Abstract
Aims
The formyl peptide receptor (FPR) subtype FPR2/ALX transduces pro-inflammatory responses and participates in the resolution of inflammation depending on activation. The aim of the present study was to unravel the role of FPR2/ALX signalling in atherosclerosis.
Methods and results
Expression of FPR2/ALX was analysed in 127 human carotid atherosclerotic lesions and revealed that this receptor was expressed on macrophages, smooth muscle cells (SMCs), and endothelial cells. Furthermore, FPR2/ALX mRNA levels were significantly up-regulated in atherosclerotic lesions compared with healthy vessels. In multiple regression, age, creatinine, and clinical signs of increased cerebral ischaemia were independent predictors of FPR2/ALX expression. To provide mechanistic insights into these observations, we generated Ldlr−/−xFpr2−/− mice, which exhibited delayed atherosclerosis development and less macrophage infiltration compared with Ldlr−/−xFpr2+/+ mice. These findings were reproduced by transplantation of Fpr2−/− bone marrow into Ldlr−/− mice and further extended by in vitro experiments, demonstrating a lower inflammatory state in Fpr2−/− macrophages. FPR2/ALX expression correlated with chemo- and cytokines in human atherosclerotic lesions and leucocytes. Finally, atherosclerotic lesions in Ldlr−/−xFpr2−/− mice exhibited decreased collagen content, and Fpr2−/− SMCs exhibited a profile of increased collagenase and decreased collagen production pathways.
Conclusion
FPR2/ALX is proatherogenic due to effects on bone marrow-derived cells, but promoted a more stable plaque phenotype through effects on SMCs. Taken together, these results suggest a dual role of FPR2/ALX signalling in atherosclerosis by way of promoting disease progression and but increasing plaque stability.
Keywords: Formyl peptide receptors, Inflammation, Lipid mediators, Smooth muscle cells
1. Introduction
The inflammatory response associated with atherosclerosis is driven both by the activation of the immune system1 and a failure in the resolution of inflammation.2 The formyl peptide receptor (FPR) subtype FPR2/ALX has the particularity of stimulating both pro-inflammatory and pro-resolution responses, depending on how the receptor is activated. For example, leucocyte chemotaxis has been described after FPR2/ALX activation by prokaryotic peptides initiated with an N-formylmethionine as well as by a number of endogenous ligands, such as amyloidogenic and antibacterial peptides.3 In contrast, ligation of this receptor with annexin A1 and related N-terminal peptides induces anti-migratory effects on neutrophils.4 In addition to these peptide agonists, also lipid mediators, such as lipoxin (LX) A4, signal through FPR2/ALX to limit neutrophil infiltration/activation and promoting non-oxidative activation of monocytes.5 It is from the latter agonist that this FPR subtype owes its name FPR2/ALX.3,6 In addition, the aspirin-induced isomer 15-epi-LXA4 and the omega-3 lipoxygenase metabolite resolvin D1 may both transduce inflammation resolution through FPR2/ALX.7–9
Several of the described FPR2/ALX ligands have been associated with atherosclerosis. For example, serum amyloid A (SAA) is a well-known biomarker of cardiovascular risk,10,11 although administrating SAA to hyperlipidaemic mice has been reported to both reduce12 and increase13 atherosclerosis burden. In addition, the antimicrobial peptide LL-37 is up-regulated in human atherosclerotic lesions,14 and genetic targeting of its murine homologue, the cathelicidin-related antimicrobial peptide (CRAMP), reduces atherosclerosis burden.15 Furthermore, blocking the production of the FPR2/ALX ligand LXA4 through disruption of either the 5- or the 12/15-lipoxgenase enzymes has generated contradictory results in mouse models of atherosclerosis, with neutral results,16 as well as both increased17 and decreased18 atherosclerotic lesion size having been reported. However, none of these studies specifically assessed FPR2/ALX signalling since the ligands used are not specific for FPR2/ALX. For example, SAA and cathelicidins also act as agonists on toll-like receptors (TLRs)19 and purinergic P2X7 receptors,20 respectively. In addition, targeting lipoxygenases inhibits not only LXA4 biosynthesis, but also alters the oxidation of other proatherogenic lipids17 and inhibits the formation of leukotrienes, another group of lipid mediators with important roles in promoting atherosclerosis development.21,22
Consequently, the exact role of FPR2/ALX signalling in atherosclerosis remains to be established. Therefore, the aim of the present study was to unravel the role of FPR2/ALX signalling in the terms of pro-inflammatory and pro-resolution pathways in atherosclerosis. To this end, we used several different approaches: histological examinations of human atherosclerotic lesions, human expression association studies, and the effect of FPR2/ALX disruption in mouse models of atherosclerosis.
2. Methods
Expanded method descriptions are available in Supplementary material online. The TaqMan Assays used are summarized in Supplementary material online, Table S1.
2.1. Human atherosclerotic samples
Atherosclerotic vascular tissue and peripheral blood mononuclear cells (PBMCs) were obtained from the BiKE biobank,23,24 including consecutive patients undergoing carotid endarterectomy (Table 1 and see Supplementary material online, Table S3). Healthy iliac arteries were used as controls. The experiments, which complied with the Declaration of Helsinki, were approved by the local ethics committee, and each participant provided informed consent. Paraffin-embedded sections were evaluated by immunohistochemistry, as described in detail in Supplementary material online. After exclusion of samples with insufficient RNA quality or quantity, global mRNA expression analysis using Affymetrix expression arrays (see Supplementary material online, Table S2) was performed on RNA from 10 iliac arteries, 127 carotid endarectomies (Table 1), and PBMC derived from 98 patients (see Supplementary material online, Table S3). Quantitative mRNA data were analysed as previously described.25 The available probe sets for FPR2/ALX exhibited similar associations, and data for probeset 210772_at are shown.
