Abstract
Background
Bone metastasis is a hallmark of advanced prostate cancer. The endothelin pathway has a mechanistic role in bone metastases. Atrasentan, an endothelin receptor antagonist, has reported activity in prostate cancer. We assessed the survival impact of atrasentan in castration resistant prostate cancer (CRPC) patients with bone metastases being treated with standard-of-care docetaxel.
Methods
Men with metastatic CRPC were stratified for progression type (PSA or radiologic), baseline pain, extra skeletal metastases and bisphosphonate use and randomised using double-blinded methodology on a 1:1 ratio to docetaxel with atrasentan or placebo for up to 12 cycles of 3 weeks and treated until progression or unacceptable toxicity. Non-progressing patients were permitted to continue atrasentan or placebo for up to 52 weeks. Co-primary endpoints were progression-free (PFS) and overall survival (OS) where 930 patients are needed to detect a 25% increase in median overall survival of 18 months with the addition of atrasentan (1-sided log-rank α=0.025, power 87%, 4 years accrual, 2.5 additional years of follow-up).
Results
1038 patents were accrued. Treatment was halted in April 2011, after an independent data safety monitoring committee pre-planned futility interim analysis. There was no significant difference in OS (HR=1.04 (95% CI 0.90,1.19) p=0.64) or PFS (HR=1.02 (95% CI 0.89,1.16) p=0.81). There was no significant difference between arms for RECIST or PSA response, treatment related deaths or grade 3 or more toxicity. Although 370 patients continued on blinded study drug after cessation of docetaxel, atrasentan did not significantly prolong post-chemotherapy OS in this subset.
Interpretation
Atrasentan, when added to docetaxel, does not improve overall or progression-free survival in men with castration-resistant prostate cancer and bone metastases.
INTRODUCTION
Prostate cancer is an androgen-dependent disease. Androgen deprivation (AD) has been standard systemic therapy for patients with metastatic hormone sensitive disease. Despite a high initial response rate, resistance to AD occurs in the vast majority of patients by 18 months 1, culminating in a disease state called castration resistant prostate cancer (CRPC). Subsequent therapy with docetaxel prolonged overall survival with no decrement in quality of life compared to the palliative agent mitoxantrone in two studies in CRPC 2, 3, resulting in the acceptance of docetaxel as standard-of-care for CRPC.
In addition, to characteristic androgen dependence, prostate cancer has other distinguishing features including a proclivity to spread to bone and produce sclerotic, osteoblast predominant metastases. More than 90% of men with fatal prostate cancer have bone metastases 4. The endothelin-pathway was delineated as critical to the initiation and maintenance of bone metastases from prostate cancer 5, 6. Increased endothelin receptor A (ETrA) expression was also seen with advancing tumor stage and grade in both primary and metastatic prostate cancer 5. Endothelin 1 - ETrA interaction proved critical to prostate cancer cell stimulation of osteoblast proliferation and migration and production of osteoblastic metastases 7. Subsequently small molecule inhibitors of this interaction were developed and demonstrated inhibition of metastases development and progression in preclinical models 8. Single agent trials of orally bioavailable small molecule inhibitors such as atrasentan and zibotentan sugggested activity against prostate cancer, particularly in patients with bone metastases. Atrasentan delayed time to disease progression in a subgroup of patients from a phase III compared to placebo while zibotentan improved overall survival compared to placebo alone in a randomized phase II study 9–11. Preclinical data from a bone metastasis model of prostate cancer suggested synergy between atrasentan and docetaxel 12. A phase I/II study of atrasentan with docetaxel and prednisone in men with mCRPC showed safety at full doses of atrasentan and docetaxel with reasonable activity based on serum PSA and RECIST criteria response 13.
On that basis, we hypothesized potential additive or synergistic effect between atrasentan and docetaxel in CRPC patients with bone metastasis. We designed a randomised, double-blind phase III trial of docetaxel and prednisone combined with either oral atrasentan or placebo in patients with CRPC who had evidence of bone metastases.
