Abstract
Objective
Childhood sexual abuse (CSA) has been associated with alterations in brain morphology using region of interest analyses that have focused on stress sensitive target regions. This study was designed to ascertain the effects on gray matter volume (GMV) of exposure to CSA in a healthy population of young adult college students selected based on the history of exposure regardless of psychiatric outcome. Voxel-based morphometry (VBM) provided unbiased delineation of most significantly affected brain regions.
Methods
High-resolution T1-weighted MRI datasets were obtained for 23 unmedicated females with CSA and 14 healthy female controls of equivalent age and socioeconomic status with no history of trauma. Cortical surface-based analysis (FreeSurfer) was performed to verify VBM results.
Results
GMV was reduced by 12.6% and 18.1% in right and left primary visual (V-1) and visual association cortices of abused subjects. This reduction was directly related to duration of CSA before age 12. GMV of left and right V-1 correlated with an overall index of visual memory (r = 0.353, P = 0.032 and r=0.448, P=0.005). Cortical surface-based analysis indicated that GMV of abused subjects was reduced in the left fusiform (P=0.004), left middle occipital (P=0.04), and right lingual (P=0.002) gyri.
Conclusions
Early visual experience exerts a strong influence on the development of the mammalian visual cortex. Present findings indicate that exposure to traumatic events may also affect the development of this region, and are even apparent even in a population of subjects who are sufficiently healthy to matriculate.
Keywords: childhood sexual abuse (CSA), voxel-based morphometry (VBM), gray matter volume (GMV), visual cortex, sensitive period
INTRODUCTION
Exposure to abuse or neglect is common throughout the world, and is a major risk factor for psychopathology (1). Childhood abuse has been associated with volume loss in the hippocampus (2–5), corpus callosum (6, 7) and prefrontal cortex (8), with altered symmetry in frontal lobes (9) and superior temporal gyrus (10), and with reduced neuronal density or integrity in the anterior cingulate (11). We have proposed that brain regions may be modified by exposure to adversity as a consequence of a cascade of events that include excessive exposure to stress hormones (cortisol, norepinephrine, vasopressin) and over-activation of monoamine neurotransmitter systems (12), particularly if the exposure occurs during a sensitive developmental period (13).
Research in this area has the potential to recast our thinking about the role of early experience in psychopathology (12, 14), but current studies are limited by their reliance on clinical samples consisting of abused subjects with a specific form of psychopathology, such as post-traumatic stress disorder (PTSD). Consequently, these studies may [1] overestimate the effects of abuse by selecting the most adversely affected subjects, [2] confound abuse-related differences with disorder-related differences, or [3] mistakenly identify preexisting brain abnormalities that were risk factors for developing a specific disorder when exposed to trauma rather than regions altered by the exposure (15). This latter point is not necessarily a concern if the focus of the study is the neurobiology of a particular disorder, but it is a problem if the focus is on the neurobiological consequences of abuse. Furthermore, current studies use region of interest (ROI) analyses and predominantly focus on target structures presumed to be vulnerable. This approach is valuable and typically hypothesis driven, but it may hinder discovery of unanticipated outcomes.
The aim of this study was to use voxel based morphometry (VBM) as an unbiased, whole brain approach to identify alterations in regional gray matter volume (GMV) in individuals recruited from the community with exposure to childhood sexual abuse (CSA), but no other forms of trauma, and enrolled regardless of psychiatric outcome.
METHOD and PARTICIPANTS
Subjects were right-handed, healthy, unmedicated young adults (18–22 years of age) with excellent hearing and visual acuity, recruited by advertisements targeted to college students, and selected based on a complete absence of exposure to trauma, or a self-reported history of forced contact CSA. This narrow age range was chosen to recruit subjects as close to the experience as possible who could provide independet informed consent, and to minimize variations in brain morphometry related to development or aging. The McLean Hospital Institutional Review Board approved all procedures. The purpose and meaning of the study were explained to subjects, who subsequently gave their written informed consent.
This was a two-phase study. During the first phase a large number of subjects interested in participating in the second (neuroimaging) phase, provided detailed information on their degree of exposure to a host of abusive or traumatic experiences, along with medical, psychiatric, developmental and family history. Applicants were aware that the neuroimaging study was on the effects of early experience on brain development, but unaware of our specific emphasis on CSA, so no candidate could fake or embellish a history to gain entry. Subjects were excluded who had any history of serious motor vehicle accident, near drowning, gang violence, muggings, natural disasters or other forms of trauma, substance abuse, any recent substance use, head trauma with loss of consciousness, significant fetal exposure to alcohol or drugs, perinatal or neonatal complications, neurological disorders, or medical conditions that could adversely affect growth and development.
History of exposure to CSA was obtained in two ways. Individuals with CSA were initially identified if they responded affirmatively to the question: “Have you ever been forced into doing more sexually than you wanted to do or were too young to understand? (By “sexually” we mean being forced against your will into contact with a sexual part of your body or of his/her body).” They also provided information on their relationship with this individual, number of times they were forced, age of first and last abuse, and whether or not they felt terrified or had their life or another person’s life threatened (16).