Table 1.
Univariate analysis of FPR2/ALX expression in human atherosclerotic lesions
| FPR2/ALX expression |
|||
|---|---|---|---|
| Spearman's rho | P-value | ||
| Number of subjects | 127 | ||
| Females (%) | 22.4 | N/A | 0.694 |
| Age (years) | 71 (46–85) | 0.188 | 0.036 |
| BMI | 25.9 (18.0–40.3) | −0.022 | 0.818 |
| Smoking | |||
| Non-smokers | 45% | N/A | 0.748 |
| Former smokers | 31% | ||
| Current smokers | 24% | ||
| Medical history | |||
| Peripheral artery disease | 10.6% | N/A | 0.766 |
| Cerebrovascular disease | 25.7% | N/A | 0.597 |
| Myocardial Infarction | 23.9% | N/A | 0.921 |
| Angina pectoris | 33.6% | N/A | 0.501 |
| Diabetes | 28.3% | N/A | 0.625 |
| Clinical signs of cerebral ischaemia (TIA 32%; stroke 39%; Amaurosis fugax 29%) | N/A | 0.213 | |
| None | 35% | ||
| >3 months | 11% | ||
| <3 months | 54% | ||
| Degree of carotid stenosis | 80% (50–95) | −0.022 | 0.813 |
| Medications | |||
| Platelet inhibitors | 93.6% | N/A | 0.868 |
| Anticoagulants | 23.2% | N/A | 0.470 |
| Anti hypertensives | 84.8% | N/A | 0.877 |
| Lipid-lowering | 84% | N/A | 0.712 |
| Laboratory parameters | |||
| Cholesterol (mmol/L) | 4.3 (2.1–7.3) | −0.0054 | 0.953 |
| LDL (mmol/L) | 2.3 (0.6–5.2) | −0.0195 | 0.844 |
| HDL | 1.1 (0.6–2.4) | 0.0594 | 0.541 |
| TG | 1.5 (0.45–7.9) | 0.0057 | 0.950 |
| Hb | 139 (87–169) | −0.151 | 0.095 |
| White blood cells | 7.4 (3.5–12.7) | 0.109 | 0.234 |
| HbA1c | 4.9 (3.9–8.7) | −0.009 | 0.931 |
| Creatinine | 87 (57–263) | 0.205 | 0.033 |
| hsCRP | 2.8 (0.2–82) | 0.167 | 0.086 |
| Fibrinogen | 3.5 (2.2–7.0) | 0.142 | 0.125 |
Binary variables were compared using Fisher's exact test.
Significant correlations (P < 0.05) are indicated in bold.
N/A: not available.
2.2. Animal experiments
All animal experiments were performed according to the institutional and national guidelines for the care and use of laboratory animals, and all procedures were approved by the local ethics committee. Fpr2-deficient mice, generated as previously described,26 were generously provided by Prof. Mauro Perretti (William Harvey Research Institute, London, UK). Of note, these knockout mice were generated through insertion of the gene cassette and a GFP reporter in reverse orientation into intron 1 of Fpr2, which prevented transcriptional read-through of the Fpr2 and Fpr3 genes,26 which are the murine homologues of human FPR2/ALX.3 These mice have also been termed Fpr2/Fpr3 knockout mice,27 but will be referred to as Fpr2−/− in the present report.
Murine aortic smooth muscle cells (mSMCs) and peritoneal macrophages were used for real-time PCR, efferocytosis, and measurements of intracellular calcium, as previously described.28,29 The experimental protocols are provided in Supplementary material online.
3. Results
3.1. FPR2/ALX is up-regulated in human atherosclerotic lesions
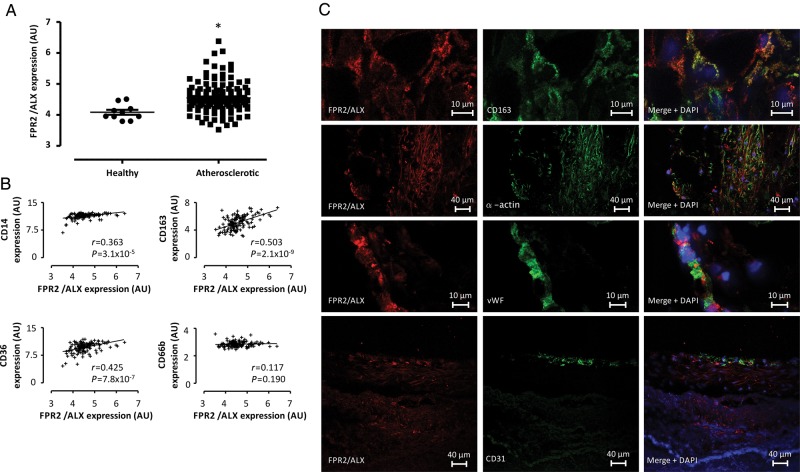
In human atherosclerotic lesions, mRNA levels of FPR2/ALX were significantly higher compared with control arteries (Figure 1A), and exhibited significant correlations with the macrophage markers CD14, CD163, and CD36 but not with the neutrophil granulocyte marker CD66b (Figure 1B). IF stainings of atherosclerotic lesions derived from the common carotid artery are shown in Figure 1C, and immunostainings provided in Supplementary material online, Figure S1 show the extent and orientation of the positive areas with abundant FPR2/ALX expression in highly cellular portions of the plaque and in the vicinity of the necrotic core (see Supplementary material online, Figure S1). FPR2/ALX co-localized with the macrophage markers CD163 and CD68, as well as with α-smooth muscle actin within the muscular arterial layer and with the endothelial cell markers von Willebrand factor (vWf) and CD31 (Figure 1C and see Supplementary material online, Figure S1). Of the clinical parameters listed in Table 1, age and serum creatinine exhibited a significant correlation with carotid artery FPR2/ALX expression in the univariate analysis. Furthermore, age- and sex-adjusted multiple regression analyses including all parameters listed in Table 1 revealed age (standardized β-coefficient [β] 0.016; P = 0.039), creatinine (β 0.0045; P = 0.036), and clinical signs of cerebral ischaemia (β −0.143; P = 0.031) as independent predictors of carotid artery FPR2/ALX expression. The latter parameter was stratified according to none (0), past (>3 months; 1), or recent (<3 months; 2) clinical signs, and the negative correlation hence indicates an inverse correlation between FPR2/ALX expression and recent clinical manifestations.
