Abstract
Enteric neural activity modulates active transepithelial ion transport in the intestine. We investigated the neural circuits mediating neurogenic secretion in mucosal explants from porcine ileum. Transmural electrical stimulation increased short-circuit current, a measure of active ion transport, by 35 ± 2 µA/cm2. The neuronal Na+ channel blocker saxitoxin, the muscarinic cholinergic receptor antagonist atropine, the 5-hydroxytryptamine3 receptor antagonist tropisetron, and the cyclooxygenase inhibitor indomethacin inhibited this response. In addition, tropisetron inhibited the atropine-resistant portion of the response, and both atropine and indomethacin attenuated the saxitoxin-resistant component. Neurogenic secretion in porcine ileum appears to be mediated by tryptaminergic and prostanoid-sensitive cholinergic pathways.
Keywords: Transmural electrical stimulation, Short-circuit current, 5-hydroxytryptamine 3 receptor, Muscarinic cholinergic receptor, Intestinal mucosa
1. Introduction
Studies conducted over the past two decades have shown that active ion transport by the small and large intestinal mucosae is regulated by the enteric nervous system. Chemical or electrical stimulation of intrinsic enteric neurons in preparations of smooth muscle-stripped intestinal mucosa with attached submucosa from several mammalian species including humans is associated with an increase in short-circuit current (Isc), a measure of active anion secretion. This mucosal response is inhibited by tetrodotoxin or other neuronal Na+ channel blockers (Brown and Miller, 1991). With the exception of the rabbit ileum (Hubel, 1984), it is also sensitive to atropine and hexamethonium, antagonists of muscarinic and nicotinic cholinergic receptors respectively, indicating that it is mediated by the release of acetylcholine, a major secretomotor neurotransmitter (Furness and Sanger, 2002). Atropine-resistant Isc responses to electrical stimulation of the guinea pig small intestinal mucosa are further reduced by a substance P receptor antagonist (Keast et al., 1985) or antisera against vasoactive intestinal peptide (VIP; Cooke et al., 1987). Other classes of neurotransmitters that may be involved in mediating transepithelial ion transport evoked by electrical stimulation have not been fully defined, particularly in mammals other than the guinea pig.
The porcine intestine is a common biomedical model for the human gut (Kararli, 1995). Swine ingest and assimilate many of the same nutrients as humans and confront a similar array of enteropathogens (Brown and Terris, 1996). Moreover, the porcine distal small intestinal mucosa responds to transmural electrical stimulation with tetrodotoxin-sensitive increases in anion secretion and short-circuit current (Hildebrand and Brown, 1990). As in the human small intestine (Hubel and Shirazi, 1982), neurogenic ion transport has been shown to be mediated in part by acetylcholine in the porcine small intestine (Hildebrand and Brown, 1990). In addition, ion transport evoked by transmural electrical stimulation of the porcine small intestine is inhibited by norepinephrine, opioids, galanin and neuropeptide Y (Brown and O’Grady, 1997).
In the present study, we examined the involvement of several non-cholinergic neurotransmitters in mediating mucosal Isc responses of the porcine distal small intestine to transmural electrical stimulation. This was accomplished through the use of selective receptor antagonists and enzyme inhibitors. The results indicate that, in addition to acetylcholine, prostanoids and 5-hydroxytryptamine appear to play a role in electrically-mediated mucosal transport in the porcine intestine.
2. Materials and Methods
2.1. Tissue isolation
A segment of distal small intestine was obtained from weaned, outbred Yorkshire pigs of each sex (6 – 10 weeks of age; 10 – 18 kg body weight). Animals were not fasted before sacrifice. They were sedated with an intramuscular injection of tiletamine hydrochloride-zolazepam (8 mg/kg each, Fort Dodge Laboratories, Fort Dodge, IA), in combination with xylazine (3 mg/kg, Phoenix Pharmaceuticals Inc, St. Joseph, MO). The animals were subsequently euthanized by barbiturate overdose in accordance with approved University of Minnesota Institutional Animal Care and Use Committee protocols. A midline laparotomy was performed and an intestinal segment, including that portion attached to the ileocecal ligament, was resected extending approximately 1.5 m orad from the ileocecal junction.