Respondents meeting eligibility requirements were further evaluated using the Traumatic Antecedents Interview (TAI) (17). This is a 100-item semi-structured interview designed to evaluate reports of physical or sexual abuse, witnessing violence, physical or emotional neglect, significant separations or losses, verbal abuse, or parental discord (17). The reliability of TAI variables ranges from acceptable to excellent (median intraclass R = 0.73) (17). Subjects needed to be consistent on both self-report and interview.
We selected subjects who reported three or more episodes of forced contact CSA accompanied by fear or terror, occurring before age 18 and at least two years prior to enrollment. Multiple episodes were required based on the assumption that CSA is typically a repeated event, and that persistent fear of recurrence may be a major factor affecting brain development.
Licensed psychiatric clinical nurse specialists conducted the assessment interviews and completed their evaluations prior to neuroimaging. Interviews included the Structured Clinical Interview for DSM-IV for Axis I psychiatric disorders (18), Revised Diagnostic Interview for Borderlines (19), Structured Clinical Interview for DSM-IV Dissociative Disorders (20), and DSM-IV ADHD Items taken from the K-SADS-E (21). A panel of three doctoral level psychiatric clinicians with extensive experience treating traumatic disorders, and blind to the neuroimaging results, reviewed questions regarding eligibility. Decisions were made by full consensus.
We also administered the Memory Assessment Scales (MAS) (22), which measures short-term, visual, verbal, and global memory. Subjects completed a Go/No-Go/Stop continuous performance attention tasks (CPT) (23) to assess components of attention including visual discrimination and response inhibition.
The initial goal was to recruit 30 subjects with CSA and 30 controls, with relatively equivalent gender ratios. Altogether 723 individuals responded to advertisements and passed an initial phone screen regarding age, handedness, health, and medications. Of these, 554 completed detailed ratings that enabled us to identify potentially eligible subjects. Ten percent (n = 53) indicated a history of exposure to CSA unaccompanied by exposure to physical abuse (PA), neglect, or witnessing domestic violence. Seventy-five percent indicated abuse by individuals outside their family. Screening for exposure to other forms of trauma and exclusionary medical history further reduced the sample. All CSA subjects meeting inclusion and exclusion criteria were invited to the laboratory for additional screening; 35 accepted. The selected neuroimaging pool included 16F/14M controls and 26F/4M with CSA. The disproportionate gender ratio in the CSA group was attributable to the lower incidence of CSA and high rate of exposure to other forms of abuse or trauma in males. Because so few men were in the abused sample, we only analyzed females. Altogether, artifact-free images suitable for VBM were available for 23 abused women and 14 female controls (Table I).
Table I.
| ANOVA | ||||
|---|---|---|---|---|
| Measures | Healthy Control Subjects | CSA Subjects | F Value | p Value |
| Subjects | 14 | 23 | ||
| Age | 19.0 ± 1.1 | 20.2 ± 1.3 | 7.59 | 0.009 |
| Socioeconomic Status | 1.93 ± .73 | 2.26 ± .92 | 1.33 | 0.26 |
| Memory Assessment Scale Short-Term | 110.5 ± 11.5 | 106.1 ± 11.0 | 1.36 | 0.25 |
| Memory Assessment Scale Verbal | 109.1 ± 14.1 | 109.8 ± 2.7 | 0.02 | 0.88 |
| Memory Assessment Scale Visual | 114.2 ± 16.3 | 118.3 ± 8.7 | 0.99 | 0.33 |
| Memory Assessment Scale Global | 114.1 ± 14.2 | 117.1 ± 10.1 | 0.56 | 0.46 |
| Scholastic Aptitude Test Math+Verbal | 1299 ± 103 | 1255 ± 142 | 0.93 | 0.34 |
| Limbic System Checklist-33 | 10.6 ± 6.1 | 29.0 ± 11.7 | 29.5 | 0.00001 |
| Dissociative Experience Scale | 3.2 ± 3.4 | 15.6 ± 12.6 | 12.69 | 0.001 |
| Kellner SQ Anxiety | 4.8 ± 3.9 | 11.6 ± 5.1 | 18.23 | 0.0001 |
| Kellner SQ Depression | 4.1 ± 4.1 | 11.5 ± 6.3 | 15.1 | 0.0004 |
| Kellner SQ Somatization | 3.7 ± 3.0 | 9.6 ± 6.7 | 9.53 | 0.004 |
| Kellner SQ Anger/Hostility | 4.2 ± 3.0 | 8.0 ± 5.9 | 4.86 | 0.03 |
ANOVA, analysis of variance; CSA, childhood sexual abuse; SQ, Symptom Questionnaire.