Figure 1.
(A) FPR2/ALX mRNA expression in healthy arteries (N = 10) and atherosclerotic plaques (N = 127). Results are expressed in arbitrary units and the horizontal line represents the mean. *P < 0.05 compared with healthy samples. (B) The associations between mRNA levels of FPR2/ALX and different leucocyte markers. (C) Representative IF staining of human atherosclerotic plaques from the carotid artery. The FPR2/ALX protein (red) co-localized with the following markers (green) in atherosclerotic plaques: CD163, α-smooth muscle actin, vWF, and CD31.
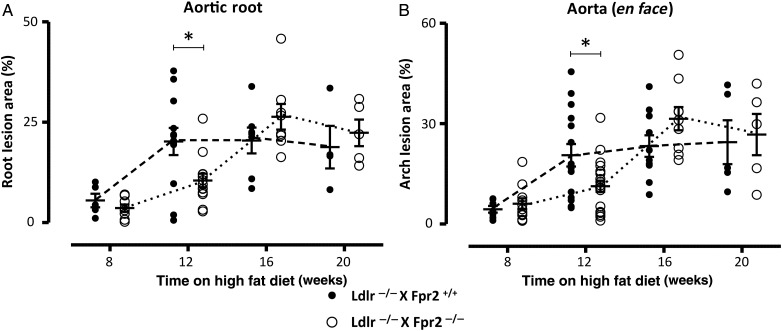
3.2. Atherosclerosis development is delayed in Ldlr−/− mice lacking Fpr2
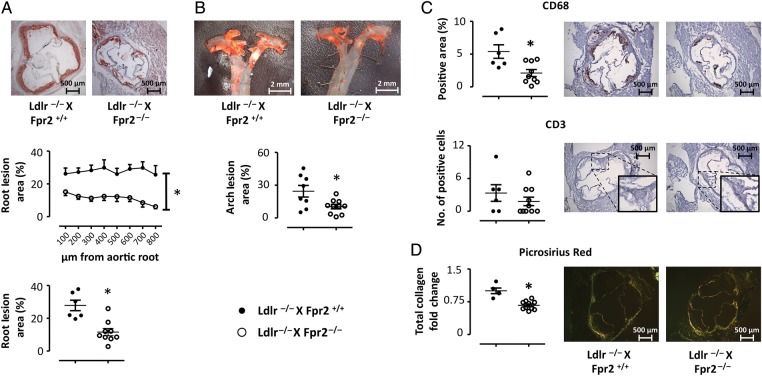
To determine the role of FPR2/ALX signalling in atherosclerosis progression, we generated double knockout mice lacking both the Ldlr and the Fpr2 genes (see Methods). Figure 2 depicts the time course of atherosclerosis development compared with Ldlr single knockout after 8–20 weeks of high-fat diet (HFD) in the aortic root (Figure 2A) and the thoracic aorta (Figure 2B). Ldlr−/−xFpr2−/− mice exhibited a delayed atherosclerosis development, with significantly less atherosclerotic lesions compared with Ldlr−/−xFpr2+/+ mice after 12 weeks of HFD, both in the aortic root (Figure 3A) and in the thoracic aorta (Figure 3B), whereas no significant differences were observed between the groups at other time points (Figure 2). To confirm these findings, a second group of mice were analysed after 12 weeks of HFD with the same results (Figure 2 including both experimental groups sacrificed after 12 weeks of HFD, whereas Figure 3 shows the first experimental group). In terms of plaque composition, the Ldlr−/−xFpr2−/− mice exhibited significantly less CD68 positivity compared with Ldlr−/−xFpr2+/+ mice, whereas the trend towards less CD3-positive cells did not reach statistical significance (Figure 3C). The collagen content of the aortic root was smaller in Ldlr−/−xFpr2−/− mice, as assessed by Picrosirius red staining (Figure 3D), with a larger proportion of thin collagen fibres (see Supplementary material online, Figure S2). There were no significant differences in plasma levels of cholesterol or triglycerides between Ldlr−/−xFpr2+/+ and Ldlr−/−xFpr2−/− mice (see Supplementary material online, Table S4).
Figure 2.
Atherosclerotic lesion size in aortic roots (A) and aortic arch (B) derived from either Ldlr−/−xFpr2+/+ (closed circles) or Ldlr−/−xFpr2−/− (open circles) after different times on HFD. Results are expressed as per cent positive staining, and lines represent mean ± SEM. *P < 0.05 compared with Ldlr−/−xFpr2+/+ at the same time point. Aortic roots: N = 5, 12, 7, and 4 for Ldlr−/−xFpr2+/+ and N = 8, 17, 8, and 5 for Ldlr−/−xFpr2−/− at 8, 12 16, and 20 weeks on HFD, respectively. Aortic arch: N = 7, 15, 10, and 5 for Ldlr−/−xFpr2+/+ and N = 11, 21, 9, and 5 for Ldlr−/−xFpr2−/− at 8, 12, 16, and 20 weeks on HFD, respectively.