2.2. Measurement of ion transport
The serosa and smooth muscle layers of an excised ileal segment were removed by blunt dissection and the remaining submucosa-mucosa was mounted between two lucite Ussing-type half chambers with 2 cm2 flux area; two strips of aluminum foil were placed diagonally on opposite sides of the tissue. Mucosal sheets were bathed in a physiological salt solution (composition in mM: NaCl, 118; KCl, 4.7; CaCl2, 3.0; MgCl2, 0.5; NaHCO3, 25; NaH2PO4, 1.0) at pH 7.4 and gassed with 5% CO2 in O2 at 39°C . d-Glucose and mannitol were added to the contraluminal and luminal media at 10 mM, respectively. The short-circuit current (Isc), a measure of net, electrogenic ion transport, was monitored by an automatic voltage clamp. Throughout each experiment, the transepithelial voltage was periodically clamped to ± 5 mV, and the resulting change in Isc was used to calculate the tissue conductance by Ohm’s law. After an equilibration period of 60 minutes, transmural electrical stimulation (300 bipolar current pulses at 10 Hz, 5 ms pulse duration, 2.1 mA/cm2) was delivered through the aluminum foil electrodes by a Model S-88 stimulator and Model SIU-5 stimulus isolation unit (AstroMed Grass Instruments, Quincy, MA). Each stimulus period was followed by the contraluminal addition of 40 µmoles of glucose. In the absence of drugs, three successive deliveries of transmural electrical stimulation produced peak Isc elevations which were averaged. One or more blocking drugs were added subsequently to the contraluminal bathing medium 10 min prior to a second series of three transmural electrical stimulation deliveries. Changes in Isc produced by drug(s) before and in response to transmural electrical stimulation were measured. In tissues desensitized to the actions of VIP or substance P, mucosal Isc responses to transmural electrical stimulation were measured before and after two additions of either neuropeptide to the contraluminal medium at a concentration of 1 µM. Because mucosal Isc responses to VIP were prolonged, responses to transmural electrical stimulation in tissues exhibiting tachyphylaxis to this peptide were also compared to those measured in time-matched tissues that were not exposed to VIP.
2.3. Drugs
All drugs were obtained from Sigma-RBI (St. Louis, MO). Stock solutions of drugs were made in distilled water, with the exception of indomethacin, which was solubilized in dimethylsulfoxide. The latter solvent did not significantly affect mucosal electrical responses to transmural electrical stimulation.
2.4. Data analysis
Data from tissues responding to three successive deliveries of transmural electrical stimulation in the absence of drugs with peak Isc elevations varying ≤ 10%) magnitude were analyzed. In each tissue preparation, inhibition of mean peak changes in Isc in response to three successive deliveries of transmural electrical stimulation in the presence of drugs is expressed as a percentage change from the average of the initial three Isc responses to transmural electrical stimulation determined prior to addition of blocking drugs or neuropeptide desensitization. Data are expressed as mean ± standard error (S.E.) of the mean. Comparisons between a control mean and a single treatment mean were made with a two-tailed, paired Student’s t-test. Comparisons of multiple means were made by one-way analysis of variance followed by Tukey’s test. In all cases, the limit for statistical significance was set at P < 0.05. These statistical analyses were performed using GraphPad Prism software (ver. 4.0a, GraphPad Software, San Diego, CA).
3. Results
3.1. Effects of transmural electrical stimulation of the mucosa-submucosa in the absence and presence of receptor antagonists and other inhibitors
The baseline Isc in muscle-stripped sheets of ileal mucosa-submucosa averaged −4.8 ± 2.9 µA/cm2 (n = 99 tissues from 52 pigs) Delivery of transmural electrical stimulation produced an increase in Isc that averaged 35.1 ± 1.9 µA/cm2 in these tissues.
The neuronal Na+ channel blocker saxitoxin decreased mucosal Isc responses to transmural electrical stimulation by 85% (Table 1). Responses to transmural electrical stimulation were also inhibited significantly by the muscarinic cholinergic receptor antagonist atropine, the cyclooxygenase inhibitor indomethacin, the eicosanoid synthesis inhibitor eicosas-5,8,11,14-tetraynoic acid (ETYA) and the 5-HT3 receptor antagonist, tropisetron (Table 1).