Four subjects with CSA (17%) had current major depression, four had PTSD, and one (4%) had depersonalization disorder. No subjects met criteria for BPD or had a history of ADHD. Controls had no history of Axis I disorders. Abused and control subjects were predominantly middle class or higher (96%), had similar measures of parental socioeconomic status (SES) (24) and cognitive abilities as evaluated using the MAS (22), and Scholastic Aptitude Test scores (Table I). Self-reported onset of CSA was 2–15 years of age, lasting for an average of 4.1 years (range 1–12). All CSA subjects had enduring memories of the abuse. No subject had “recovered memories”, nor were any pursuing legal action against the abuser.
MRI images were acquired on a General Electric Medical Systems (Milwaukee, WI) 1.5T Horizon LX Echo Speed scanner with a prototype Pathway MRI quadrature, receive-only, volume head coil. The anatomical image series consisted of T1- and T2-weighted sagittals, T2-weighted axials, volumetric T1-weighted coronals, and anatomical dual echo axials (proton and T2-weighted). Parameters for the volumetric T1-weighted coronal images were three-dimensional, Fourier transform, spoiled gradient recalled acquisition (3DFT, SPGR) pulse sequence (TR = 35, TE = 5 msec/Fr; Flip angle = 45 degrees, FOV = 22 x16 cm, 1.5 mm slice with no skip, 256 × 192 matrix, 1 NEX).
VBM was performed using SPM5 (25–28) running in MATLAB 6.5 (The MathWorks Inc., Natick, MA, USA). Images were segmented coarsely into gray matter, white matter, cerebrospinal fluid, and skull/scalp compartments using tissue probability maps. We used a standard template (Ashburner & Friston) (25, 29) which conforms to the space defined by the ICBM, NIH P-20 project. It approximates the space described in the Talairach and Tournoux atlas (30). The transform for this normalization was used to rewrite the original image into standard space. Volume changes induced by normalization were adjusted via a modulation algorithm. Spatially normalized images were segmented into gray and white matter and then smoothed using a 12-mm full-width half-maximum isotropic Gaussian kernel. Regional differences in GMV between groups were analyzed statistically using the general linear model. Potential confounding effects of SES and whole segment GMV differences were modeled, and variances attributable to them excluded. The resulting set of voxel values used for comparison generated a statistical parametric map of t-statistic SPM{t} that was transformed to a unit normal distribution (SPM{Z}). Statistical threshold was set at P < 0.05 with correction for multiple comparisons at cluster level (height threshold of Z > 3.09) because of the increased sensitivity of clusters to detect spatially extended signal changes (31, 32). Inference testing was based on the theory of Gaussian fields (33). We corrected for potential problems relating to non-isotropic smoothness, which can invalidate cluster level comparisons (25), by adjusting cluster size from the resel per voxel image (31, 34). Exploratory correlation analyses between neuropsychiatric measures and regions of reduced GMV were performed to identify potential functional correlates, with correction for multiple comparisons.
VBM is a potentially powerful technique for identifying morphometric differences, but it hinges on a number of assumptions, particularly the accuracy of image co-registration (35). Hence, VBM findings were reevaluated using an independent technique that does not rely on image co-registration. Cortical surface-based analysis was performed using the FreeSurfer program distributed by the Massachusetts General Hospital NMR Center and CorTechs® (Boston, MA). (36–38). Each subject’s reconstructed brain was converted to an average spherical surface representation that optimally aligned sulcal and gyral features for the individual subject (36, 37). Subdivision of the cortical ribbon into gyral-based subdivisions caused in the identification of 82 validated cortical parcellation units per hemisphere. By application of the original deformation algorithms in reverse, ROIs were mapped back on to each unfolded surface (37, 39). Differences between abused and control groups were assessed using analysis of covariance with SES and total brain volume as covariates. Parcellation regions selected for analysis were located in and around the areas of greatest difference identified by VBM. They included the lingual, fusiform, middle occipital, and inferior occipital gyri, plus the cuneus and occipital pole. A comprehensive report of FreeSurfer measures of thickness, surface area, and GMV across the myriad parcellation regions will be published separately.
RESULTS
There were two significant clusters of reduced GMV in CSA subjects (Fig 1). The largest involved left primary (V1) and secondary (V2) visual cortex (Brodmann’s Area [BA] 17 to 18; Talairach’s coordinates x= −30 - −14, y= −89 - −70, z= −9 - 2) (Z = 4.03, corrected cluster level). A slightly smaller cluster was seen in the same regions on the right side (Talairach’s coordinates x= 16–40, y= −89 – −72, z= 1–5) (Z = 3.87, corrected cluster level). Compared to healthy controls, there was an 18.1% and 12.6% average reduction of GMV in left and right visual cortex clusters of CSA subjects, respectively. Since the mammalian visual cortex appears susceptible to the effects of visual experience that occurs prior to puberty, data were analyzed to ascertain whether reduction in GMV in V1 was related to abuse prior to age 12, or at later ages. Multiple regression analysis, including duration of abuse prior to age 12, from 12 years on, and total GMV, indicated that GMV in left and right V1 correlated with the duration of CSA that occurred prior to age 12 (β = −0.362, P = 0.02; β = −0.323, P < 0.03). V1 GMV did not significantly correlate with the duration of CSA from 12 years of age on (β = −0.303, P = 0.06; β = −0.146, P > 0.3). There was no significant correlation between age of onset of CSA and GMV in left or right V1 (r = 0.242, P > 0.2; r = 0.099, P > 0.6).
