Abstract
The cyanobacterium Nostoc strain ATCC 53789, a known cryptophycin producer, was tested for its potential as a source of natural pesticides. The antibacterial, antifungal, insecticidal, nematocidal, and cytotoxic activities of methanolic extracts of the cyanobacterium were evaluated. Among the target organisms, nine fungi (Armillaria sp., Fusarium oxysporum f. sp. melonis, Penicillium expansum, Phytophthora cambivora, P. cinnamomi, Rhizoctonia solani, Rosellinia, sp., Sclerotinia sclerotiorum, and Verticillium albo-atrum) were growth inhibited and one insect (Helicoverpa armigera) was killed by the extract, as well as the two model organisms for nematocidal (Caenorhabditis elegans) and cytotoxic (Artemia salina) activity. No antibacterial activity was detected. The antifungal activity against S. sclerotiorum was further studied with both extracts and biomass of the cyanobacterium in a system involving tomato as a host plant. Finally, the herbicidal activity of Nostoc strain ATCC 53789 was evaluated against a grass mixture. To fully exploit the potential of this cyanobacterium in agriculture as a source of pesticides, suitable application methods to overcome its toxicity toward plants and nontarget organisms must be developed.
The reduction of crop losses is a major goal in agriculture, and until now synthetic chemicals have played a fundamental role in suppressing pests and maintaining high crop yields. The world trade of pesticides in 1999 amounted to more than $22 billion, of which about 25% was for fungicides, 35% was for herbicides, and 21% was for insecticides (Food and Agriculture Organization of United Nations, FAOSTAT-Agriculture Data, http://apps.fao.org). Starting from the mid-1970s, however, the adverse environmental impact of pesticides has become a major concern, not only because of the persistence of the chemicals in topsoils or their leaching to groundwater but also because of their undesired effects on nontarget areas such as towns or inhabited areas reached by volatilized pesticides (4). Furthermore, the massive use of synthetic pesticides has led to the development of resistance in all classes of pests (33). Natural products are considered to be less harmful, mainly because they have higher biodegradability and are often active at lower doses (33). Moreover, natural molecules can provide novel structures and mechanisms of action for the discovery of safer synthetic pesticides. The search for new natural compounds is of great importance for the development of organic agriculture, which at present is applied to more than 17 million ha worldwide, mostly in Oceania, Europe, and Latin America, with a market of about $1.7 billion (39).
Cyanobacteria are an important source of novel antifungal and, to a lesser extent, antibacterial compounds (5, 6, 17, 26, 30). However, most of the research has been focused on pharmaceutical applications of cyanobacteria-derived products, mainly for economic reasons. The potential use of cyanobacteria in agriculture is often reported as a secondary goal, and, except in a few cases (19, 37), research in this field has been limited to in vitro screening. Some antifungal molecules derived from cyanobacteria have been patented for agricultural use, but research on this topic has not been continued (14, 21). The insecticidal and nematocidal activity of cyanobacteria is still largely unexplored and only a few studies of this subject have been published (15, 28, 32). This is true also for their phytotoxic and herbicidal properties (8, 13, 18); one herbicidal compound, cyanobacterin, from Scytonema hofmanni, has been patented (11) but not commercially developed.
In 1990, researchers at Merck & Co., Inc. (Rahway, N.J.) isolated a novel depsipeptide from a cyanobacterium of the genus Nostoc that showed potent activity against filamentous fungi and yeasts, mainly of the genus Cryptococcus (34). The strain was deposited as ATCC 53789, and its potential use in the pharmaceutical and agricultural field was patented (14). The structure of the active compound, named cryptophycin 1, was determined later by Moore and coworkers (2, 38), who isolated it from Nostoc strain GSV224, which was discovered to be a cryptophycin producer during screening for cyanobacterial anticancer activity at the University of Hawaii (20, 25). Cryptophycin 1 exerts antiproliferative and antimitotic activity by binding to the ends of the microtubules, thus blocking the cell cycle at the metaphase of mitosis. Cryptophycin 1 is the most potent suppressor of microtubule dynamics yet described (24). The agricultural use of cryptophycin has not been further investigated because research has been focused on its potent antitumor activity (12, 22). A synthetic analogue, cryptophycin-52, is at present in phase II clinical trials by Eli Lilly & Co, Inc., Indianapolis, Ind. (10, 35).
In this work, methanolic extracts obtained from Nostoc strain ATCC 53789 were tested against a variety of pathogens of agricultural importance: 12 fungi (Armillaria sp., Colletotrichum coffeanum, C. trifolii, Fusarium solani, F. oxysporum f. sp. melonis, Penicillium expansum, Phytophthora cambivora, P. cinnamomi, Rhizoctonia solani, Rosellinia sp., Sclerotinia sclerotiorum, and Verticillium albo-atrum), 2 bacteria (Agrobacterium tumefaciens and Pseudomonas aeruginosa), and 2 insects (Galleria mellonella and Helicoverpa armigera), as well as against Caenorhabditis elegans and Artemia salina as model organisms for nematocidal and cytotoxic activity, respectively. As a control, extracts of Nostoc strain UTEX 2493, a nontoxic strain (29), were tested against the same target organisms. In view of a possible application of Nostoc strain ATCC 53789 in agriculture, its activity against Sclerotinia sclerotiorum was also verified in the presence of a host plant (tomato). Finally, the herbicidal potential of the strain was evaluated against a mixture of Graminaceae, composed of Festuca arundinacea (75%), Lolium perenne (15%), and Poa pratensis (10%).