Figure 3.
Atherosclerotic lesion size in aortic roots (A) and aortic arch (B) derived from either Ldlr−/−xFpr2+/+ (closed circles, N = 8) or Ldlr−/−xFpr2−/− (open circles, N = 13) after 12 weeks on HFD. Representative micrographs are shown on top. The plaque composition of the aortic roots in terms of CD68 and CD3 is shown in (C). For Picrosirius red collagen staining (D), each symbol represents the average of at least four sections derived from each mouse expressed as fold change relative Ldlr−/−xFpr2+/+. Representative micrographs of each staining are shown in the right side. Results are expressed as mean ± SEM for the indicated units. *P < 0.05.
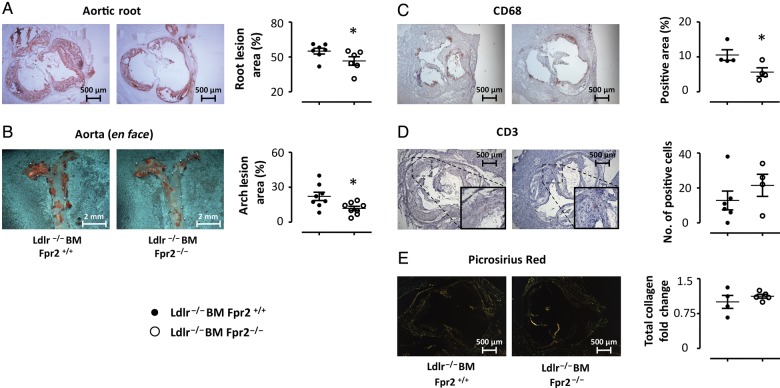
3.3. Bone marrow transplantation mimics decreased atherosclerosis by Fpr2 deletion in Ldlr−/− mice
To address the role of leucocyte Fpr2 signalling in atherosclerosis progression, bone marrow transplantation (BMT) was performed. After 20 weeks of HFD, lesion size in the en face analysis was comparable with that observed after 12 weeks of HFD in the Ldlr−/−xFpr2−/− mice (cf. Figures 3B and 4B). At this time point, Ldlr−/− mice receiving Fpr2−/− bone marrow exhibited significantly smaller atherosclerotic lesions in the aortic root (Figure 4A) and in the thoracic aorta (Figure 4B), when compared with those receiving Fpr2+/+ bone marrow. The latter findings were accompanied by a reduced number of macrophages in the lesions, as measured by CD68 expression by immunohistochemistry (Figure 4C), whereas CD3 did not significantly differ between the groups (Figure 4D). No differences in collagen content were observed in the lesions derived from mice receiving Fpr2−/− compared with wild-type bone marrow (Figure 4E). There were no significant differences in plasma lipids between mice receiving BM either from Fpr2+/+ or Fpr2−/− mice (see Supplementary material online, Table S4). The levels of SAA were almost 10 000-fold higher compared with LXA4 (SAA: 296 ± 62 µg/mL; LXA4: 34 ± 3.3 ng/mL; P < 0.05, n = 8 in each group).
Figure 4.
Atherosclerotic lesion size in aortic roots (A) and aortic arch (B) derived from Ldlr−/− mice receiving BM from either Fpr2+/+ (Ldlr−/−BM Fpr2+/+, closed circles, N = 8) or Fpr2−/− (Ldlr−/−BM Fpr2−/−, open circles, N = 8) donors. Tissue was collected after 20 weeks of HFD. Representative micrographs are also shown on top. The plaque composition of the aortic roots in terms of CD68 and CD3 is shown in (C and D). For Picrosirius red collagen staining, each symbol represents the average of at least four sections derived from each mouse expressed as fold change relative Ldlr−/−BM Fpr2+/+. Representative micrographs of each staining are also shown. Results are expressed as mean ± SEM for the indicated units. *P < 0.05.