Figure 1.
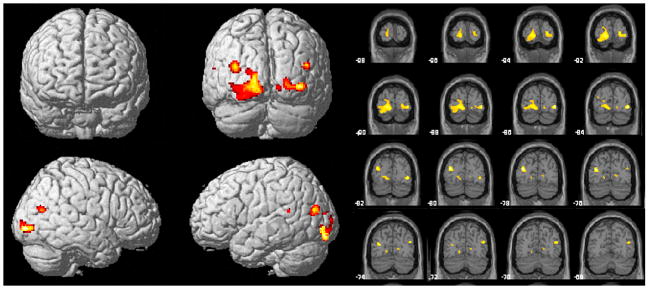
Portrayal of the locations of significant differences between abused subjects and controls in regional gray matter volume as revealed by voxel-based morphometry. Significantly lower gray-matter densities in abused subjects were measured in the left and right visual cortex. Color scale: 0–5 represents t-values.
To ascertain whether the association between alterations in visual cortex GMV was a consequence of psychiatric illness, abused subjects who did not meet criteria for an Axis I psychiatric disorder (n=14) were analyzed separately versus controls. A significant reduction in GMV of left BA17 to 18 (x= −26 – −15, y= −89, z= −12 – −3; Z = 4.42, uncorrected) was observed in abused subjects without Axis I psychopathology.
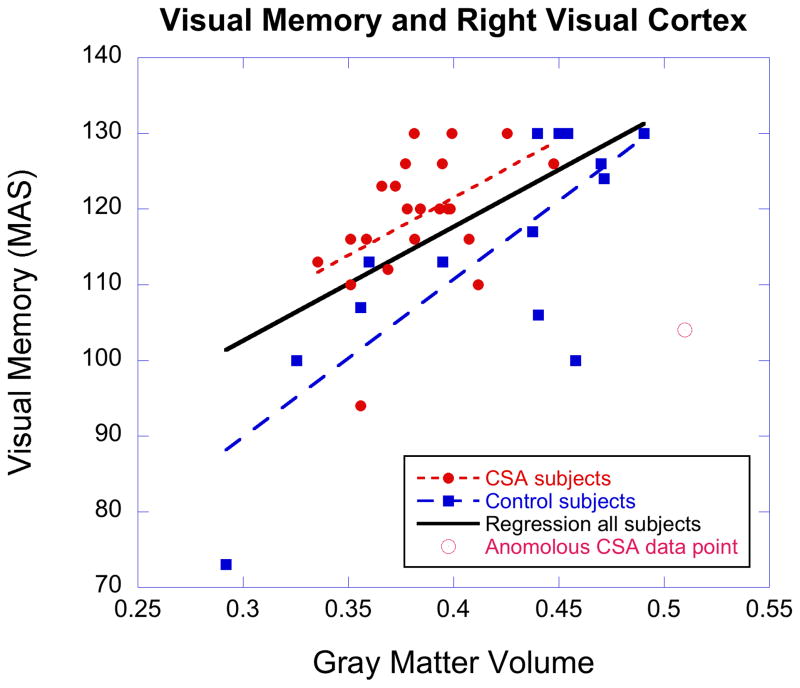
There were significant correlations between visual cortex GMV and measures of visual memory on the MAS. Left and right BA17–18 GMV correlated with visual memory across all subjects, respectively (r = 0.353, P = 0.032; x= −12, y= −73, z= 17; r = 0.448, P = 0.005; x= 12, y= −85, z= 17). This relationship was quite apparent in healthy controls (r= 0.629, P = 0.016; r= 0.778, P = 0.001), but was not apparent in subjects with CSA (r= 0.135, P > 0.5; r= 0.061, P > 0.7). However, lack of correlation in CSA subjects may have been secondary to an anomalous data point (Fig. 2). Excluding that point revealed a significant correlation in CSA subjects (r = 0.484, P = 0.022), and parallel regression slopes across groups (F = 0.456, df=1,33, P > 0.5). GMV in left V1 correlated marginally (r = 0.361 P = 0.054, n = 29) with capacity to distinguish targets from non-targets on the Go/No-Go/Stop CPT, across all subjects tested.
Figure 2.
Scatter plot portraying the relation between gray matter volume and visual memory on the Memory Assessment Scale at the cluster location of maximal correlation in right lingual gyrus (x= 12, y= −85, z= 17). Linear regression for all subjects, and for controls only, shown as solid black line and dashed blue line. Regression in CSA subjects (red dotted line) shown excluding anomalous data point (r = 0.484). There was no significant correlation between visual memory and right V1 GMV in CSA subjects with all points included (r = 0.061).
