] [Google Scholar]
- 34. Levens NR, Peach MJ, Carey RM. Role of the intrarenal reninangiotensin system in the control of renal function. Circ Res. 1981;48(2):157–167. doi: 10.1161/01.res.48.2.157.. This manuscript assesses the state of science concerning the renal RAS in the seventies and eighties.
- 35.Campbell DJ, Alexiou T, Xiao HD, Fuchs S, McKinley MJ, Corvol P, et al. Effect of reduced angiotensin-converting enzyme gene expression and angiotensin-converting enzyme inhibition on angiotensin and bradykinin peptide levels in mice. Hypertension. 2004;43(4):854–859. doi: 10.1161/01.HYP.0000119190.06968.f1. [DOI] [PubMed] [Google Scholar]
- 36.Cole J, Quach DL, Sundaram K, Corvol P, Capecchi MR, Bernstein KE. Mice lacking endothelial angiotensin-converting enzyme have a normal blood pressure. Circ Res. 2002;90(1):87–92. doi: 10.1161/hh0102.102360. [DOI] [PubMed] [Google Scholar]
- 37.Shen XZ, Li P, Weiss D, Fuchs S, Xiao HD, Adams JA, et al. Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am J Pathol. 2007;170(6):2122–2134. doi: 10.2353/ajpath.2007.061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123(5):2011–2023. doi: 10.1172/JCI65460.. This manuscript supports the concept of an obligatory role of renal angiotensin II in the development of hypertension. A detailed analysis of the renal ACE/angiotensin II pathway actions during angiotensin II-dependent hypertension is offered.
- 39. Guyton AC. Blood pressure control–special role of the kidneys and body fluids. Science. 1991;252(5014):1813–1816. doi: 10.1126/science.2063193.. This manuscript describes the role of the renal ACE/angiotensin II pathway in the hypertension induced by L-NAME, a low renin form of hypertension.
- 40.Giani JF, Janjulia T, Kamat N, Seth DM, Blackwell W-LB, Shah KH, et al. Renal angiotensin-converting enzyme is essential for the Hypertension induced by Nitric Oxide Synthesis Inhibition. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013091030. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schreiber A, Shulhevich Y, Geraci S, Hesser J, Stsepankou D, Neudecker S, et al. Transcutaneous measurement of renal function in conscious mice. Am J Physiol Renal Physiol. 2012;303(5):F783–F788. doi: 10.1152/ajprenal.00279.2012. [DOI] [PubMed] [Google Scholar]