PATIENTS AND METHODS
Key Eligibility Criteria
Patients were required to have pathologically confirmed prostate adenocarcinoma with evidence of bone metastases on bone scan deemed to be unresponsive or refractory to hormone therapy by one or more of the following (despite androgen deprivation and antiandrogen withdrawal when applicable by one of the following criteria):
Progression of one-dimensionally measurable disease assessed within 28 days prior to registration.
Progression of evaluable but not measurable disease (i.e., bone scan) assessed within 42 days prior to registration.
Rising PSA is defined as at least two consecutive rises in PSA to be documented over a reference value (measure 1). The first rising PSA (measure 2) must have been taken at least 7 days after the reference value. A third confirmatory PSA measure was required (2nd beyond the reference level) to be greater than the second measure, and it must have been obtained at least 7 days after the 2nd measure. If this is not the case, a fourth PSA is required to be taken and be greater than the second measure. The patient must have a PSA ≥ 5 ng/ml in addition to increasing PSA to be eligible by rising PSA criteria.
No minimum PSA was required for patients with progression based on measurable or non-PSA evaluable disease. In a case, where the bone scan result was considered equivocal then magnetic resonance imaging was used to discriminate between malignant and other causes of bone scan abnormality.
Patients were required to have been previously surgically or medically castrated with a serum testosterone less than 50ng/ml. If method of castration was LHRH agonists, then the patient should be willing to continue the use of LHRH agonists. If the patient had been treated with non-steroidal antiandrogens (flutamide, bicalutamide, nilutamide or ketoconazole), they must have been stopped at least 28 days prior to enrollment. Prior radiation therapy to less than 30% of the bone marrow volume was permitted as was prior samarium but prior strontium was not permitted. One prior systemic therapy (vaccine or biologic therapy) was allowed and at least 28 days must have elapsed since completion of therapy and patient must have recovered from all side effects. Prior therapy must not have included cytotoxic chemotherapy for metastatic prostate cancer. Prior adjuvant therapy with a single non-taxane containing cytotoxic regimen was permitted provided more than 2 years had elapsed since its completion. Patients were permitted to take bisphosphonates provided they commenced therapy before registration and that they continue them as per the manufacturer's guidelines and/or as per institutional practice. Patients not taking ongoing bisphosphonate therapy were not permitted to start such therapy until they had completed 12 weeks of study treatment. SWOG performance status of 0 – 3 was required. Patients with PS of 3 because of pain secondary to bone metastases were eligible. Patients with brain metastases, active infection and significant ascites or pleural effusions were excluded. Completion of a baseline quality of life questionnaire for men who could complete them in English or Spanish and the offering of participation in correlative studies were required. Written informed consent from the patient and institutional or national review board approval of the protocol were required.
Treatment Plan
All patients received docetaxel 75mg/m2 intravenously every 21 days with dexamethasone and 5HT3 anti emetic therapy and prednisone 5mg twice daily orally every day. Patients were stratified by type of progression, presence of pain, extra skeletal disease and bisphosphonate usage status at study entry and randomized in a one to one ratio to either atrasentan 10mg daily orally every day (Arm 1) or an identical placebo (arm 2). Imaging was undertaken at baseline and every 4 cycles or 12 weeks with CT or MRI and technetium bone scan. Treatment was continued every 3 weeks for up to 12 cycles or until RECIST soft tissue progression, confirmed bone scan progression, pain progression, unacceptable toxicity, patient election or dose delay of more than 3 weeks. Confirmed bone scan progression required development of definitive new lesions on bone scan confirmed with the development of further new lesions on a subsequent bone scan undertaken not less than 6 weeks after the first. If bone scan progression was confirmed than the patients was censored for progression at the date of the first scan showing new bone metastases. Pain progression was determined by a two-point increase 14 in the Brief Pain Inventory (BPI) Worst Pain score 15 or increased opioid analgesia score on a Pain Medication Log 16 or both on the day of chemotherapy. Patients were not deemed to have progressed or to be removed from study for increased serum PSA alone although this was obtained with each cycle and available to the clinician and the patient. The sequence in which different forms of progression occurred (RECIST soft tissue, confirmed bone scan and pain progression) was recorded to compare sequences of initial and subsequent progression with outcome. Patients who completed 12 cycles of docetaxel therapy or who stopped chemotherapy because of toxicity were permitted to continue atrasentan or placebo for a total of up to 52 weeks from its commencement on the study. Patients did not receive routine prophylactic colony stimulating factors to prevent neutropenia but were permitted to have such treatment based on National Risk guidelines during the study.