MATERIALS AND METHODS
Nostoc strain ATCC 53789 was cultivated in 1- and 10-liter glass tubes bubbled with an air-CO2 mixture (98:2, vol/vol) in BG110 medium (30), under continuous illumination (ca. 200 μmol of photons m−2 s−1 photosynthetically active radiation) provided by “daylight” fluorescent lamps, at 25 to 28°C. Nostoc strain UTEX 2493 was cultivated under the same conditions, but only in 1-liter glass tubes. The biomass of the two cyanobacteria was harvested by filtration and frozen. For the screening, the strains were cultivated axenically in 1-liter tubes, whereas Nostoc strain ATCC 53789 for the experiments involving plants was grown under nonaxenic conditions in a 10-liter tube since it was necessary to obtain larger amounts of biomass. Biomass produced in the 10-liter tube showed the same level of bioactivity as that of the biomass produced for the screening.
To obtain the extracts, the frozen biomass was thawed, separated from the thawing water (water released from the thawed biomass plus water used to wash the biomass) by filtration, and extracted overnight with 50 ml of pure methanol per g of dry biomass. Methanol was used since it is a common solvent for cryptophycin extraction, although it can result in the formation of small amounts of cryptophycins with modified structure, which are considered artifacts since they cannot be detected in cells extracted with other solvents (38). The thawing water was frozen, lyophilized, and then extracted using the same procedure. The solvent was evaporated under vacuum, and the residue was resuspended in a small volume of methanol to obtain the crude extracts.
For evaluation of antifungal and antibacterial activities, aliquots (100 μl) of the crude methanolic extracts were distributed in 96-well microplates and evaporated; the residue was then resuspended in 100 μl of dimethyl sulfoxide-water (9:1) and serially diluted 10-fold with the same solvent. A 10-μl volume of each serial dilution was then distributed in a second 96-well microplate, and 90 μl of potato dextrose broth inoculated with the target organism was added so that the final dimethyl sulfoxide concentration was 1%. Each microplate also included two controls without extract, one with 1% dimethyl sulfoxide and one with water. An extract was considered active if it inhibited the growth of the target organism at least 75% compared to the control with only dimethyl sulfoxide, after 72 h (for fungi) or 24 h (for bacteria) of incubation.
Activity against insects was tested using two different methods: (i) direct injection of the extract under the skin of the larvae for G. mellonella and (ii) feeding assay for H. armigera, in which the agarized food for the larvae was distributed in 24-well plates and poisoned with different concentrations of the extract spread on the surface of the feeding substrate. In both cases, the viability of the larvae was checked daily. After 8 days, H. armigera larvae were counted and, if alive, weighed. The significance of the difference between the means of the weights of the treated and control larvae was evaluated statistically. The feeding assay method was used also for C. elegans, and bioactivity was evaluated both as loss of viability of the larvae and as alteration of the nematode life cycle, which was evidenced as a longer time required to complete different stages and release the eggs. Cytotoxic activity was evaluated against A. salina in 96-well microplates (36) in the presence of serial dilutions of 10 μl of extract to which 90 μl of artificial seawater (24‰ salinity) containing four to six nauplii was added per well. An extract was considered active if it caused an increase in mortality at least of 75% in comparison with the control after 24 h of incubation.
For antifungal activity in the presence of the host plant and for herbicidal activity, experiments were carried out with 580-ml sterile glass jars containing 50 ml of agarized and autoclaved medium and 10 (tomato) or 15 (grass mixture) sterilized seeds. The medium used was one-quarter-strength Hoagland solution (16) to which 5 g of agar per liter and, for antifungal tests, also 5 g of glucose per liter had been added. For sterilization, tomato seeds were immersed twice in H2O2 (30%, vol/vol) for 5 min and rinsed after each immersion with sterile demineralized water. Grass seeds were immersed for 10 min in sterile water, treated with H2O2 (30%, vol/vol), for 10 min, and then rinsed for 10 min in sterile water. For these experiments, the cyanobacterial extract was obtained only from the lyophilized biomass which had been extracted overnight with pure methanol and processed as described above.
The test for antifungal activity in the presence of the host plant was structured as follows. Ten tomato seeds were placed in each of the 580-ml jars, to which one of the following additions was made: (i) Sclerotinia sclerotiorum inoculum (plant and fungal control); (ii) lyophilized cyanobacterial biomass at a concentration of 0.2, 2, or 20 g liter−1; (iii) lyophilized cyanobacterial biomass at a concentration of 0.2, 2, or 20 g liter−1 plus fungal inoculum; (iv) cyanobacterial extracts at a concentration of 0.2, 2, or 20 g/liter−1 (note that the concentration refers to the amount of dry biomass extracted); (v) cyanobacterial extracts at a concentration of 0.2, 2, or 20 g/liter−1 plus fungal inoculum; or (vi) no addition (plant control). To a jar without seeds, S. sclerotiorum alone was added. After the additions, the agarized medium was mixed and left to solidify. Each control and treatment was repeated three times.
Before the trial with plants, aliquots (0.2 g) of biomass were autoclaved for 20 min or heated in an oven at 80°C for 3 h. The treated biomasses and a corresponding quantity of nontreated biomass (control) were extracted with methanol and tested for bioactivity against S. sclerotiorum by the procedure described above. No difference among the control and the two treated biomasses was observed. Thus, autoclaved biomass was used in all the trials with plants (tomato and grass mixture) to avoid contamination of the system since the biomass was not axenic. Both the autoclaved biomass and the extract were directly mixed with the liquid agar at the required concentration. The fungus was added in the form of small agar beads prepared from an inoculated petri dish.