No other areas of reduction were found with a corrected cluster probability value that approached significance. Examination of voxels with increased GMV in CSA subjects identified no significant corrected voxel level-cluster regions. There was one small region of increased GMV in CSA subjects in the left middle frontal gyrus (BA8, x= −38 y= 30 z= 50, cluster size = 159) that was significant (Z = 3.77) at the uncorrected voxel level.
FreeSurfer results were highly complementary. As illustrated in Figure 3, the analysis revealed an 8.0% reduction in left visual cortex GMV (F = 8.3, df = 1,34, P = 0.007). This was specifically related to an 18.0% reduction in the left fusiform gyrus (F = 9.5, df = 1,34, P = 0.004) and a 9.5% reduction in the left middle occipital gyrus (F = 4.5, df = 1,34, P = 0.041). A 5% lower value was found for GMV for the entire right visual cortex (F = 4.7, df = 1,34, P = 0.038), which was attributable to an 8.9% reduction in the right lingual gyrus (F = 11.2, df = 1,34, P = 0.002). GMV in the left V1 cluster identified by VBM correlated significantly with GMV in left fusiform (r = 0.441, P = 0.006) and left middle occipital (r = 0.452, P = 0.005) gyri. Similarly, the right V1 cluster correlated strongly with GMV in right lingual gyrus (r = 0.570, P < 0.001).
Figure 3.
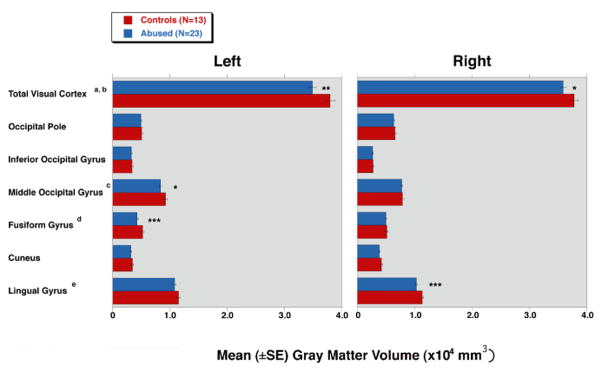
Mean (±SE) gray matter volume (GMV) in the visual cortex acquired by cortical surface-based analysis showing differences between healthy controls and subjects with repeated exposure to childhood sexual abuse. *, P < 0.05; **, P < 0.01; and ***, P < 0.005.
aSignificant difference between two groups in the left visual cortex (abused 34838±627 versus vs. controls 37886±838; F = 8.3, df = 1,34, P = 0.007).
bSignificant difference between two groups in the right visual cortex (35887±518 vs. 37773±693; F = 4.7, df = 1,34, P = 0.038).
cSignificant difference between two groups in the left middle occipital gyrus (8347±243 vs. 9219±325; F = 4.5, df = 1,34, P = 0.041).
dSignificant difference between two groups in the left fusiform gyrus (4285±181 vs. 5226±242; F = 9.5, df = 1,34, P = 0.004).
eSignificant difference between two groups in the right lingual gyrus (10289±179 vs. 11300±239; F = 11.2, df = 1,34, P = 0.002).
DISCUSSION
GMV was significantly reduced in left and right lingual (BA17) and inferior occipital gyri (BA18) of young adults with CSA. This unexpected finding emerged from a global VBM analytical approach. Cortical surface-based analyses confirmed a significantly lower GMV in the left > right visual cortex. Previous studies on the effects of early abuse focused on ROIs and did not report results for occipital cortex, with one notable exception. Fennema-Notestine et al. (40) conducted a volumetric MRI study of victims of intimate-partner violence (IPV). These individuals had significantly lower occipital GMV that was associated with exposure to childhood abuse rather than IPV. Together, these studies suggest that exposure to abuse affects visual cortex development, but that vulnerability is limited to an early sensitive period.
We found that reduced right and left V1 GMV was significantly associated with duration of CSA prior to age 12 but not after. This age cutoff was selected for three reasons. First, Hubel and Wiesel (41) reported that the sensitive period for the effects of visual experience on the visual cortex of kittens extended until about 3 months of age, which is the earliest onset point for puberty in that species. Second, Lewis and Maurer (42) reported that human perceptual development remains vulnerable to damage from adverse visual experience until 10–13 years. Third, Garey et al. (43) reported that synaptogenesis in V1 is rapid after birth with maximal synaptic density occurring at about 8 months. Thereafter synapses are eliminated to reach “adult” levels at about 11 years. Hence, we hypothesized that the visual cortex should be relatively plastic through about 11 years of age, with menses begin on average at about age 12. Unfortunately, we did not collect data on menarche, which may have provided a more meaningful demarcation point.