3.4. Macrophages lacking Fpr2 exhibit a lower inflammatory state
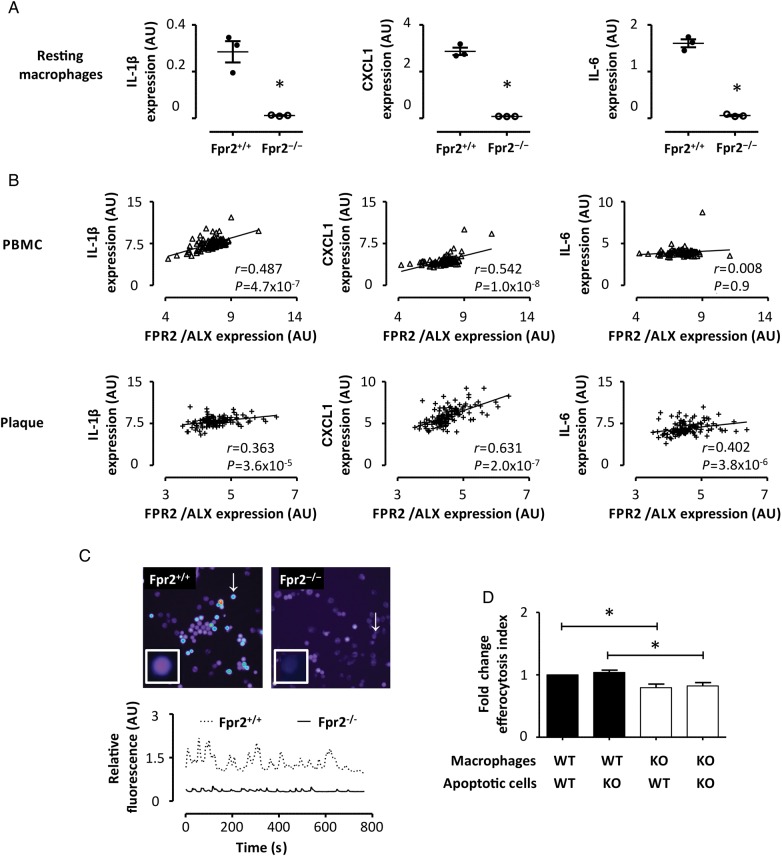
Based on the above findings that Fpr2−/− delayed atherosclerosis and reduced the number of macrophages in the lesions, we next sought to assess the characteristics of macrophages derived from these mice. Peritoneal macrophages derived from Fpr2−/− mice exhibited substantially lower mRNA levels of IL-6, IL-1β, and chemokine (C-X-C motif) ligand 1 (CXCL-1) when compared with Fpr2+/+ macrophages (Figure 5A), whereas collagenase expression was not significantly different (see Supplementary material online, Figure S3). Furthermore, FPR2/ALX expression was significantly correlated with IL-6, IL-1β, and CXCL-1 in atherosclerotic lesions, and with IL-1β and CXCL-1 in PBMC derived from atherosclerotic subjects (Figure 5B). Changes in intracellular calcium exhibited a characteristic oscillatory pattern. Representative tracings of these experiments are presented in Figure 5C and videos of the calcium oscillations in the corresponding cells are available in Supplementary material online. Macrophages derived from Fpr2−/− mice exhibited significantly lower amplitudes of calcium oscillations compared with Fpr2+/+ cells (see Supplementary material online, Figure S3). In contrast, the frequency of macrophage intracellular calcium oscillations was similar in the different genotypes (data not shown). Fpr2−/− macrophages exhibited 20% less uptake of apoptotic splenocytes compared with those derived from Fpr2+/+ mice. In contrast, this efferocytosis was not significantly altered by the use of either Fpr2+/+ or Fpr2−/− apoptotic splenocytes (Figure 5D).
Figure 5.
(A) The results of real-time PCR for IL-1β, CXCL1, and IL-6 expression in peritoneal macrophages derived from either Fpr2+/+ (close circles) or Fpr2−/− (open circles). Each symbol represents three different experiments using pooled samples from three mice. (B) The correlations of FPR2/ALX mRNA levels with the respective cyto/chemokine mRNA in peripheral blood monocytic cells (PMBC, n = 98) and atherosclerotic lesions (n = 127) derived from patients undergoing carotid endarterectomy. In (C), representative tracings of calcium oscillations in peritoneal macrophages from Fpr2+/+ (dotted line) and Fpr2−/− (continuous line) are shown. These tracings represent the oscillations in the respective cells marked with a white arrow in the right panels, and can be visualized in Supplementary material online, Videos S1 and S2, respectively. (C) The uptake of apoptotic splenocytes by peritoneal macrophages (1 : 2 ratio efferocyte : apoptotic cell), derived from either Fpr2+/+ (black bars) or Fpr2−/− (white bars) mice. Results (mean ± SEM) are expressed as fold change compared with Fpr2+/+ macrophages; n = 5. *P < 0.05.
3.5. Fpr2 signalling favours collagen accumulation
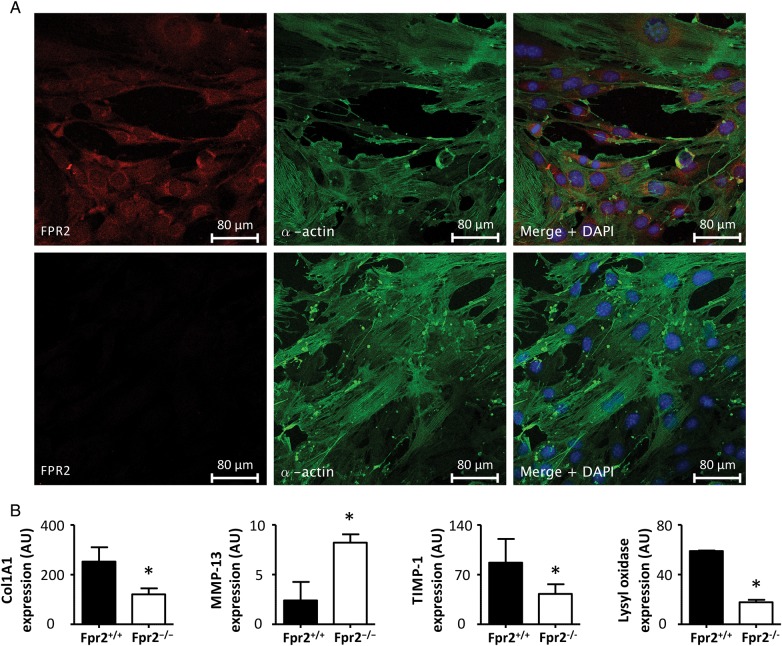
Since the immunohistochemical analysis of human atherosclerotic lesions indicated that SMCs express the FPR2/ALX receptor, and since Fpr2 deletion, but not Fpr2−/− BMT, affected collagen content in murine atherosclerotic lesions, Fpr2 signalling in SMC was subsequently addressed. IF staining confirmed the co-localization of the murine Fpr2 with α-smooth muscle actin in aortic SMCs isolated from Fpr2+/+ mice, whereas those obtained from Fpr2−/− mice stained negative for the Fpr2 protein (Figure 6A). Furthermore, mSMCs derived from Fpr2+/+ produced higher levels of collagen 1A mRNA compared with those derived from Fpr2−/− mice (Figure 6B). In contrast, Fpr2−/− mSMCs exhibited higher levels of the collagenase MMP-13, whereas the mRNA levels encoding the endogenous MMP inhibitor TIMP1, and the collagen cross-linking enzyme lysyl oxidase, were lower (Figure 6B).