For herbicidal activity, two replicas of the control and of three concentrations of extract (1, 10, and 100 g liter−1) were tested. Both antifungal and herbicidal tests were performed under axenic conditions. The jars were kept in the laboratory under natural illumination and at room temperature (20 to 25°C).
Development of the seedlings was monitored by periodically measuring their height, without opening the jars, and assigning the values to growth classes in the range from 0 to 11 cm with an interval of 1 cm. At the end of the antifungal tests (day 39), the dry weight, height, and diameter of the stem of each seedling, as well as the length and dry weight of the roots, were determined. For herbicidal activity, only the height of the culms and the length of the roots were measured. The differences between the treatments and the control were evaluated by analysis of variance (ANOVA) (7).
RESULTS
Methanolic extracts of Nostoc strain ATCC 53789 obtained from both thawed biomass and thawing water were active against 9 of the 12 fungi tested (Table 1). The most sensitive fungi were Armillaria sp., Penicillium expansum, Rosellinia sp., and Sclerotinia sclerotiorum, which were inhibited by biomass extract (BE) at 0.25 g liter−1 (grams of dry biomass per liter of inoculated medium). None of the extracts showed antibacterial activity. One of the two insects tested, G. mellonella, was not susceptible, while all H. armigera larvae were killed by BE at the dose of 2.20 mg cm−2, with a 50% lethal concentration of 0.44 mg cm−2. After 8 days, no mortality was observed among the H. armigera larvae of the control. BE caused a significant (P < 0.01) reduction in the weight of the larvae with respect to the control (39.38 ± 12.04 mg and 147.76 ± 10.28 mg, respectively) when present at concentrations as low as 0.02 mg cm−2. Although the thawing-water extract (WE) was unable to kill the larvae, at a concentration of 2.20 mg cm−2 it caused a significant (P < 0.01) reduction in their weight (19.08 ± 9.39 mg and 147.76 ± 10.28 mg for the treated and control larvae, respectively). No effect was observed at lower concentrations of WE. The model organism for nematocidal activity, C. elegans, was killed at concentrations of about 0.02 mg cm−2 if treated with BE and of 1.82 mg cm−2 if treated with WE. Concentrations about one-half of these slowed the nematode life cycle. BE was strongly cytotoxic in the Artemia salina test. In contrast, the methanolic extracts obtained from the control strain, Nostoc strain UTEX 2493, did not show any activity with the exception of an inhibitory effect observed at very high concentrations (34.5 g liter−1 for BE and 100 g liter−1 for WE) against the fungus Rosellinia sp., which was one of the most susceptible.
TABLE 1.
Bioactivity of methanolic extracts of Nostoc strains ATCC 53789 and UTEX 2493
| Target organism | Bioactivity ofa:
|
|||
|---|---|---|---|---|
|
Nostoc strain ATCC 53789
|
Nostoc strain UTEX 2493
|
|||
| BE | WE | BE | WE | |
| Fungi | ||||
| Armillaria sp. | 0.25 g liter−1 | 1 g liter−1 | NA | NA |
| Colletotrichum coffeanum | NAb | NA | NA | NA |
| Colletotrichum trifolii | NA | NA | NA | NA |
| Fusarium solani | NA | NA | NA | NA |
| Fusarium oxysporum f. sp. melonis | 2.50 g liter−1 | 10 g liter−1 | NA | NA |
| Penicillium expansum | 0.25 g liter−1 | 10 g liter−1 | NA | NA |
| Phytophthora cambivora | 25 g liter−1 | 100 g liter−1 | NA | NA |
| Phytophthora cinnamomi | 25 g liter−1 | 100 g liter−1 | NA | NA |
| Rhizoctonia solani | 2.50 g liter−1 | 10 g liter−1 | NA | NA |
| Rosellinia sp. | 0.25 g liter−1 | 10 g liter−1 | 34.5 g liter−1 | 100 g liter−1 |
| Sclerotinia sclerotiorum | 0.25 g liter−1 | 1 g liter−1 | NA | NA |
| Verticillium albo-atrum | 2.50 g liter−1 | 10 g liter−1 | NA | NA |
| Bacteria | ||||
| Agrobacterium tumefaciens | NA | NA | NA | NA |
| Pseudomonas aeruginosa | NA | NA | NA | NA |
| Insects | ||||
| Galleria mellonella | NA | NA | NA | NA |
| Helicoverpa armigera | 2.20 mg cm−2 | NA | NA | NA |
| Nematodes | ||||
| Caenorhabditis elegans | 0.02 mg cm−2 | 1.82 mg cm−2 | NA | NA |
| Crustaceans | ||||
| Artemia salina | 0.27 g liter−1 | NA | NA | NA |
Expressed as grams (dry weight) of extracted biomass which inhibit the growth of the target organism in 1 liter of inoculated medium of at least 75% compared to the control after 24 h (bacteria and A. salina) or 72 h (fungi) of incubation, or expressed as milligrams (dry weight) of extracted biomass per square centimeter of agarized medium which causes 100% mortality.
NA, no activity.