Table 1.
Effects of receptor antagonists and pharmacological inhibitors on mucosal responses to transmural electrical stimulation of the mucosa-submucosa
| Treatment | Average ΔIsc after delivery of transmural electrical stimulation (µA/cm2, mean ± SE) | n tissues | P value (two-tailed paired t test) |
|---|---|---|---|
| Pre-drug | 24. 7 ± 0.9 | 5 | 0.002 |
| Saxitoxin, 0.1 µM | 3.4 ± 0.4 | ||
| Pre-drug | 56.6 ± 1.1 | 7 | 0.002 |
| Atropine, 0.1 µM | 34.2 ± 2.1 | ||
| Pre-drug | 45.5 ± 1.4 | 7 | 0.002 |
| Indomethacin, 10 µM | 30.8 ± 2.3 | ||
| Pre-drug | 29.7 ± 0.7 | 5 | 0.005 |
| ETYA, 100 µM | 17.3 ± 1.7 | ||
| Pre-drug | 41.6 ± 6.4 | 9 | 0.06 |
| Tropisetron, 1 µM‡ | 34.3 ± 7.8 | ||
One tissue did not respond to tropisetron. If data from this tissue are excluded, the mean electrically-induced ΔIsc before and after tropisetron administration was respectively 39.4 ± 6.9 and 29.1 ± 6.6 µA/cm2, P = 0.0006.
Desensitization of the ileal mucosa to the Isc-elevating actions of VIP or substance P did not affect the subsequent mucosal response to transmural electrical stimulation (transmural electrical stimulation-evoked ΔIsc before and after two applications of VIP = 29.2 ± 0.8 and 21.2 ± 1.0 µA/cm2, respectively, n = 10 tissues, P > 0.05; ΔIsc before and after two applications of substance P = 23.3 ± 0.6 and 19.6 ± 1.3 µA/cm2, respectively, n = 6 tissues, P > 0.05, paired t tests). Moreover, mucosal responses to transmural electrical stimulation were not significantly altered (data not shown; n = 3 tissues from 3 pigs/treatment) by the glutaminergic NMDA receptor antagonist (−)-3-(2-carboxypiperazine-4-yl) propyl-1-phosphonic acid (CPP; 100 µM), the purinergic receptor antagonist pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS; 10 µM), the calcitonin gene-related peptide (CGRP) receptor antagonist α-CGRP8–37 (1 µM) or the endopeptidase inhibitor thiorphan (10 µM).
3.2. Examination of the saxitoxin- and atropine-resistant components of neurogenic mucosal responses to transmural electrical stimulation
The residual mucosal response to transmural electrical stimulation in tissues pretreated with saxitoxin was inhibited further by either atropine or indomethacin (Fig. 1, left). Tissues pretreated with atropine displayed a attenuated mucosal response to transmural electrical stimulation as well. In these tissues, tropisetron but not indomethacin further reduced transmural electrical stimulation-evoked Isc elevations (Fig. 1, right).
Figure 1.
