Abstract
The JmjC protein Mina is an important immune response regulator. Classical forward genetics first discovered its immune role in 2009 in connection with the development of T helper 2 (Th2) cells. This prompted investigation into Mina’s role in the two best-studied contexts where Th2 responses are essential: atopic asthma and helminth expulsion. In work focused on a mouse model of atopic asthma, Mina deficiency was found to ameliorate airway hyper-resistance and pulmonary inflammation. And, in a case-control study genetic variation at the human MINA locus was found to be associated with the development of childhood atopic asthma. Although the underlying cellular and molecular mechanism of Mina’s involvement in pulmonary inflammation remains unknown, our recent work on parasitic helminth expulsion suggests the possibility that, rather than T cells, epithelial cells responding to TGFβ may play the dominant role. Here we review the growing body of literature on the emerging Mina pathway in T cells and epithelial cells and attempt to set these into a broader context.
Introduction
Mina’s encoding gene was mapped to a locus regulating Th2-bias [1–3], a genetic trait defined as the propensity of naïve T helper cells to develop in vitro under neutral conditions into Th2 effector cells [2,4]. Th2 cells play a critical role in host protection from multicellular parasites such as intestinal helminths and when disregulated contribute to atopic diseases such as asthma [5,6]. The Th2-bias trait is known to vary across different inbred mouse strains [1,4] and correlates inversely with the relative abundance of intracellular Mina protein [3]. Consistent with its genetic association with Th2 bias, Mina was shown to act as a dose-dependent transcriptional corepressor of the gene encoding interleukin-4 (IL4) [3], a key regulator of Th2 development [2,4,7,8]. Despite this evidence linking Mina to Th2 biology, we showed recently that CD4 T cells from Mina KO mice, predicted to exhibit a hyper-Th2 phenotype, developed normally into Th2 cells [9]. One possible explanation for this apparent inconsistency may be functional redundancy with NO66, Mina’s close structural and evolutionary paralog which is also expressed in CD4 T cells and may share substrates with Mina [10]. Notwithstanding Mina’s dispensability for Th2 cell development, recent work reveals an unexpected and critical TGFβ-regulated, epithelial cell role of Mina in modulating gut and lung Th2 responses. Here we review the most recent developments connecting the Mina pathway to Th2 responses in parasitic worm expulsion and the development of atopic pulmonary inflammation, highlighting the need to relate Mina’s biochemical functions to its various physiological roles.
Mina structure and enzymatic activity
Mina and NO66 belong to the phylogenetically conserved JmjC domain-containing protein family. Many of its constituent members function as epigenetic transcriptional regulators by virtue of histone demethylase activity contained in their JmjC domains [11]. Mina and NO66 are widely expressed, relatively small proteins (53 and 66 Kd, respectively) exhibiting strong nuceolar and weaker nucleoplasmic and cytoplasmic localization [12,13]. Both possess only a single recognizable structural motif corresponding to the hallmark JmjC domain. Sequence analysis of their JmjC domains reveals the presence of amino acid residues compatible with enzymatic activity at critical cofactor binding sites for Fe(II) and 2-oxoglutarate, including histidine at position 179 and lysine at position 194 [3,11]. Consistent with this finding, both Mina and NO66 have been reported to exhibit histone demethylase activity [14–16]. siRNA knockdown of Mina in bronchial epithelial cells resulted in enhanced H3K9me3 and a marginal decrease in H3K9me2 as determined by immunofluorescence staining [15]. While in transfected cell lines deletion of the JmjC domain and catalytically inactivating point mutations (H179Y and K194A) in the JmjC domain abrogated Mina’s demethylase activity [15]. This evidence notwithstanding, an indirect as opposed to a direct demethylation role for Mina cannot be ruled out.
Indeed, the direct histone demethylation role of Mina and NO66 is controversial [17,18]. A recent structural analysis of JmjC domain-containing proteins identified a novel protein fold that subdivides family members into histone demethylases and histidine hydroxylases [17]. Based on this analysis Mina and NO66 fall into the latter, non-demethylase class. Further, a recent proteomic study found the 60S ribosomal subunit proteins L27a (Rpl27a) and L8 (Rpl8) to undergo hydroxylation, respectively, by Mina on His39 and by NO66 on His216 [18]. Gene knockdown experiments confirmed the requirement for Mina and NO66, respectively, in Rpl27a and Rpl8 hydroxylation, while co-immunoprecipitation experiments confirmed the direct specificity of Mina and NO66 for their respective ribosomal substrates. Additional hydroxylation substrates for Mina and NO66 may remain to be discovered.