Statistical Considerations
S0421 was designed with co-primary endpoints. We sought to evaluate whether survival with the addition of atrasentan to standard docetaxel with prednisone chemotherapy improved progression free survival or overall survival. Progression required increased pain and/or analgesia, progression in soft tissue disease as per RECIST 1.0 criteria, or the development of new lesions on Technetium bone scan, confirmed with further new lesions on a confirmation bone scan not less than 6 weeks after that scan. An intent-to-treat analysis was specified as primary. The Hochberg procedure was used to handle multiple testing. If the larger of the two final p-values were less than 0.025 (PFS) or 0.022 (OS to account for interim testing), then both null hypotheses would be rejected. Otherwise, if the smaller of the two were less than 0.0125 (if PFS) or 0.011 (if OS) then the corresponding null hypothesis would be rejected. Assuming a 4 year accrual period, 2.5 years of follow-up, and 930 eligible patients, the study was designed to have 87% power to detect a 25% (6 to 7.5 months) increase in median progression-free survival using a one-sided log-rank with an alpha of 0.0125 and 87% power to detect a 25% (18 to 22.5 months) increase in median survival using a one sided log rank with an alpha of 0.0125.
The study was overseen by the SWOG Data Safety Monitoring Committee (DSMC) on a biannual basis. In addition, three formal interim analyses were planned after half of the patients were enrolled, after 75% of accrual (n=698) and 40% of the expected deaths had occurred, and after completion of accrual (n=930). An evaluation of the alternative hypothesis of a 25% improvement in survival was tested at the one-sided 0.005 level and the null hypothesis of no survival difference between arms was tested at the one-sided 0.001 level at each interim analysis time point. There were no formal stopping rules for the composite PFS endpoint, but analyses were included in the DSMC report to use as supportive data. The recommendation to terminate accrual and report early fell to the DSMC, based on progression, toxicities and other factors in addition to survival. The final analysis was to be conducted when the pre-specified number of deaths had occurred. A comprehensive quality of life assessment was conducted in S0421; these results will be reported in a separate manuscript.
The graphical and numerical methods of Lin, Wei, and Ying (1993) were used for checking the adequacy of the Cox regression model 17. SAS version 9.2 was used for all statistical analyses.
RESULTS
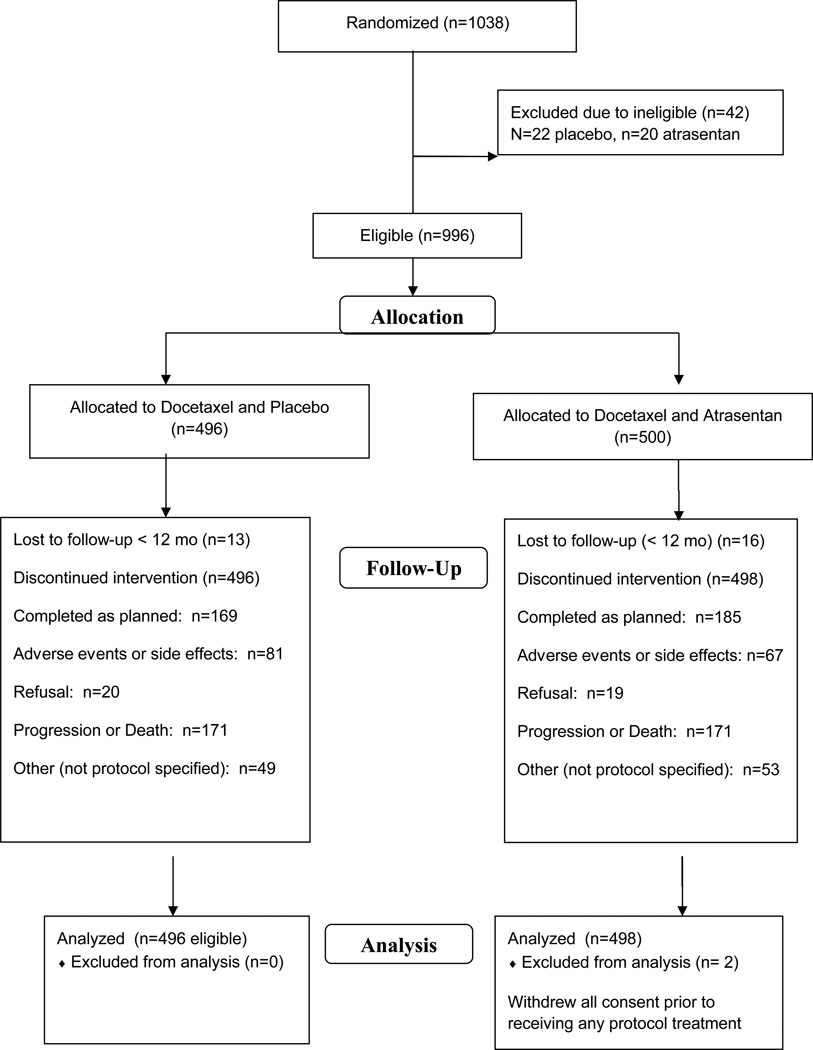