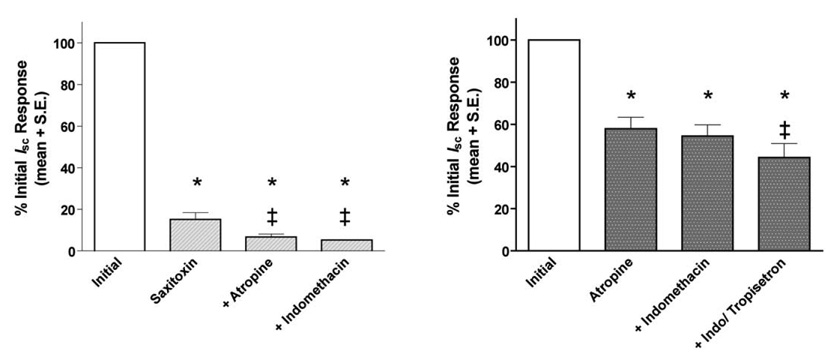
(Left) Effects of atropine (0.1 µM) or indomethacin (10 µM) on the saxitoxin-resistant portion of the mucosal response to transmural electrical stimulation. Each drug produced a significant additional decrease in the peak Isc elevation elicited by transmural electrical stimulation. Each bar represents the mean ± S.E. percentage decrease in the peak Isc elevation in 3 – 5 tissues from 3–5 pigs that was evoked by transmural electrical stimulation. The symbols “*” and “‡” appearing over the bars indicate a significant difference (P < 0.05, Tukey test) from the mean response determined in tissues prior to drug treatment (initial) or pretreated with 0.1 µM saxitoxin, respectively. (Right) Effects of atropine (0.1 µM) in the absence and presence of indomethacin (10 µM) and tropisetron (1 µM; bar labeled “+Indo/tropisetron”) on the peak mucosal response to transmural electrical stimulation. Each bar represents the mean ± S.E. percentage decrease in the peak Isc elevation in 4 – 7 tissues from 4–6 pigs that was evoked by transmural electrical stimulation. The symbols “*” and “‡” appearing over the bars indicate a significant difference (P < 0.05, Tukey test) from the mean response determined in tissues prior to drug treatment (initial) and tissues pretreated with atropine and indomethacin, respectively.
4. Discussion
As in intestinal preparations from rodents, guinea pigs and humans, transmural electrical stimulation produced a transient, saxitoxin-sensitive elevation in Isc in muscle-stripped mucosal sheets from the porcine ileum. In previous studies from our laboratory, transmural electrical stimulation delivered under similar stimulus parameters produced Isc elevations in mucosal sheets from the porcine distal small intestine of equal or somewhat greater magnitude (Hildebrand and Brown, 1990). This mucosal response to transmural electrical stimulation has been attributed to active chloride and bicarbonate secretion (Hildebrand and Brown, 1992).
Presumptive cholinergic, tachykininergic and VIPergic neurons constitute the majority of secretomotor nerves innervating the mucosa of the porcine small intestine (Hens et al., 2000). Interruption of cholinergic neurotransmission by pretreatment with atropine nearly halved peak changes in Isc evoked by transmural electrical stimulation. Moreover, in the presence of saxitoxin, atropine produced a smaller, but significant additional decrease in transmural electrical stimulation-evoked Isc, a result suggesting that cholinergic nerves both sensitive and insensitive to the axonal conduction blocker mediate this effect. Indeed, neurons expressing saxitoxin-resistant NaV1.9 sodium channel subtype have been characterized recently in the rodent intestine (Rugiero et al., 2003). On the other hand, mucosal responses to transmural electrical stimulation were not significantly reduced in tissues exhibiting tachyphylaxis to the prosecretory actions of VIP. The extended time course of these experiments, encompassing two VIP treatments and subsequent return of the Isc to baseline levels, before a second round of electrically-evoked Isc elevations could be measured introduces an additional variable because mucosal responses to transmural electrical stimulation spontaneously decline over time. However, electrically-evoked changes in Isc in tissues desensitized to VIP were not significantly different from time-matched control tissues untreated with the peptide. -Supporting this conclusion, a previous study using a specific VIP receptor antagonist in the porcine small intestine indicated that electrically-evoked Isc elevations were not altered; however, this receptor antagonist did inhibited the secretory action of exogenous VIP (Hildebrand and Brown, 1990). In the guinea pig small intestine, Isc responses to electrical stimulation were not altered in tissues desensitized to VIP (MacNaughton et al., 2004), but were inhibited by anti-VIP antiserum in tissues pretreated with atropine to abolish cholinergic influences (Cooke et al., 1987). Tachyphylaxis to substance P was not associated with a significant change in electrically-evoked responses in the porcine ileum. This finding is in agreement with an earlier study showing that substance P receptor antagonists did not alter electrically-evoked secretion in the guinea pig small intestinal mucosa (Reddix and Cooke, 1992). On the other hand, atropine-resistant Iscresponses to electrical stimulation of the guinea pig small intestinal mucosa are reportedly reduced by a substance P receptor antagonist (Keast et al., 1985). The involvement of tachykinins in electrically-induced neurogenic secretion may vary with the intestinal segment examined however, as neurally-evoked mucosal responses in the guinea pig jejunum are reduced by substance P tachyphylaxis and anti-substance P antiserum (Perdue et al., 1987). It is possible that the involvement of VIP or substance P, however limited, in mediating electrically-evoked Isc responses in porcine ileum would be revealed in tissues pretreated with atropine and treated with selective VIP and neurokinin receptor antagonists.