The requirement for 2-oxoglutarate and Fe(II) as cofactors in Mina’s enzymatic activity raises the possibility of functional linkages with metabolism and hypoxia. Thus, it is tempting to speculate that Mina activity may be modulated by oxygen and/or nutrient restriction as may occur at sites of infection, within tumors and in naturally anaerobic compartments such as the lumen and mucosa of the gastrointestinal tract as compared to the lung.
Mina as a transcriptional regulatory protein
Targeted Mina ablation leads to dramatic changes in specific mRNA expression levels (Meenu Pillai, submitted). This raises the central question whether Mina’s role in gene regulation is direct or indirect. If indeed Mina possess histone demethylation activity, a direct gene regulatory role is easy to envision, with Mina being recruited to specific genes by locus-specific transcription factors [3]. However, if Mina functions exclusively as a ribosomal protein hydroxylase, it is difficult to imagine how regulatory effects on specific mRNA levels could be directly mediated.
One possibility is that combinatorial ribosomal protein hydroxylation may contribute to the elaboration of a repertoire of functionally distinct ribosomes [19,20]. Distinct ribosomal hydroxylation patterns may then impose specificity for select groups of mRNAs. One problem with this idea is that in a variety of cell lines and primary tissues the majority (>90%) of Rpl27a occurred in its His39 hydroxylated form, potentially precluding this molecular tag from contributing to ribosomal heterogeneity [18]. However, it is possible that a small fraction of Rpl27a escapes Mina-dependent hydroxylation and contributes to ribosomal heterogeneity. Nevertheless, the model is still challenged to explain how ribosomal hydroxylation could directly affect the level of specific mRNAs (as distinct from specific proteins). Scenarios involving mRNA sequestration by ribosomes could be invoked. However, a simpler possibility is that Mina modulates specific mRNA levels by hydroxylating or demethylating a distinct class of direct transcriptional regulatory substrates, including nucleosomal histones. Comparative proteomic and transcriptomic analysis of Mina in WT and KO cells may help distinguish these possibilities. Notwithstanding these exciting advances, how Mina’s currently known biochemical activities and direct substrates relate (if at all) to its physiological roles remains an urgent question in the field.
Mina promotes atopic pulmonary inflammation
Despite the conflicting link with Th2 development, Mina has been found to impact the two best studied disease states where Th2 responses are known to be important: atopic asthma and parasitic helminth infection. Atopic asthma is a major global health problem with ~300 million people currently affected, an estimated national prevalence as high as 18% (11% in the USA) and annual global mortality at ~250,000 [21]. The development of airway hyper-reactivity and fibrosis characteristic of human atopic asthma is driven by pulmonary inflammation in response to aeroallergens. The Th2 cytokines IL13, IL4 and IL5 have been shown to play critical roles in this process, driving the recruitment of CD4 T cells, eosinophils, macrophages and dendritic cells to peribronchial and alveolar regions of the lung.
The first evidence of a role for Mina in pulmonary inflammatory disease came from a genetic case-control study focused on a population of Han Chinese children. It found that the T allele of MINA rs4857304 (in intron 2) is associated with a significant increase in the risk of developing atopic asthma and that the TT genotype is significantly associated with elevated serum IgE and IL4 [22]. Modeled in mice, human-like disease can be triggered via repeated intranasal administration with aeroallergens such as those present in extracts of house dust mites (HDM) or cockroaches [23]. A recent report examining the pulmonary response of C57BL/6 background mice challenged intranasaly with HDM extract found that Mina ablation significantly ameliorated airway hyper-responsiveness, pulmonary inflammation and IL4 and IL5 levels in bronchoalveolar lavage fluid [24]. Taken together, these results indicate that Mina acts in a pathway that promotes the development of pulmonary inflammation. What remains to be elucidated however, is the underlying cellular and biochemical mechanism of Mina’s involvement.