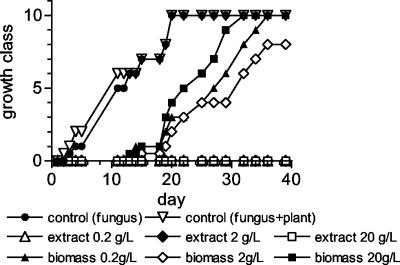
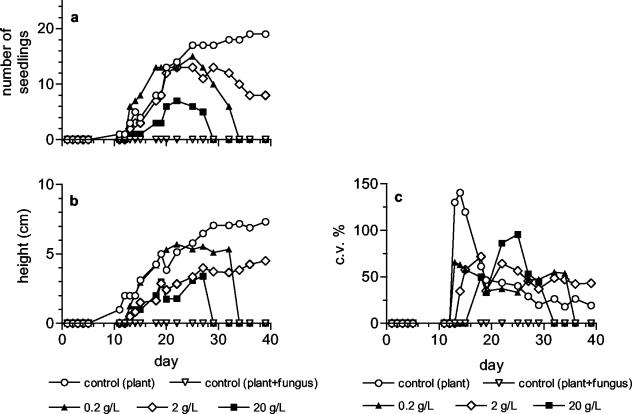
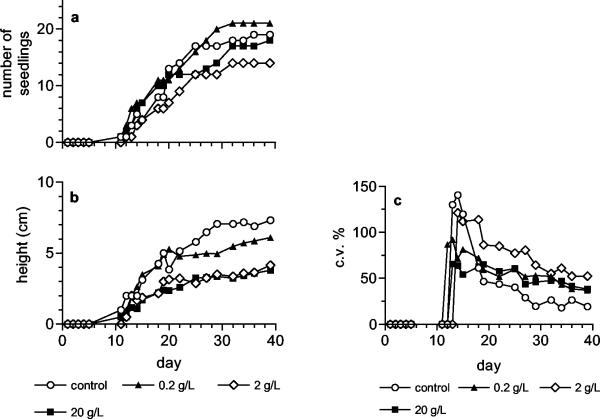
In the second phase of the research, the antifungal activity of Nostoc strain ATCC 53789 was tested in a system including Sclerotinia sclerotiorum on tomato plants. In Fig. 1, the development of the fungus in the presence of different concentrations of extract and biomass is shown. The fungal growth was completely inhibited by the extract at all the concentrations tested. The treatment with biomass was not so effective; it halted fungal growth only for 10 days. Unexpectedly, after this lag period the development of the fungus was faster at the highest biomass concentration tested. The lack of complete inhibition of fungal growth when biomass instead of extract was used resulted in progressive infection of the seedlings, which started to die from the third week (Fig. 2a). It is noteworthy that the control plants inoculated with the fungus (without biomass addition) were killed already at the rootlet stage. In the presence of biomass with the fungus, the growth of the seedlings, expressed as mean height, was similar to that of the seedlings grown without fungus, but only until they were killed by the fungal infection (Fig. 2b and 3b). The seedlings treated with biomass but not inoculated with the fungus were viable throughout the trial (Fig. 3a), but their development, measured as mean height, was slower with respect to the control (plants grown without neither biomass nor fungus), in particular at the intermediate and highest concentrations (Fig. 3b). The seedlings treated at the lowest concentration (0.2 g liter−1) grew as much as the control plants for the first 20 days and then went through a 10-day growth arrest, after which growth restarted, but the gap between these seedlings and the control plants was not recovered. The coefficients of variation of the height of either the plants inoculated with the fungus or not inoculated (Fig. 2c and 3c) show a high variability in the first few days after the beginning of germination, when the mean height is highly affected by the shortness of the newly germinated seedlings, and tend to decrease and stabilize as the seeds stop to germinate. At the end of the curve, the c.v. values are about 50% for the treated plants and 25% for the control plants. The extract completely inhibited the growth of the fungus (Fig. 1) but did not alter the development of the seedlings (data not shown), which was similar to that of the seedlings grown without fungal inoculum (Fig. 4a). The height of the treated seedlings was greatly reduced compared to the control; furthermore, their growth ceased after only 20 days. No differences were detected among the concentrations tested. The coefficient of variation of the mean height was quite constant (about 25%) for the treated plants, which grew to very low heights, whereas the coefficient of variation of the control was much higher (about 150%) in the first few days after germination and decreased at the same level as that of the treated seedlings after the end of germination (Fig. 4b).
FIG. 1.
Effect of extract or biomass of Nostoc strain ATCC 53789 on the growth of Sclerotinia sclerotiorum in the presence of tomato. Fungal development was attributed to arbitrary classes in the range from 1 to 10 on the basis of macroscopic observations of the colony size.
FIG. 2.
Effect of different concentrations of biomass of Nostoc strain ATCC 53789 on tomato seedlings, in the presence of the Sclerotinia sclerotiorum inoculum, during the 39 days of the test. (a) Number of viable seedlings; (b) growth of the seedlings expressed as mean height; (c) coefficient of variation (c.v.) of the height.
FIG. 3.
Effect of different concentrations of biomass of Nostoc strain ATCC 53789 on tomato seedlings, without fungal inoculum, during the 39 days of the test. (a) Number of viable seedlings; (b) growth of the seedlings expressed as mean height; (c) coefficient of variation (c.v.) of the height.
FIG. 4.
Effect of different concentrations of extract of Nostoc strain ATCC 53789, without fungal inoculum, on tomato seedlings. (a) Growth of the seedlings expressed as mean height; (b) coefficient of variation (c.v.) of the height.
At the end of the experiment (day 39), the germinated tomato seeds were counted (Table 2). No significant differences appeared between the control and the treated plants (with both extract and biomass) without fungal inoculum. S. sclerotiorum seemed to favour germination of the control seeds but not of the treated seeds. The latter showed germination values similar to those of the fungus-free seeds, with the exception of the 20-g-liter−1 treatments (biomass and extract), which apparently suffered in the presence of the fungus. We have no explanation for this behavior.