Between August 2006 and May 2010, 1038 patients were enrolled by SWOG, CALGB and ECOG. Forty-five patients were ineligible (4%). Characteristics of the 994 eligible patients (also excluding two patients who withdrew all consent immediately after randomization) are summarized in Table 1. Notably, 80% (n=795) of patients had progressed with measurable or evaluable disease to enter the trial, while only 20% (n=199) were eligible with PSA increase alone in the presence of metastatic disease. At randomization 61% (n=606) were already on bisphosphonate therapy, 56% had extra skeletal metastases in addition to bone involvement, 43% (n=221) had significant pain with a Brief Pain Inventory score >=4 and 311 (31%) had prior prostatectomy. The median number of cycles of docetaxel delivered was 9, and 34% of the placebo patients (n=169) and 37% of the atrasentan patients (n=185) completed all 12 cycles of chemotherapy. Patients’ characteristics were well balanced between the 2 treatment arms. (Table 1) Patient disposition is summarized in the CONSORT diagram (Figure 1). The trial was terminated for futility in April 2011 based on the recommendation of the DSMC following review of the third interim futility analysis. At that time, 18 patients were still on atrasentan or placebo. Clinicians and patients were informed of the results and atrasentan therapy stopped in those still on it. The trial was released to the investigators to report one year prior to the scheduled final analysis time.
Table 1.
Patient Demographics for the 994 Eligible, Consenting Patients
| Docetaxel + Placebo (n=496) |
Docetaxel + Atrasentan (n=498) |
|
|---|---|---|
| Age in years, median (min, max) | 69 (43, 89) | 69 (40, 92) |
| Serum PSA in ng/ml, median (25%, 75%) | 67.7 (24.6, 202.4) | 79 (23.5, 228.3) |
| Hispanic n (%) | 20 (4%) | 21 (4%) |
| Race White n (%) Black n (%) Asian n (%) Unknown n (%) |
403 (81%) 64 (13%) 12 (2%) 14 (3%) |
403 (81%) 73 (15%) 8 (2%) 10 (2%) |
| Type of Progression at Study Entry Measureable/Evaluable (vs. PSA Only) n (%) |
394 (79%) | 407 (81%) |
| Bisphosphonate Usage at Study Entry n (%) | 305 (61%) | 304 (61%) |
| Worst Pain: BPI ≥4 (vs. < 4) 15 n (%) | 213 (43%) | 210 (42%) |
| Disease Extent Skeletal Mets Only n (%) Lymph Nodes n (%) Lung, Liver or Brain Mets n (%) Extra-skeletal but not enough information** n (%) |
206 (42%) 148 (30%) 94 (19%) 48 (10%) |
203 (41%) 149 (30%) 101 (20%) 45 (9 %) |
| Prior Prostatectomy n (%) | 145 (29%) | 168 (34%) |
| Performance Status 2–3 (vs. 0–1) n (%) | 39 (8%) | 36 (7%) |
| Gleason Score 5–6 n (%) 7 n (%) 8–10 n (%) Missing n (%) |
49 (10%) 137 (28%) 272 (55%) 38 (8%) |
52 (10%) 141 (28%) 275 (55%) 30 (6%) |
Unless noted, All 994 patients had a valid value for the factor.