Mina constrains parasitic worm expulsion
The other major physiological setting where Th2 responses are known to play a critical role is in the expulsion of parasitic worms. According to WHO estimates, helminth parasitization inflicts enhanced mortality and morbidity on over one billion people. Within this number, Trichuris trichiura, a soil-transmitted whipworm, causes gastrointestinal inflammatory disorders and nutritional deficits in over ~700 million people globally [25], constituting a huge socioeconomic burden in developing countries. Extensive studies of T. trichiura and its closely related and well studied mouse model T. muris have revealed that a Th2 response is required for rapid expulsion while a Th1 response promotes chronic infection [26]. The distinct cytokine milieus differentially regulate multiple worm expulsatory effector mechanisms to exert their disparate influence on disease course [27,28]. One such mechanism involves the secretion of Resistin-like beta (Relmβ) by intestinal epithelial cells in response to stimulation by the Th2 cytokines IL13 and IL4 [28]. Relmβ is an orphan cytokine with known pro-inflammatory roles in the gut and the lung [29,30] and is required to promote efficient T. muris expulsion, in part by direct interaction with and perturbation of T. muris sensory apparatus [31].
Our recent studies of worm expulsion have revealed that C57BL/6 background Mina KO mice clear T. muris faster than littermate controls (Meenu Pillai, submitted). Using bone marrow chimera and tissue-specific genetic ablation experiments with Mina KO and Villin-Cre::Mina(flox) mice, we pinpointed intestinal epithelial cells rather than hematopoietic cells as the locus of Mina’s essential role in restraining helminth expulsion. In the absence of Mina, Th2 responses appeared unperturbed but Relmβ expression was nonetheless elevated in the intestinal mucosa of infected Mina KO mice, providing an explanation for their accelerated worm expulsion.
TGFβ switches Mina activity from an activator to a repressor
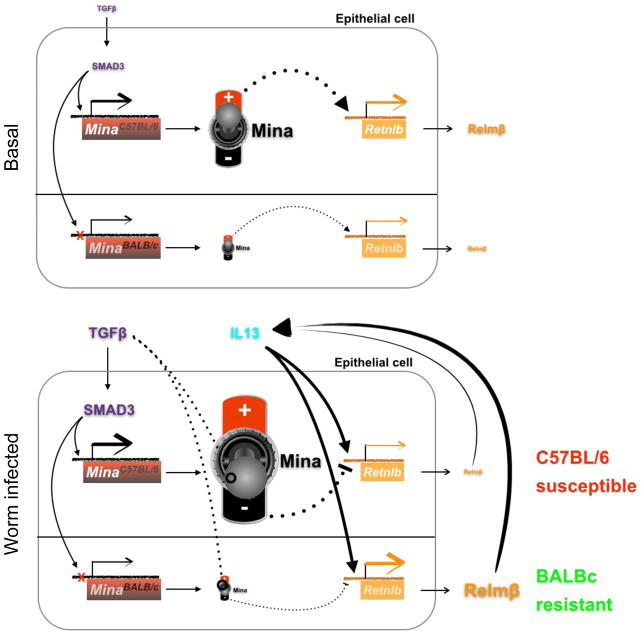
Perhaps most interestingly, Mina’s regulatory role toward Relmβ expression was plastic (Figure 1) (Meenu Pillai, submitted). Under the influence of TGFβ signaling, Mina toggled from acting as an enhancer to behaving like a repressor of Relmβ expression. Thus, Mina ablation in low TGFβ conditions (in uninfected mice or untreated epithelial cells in vitro) caused a decrease in Relmβ expression; by contrast, Mina ablation in the presence of TGFβ (in worm infected mice or TGFβ-treated epithelial cells in vitro) led to elevated Relmβ expression. The molecular mechanism underlying this switch-like behavior of TGFβ on Mina’s activity toward Relmβ is currently under investigation.
Figure 1. Shown is a model of TGFβ promoting Mina gene expression and switching Mina from acting as an enhancer to a repressor of Retnlb, encoding the pro-expulsatory cytokine Relmβ.
SNP rs4857304 (red X, shown for visual clarity upstream of the TSS) in Mina enhancer 2 renders the C57BL/6 but not the BALB/c allele of Mina sensitive to TGFβ/SMAD3-dependent activation. Under basal conditions (top) the relatively low level of TGFβ present is insufficient to toggle Mina activity from an activator to a repressor of Retnlb transcription. Together, these conspire to elevate expression of both Mina and Retnlb in epithelial cells from C57BL/6 as compared to BALB/c. Following parasitic worm infection (bottom), two parameters change. The first change is an increase in TGFβ level. This switches Mina from an activator to a repressor of Retnlb. The second change is the initiation of a Th2 response whose constituent IL13 elevation independently promotes Retnlb transcription. This has the potential to establish a positive feedback loop as Relmβ promotes the Th2 response. The net result is that in C57BL/6 cells expressing a high level of — now repressive — Mina, Retnlb expression is greatly attenuated. By contrast, the low level of repressive Mina in BALB/c cells is insufficient to attenuate IL13-induced Retnlb expression. Thus, in worm infected animals Relmβ level is high in BALB/c and low in C57BL/6, likely contributing to the enhanced resistance to Trichuris muris of BALB/c relative to C57BL/6. Dashed lines represent mRNA transcripts. Solid and dotted lines represent, respectively, direct and indirect effects.