Why the visual cortex may be affected is an interesting question. We have proposed that exposure to different forms of abuse may have shared neurobiological consequences (related to their common action as stressors) and unique consequences related to sensory systems activated by the stress (44, 45). Specifically, the child’s brain may endeavor to reduce distress by attenuating the development of sensory systems and pathways relaying recurrent aversive or traumatic experiences (44). This may emerge as a form of experience-dependent plasticity that occurs during a sensitive period (13). Such periods allow experience to instruct neural circuits to process or represent information in ways that are adaptive for the individual (46). This is consonant with our hypothesis that abuse-associated neurobiological alterations may not simply reflect damage, but may serve some adaptive purpose (47). The effect of experience on the brain during periods of high plasticity can alter axonal or dendritic morphologies, produce or eliminate synapses, and change the strength of synaptic connections (46). This hypothesis applies to other sensory systems as well. We recently reported that exposure to parental verbal abuse was associated with reduced fractional anisotropy in the left arcuate fasciculus, which connects Wernike’s and Broca’s areas, and is important for verbal comprehension (45). Conversion disorders, including loss of vision or hearing, and dissociative disorders can also result from abuse (48, 49), suggesting an array of potential mechanisms that may serve to protect the individual by reducing the biological or psychological impact of exposure.
Although we have discussed this finding in terms of a potential cause and effect mechanism, it must be emphasized that we only have evidence of an association. A possible alternative explanation for reduced occipital GMV is that children with ADHD may be at increased risk for CSA, and that ADHD has been associated with altered occipital GMV. However, no subject in this sample had a history of ADHD. Moreover, the association between ADHD and exposure to CSA is modest and may apply only to the inattentive subtype (50). Another alternative explanation, that we cannot reject, is that reduced visual cortex GMV may be a preexisting abnormality that enhances risk of CSA. However, there is no other evidence to support this conjecture. Another unsupported possibility is that reduced occipital GMV runs in families and is associated with increased likelihood of pedophilic or incestuous behaviors. Most subjects in this study experienced CSA from unrelated individuals. No subject in this sample reported CSA by biological parents. The predicted observation that V1 GMV correlated with the duration of abuse up to 11 years of age, but not after, is consistent with a causal relation.
FreeSurfer revealed that the most strongly affected components in this area were the left fusiform and right lingual gyri. The fusiform or occipitotemporal gyrus plays an important role in the recognition of faces (52), words (53), objects (54), and colors (55). Activity in the fusiform gyrus tends to be right lateralized for unfamiliar faces, bilateral for objects, and left lateralized for printed words (56). The right fusiform appears to be specialized for processing a face as a whole, and the left fusiform is apparently specialized for processing based on facial features (57). Moreover, the left fusiform gyrus was found to be activated specifically when viewing one’s own face (58) and remembered faces (59). It is conceivable that reduced GMV in left but not right fusiform gyrus may bias facial perception and help explain the tendency of some patients to interpret ambiguous facial expressions as angry (60).
The right lingual gyrus appears to be involved in the global aspects of figure recognition (distinguishing a forest from trees) (61) and object naming (62). It may also be a critical substrate for dreaming (63), and is a brain region that consistently shows reduced cerebral blood flow after sleep deprivation or disruption (64, 65). Nightmares and sleep disruption are frequently reported sequelae of CSA (66–68). Sleep disruption caused by CSA may diminish activity and blood flow to this region, and consequently alter its developmental trajectory.
Overall, the association between exposure to CSA and reduced GMV in the visual cortex is particularly intriguing, given the historic importance of the visual cortex in elucidating the role of early experience on brain development (41). Furthermore, the observation of a potential sensitive period during which the visual cortex is maximally vulnerable to CSA argues for the value of early intervention or prevention strategies.
The main limitation of this study is the small sample size, particularly that of controls. The cleanliness of the sample (all unmedicated, very narrow age range, no other forms of traumatic exposure, high SES, minimal exposure to alcohol or drugs of abuse) might have compensated that shortcoming, at least in part, by reducing error variance. Global analytical techniques, such as VBM, are limited by the need to adjust for multiple comparisons to minimize the risk of detecting chance related differences. Consequently, only the most robust differences tend to emerge. We recently published results of an ROI analysis on these subjects, and identified alterations in their hippocampus, corpus callosum, and frontal cortex (13). The focus of that paper was the identification of sensitive periods for effects of CSA on structures previously identified as susceptible.
These studies differ considerably from prior reports on the association between childhood abuse and brain morphometry. Previous studies recruited abused subjects meeting criteria for specific psychiatric disorders. This strategy is useful when the primarily focus is on the neurobiology of the disorder. However, this strategy does not provide an unbiased perspective of the effects of exposure to childhood abuse. The alternative approach of selecting only subjects without psychopathology is equally problematic because it might underestimate the effects of exposure, and confound consequences with preexisting morphometric differences that enhance resilience. The only way around this dilemma, in our opinion, was to recruit subjects with a history of exposure, regardless of psychiatric outcome. Including abused subjects with and without psychopathology avoids overestimating or underestimating consequences, and accepting virtually all types of outcomes eliminates concern that an identified abnormality was actually a preexisting risk factor for a specific disorder. Consequently, imaging differences observed in these subjects may generalize better to the population at large, as they are outcome independent.