Patient was stratified as having extra-skeletal disease, but not enough information was available to classify into a sub-category
Figure 1.
SWOG S0421 CONSORT diagram
Response and outcomes summary
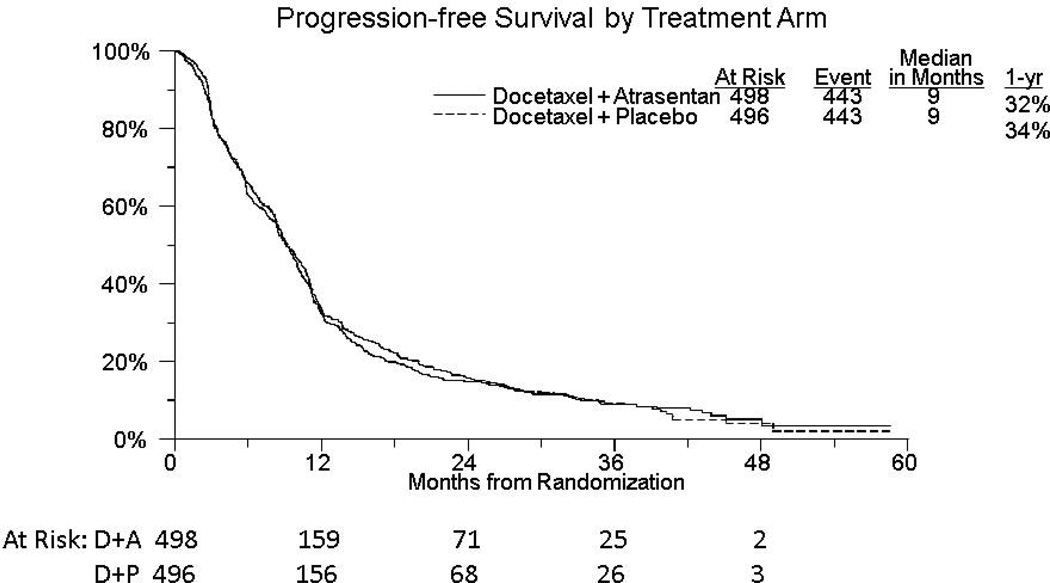
The median time to composite disease progression or death due to any cause was 9 months in both arms (HR 1.02 (95% CIs: 0.89–1.16; see Figure 2). 15% (n=55) of patients in the experimental arm and 16% (n=53) of patients in the placebo arm had not progressed or died at 2 years. In 28% of patients with an event (n=133), the date of progression is the date of death. PSA response with a fall to below 50% of the baseline value was seen in 50% (n=249) and 49% (n=243) of patients in the atrasentan and placebo arms, respectively (p=0.75). 461 patients were assessable for RECIST response based on measurable disease at trial enrolment. Partial responses were seen in 14% of patients on both arms (n=31 placebo, n=32 atrsesentan; p=0.97), although unconfirmed partial responses were seen in slightly more patients on atrasentan than placebo (unconfirmed response: 26% vs. 21%, respectively; p=0.28). Table 2 lists adverse events with at least one Grade 4 or 5 event. 57% (n=278) of patients on the atrasentan arm manifested grade 3 or greater toxicity compared to 60.4% (n=294) on the placebo arm (p=0.22). There were 10 deaths deemed possibly or probably due to protocol therapy, 3 in the atrasentan arm and 7 in the placebo arm. Other secondary objectives including serum bone markers and circulating tumor cell number will be the subject of concurrent or later reports.
Figure 2.
Kaplan Meier curves for composite progression-free survival in each arm.
Table 2.