Our work indicates that Mina restrains T. muris expulsion by acting in intestinal epithelial cells to repress Relmβ expression. Mina could accomplish this directly by, for example, recruitment to the Relmβ-encoding Retnlb promoter or indirectly by regulation of a Retnlb regulator, such as IL13 or miR375 [32]. Regardless of the precise mechanism, it is clear that TGFβ exerts a powerful influence on the nature of Mina activity such that at low level Mina acts as an enhancer while at high level Mina converts to a repressor. In the TGFβ rich mucosa of T. muris-infected mice, Mina may thus act as an important counterbalance to the induction of Relmβ by the Th2 cytokines IL4 and IL13. While in uninfected mice with low levels of intestinal TGFβ and in the absence of a Th2 response, Mina-dependent maintenance of a basal level of Relmβ may be important for normal gut homeostasis and commensal microbiota diversity.
Both the cellular locus of Mina’s pro-inflammatory activity in the lung and its biochemical mechanism there are unknown. One possibility is that Mina acts in T cells to promote a pulmonary Th2 response [24]. However, given the normal in vitro Th2 development exhibited by Mina KO T cells [9], it is worth considering another possibility that Mina may promote pulmonary inflammation by triggering Relmβ expression from pulmonary epithelial cells. Indeed, we have found that Relmβ expression is significantly lower in primary 3D air-liquid interface lung epithelial cell cultures seeded from Mina KO as opposed to WT control mice (Meenu Pillai, unpublished data) [33,34]. If, as we hypothesize, Relmβ levels are found to be lower in the lungs of HDM-treated Mina KO as opposed to WT control mice, how could we reconcile the opposing effects of Mina ablation on Relmβ expression in T. muris infected intestinal epithelial cells (where it is elevated) versus in HDM-inflamed pulmonary epithelial cells (where it is depressed)? The answer may be found in differential TGFβ expression, such that in HDM-inflamed lungs (as opposed to T. muris-infected intestines) levels may be below the threshold required to switch Mina’s activity from an enhancer to a repressor of Relmβ.
TGFβ also regulates Mina gene expression
In this context where Mina activity toward Relmβ expression is qualitatively modulated by TGFβ, it is interesting that Mina gene expression itself is also regulated by the TGFβ signaling pathway (Shangli Lian, submitted). Using DNAseI hypersensitivity mapping, expression reporter, EMSA and ChIP assays, we identified 4 enhancers spanning the ~20 kb Mina locus. Enhancer 2 (E2), the strongest of the four, functioned as a SMAD3-binding, TGFβ response element required for efficient Mina mRNA expression in differentiating Th17 cells. Interestingly, in developing Th17 cells the BALB/c (A) but not the C57BL/6 (G) allele of a naturally occurring SNP (rs4191790) located in the center of the E2 SMAD consensus sequence abolished SMAD3 binding, TGFβ responsiveness and Mina mRNA expression. The E2 SNP likely accounts for the strain dependent difference in Mina expression level [3] (Figure 1). It will be interesting to determine whether the elevation of this SNP to a detectable population frequency arose through a process of evolutionary positive selection. If so, this may be a useful system to study the nature of the potentially competing selective forces that drove to fixation a genetic variant functioning in multiple cell types, tissues and organ systems.
Conclusion
This article has emphasized Mina’s new Th2-linked role in gut and lung epithelial cells, yet it is important not to lose sight of Mina’s role in T cells. Transcriptional profiling and perturbation analyses recently revealed Mina’s involvement in regulating T helper 17 (Th17) and induced T regulatory (iTreg) cell balance during in vitro Th17-differentiation in response to TGFβ and IL6 [9]. It is likely that in this Th17/iTreg balancing role Mina will have an important impact in autoimmune diseases such as multiple sclerosis, and rheumatoid arthritis as well as in bacterial and fungal diseases [35]. Finally, given Mina’s ubiquitous expression pattern, it is likely that additional new and unexpected roles will be discovered as research progresses into Mina’s physiological impact from more cell types.
Highlights (for review).
Mina is associated with the development of atopic asthma in humans and mice.
Mina impairs helminth expulsion by modulating Relmβ expression in intestinal epithelial cells.