Another unique feature of the present study is that these CSA subjects were unexposed to other forms of abuse or trauma. Prior studies included subjects who experienced different or multiple types of abuse (e.g., physical or sexual abuse) (2, 5, 8, 69–73), or selected subjects exposed to CSA without excluding subjects who experienced multiple forms of abuse (3). Results from the present sample provide the only data available on the specific associations between CSA and brain structure and function. Such subjects are somewhat atypical (only 1/3 of subjects reporting CSA), but are not rare (10% of the screened sample). Hence, this study may identify neurobiological differences that are specifically associated with exposure to CSA, but may not apply to other forms of maltreatment.
Acknowledgments
This study was supported by RO1 awards from the U.S.A. National Institute of Mental Health (MH-53636, MH-66222) and National Institute of Drug Abuse (DA-016934, DA-017846) to MHT. We thank Hanako Suzuki, Anthony Mullin, Kumiko Suzuki, Jordan Deifik and Ray Fix for their assistance in data analysis, and Cynthia McGreenery, Danielle Webster and Dr. Carol A. Glod for recruitment and interviewing of subjects.
References
- 1.Lange A, de Beurs E, Dolan C, Lachnit T, Sjollema S, Hanewald G. Long-term effects of childhood sexual abuse: objective and subjective characteristics of the abuse and psychopathology in later life. J Nerv Ment Dis. 1999;187:150–158. doi: 10.1097/00005053-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 4.Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 5.Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. A.E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 7.Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 8.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 9.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 10.De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Iyengar S, Beers SR, et al. Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biol Psychiatry. 2002;51:544–552. doi: 10.1016/s0006-3223(01)01374-9. [DOI] [PubMed] [Google Scholar]
- 11.De Bellis MD, Keshavan MS, Spencer S, Hall J. N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am J Psychiatry. 2000;157:1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- 12.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 13.Andersen SL, Tomoda A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am. 2002;25:397–426. vii–viii. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 15.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am J Psychiatry. 2006;163:993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- 17.Roy CA, Perry JC. Instruments for the assessment of childhood trauma in adults. J Nerv Ment Dis. 2004;192:343–351. doi: 10.1097/01.nmd.0000126701.23121.fa. [DOI] [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders - clinician version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 19.Gunderson JG, Kolb JE, Austin V. The diagnostic interview for borderline patients. Am J Psychiatry. 1981;138:896–903. doi: 10.1176/ajp.138.7.896. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg M. Interviewer’s guide to the structured clinical interview for DSM-IV dissociative disorder (SCID-D) Arlington, VA: American Psychiatric Press; 1994. [Google Scholar]
- 21.Orvaschel H, Puig-Antich J. Schedule for Affective Disorder and Schizophrenia for School-Age Children, Epidemiologic Version, Fifth Revision. Fort Lauderdale, FL: Nova Southeastern University; 1994. [Google Scholar]
- 22.Williams JM. Psychological Assessment Resources. Odessa, FL: 1991. Memory Assessment Scales: Professional Manual. [Google Scholar]
- 23.Navalta CP, Polcari A, Webster DM, Boghossian A, Teicher MH. Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. J Neuropsychiatry Clin Neurosci. 2006;18:45–53. doi: 10.1176/jnp.18.1.45. [DOI] [PubMed] [Google Scholar]
- 24.Hollingshead AB. Hollingshead two factor index of social position, occupational categories. Rockville, MD: National Institute of Health, Psychopharmacology Research Branch; 1965. [Google Scholar]
- 25.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 26.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 27.Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 28.Okada T, Tanaka M, Kuratsune H, Watanabe Y, Sadato N. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol. 2004;4:14. doi: 10.1186/1471-2377-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to medical cerebral imaging. Stuttgart: Thieme; 1988. [Google Scholar]
- 31.Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 32.Moorhead TW, Job DE, Spencer MD, Whalley HC, Johnstone EC, Lawrie SM. Empirical comparison of maximal voxel and non-isotropic adjusted cluster extent results in a voxel-based morphometry study of comorbid learning disability with schizophrenia. Neuroimage. 2005;28:544–552. doi: 10.1016/j.neuroimage.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 33.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 34.Worsley KJ, Andermann M, Koulis T, MacDonald D, Evans AC. Detecting changes in nonisotropic images. Hum Brain Mapp. 1999;8:98–101. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<98::AID-HBM5>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- 36.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 37.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 38.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 39.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 41.Hubel DH, Wiesel TN. Early exploration of the visual cortex. Neuron. 1998;20:401–412. doi: 10.1016/s0896-6273(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 42.Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Dev Psychobiol. 2005;46:163–183. doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- 43.Garey LJ. Structural development of the visual system of man. Hum Neurobiol. 1984;3:75–80. [PubMed] [Google Scholar]