Figure 6.
(A) Representative confocal IF stainings of FPR2 (red) and α-smooth muscle actin (green) in mSMC derived from either Fpr2+/+ (top) or Fpr2−/− mice (bottom). (B) The results of real-time PCR for collagen 1A (Col1A1), lysyl oxidase (LOX), MMP–13, and tissue inhibitor of MMP (TIMP)-1 in mSMC derived from Fpr2+/+ and Fpr2−/− mice (B; N = 3). *P < 0.05 compared with the respective control in each graph.
4. Discussion
Three major findings emerge from the present study. First, Fpr2 expression accelerated atherosclerosis development through effects on bone marrow-derived cells. Secondly, carotid artery Fpr2 expression was associated with clinical signs of cerebral ischaemia in the human observational study and a more stable atherosclerosis plaque phenotype in murine models. Thirdly, Fpr2 expression on mSMC was coupled with increased collagen production and maturation, and pathways of decreased collagen degradation. Taken together, these results suggest that Fpr2 signalling has important vascular effects and contributes to increased atherosclerotic lesion size but a more stable plaque phenotype.
Through the exploration of mRNA expression levels in human atherosclerotic plaques derived from the BiKE study of carotid endarterectomy, we show that FPR2/ALX is up-regulated in atherosclerotic, compared with normal arterial, samples. Those expression levels were significantly correlated with macrophage markers. Immunohistological examinations confirmed FPR2/ALX expression on macrophages in human atherosclerotic lesions, and in addition revealed that SMC and endothelial cells express FPR2/ALX. Multiple regression revealed that, in addition to age and creatinine, clinical signs of cerebral ischaemia were independent predictors of FPR2/ALX mRNA levels. Based on our previous characterizations of this cohort,23,24 these results suggest that FPR2/ALX expression in human atherosclerosis was associated with clinical features of a more stable phenotype. Nevertheless, certain limitations should be acknowledged. For example, the BiKE biobank lacks quantitative data on protein expression. Since data on non-cardiovascular co-morbidities are also missing, it can also not be completely excluded that the observed associations may be driven by unknown confounding factors. Finally, the possibility of overfitting should also be considered when interpreting multiple regression analysis.
Since the observational design of the clinical part of the present study means that no causality can be definitely attributed to the observed associations, we subsequently explored the role of FPR2/ALX signalling in atherosclerosis using mice deficient in the murine homologues of this receptor (Fpr2−/−). Interestingly, Ldlr−/− mice lacking Fpr2 exhibited delayed atherosclerosis development and reached the full spectrum of aortic atherosclerosis at a later stage compared with Ldlr−/−xFpr2+/+ mice. Transplantation of Fpr2-deficient bone marrow into Fpr2 expressing Ldlr−/− mice replicated the decreased atherosclerosis, supporting the human observational data that Fpr2 signalling in haematopoietic cells is of major importance for its proatherogenic effect. However, the effects of tissue disruption of Fpr2 in the presence of Fpr2+/+ bone marrow remain to be established.
Histologically, atherosclerotic lesions of Fpr2−/− mice contained fewer macrophages compared with lesions in wild-type mice, which is in line with the chemotactic effects of different peptide ligands for FPR2/ALX,3 and that signalling through this receptor mediates integrin activation in monocytes.30 The present study extends those findings by showing that macrophages derived from Fpr2−/− mice exhibited lower levels of chemo- and cytokines compared with wild-type cells. Interestingly, these observations were replicated in human samples of both atherosclerotic lesions and circulating cells, hence proving a similar FPR2/ALX activity parameter in human samples, and reinforcing the parallels between the animal models used with the human atherosclerosis.
Fpr2−/− macrophages also exhibited lower intracellular calcium oscillations, which is a marker of cell surface receptor-mediated macrophage activation coupled with transcription factors such as nuclear factor of activated T-cells and NF-κB,31 as well as phagocytosis and oxLDL uptake.32 In further support of an activation of proatherogenic pathways through FPR2/ALX signalling, SAA has previously been demonstrated to induce foam cell formation.33 Although SAA may activate several pathways,19 the stimulation of foam cell formation appears to be specific for FPR2/ALX since this SAA-induced response was abolished by either FPR2/ALX antagonism or siRNA against FPR2/ALX and not altered in TLR4−/− cells.33 In contrast, other FPR2/ALX agonists, such as annexin A1-derived peptides and LXA4, promote the uptake of apoptotic cells, which is referred to as efferocytosis and is a hallmark of the resolution of inflammation34,35 associated with anti-atherogenic macrophage signalling, decreased necrotic core formation, and increased plaque stability.36 In the present study, Fpr2−/− macrophages exhibited a defective efferocytosis, which corroborates previous studies in these mice.35
The above-discussed differential FPR2/ALX signalling may depend on the ligand composition in the milieu,37 although the co-expression of different FPRs may also be a decisive factor in this biased agonism.38 Comparing the endogenous levels of two FPR2/ALX ligands in the current mouse model of atherosclerosis revealed that SAA was present in almost 10 000-fold higher concentrations compared with LXA4 and suggests that potential inflammation resolution induced by LXA4 signalling through leucocyte FPR2/ALX may already be impaired in this atherosclerosis model as a result of low biosynthesis of this agonist. A defective resolution of inflammation has indeed been established in atherosclerosis,2 and the present study supports this notion in terms of the LXA4–FPR2/ALX pathway.