TGFβ signaling converts Mina from an activator to a repressor of Retnlb, encoding Relmβ.
TGFβ promotes Mina gene expression via an intronic enhancer element, E2.
E2 contains a SNP whose BALB/c but not C57BL/6 allele abolishes SMAD3 binding and enhancer activity.
Acknowledgments
I thank Mathew Coleman and Belgacem Mihi for constructive comments on the manuscript. This work was supported by ALSAC (MB), NIH 1R56AI06875-01A1 (MB) and 5R21AI101853-02 (MB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1•.Baguet A, Epler J, Wen KW, Bix M. A Leishmania major Response Locus Identified by Interval-specific Congenic Mapping of a T Helper Type 2 Cell Bias-controlling Quantitative Trait Locus. J Exp Med. 2004;200:1605–1612. doi: 10.1084/jem.20040334. This report describes the forward genetic mapping of the Th2-regulatory locus Dice1.2, subsequently found to contain the Mina gene locus, differentially expressed in BALB/c and C57BL/6 mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bix M, Wang ZE, Thiel B, Schork NJ, Locksley RM. Genetic regulation of commitment to interleukin 4 production by a CD4(+) T cell-intrinsic mechanism. J Exp Med. 1998;188:2289–2299. doi: 10.1084/jem.188.12.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Okamoto M, Van Stry M, Chung L, Koyanagi M, Sun X, Suzuki Y, Ohara O, Kitamura H, Hijikata A, Kubo M, et al. Mina, an Il4 repressor, controls T helper type 2 bias. Nature Immunology. 2009;10:872–879. doi: 10.1038/ni.1747. This is the first report of an immune role for Mina, in connection with Th2 responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yagi R, Suzuki W, Seki N, Kohyama M, Inoue T, Arai T, Kubo M. The IL-4 production capability of different strains of naive CD4(+) T cells controls the direction of the T(h) cell response. Int Immunol. 2002;14:1–11. doi: 10.1093/intimm/14.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Urban JF, Jr, Madden KB, Svetic A, Cheever A, Trotta PP, Gause WC, Katona IM, Finkelman FD. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev. 1992;127:205–220. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 6.Meyer EH, DeKruyff RH, Umetsu DT. T cells and NKT cells in the pathogenesis of asthma. Annu Rev Med. 2008;59:281–292. doi: 10.1146/annurev.med.59.061506.154139. [DOI] [PubMed] [Google Scholar]
- 7.Seder RA, Paul WE, Davis MM, Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noben-Trauth N, Hu-Li J, Paul WE. IL-4 secreted from individual naive CD4+ T cells acts in an autocrine manner to induce Th2 differentiation. Eur J Immunol. 2002;32:1428–1433. doi: 10.1002/1521-4141(200205)32:5<1428::AID-IMMU1428>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9•.Yosef N, Shalek AK, Gaublomme JT, Jin H, Lee Y, Awasthi A, Wu C, Karwacz K, Xiao S, Jorgolli M, et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature. 2013;496:461–468. doi: 10.1038/nature11981. This report describes Mina's role in Th17 differentiation in response to TGFβ and IL6 where it regulates the balance of Th17 and iTreg cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heng TS, Painter MW Immunological Genome Project C. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 11.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7:715–727. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- 12.Eilbracht J, Reichenzeller M, Hergt M, Schnölzer M, Heid H, Stöhr M, Franke WW, Schmidt-Zachmann MS. NO66, a highly conserved dual location protein in the nucleolus and in a special type of synchronously replicating chromatin. Mol Biol Cell. 2004;15:1816–1832. doi: 10.1091/mbc.E03-08-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eilbracht J, Kneissel S, Hofmann A, Schmidt-Zachmann MS. Protein NO52--a constitutive nucleolar component sharing high sequence homologies to protein NO66. Eur J Cell Biol. 2005;84:279–294. doi: 10.1016/j.ejcb.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Yu M, Chang Q, Lu Y, Thakur C, Ma D, Yi Z, Chen F. Mdig de-represses H19 large intergenic non-coding RNA (lincRNA) by down-regulating H3K9me3 and heterochromatin. Oncotarget. 2013;4:1427–1437. doi: 10.18632/oncotarget.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Y, Chang Q, Zhang Y, Beezhold K, Rojanasakul Y, Zhao H, Castranova V, Shi X, Chen F. Lung cancer-associated JmjC domain protein mdig suppresses formation of tri-methyl lysine 9 of histone H3. Cell Cycle. 2009;8:2101–2109. doi: 10.4161/cc.8.13.8927. [DOI] [PubMed] [Google Scholar]