Inhibitors of prostanoid synthesis, including indomethacin and ETYA, significantly reduced electrically-evoked ion transport indicating that endogenous prostanoids may modulate neurogenic secretion in the porcine intestine. They appear to do so primarily through interactions with submucosal cholinergic nerves, as indomethacin failed to further decrease electrically-evoked mucosal responses in the presence of atropine. The interaction between prostanoids and cholinergic secretomotor nerves in the intestinal wall have been the subject of a recent review (Karaki and Kuwahara, 2004). In the piglet small intestine, prostacyclin and likely other cyclooxygenase products, which are synthesized and released in Cryptosporidium-elicited enteritis, have been hypothesized to stimulate cholinergic interneurons. This effect is associated with decreased epithelial sodium chloride absorption (Argenzio et al., 1996). We have found in previous studies of the porcine ileal mucosa that agonists at kinin (Green et al., 2003), 5-hydroxytryptamine (Green and Brown, 2002) and type 2 proteinase receptors (Green et al., 2000) produce saxitoxin-sensitive elevations in Isc that are reduced by indomethacin, but are resistant to atropine. Mucosal responses associated with the activation of these receptors, and to transmural electrical stimulation (Townsend and Brown, 2003), are inhibited by δ-opioid receptor agonists, such as d-Pen2,5-enkephalin. Delta-opioid receptors may be expressed on submucosal nerves linked to epithelial ion transport that mediate the effects of enteric prostanoids and acetylcholine.
5-Hydroxytryptamine is an important enteric neurohormone that, like prostanoids, is released in enteric infections and inflammation and elicits intestinal secretion in several mammalian species (Brown, 1996). In the pig small intestine, it appears to interact with epithelial type two 5-hydroxytryptamine (5-HT2) receptors as well as neuronal 5-HT3 and 5-HT4 receptors which are linked to anion secretion (Hansen and Skadhauge, 1994). Tropisetron, a 5-HT3 receptor antagonist, significantly decreased mucosal responses to transmural electrical stimulation alone and in the presence of atropine and indomethacin. These results are in agreement with previous studies demonstrating the antisecretory effects of 5-HT3 receptor antagonists in the porcine ileum (Grondahl et al., 1998). On the other hand, antagonists of purinergic, CGRP or N-methyl-d-aspartate (NMDA)-type glutaminergic receptors or of neutral endopeptidase, an enzyme which catalyzes the breakdown of several classes of enteric neuropeptides, did not significantly alter Isc responses evoked by transmural electrical stimulation. These results indicate that, despite their importance as enteric neurotransmitters, glutamate, adenosine 5’-triphosphate or CGRP play little or no role in mediating this neurogenic effect. As PPADS has some selectivity for P2-purinoceptors, we cannot rule out the possibility that electrically-evoked mucosal responses are mediated at least in part by ATP acting through P1-purinoceptors. However, active intestinal ion transport has been shown to be modulated predominately by P2-purinoceptors (Cressman et al., 1999; Christofi et al., 2004). The lack of effect of thiorphan on this response lends further support for the hypothesis that VIP, substance P and other classes of pro-secretory neuropeptides are not substantially involved in mediating this effect.
Acetylcholine, cyclooxygenase products and 5-hydroxytryptamine appear to mediate a portion of the prosecretory effects of transmural electrical stimulation in the porcine ileum. Prostanoids and muscarinic cholinergic receptors may function within a common neural pathway, whereas 5-HT3 receptors seem to be expressed in a separate electrically-evoked neural circuit. Clearly, one or more other as yet unidentified non-cholinergic, non-adrenergic transmitter substances present in enteric nerves innervating the porcine ileal mucosa mediate a substantial portion of the secretory response to transmural electrical stimulation.