TABLE 2.
Germination of tomato seeds treated with different concentrations of extract or biomass of Nostoc strain ATCC 53789, with or without Sclerotinia sclerotiorum inoculuma
| Without fungal inoculum | With fungal inoculum
|
||
|---|---|---|---|
| Sample | No. of germinated seeds | Sample | No. of germinated seeds |
| Control (plant) | 6.3 ± 1.2a | Control (plant) | 9.7 ± 0.6 |
| Extract, 0.2 g liter−1 | 6.7 ± 1.5abc | Extract, 0.2 g liter−1 | 6.0 ± 1.0b |
| Extract, 2 g liter−1 | 6.0 ± 0.0abdef | Extract, 2 g liter−1 | 6.0 ± 1.7fi |
| Extract, 20 g liter−1 | 6.3 ± 0.6abdg | Extract, 20 g liter−1 | 3.0 ± 1.0l |
| Biomass, 0.2 g liter−1 | 7.0 ± 1.0ac | Biomass, 0.2 g liter−1 | 6.0 ± 2.7hm |
| Biomass, 2 g liter−1 | 4.7 ± 3.2ae | Biomass, 2 g liter−1 | 6.0 ± 1.0imn |
| Biomass, 20 g liter−1 | 6.0 ± 0.0ag | Biomass, 20 g liter−1 | 4.0 ± 0.0lmn |
Evaluation was made at the end of the test (day 39). The same letter beside the mean ± standard deviation values indicates nonsignificant difference between the data (P > 0.05) by ANOVA.
In Table 3, the effects of extract and biomass on stems and roots of the seedlings are reported. With the extract, the stem height was strongly reduced compared to that of the control at all the concentrations tested; a significant difference was only observed between the lowest and highest concentrations. The effect of biomass was less marked and more dependent on concentration. The diameter of the stems increased with respect to the control, and independently of concentration, only with the extract. The root system appeared highly altered in both treatments. The roots were significantly shorter than in the control, with a slight dependence on concentration. Moreover, the number of secondary roots decreased with increasing concentration while the number of adventitious roots increased. The effects of both extract and biomass on the morphology of the seedlings are shown in Fig. 5.
TABLE 3.
Development of stems and roots of tomato seedlings treated with different concentrations of extract or biomass of Nostoc strain ATCC 53789a
| Characteristic | Value for:
|
||||||
|---|---|---|---|---|---|---|---|
| Control | Extract
|
Biomass
|
|||||
| 0.2 g liter−1 | 2 g liter−1 | 20 g liter−1 | 0.2 g liter−1 | 2 g liter−1 | 20 g liter−1 | ||
| Stems | |||||||
| Height (cm) | 6.8 ± 1.4 | 1.8 ± 0.4a | 1.5 ± 0.4ab | 1.2 ± 0.3b | 5.5 ± 2.1c | 3.6 ± 1.9 | 3.2 ± 1.4c |
| Diameter (mm) | 0.8 ± 0.1a | 1.7 ± 0.3b | 1.7 ± 0.2bc | 1.5 ± 0.2c | 0.9 ± 0.2ade | 0.9 ± 0.2ad | 0.8 ± 0.3ae |
| Roots | |||||||
| Length (cm) | 6.4 ± 2.2a | 2.5 ± 1.0b | 2.4 ± 1.0bed | 1.4 ± 0.5ce | 4.1 ± 1.2af | 3.3 ± 0.8g | 2.5 ± 1.2defg |
| No. of secondary roots | 4.4 ± 1.7a | 3.9 ± 2.1ab | 1.7 ± 2.0cd | 0.5 ± 0.9ce | 4.0 ± 1.1ab | 2.7 ± 2.0d | 1.3 ± 1.2e |
| No. of adventitious roots | 0.5 ± 0.7a | 1.6 ± 1.7abc | 2.3 ± 1.3bde | 2.3 ± 2.3df | 1.1 ± 1.0ac | 2.4 ± 1.1eg | 2.7 ± 2.1fg |
Evaluation was made at the end of the test (day 39). Within each row, the same letter beside the mean ± standard deviation values indicates nonsignificant difference between the data (P > 0.05) by ANOVA.
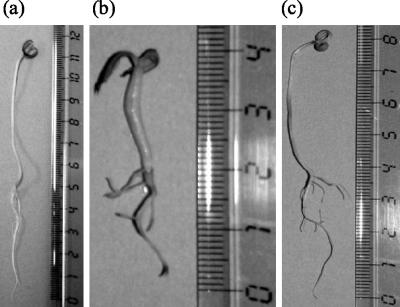
FIG. 5.
Effect of extract (b) or biomass (c) of Nostoc strain ATCC 53789 (2 g liter−1) on the development of tomato seedlings at the end of the test (day 39), relative to the control (a).
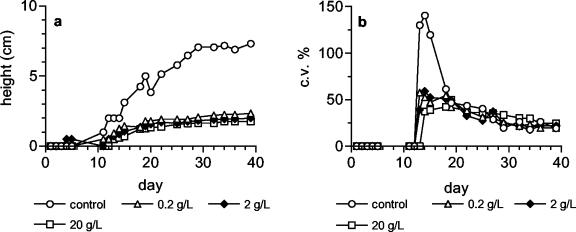
On the basis of these results, the extract of Nostoc strain ATCC 53789 was tested against a mixture of grasses for herbicidal activity. The extract did not affect the germination of seeds (Table 4), but it had a strong effect on the development of the plants. At 1 and 10 g liter−1, culm height was reduced by about 65% compared with the control. At the highest concentration tested (100 g liter−1), the reduction was slight and the difference from the control was not significant. However, the roots were affected to the same extent at all the concentrations, with an almost complete inhibition of their development (also shown in Fig. 6).