- 16.Sinha KM, Yasuda H, Coombes MM, Dent SYR, De Crombrugghe B. Regulation of the osteoblast-specific transcription factor Osterix by NO66, a Jumonji family histone demethylase. EMBO J. 2009:1–12. doi: 10.1038/emboj.2009.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Chowdhury R, Sekirnik R, Brissett NC, Krojer T, Ho CH, Ng SS, Clifton IJ, Ge W, Kershaw NJ, Fox GC, et al. Ribosomal oxygenases are structurally conserved from prokaryotes to humans. Nature. 2014 doi: 10.1038/nature13263. This report describes a series of structural analyses that support the assignment of Mina and NO66 to a novel subfamily of JmjC-containing proteins predicted to possess hydroxylase but not demethylase activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Ge W, Wolf A, Feng T, Ho CH, Sekirnik R, Zayer A, Granatino N, Cockman ME, Loenarz C, Loik ND, et al. Oxygenase-catalyzed ribosome hydroxylation occurs in prokaryotes and humans. Nat Chem Biol. 2012;8:960–962. doi: 10.1038/nchembio.1093. This report describes Rpl27a and Rpl8 as hydroxylation substrates of Mina and NO66, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauro VP, Edelman GM. The ribosome filter hypothesis. Proc Natl Acad Sci U S A. 2002;99:12031–12036. doi: 10.1073/pnas.192442499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrgazov K, Vesper O, Moll I. Ribosome heterogeneity: another level of complexity in bacterial translation regulation. Curr Opin Microbiol. 2013;16:133–139. doi: 10.1016/j.mib.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Chen Y, Yang X, Huang Y, Liu E, Wang L. Associations of the single-nucleotide polymorphisms of the Mina gene with the development of asthma in Chinese Han children: a case-control study. Genetic testing and molecular biomarkers. 2011;15:531–536. doi: 10.1089/gtmb.2010.0240. This report describes the association of a MINA SNP with childhood atopic asthma. [DOI] [PubMed] [Google Scholar]
- 23.Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech. 2008;1:213–220. doi: 10.1242/dmm.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Mori T, Okamoto K, Tanaka Y, Teye K, Umata T, Ohneda K, Tokuyama K, Okabe M, Tsuneoka M. Ablation of Mina53 in mice reduces allergic response in the airways. Cell Struct Funct. 2013 doi: 10.1247/csf.13006. This report describes the ameliorated inflammatory phenotype of Mina KO mice in a model of atopic asthma. [DOI] [PubMed] [Google Scholar]
- 25.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 26.Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 28.Patel N, Kreider T, Urban JF, Jr, Gause WC. Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. Int J Parasitol. 2009;39:13–21. doi: 10.1016/j.ijpara.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra A, Wang M, Schlotman J, Nikolaidis NM, DeBrosse CW, Karow ML, Rothenberg ME. Resistin-like molecule-beta is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. American journal of physiology Lung cellular and molecular physiology. 2007;293:L305–313. doi: 10.1152/ajplung.00147.2007. [DOI] [PubMed] [Google Scholar]
- 30•.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206:2947–2957. doi: 10.1084/jem.20091268. This report describes the pro-expulsatory role of Relmβ with respect to infection by Trichuris muris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biton M, Levin A, Slyper M, Alkalay I, Horwitz E, Mor H, Kredo-Russo S, Avnit-Sagi T, Cojocaru G, Zreik F, et al. Epithelial microRNAs regulate gut mucosal immunity via epithelium-T cell crosstalk. Nat Immunol. 2011;12:239–246. doi: 10.1038/ni.1994. [DOI] [PubMed] [Google Scholar]
- 33.Dash P, Barnett PV, Denyer MS, Jackson T, Stirling CM, Hawes PC, Simpson JL, Monaghan P, Takamatsu HH. Foot-and-mouth disease virus replicates only transiently in well-differentiated porcine nasal epithelial cells. J Virol. 2010;84:9149–9160. doi: 10.1128/JVI.00642-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Stry M, Oguin TH, 3rd, Cheloufi S, Vogel P, Watanabe M, Pillai MR, Dash P, Thomas PG, Hannon GJ, Bix M. Enhanced susceptibility of Ago1/3 double-null mice to influenza A virus infection. Journal of virology. 2012;86:4151–4157. doi: 10.1128/JVI.05303-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-) evolutionary perspective. Nat Rev Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]