Acknowledgements
This investigation was supported in part by National Institutes of Health grants R01 DA-10200 and T32 DA-07234.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argenzio RA, Armstrong M, Rhoads JM. Role of the enteric nervous system in piglet cryptosporidiosis. J. Pharmacol. Exp. Ther. 1996;279:1109–1115. [PubMed] [Google Scholar]
- Brown DR. Mucosal protection through active intestinal secretion: neural and paracrine modulation by 5-hydroxytryptamine. Behav. Brain Res. 1996;73:193–197. doi: 10.1016/0166-4328(96)00095-2. [DOI] [PubMed] [Google Scholar]
- Brown DR, Miller RJ. Neurohormonal control of fluid and electrolyte transport in intestinal mucosa. In: Field M, Frizzell RA, editors. Handbook of Physiology. Section 6: The Gastrointestinal System. Vol. 4: Intestinal Absorption and Secretion, chapter 24. Bethesda, MD: American Physiol. Soc.; 1991. pp. 527–589. [Google Scholar]
- Brown DR, O’Grady SM. Regulation of ion transport in the porcine intestinal tract by enteric neurotransmitters and hormones. Comp. Biochem. Physiol. 1997;118A:309–317. doi: 10.1016/s0300-9629(96)00311-8. [DOI] [PubMed] [Google Scholar]
- Brown DR, Terris JM. Swine in physiological and pathophysiological research. In: Tumbleson M, Schook LB, editors. Advances in Swine in Biomedical Research. Vol. 1. New York, NY: Plenum Press; 1996. pp. 5–6. [Google Scholar]
- Christofi FL, Wunderlich J, Yu JG, Wang YZ, Xue J, Guzman J, Javed N, Cooke H. Mechanically evoked reflex electrogenic chloride secretion in rat distal colon is triggered by endogenous nucleotides acting at P2Y1, P2Y2, and P2Y4 receptors. J. Comp. Neurol. 2004;469:16–36. doi: 10.1002/cne.10961. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Zafirova M, Carey HV, Walsh JH, Grider J. Vasoactive intestinal polypeptide actions on the guinea pig intestinal mucosa during neural stimulation. Gastroenterology. 1987;92:361–370. doi: 10.1016/0016-5085(87)90129-6. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Lazarowski E, Homolya L, Boucher RC, Koller BH, Grubb BR. Effect of loss of P2Y(2) receptor gene expression on nucleotide regulation of murine epithelial Cl(−) transport. J. Biol. Chem. 1999;274:26461–26468. doi: 10.1074/jbc.274.37.26461. [DOI] [PubMed] [Google Scholar]
- Furness JB, Sanger GJ. Intrinsic nerve circuits of the gastrointestinal tract: identification of drug targets. Curr. Opin. Pharmacol. 2002;2:612–622. doi: 10.1016/s1471-4892(02)00219-9. [DOI] [PubMed] [Google Scholar]
- Green BT, Brown DR. Active bicarbonate-dependent secretion evoked by 5-hydroxytryptamine in porcine ileal mucosa is mediated by opioid-sensitive enteric neurons. Eur. J. Pharmacol. 2002;451:185–190. doi: 10.1016/s0014-2999(02)02249-5. [DOI] [PubMed] [Google Scholar]
- Green BT, Bunnett NW, Kulkarni-Narla A, Steinhoff M, Brown DR. Intestinal type 2 proteinase-activated receptors: expression in opioid-sensitive secretomotor neural circuits that mediate epithelial ion transport. J. Pharmacol .Exp. Ther. 2000;295:410–416. [PubMed] [Google Scholar]
- Green BT, Calvin A, O'Grady SM, Brown DR. Kinin-induced anion-dependent secretion in porcine ileum: characterization and involvement of opioid- and cannabinoid-sensitive enteric neural circuits. J. Pharmacol. Exp. Ther. 2003;305:733–739. doi: 10.1124/jpet.102.047829. [DOI] [PubMed] [Google Scholar]