TABLE 4.
Germination of seeds and growth of stems and roots of seedlings of a grass mixture treated with different concentrations of extract of Nostoc strain ATCC 53789a
| Characteristic | Value for:
|
|||
|---|---|---|---|---|
| Control | Extract
|
|||
| 1 g liter−1 | 10 g liter−1 | 100 g liter−1 | ||
| Germination | ||||
| No. of germinated seeds | 13.7 ± 1.5a | 12.0 ± 0.0a | 12.5 ± 0.7a | 13.5 ± 2.1a |
| Stems | ||||
| Height (cm) | 7.6 ± 4.3a | 2.5 ± 1.9 | 2.7 ± 2.0 | 5.1 ± 2.8a |
| Roots | ||||
| Length (cm) | 1.6 ± 0.9 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
Evaluation was made at the end of the test (day 39). Within each row, the letter beside the mean ± standard deviation values indicates nonsignificant difference between the data (P > 0.05) by ANOVA.
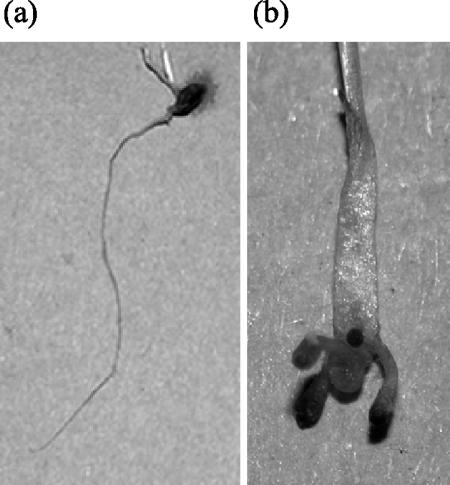
FIG. 6. .
Effect of extract (b) of Nostoc strain ATCC 53789 (10 g liter−1) on the development of roots in grass seedlings, at the end of the test (day 39), as compared to the control (a). Panel b is magnified threefold with respect to panel a.
DISCUSSION
Nostoc strain ATCC 53789 has shown wide-ranging and potent activity against several eukaryotic plant pathogens and model organisms, whereas the control strain Nostoc strain UTEX 2493 completely lacks activity except for causing a very weak inhibition of the most sensitive fungus. The known ability of Nostoc strain ATCC 53789 to produce cryptophycins, the action spectrum and the potency of its extract found in our experiments, together with the effects observed in this work on the roots of tomato and grass seedlings, which are very similar to those reported for other antimitotic substances with goosegrass (1), support the hypothesis that the activity is due to a cryptophycin. Additional indication that cryptophycins are involved is obtained from experiments that showed the high stability of the active molecule(s). Heat-treated extracts from Nostoc strain ATCC 53789 retained their activity; similarly, cytotoxic activity from the culture medium of the cryptophycin producer Nostoc strain GSV 224 was eliminated only after a prolonged period of autoclaving (38), and, as reported for Nostoc strain GSV 224 (38), contamination of extracts by glassware previously used for Nostoc strain ATCC 53789 was often observed until the glassware was washed with a solution of NaOH in ethanol-water.
Our screening confirms the high antifungal activity and the absence of antibacterial activity, previously described for this strain (14), and points out its insecticidal and nematocidal potential, for which no data were available. Unfortunately, a strong phytotoxicity has also been observed, which makes application of this cyanobacterium in agriculture problematic.
The thickening of both stems and roots observed in plants treated with the extract of Nostoc strain ATCC 53789 might be due to an abnormal increase in cell size as a result of the blockage of cell division, presumably caused by cryptophycins. The development of numerous adventitious roots probably occurs to increase nutrient uptake, which is strongly hampered by the reduction of the primary and secondary roots in treated plants. The roots are sensitive to both extract and biomass. Stems are strongly affected by the extract, but not by the biomass, which causes only a slight thickening confined to the collar region. With biomass, which must undergo lysis to release the active molecule, a concentration high enough to interfere with stem development is not reached. Moreover, with biomass, which is not as homogeneously dispersed as the extract within the agarized medium, a limited diffusion could also be involved. The slow release of the active molecule from the biomass may also explain the reduced effect on fungal growth compared with that of the extract. The time necessary to release the active principle in the medium and to reach the inhibitory concentration can also explain the 10-day growth arrest of the plants, which were growing as much as the control for the first 20 days, at the lower concentration tested.
The tests for herbicidal activity, besides confirming the high toxicity of the extract toward the root system (ca. 90% reduction in length), have shown another interesting property of Nostoc strain ATCC 53789. At the highest extract concentration (100 g liter−1), the stem height is less strongly affected than at the lower concentrations tested. This could be explained by the presence of stimulating substances, which, at high concentration, mask or counteract the effects of the inhibitory molecule(s). Also, our experiments with plants suggest a saturation dynamic of the uptake of the active molecule (probably a cryptophycin), as found for cryptophycin-52 in human leukemia cells (9). This could also be a cause of the smaller effect of the extract at high concentrations.