Interestingly, atherosclerotic lesions of Ldlr−/−xFpr2−/− mice exhibited less collagen content with a larger proportion of thin fibres compared with lesions in Ldlr−/−xFpr2+/+ mice. This finding was not replicated by BMT, and hence suggests direct effects mediated though FPR2/ALX on non-myeloid cells. The FPR2/ALX ligands, annexin A1 and LXA4, have previously been shown to reduce pro-inflammatory cytokines and MMP activity in SMC and fibroblasts, respectively.39,40 The present study extends those findings by providing a first indication that FPR2/ALX signalling also promotes increased collagen production and maturation, which in addition to a decreased collagen degradation could participate in rendering the atherosclerotic plaque more stable.41 In addition, those findings provide a potential mechanism for the present study's observational data associating FPR2/ALX expression in carotid atherosclerosis with clinical signs of cerebral ischaemia. Further studies are needed to establish the exact role of FPR2/ALX signalling in SMC, and its implications for cardiovascular diseases.
Previous in vivo observations in Fpr2−/− mice have demonstrated that this receptor transduces both inflammation resolution26,27 and inflammation activation.42 The present finding of effects promoting both inflammation (atherosclerosis progression) and its resolution (increased plaque stability) is supported by a model of dextran sulphate-induced colitis, in which Fpr2−/− confers disease protection in the acute phase, but a delayed healing and increased mortality compared with wild-type mice at later stages.43 Taken together, these findings support that the signalling through FPR2/ALX may, in addition to differential effects of different ligands, also depend on the stage of disease and the target cell involved.
In summary, FPR2/ALX was up-regulated in human carotid atherosclerotic lesions, in which expression levels were associated with clinical signs of cerebral ischaemia. Explorations in murine models revealed pro-inflammatory signalling through FPR2/ALX in leucocytes transducing accelerated atherosclerosis, whereas direct effects on SMCs potentially increased plaque stability. In conclusion, given its potential roles in inhibiting disease progression and increasing plaque stability, FPR2/ALX may be an important therapeutic target in atherosclerosis.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Swedish Research Council (grant nos 2011-2988 to M.B. and 2012-2577 to G.K.H.); the Swedish Heart and Lung Foundation (grant nos 20120474 and 20120827 to M.B.; 20110449 to G.K.H., and 20080482 to G.P-B.), and the Stockholm County Council (grant no. 20120297 to M.B. and 20110260 to G.K.H.).
Acknowledgements
The authors thank Mauro Perretti (London, UK) for providing the Fpr2−/−mice, and Teodora Andonova and Robert Badeau for their valuable participation in the experimental work.
Conflict of interest: none declared.
References
- 1.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D, Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107:2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- 5.Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 6.Bäck M, Powell WS, Dahlen SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7. Br J Pharmacol. 2014;171:3551–3574. doi: 10.1111/bph.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci USA. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 11.Fyfe AI, Rothenberg LS, DeBeer FC, Cantor RM, Rotter JI, Lusis AJ. Association between serum amyloid A proteins and coronary artery disease: evidence from two distinct arteriosclerotic processes. Circulation. 1997;96:2914–2919. doi: 10.1161/01.cir.96.9.2914. [DOI] [PubMed] [Google Scholar]
- 12.Tam SP, Ancsin JB, Tan R, Kisilevsky R. Peptides derived from serum amyloid A prevent, and reverse, aortic lipid lesions in apoE−/− mice. J Lipid Res. 2005;46:2091–2101. doi: 10.1194/jlr.M500191-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Dong Z, An F, Wu T, Zhang C, Zhang M, Zhang Y, An G, An F. PTX3, a key component of innate immunity, is induced by SAA via FPRL1-mediated signaling in HAECs. J Cell Biochem. 2011;112:2097–2105. doi: 10.1002/jcb.23128. [DOI] [PubMed] [Google Scholar]
- 14.Edfeldt K, Agerberth B, Rottenberg ME, Gudmundsson GH, Wang XB, Mandal K, Xu Q, Yan ZQ. Involvement of the antimicrobial peptide LL-37 in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:1551–1557. doi: 10.1161/01.ATV.0000223901.08459.57. [DOI] [PubMed] [Google Scholar]
- 15.Doring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res. 2012;110:1052–1056. doi: 10.1161/CIRCRESAHA.112.265868. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L, Moos MP, Grabner R, Pedrono F, Fan J, Kaiser B, John N, Schmidt S, Spanbroek R, Lotzer K, Huang L, Cui J, Rader DJ, Evans JF, Habenicht AJ, Funk CD. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004;10:966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]
- 17.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest. 1999;103:1597–1604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He RL, Zhou J, Hanson CZ, Chen J, Cheng N, Ye RD. Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood. 2009;113:429–437. doi: 10.1182/blood-2008-03-139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 21.Bäck M, Hansson GK. Leukotriene receptors in atherosclerosis. Ann Med. 2006;38:493–502. doi: 10.1080/07853890600982737. [DOI] [PubMed] [Google Scholar]
- 22.Bäck M. Inhibitors of the 5-lipoxygenase pathway in atherosclerosis. Curr Pharm Des. 2009;15:3116–3132. doi: 10.2174/138161209789058020. [DOI] [PubMed] [Google Scholar]