- Grøndahl ML, Jensen GM, Nielsen CG, Skadhauge E, Olsen JE, Hansen MB. Secretory pathways in Salmonella Typhimurium-induced fluid accumulation in the porcine small intestine. J. Med. Microbiol. 1998;47:151–157. doi: 10.1099/00222615-47-2-151. [DOI] [PubMed] [Google Scholar]
- Hansen MB, Skadhauge E. Signal transduction pathways for serotonin as an intestinal secretagogue. Comp. Biochem. Physiol. 1997;118A:283–290. doi: 10.1016/s0300-9629(97)00085-6. [DOI] [PubMed] [Google Scholar]
- Hens J, Schrodl F, Brehmer A, Adriaensen D, Neuhuber W, Scheuermann DW, Schemann M, Timmermans JP. Mucosal projections of enteric neurons in the porcine small intestine. J. Comp. Neurol. 2000;421:429–436. [PubMed] [Google Scholar]
- Hildebrand KR, Brown DR. Intrinsic neuroregulation of ion transport in porcine distal jejunum. J. Pharmacol. Exp. Ther. 1990;255:285–292. [PubMed] [Google Scholar]
- Hildebrand KR, Brown DR. Norepinephrine and alpha-2 adrenoceptors modulate active ion transport in porcine small intestine. J. Pharmacol. Exp. Ther. 1992;263:510–519. [PubMed] [Google Scholar]
- Hubel KA. Electrical stimulus-secretion coupling in rabbit ileal mucosa. J. Pharmacol. Exp. Ther. 1984;231:577–582. [PubMed] [Google Scholar]
- Hubel KA, Shirazi S. Human ileal ion transport in vitro: changes with electrical field stimulation and tetrodotoxin. Gastroenterology. 1982;83:63–88. [PubMed] [Google Scholar]
- Karaki SI, Kuwahara A. Regulation of intestinal secretion involved in the interaction between neurotransmitters and prostaglandin E2. Neurogastroenterol. Motil. 2004;16(Suppl 1):96–99. doi: 10.1111/j.1743-3150.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995;16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- Keast JR, Furness JB, Costa M. Investigations of nerve populations influencing ion transport that can be stimulated electrically, by serotonin and by a nicotinic agonist. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1985;331:260–266. doi: 10.1007/BF00634247. [DOI] [PubMed] [Google Scholar]
- MacNaughton WK, Van Sickle MD, Keenan CM, Cushing K, Mackie K, Sharkey KA. Distribution and function of the cannabinoid-1 receptor in the modulation of ion transport in the guinea pig ileum: relationship to capsaicin-sensitive nerves. Am. J. Physiol. 2004;286:G863–G871. doi: 10.1152/ajpgi.00482.2003. [DOI] [PubMed] [Google Scholar]
- Perdue MH, Galbraith R, Davison JS. Evidence for substance P as a functional neurotransmitter in guinea pig small intestinal mucosa. Regul. Pept. 1987;18:63–74. doi: 10.1016/0167-0115(87)90036-x. [DOI] [PubMed] [Google Scholar]
- Reddix RA, Cooke HJ. Neurokinin 1 receptors mediate substance P-induced changes in ion transport in guinea-pig ileum. Regul. Pept. 1992;39:215–225. doi: 10.1016/0167-0115(92)90542-3. [DOI] [PubMed] [Google Scholar]
- Rugiero F, Mistry M, Sage D, Black JA, Waxman SG, Crest M, Clerc N, Delmas P, Gola M. Selective expression of a persistent tetrodotoxin-resistant Na+ current and NaV1.9 subunit in myenteric sensory neurons. J. Neurosci. 2003;23:2715–2725. doi: 10.1523/JNEUROSCI.23-07-02715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D, Brown DR. Pharmacological characterization of a 7-benzylidenenaltrexone-preferring opioid receptor in porcine ileal submucosa. Brit. J. Pharmacol. 2003;140:691–700. doi: 10.1038/sj.bjp.0705485. [DOI] [PMC free article] [PubMed] [Google Scholar]