It is worth mentioning that although studies have been carried out in the past on synthetic antimitotic agents with inhibitory effects on roots and stems, and thus showing herbicidal potential (1, 3, 23, 27), no data on the effect on plants of cryptophycins or cryptophycin-containing extracts were available in the literature.
If Nostoc strain ATCC 53789 was to be exploited in agriculture, suitable application methods (e.g. foliar spraying) would be needed to prevent root damage. Another possible solution is the selection of plants resistant to the cryptophycins. This means finding species or varieties or even individuals that have natural resistance to the molecule and are able to transmit it. Natural resistance has been found for other antimitotic substances (dinitroanilines), for which spontaneous mutants become resistant as a result of a modification in the α-subunit of tubulin (23). To explain why certain tumor cell lines are less susceptible to cryptophycin-52 than others, it has been suggested that multiple cellular components and pathways are involved in its mechanism of action (9). The existence of multiple pathways in the inhibition of plant meristematic tissues is also plausible and justifies the search for resistant plants. Studies are also necessary to assess the toxicity of the final formulation against nontarget organisms and humans.
Acknowledgments
This research was partially supported by Sogesca S.r.l. (Padua, Italy).
REFERENCES
- 1.Anthony, R. G., and P. J. Hussey. 1999. Dinitroaniline herbicide resistance and the microtubule cytoskeleton. Trends Plant Sci. 4:112-116. [DOI] [PubMed] [Google Scholar]
- 2.Barrow, R. A., T. Hemscheidt, J. Liang, S. Paik, R. E. Moore, and R. A. Tius. 1995. Total synthesis of cryptophycins. Revision of the structure of cryptophycins A and C. J. Am. Chem. Soc. 117:2479-2490. [Google Scholar]
- 3.Batra, J. K., L. J. Powers, F. D. Hess, and E. Hamel. 1986. Derivatives of 5,6- diphenylpyridazin-3-one: synthetic antimitotic agents which interact with plant and mammalian tubulin at a new drug-binding site. Cancer Res. 46:1889-1893. [PubMed] [Google Scholar]
- 4.Boesten, J. J. T. I. 2000. From laboratory to field: uses and limitations of pesticide behaviour models for the soil/plant system. Weed Res. 40:23-138. [Google Scholar]
- 5.Borowitzka, M. A. 1995. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 7:3-15. [Google Scholar]
- 6.Burja, A. M., B. Banaigs, E. Abou-Mansour, J. G. Burgess, and P. C. Wright. 2001. Marine cyanobacteria—a profile source of natural products. Tetrahedron 57:9347-9377. [Google Scholar]
- 7.Campbell, R. C. 1989. Statistics for biologists. Cambridge University Press, Cambridge, United Kingdom.
- 8.Chauhan, V. S., J.B. Marwah, and S. N. Bagchi. 1992. Effect of an antibiotic from Oscillatoria sp. on phytoplankters, higher plants and mice. New Phytol. 120:251-257. [Google Scholar]
- 9.Chen, B. D. M., A. Nakeff, and F. Valeriote. 1998. Cellular uptake of a novel cytotoxic agent, cryptophycin-52, by human THP-1 leukemia cells and H-125 lung tumor cells. Int. J. Cancer 77:869-873. [DOI] [PubMed] [Google Scholar]
- 10.Edelman, M. J., D. R. Gandara, P. Hausner, V. Israel, D. Thornton, J. DeSanto, and L. A. Doyle. 2003. Phase 2 study of cryptophycin 52 (LY355703) in patients previously treated with platinum based chemotherapy for advanced non-small cell lung cancer. Lung Cancer 39:97-199. [DOI] [PubMed] [Google Scholar]
- 11.Gleason, F. K. December 1986. Cyanobacterin herbicide. U.S. patent 4,626,271.
- 12.Grossman, C. S., J. M. Gruber, and C. Shih. March 1998. Pharmaceutical compounds. World patent 98/08829.
- 13.Hagmann, L., and F. Jüttner. 1996. Fischerellin A, a novel photosystem-II-inhibiting allelochemical of the cyanobacterium Fischerella muscicola with antifungal and herbicidal activity. Tetrahedron Lett. 37:6539-6542. [Google Scholar]
- 14.Hirsch, C. F., J. M. Liesch, M. J. Salvatore, R. E. Schwartz, and D. F. Sesin. August 1990. Antifungal fermentation product and method. U.S. patent 4,946,835.
- 15.Kiviranta, J., and A. Abdel-Hameed. 1994. Toxicity of the blue-green alga Oscillatoria agardhii to the mosquito Aedes aegytpi and the shrimp Artemia salina. World J. Microbiol. Biotechnol. 10:517-520. [DOI] [PubMed] [Google Scholar]
- 16.Knowlton, S., A. Berry, and J. G. Torrey. 1980. Evidence that associated soil bacteria may influence root hair infection of actinorhizal plants by Frankia. Can. J. Microbiol. 26:971-977. [DOI] [PubMed] [Google Scholar]
- 17.Kulik, M. M. 1995. The potential for using cyanobacteria (blue-green algae) and algae in the biological control of plant pathogenic bacteria and fungi. Eur. J. Plant Pathol. 101:585-599. [Google Scholar]
- 18.Kurki-Helasmo, K., and J. Meriluoto. 1998. Microcystin uptake inhibits growth and protein phosphatase activity in mustard (Sinapis alba L.) seedlings. Toxicon 36:1921-1926. [DOI] [PubMed] [Google Scholar]
- 19.Mahakhant, A., P. Padungwong, V. Arunpairojana, P. Atthasampunna. 1998. Control of the plant pathogenic fungus Macrophomina phaseolina in mung bean by microalgal extract. Phycol. Res. 46(Suppl.):3-7. [Google Scholar]
- 20.Moore, R. E. 1996. Cyclic peptides and depsipeptides from cyanobacteria: a review. J. Ind. Microbiol. 16:134-143. [DOI] [PubMed] [Google Scholar]
- 21.Moore, R. E., E. Furusawa, T. R. Norton, G. M. L. Patterson, and J. S. Mynderse. February 1991. Scytophycins. U.S. patent 4,996,229.