- 44.Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- 45.Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry. 2009;65:227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 47.Teicher MH. Scars that won’t heal: the neurobiology of child abuse. Sci Am. 2002;286:68–75. doi: 10.1038/scientificamerican0302-68. [DOI] [PubMed] [Google Scholar]
- 48.Sar V, Akyuz G, Kundakci T, Kiziltan E, Dogan O. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161:2271–2276. doi: 10.1176/appi.ajp.161.12.2271. [DOI] [PubMed] [Google Scholar]
- 49.Chu JA, Frey LM, Ganzel BL, Matthews JA. Memories of childhood abuse: dissociation, amnesia, and corroboration. Am J Psychiatry. 1999;156:749–755. doi: 10.1176/ajp.156.5.749. [DOI] [PubMed] [Google Scholar]
- 50.Ouyang L, Fang X, Mercy J, Perou R, Grosse SD. Attention-deficit/hyperactivity disorder symptoms and child maltreatment: a population-based study. J Pediatr. 2008;153:851–856. doi: 10.1016/j.jpeds.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Super H. Working memory in the primary visual cortex. Arch Neurol. 2003;60:809–812. doi: 10.1001/archneur.60.6.809. [DOI] [PubMed] [Google Scholar]
- 52.Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain. 1992;115(Pt 1):15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- 53.Devlin JT, Jamison HL, Gonnerman LM, Matthews PM. The role of the posterior fusiform gyrus in reading. J Cogn Neurosci. 2006;18:911–922. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tyler LK, Stamatakis EA, Dick E, Bright P, Fletcher P, Moss H. Objects and their actions: evidence for a neurally distributed semantic system. Neuroimage. 2003;18:542–557. doi: 10.1016/s1053-8119(02)00047-2. [DOI] [PubMed] [Google Scholar]
- 55.Barrett NA, Large MM, Smith GL, Michie PT, Karayanidis F, Kavanagh DJ, et al. Human cortical processing of colour and pattern. Hum Brain Mapp. 2001;13:213–225. doi: 10.1002/hbm.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossion B, Joyce CA, Cottrell GW, Tarr MJ. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage. 2003;20:1609–1624. doi: 10.1016/j.neuroimage.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Rossion B, Dricot L, Devolder A, Bodart JM, Crommelinck M, De Gelder B, et al. Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. J Cogn Neurosci. 2000;12:793–802. doi: 10.1162/089892900562606. [DOI] [PubMed] [Google Scholar]
- 58.Sugiura M, Kawashima R, Nakamura K, Okada K, Kato T, Nakamura A, et al. Passive and active recognition of one’s own face. Neuroimage. 2000;11:36–48. doi: 10.1006/nimg.1999.0519. [DOI] [PubMed] [Google Scholar]
- 59.Druzgal TJ, D’Esposito M. A neural network reflecting decisions about human faces. Neuron. 2001;32:947–955. doi: 10.1016/s0896-6273(01)00519-0. [DOI] [PubMed] [Google Scholar]
- 60.Domes G, Czieschnek D, Weidler F, Berger C, Fast K, Herpertz SC. Recognition of facial affect in Borderline Personality Disorder. J Personal Disord. 2008;22:135–147. doi: 10.1521/pedi.2008.22.2.135. [DOI] [PubMed] [Google Scholar]
- 61.Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382:626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- 62.Kiyosawa M, Inoue C, Kawasaki T, Tokoro T, Ishii K, Ohyama M, et al. Functional neuroanatomy of visual object naming: a PET study. Graefes Arch Clin Exp Ophthalmol. 1996;234:110–115. doi: 10.1007/BF00695250. [DOI] [PubMed] [Google Scholar]
- 63.Bischof M, Bassetti CL. Total dream loss: a distinct neuropsychological dysfunction after bilateral PCA stroke. Ann Neurol. 2004;56:583–586. doi: 10.1002/ana.20246. [DOI] [PubMed] [Google Scholar]
- 64.Joo EY, Tae WS, Han SJ, Cho JW, Hong SB. Reduced cerebral blood flow during wakefulness in obstructive sleep apnea-hypopnea syndrome. Sleep. 2007;30:1515–1520. doi: 10.1093/sleep/30.11.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joo EY, Seo DW, Tae WS, Hong SB. Effect of modafinil on cerebral blood flow in narcolepsy patients. Sleep. 2008;31:868–873. doi: 10.1093/sleep/31.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krakow B, Sandoval D, Schrader R, Keuhne B, McBride L, Yau CL, et al. Treatment of chronic nightmares in adjudicated adolescent girls in a residential facility. J Adolesc Health. 2001;29:94–100. doi: 10.1016/s1054-139x(00)00195-6. [DOI] [PubMed] [Google Scholar]
- 67.Agargun MY, Kara H, Ozer OA, Selvi Y, Kiran U, Kiran S. Nightmares and dissociative experiences: the key role of childhood traumatic events. Psychiatry Clin Neurosci. 2003;57:139–145. doi: 10.1046/j.1440-1819.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 68.Noll JG, Trickett PK, Susman EJ, Putnam FW. Sleep disturbances and childhood sexual abuse. J Pediatr Psychol. 2006;31:469–480. doi: 10.1093/jpepsy/jsj040. [DOI] [PubMed] [Google Scholar]
- 69.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 70.Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119:509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- 71.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 72.Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD. Hippocampal and amygdalar volumes in dissociative identity disorder. Am J Psychiatry. 2006;163:630–636. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, et al. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Res. 2008;162:256–261. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]