- 23.Gabrielsen A, Qiu H, Bäck M, Hamberg M, Hemdahl AL, Agardh H, Folkersen L, Swedenborg J, Hedin U, Paulsson-Berne G, Haeggstrom JZ, Hansson GK. Thromboxane synthase expression and thromboxane A2 production in the atherosclerotic lesion. J Mol Med (Berl) 2010;88:795–806. doi: 10.1007/s00109-010-0621-6. [DOI] [PubMed] [Google Scholar]
- 24.Agardh HE, Folkersen L, Ekstrand J, Marcus D, Swedenborg J, Hedin U, Gabrielsen A, Paulsson-Berne G. Expression of fatty acid-binding protein 4/aP2 is correlated with plaque instability in carotid atherosclerosis. J Intern Med. 2011;269:200–210. doi: 10.1111/j.1365-2796.2010.02304.x. [DOI] [PubMed] [Google Scholar]
- 25.Gisterå A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, Lundberg AM, Li MO, Flavell RA, Hansson GK. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013;5:196ra100. doi: 10.1126/scitranslmed.3006133. [DOI] [PubMed] [Google Scholar]
- 26.Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, Gray M, D'Acquisto F, Buckingham JC, Perretti M, Flower RJ. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–2619. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brancaleone V, Gobbetti T, Cenac N, le Faouder P, Colom B, Flower RJ, Vergnolle N, Nourshargh S, Perretti M. A vasculo-protective circuit centered on lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 operative in murine microcirculation. Blood. 2013;122:608–617. doi: 10.1182/blood-2013-04-496661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy E, Andersson DC, Caidahl K, Eriksson MJ, Eriksson P, Franco-Cereceda A, Hansson GK, Bäck M. Upregulation of the 5-lipoxygenase pathway in human aortic valves correlates with severity of stenosis and leads to leukotriene-induced effects on valvular myofibroblasts. Circulation. 2011;123:1316–1325. doi: 10.1161/CIRCULATIONAHA.110.966846. [DOI] [PubMed] [Google Scholar]
- 29.Miksa M, Komura H, Wu R, Shah KG, Wang P. A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. J Immunol Methods. 2009;342:71–77. doi: 10.1016/j.jim.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wantha S, Alard JE, Megens RT, van der Does AM, Doring Y, Drechsler M, Pham CT, Wang MW, Wang JM, Gallo RL, von Hundelshausen P, Lindbom L, Hackeng T, Weber C, Soehnlein O. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ Res. 2013;112:792–801. doi: 10.1161/CIRCRESAHA.112.300666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vukcevic M, Zorzato F, Spagnoli G, Treves S. Frequent calcium oscillations lead to NFAT activation in human immature dendritic cells. J Biol Chem. 2010;285:16003–16011. doi: 10.1074/jbc.M109.066704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen JH, Riazy M, Smith EM, Proud CG, Steinbrecher UP, Duronio V. Oxidized LDL-mediated macrophage survival involves elongation factor-2 kinase. Arterioscler Thromb Vasc Biol. 2009;29:92–98. doi: 10.1161/ATVBAHA.108.174599. [DOI] [PubMed] [Google Scholar]
- 33.Lee HY, Kim SD, Baek SH, Choi JH, Bae YS. Role of formyl peptide receptor 2 on the serum amyloid A-induced macrophage foam cell formation. Biochem Biophys Res Commun. 2013;433:255–259. doi: 10.1016/j.bbrc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Dalli J, Consalvo AP, Ray V, Di Filippo C, D'Amico M, Mehta N, Perretti M. Proresolving and tissue-protective actions of annexin A1-based cleavage-resistant peptides are mediated by formyl peptide receptor 2/lipoxin A4 receptor. J Immunol. 2013;190:6478–6487. doi: 10.4049/jimmunol.1203000. [DOI] [PubMed] [Google Scholar]
- 35.Maderna P, Cottell DC, Toivonen T, Dufton N, Dalli J, Perretti M, Godson C. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 2010;24:4240–4249. doi: 10.1096/fj.10-159913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A, Mallat Z. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1429–1431. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- 37.Cattaneo F, Parisi M, Ammendola R. Distinct signaling cascades elicited by different formyl peptide receptor 2 (FPR2) agonists. Int J Mol Sci. 2013;14:7193–7230. doi: 10.3390/ijms14047193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci USA. 2013;110:18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. Lipoxin A4 inhibits IL-1 beta-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J Immunol. 2000;164:2660–2666. doi: 10.4049/jimmunol.164.5.2660. [DOI] [PubMed] [Google Scholar]
- 40.Viiri LE, Full LE, Navin TJ, Begum S, Didangelos A, Astola N, Berge RK, Seppala I, Shalhoub J, Franklin IJ, Perretti M, Lehtimaki T, Davies AH, Wait R, Monaco C. Smooth muscle cells in human atherosclerosis: proteomic profiling reveals differences in expression of Annexin A1 and mitochondrial proteins in carotid disease. J Mol Cell Cardiol. 2013;54:65–72. doi: 10.1016/j.yjmcc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Bäck M, Ketelhuth DF, Agewall S. Matrix metalloproteinases in atherothrombosis. Prog Cardiovasc Dis. 2010;52:410–428. doi: 10.1016/j.pcad.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Chen K, Le Y, Liu Y, Gong W, Ying G, Huang J, Yoshimura T, Tessarollo L, Wang JM. A critical role for the g protein-coupled receptor mFPR2 in airway inflammation and immune responses. J Immunol. 2010;184:3331–3335. doi: 10.4049/jimmunol.0903022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen K, Liu M, Liu Y, Yoshimura T, Shen W, Le Y, Durum S, Gong W, Wang C, Gao JL, Murphy PM, Wang JM. Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J Clin Invest. 2013;123:1694–1704. doi: 10.1172/JCI65569. [DOI] [PMC free article] [PubMed] [Google Scholar]