- 22.Moore, R. E., C. D. Smith, G. M. L. Patterson, S. L. Mooberry, T. H. Corbett, F. A. Valeriote, and T. Golakoti. September 1999. Cryptophycins. U.S. patent 5,952,298.
- 23.Nyporko, A. Y., A. I. Yemets, L. A. Klimkina, and Y. B. Blume. 2002. Sensitivity of Eleusine indica callus to Trifluralin and Amiprophosmethyl in correlation to the binding of these compounds to tubulin. Russ J. Plant Physiol. 49:413-418. [Google Scholar]
- 24.Panda, D., R. H. Himes, R. E. Moore, L. Wilson and M. A. Jordan. 1997. Mechanism of action of the unusually potent microtubule inhibitor cryptophycin 1. Biochemistry 36:12948-12953. [DOI] [PubMed] [Google Scholar]
- 25.Patterson, G. M. L., C. L. Baldwin, C. M. Bolis, F. R. Caplan, H. Karuso, L. K. Larsen, I. A. Levine, R. E. Moore, C. S. Nelson, K. D. Tschappat, G. D. Tuang, E. Furusawa, S. Furusawa, T. R. Norton, and R. B. Raybourne. 1991. Antineoplastic activity of cultured blue-green algae (Cyanophyta). J. Phycol. 27:530-536. [Google Scholar]
- 26.Patterson, G. M. L., L. K. Larsen, and R. E. Moore. 1994. Bioactive natural products from blue-green algae. J. Appl. Phycol. 6:151-157. [Google Scholar]
- 27.Pyne, W. J., J. Lowbridge, I.-K. Chang, F. Knotz, and I. J. Powers. October 1985. Herbicidal and plant growth regulant diphenylpyridaziniones. U.S. patent 4,545,810.
- 28.Pushparaj, B., E. Pelosi, and S. Caroppo. 2000. Effect of Nodularia harveyana biomass on the incidence of root-knot nematode (Meloidogyne incognita) in tomato. J. Appl. Phycol. 12:489-492. [Google Scholar]
- 29.Piccardi, R. 2001. Uso di cianobatteri bioattivi nel controllo di patogeni di piante di interesse agrario. Ph.D. thesis. University of Florence, Florence, Italy.
- 30.Piccardi, R., A. Frosini, M. R. Tredici, and M. C. Margheri. 2000. Bioactivity in free-living and symbiotic cynaobacteria of the genus Nostoc. J. Appl. Phycol. 12:553-556. [Google Scholar]
- 31.Rippka, R., J. Deruelles, J. B. Waterbury, M. Herdman, and R. Y. Stanier. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1-61. [Google Scholar]
- 32.Sathiyamoorthy, P., and S. Shanmugasundaram. 1996. Preparation of cyanobacterial peptide toxin as a biopesticide against cotton pests. Appl. Microbiol. Biotechnol. 46:511-513. [Google Scholar]
- 33.Saxena, S., and A. K. Pandey. 2001. Microbial metabolites as eco-friendly agrochemicals for the next millennium. Appl. Microbiol. Biotechnol. 55:395-403. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz, R. E., C. F. Hirsch, D. F. Sesin, J. E. Flor, M. Chartrain, R. E. Fromtling, G. H. Harris, M. J. Salvatore, J. M. Liesch, and K. Yudin. 1990. Pharmaceuticals from cultured algae. J. Ind. Microbiol. 5:113-124. [Google Scholar]
- 35.Shih, C., and B. A. Teicher. 2001. Cryptophycins: a novel class of potent antimitotic antitumor depsipeptides. Curr. Pharm. Des. 7:1259-1276. [DOI] [PubMed] [Google Scholar]
- 36.Solis, P. N., C. W. Wright, M. M. Anderson, M. P. Gupta, and J. D. Phillipson. 1993. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med. 59:50-252. [DOI] [PubMed] [Google Scholar]
- 37.Tassara, C. A., M. V. Lopez, and E. R. Wright. 2001. Efectos de extractos de una cianofita (Nostoc muscorum) sobre Sclerotinia sclerotiorum en plantulas de lechuga. Rev. Fac. Agron. Univ. B. Aires 21:1-4. [Google Scholar]
- 38.Trimurtulu, G., J. Ogino, C. E. Heltzel, T. Le Husebo, C. M. Jensen, L. K. Larsen, G. M. L. Patterson, R. E. Moore, S. L. Mooberry, T. H., Corbett, and F. A. Valeriote. 1995. Structure determination, conformational analysis, chemical stability studies, and antitumor evaluation of the cryptophycins. Isolation of 18 new analogs from Nostoc sp. strain GSV 224. J. Am. Chem. Soc. 117:12030-12049. [Google Scholar]
- 39.Youssefi, M., and H. Willer. 2002. Organic agriculture worldwide 2002. Statistics and future prospects. Söl-Sonderausgabe no. 74. Söl, Bad Dürkheim, Germany.