Adverse Event Categories Where at Least One Grade 4 was Reported* Among Patients Who Received Any Protocol Treatment
| Docetaxel + Placebo (n=486) | Docetaxel + Atrasentan (n=492) | |||||
|---|---|---|---|---|---|---|
| Grade | Grade | |||||
| Adverse event | ≤3 | 4 | 5 | ≤3 | 4 | 5 |
| Allergy/Immunology | 486 | 0 | 0 | 491 | 1 | 0 |
| Blood/Bone Marrow | 396 | 90 | 0 | 405 | 87 | 0 |
| Cardiac Arrhythmia | 485 | 1 | 0 | 491 | 1 | 0 |
| Cardiac General | 482 | 3 | 1 | 489 | 3 | 0 |
| Constitutional symptoms | 481 | 5 | 0 | 491 | 1 | 0 |
| Dermatogical | 484 | 0 | 2 | 492 | 0 | 0 |
| Gastrointestinal | 485 | 1 | 0 | 490 | 2 | 0 |
| Hemorrhage/Bleeding | 485 | 1 | 0 | 491 | 0 | 1 |
| Infection | 478 | 7 | 1 | 485 | 5 | 2 |
| Metabolic/Laboratory | 479 | 7 | 0 | 487 | 5 | 0 |
| Musculoskeletal/Soft Tissue | 485 | 1 | 0 | 492 | 0 | 0 |
| Neurology | 484 | 2 | 0 | 491 | 1 | 0 |
| Pain | 486 | 0 | 0 | 491 | 1 | 0 |
| Pulmonary/Upper Respiratory | 473 | 10 | 3 | 490 | 2 | 0 |
| Renal/Genitourinary | 485 | 1 | 0 | 492 | 0 | 0 |
| Vascular | 481 | 5 | 0 | 485 | 7 | 0 |
Treatment attribution: possible, probable, or definite, No Grade 5 reported
For all patients, including all AE categories.
Survival
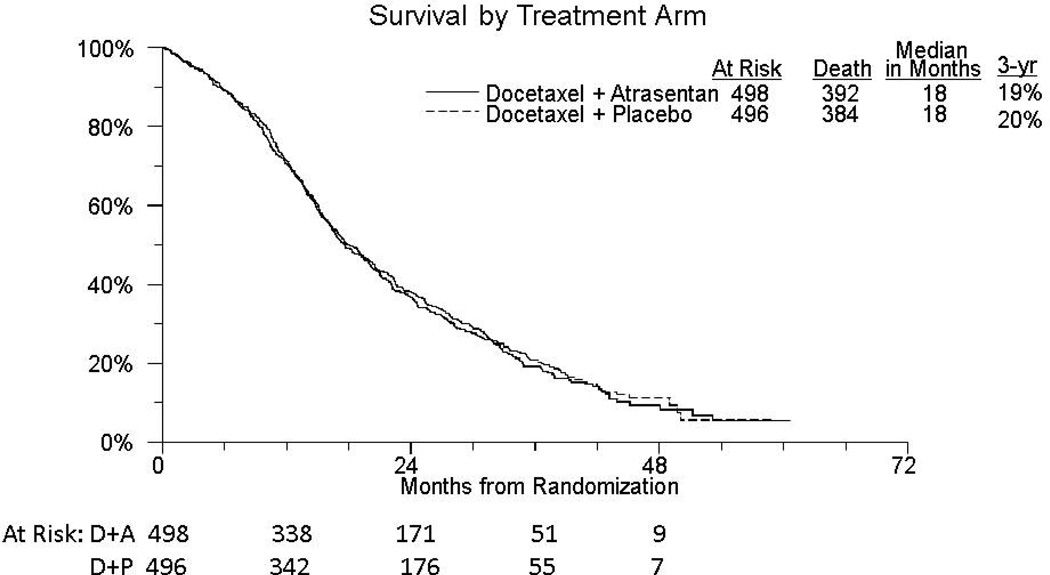
There have been 776 deaths; 384 on the placebo arm and 392 on the atrasentan arm. Median survival of patients treated with docetaxel, prednisone and atrasentan was 18 months compared with 18 months in the placebo are (HR=1.04 (95% CI 0.90,1.19) p=0.64; Figure 3). 21% of patients in the placebo group and 19% on the atrasentan arm were alive at 3 years. A multivariable proportional hazards analysis was used to assess the impact of treatment after conditioning on stratification factors and other patient and disease characteristics. Hazard ratios for the risk factors were in the expected direction with worse overall survival in patients with radiologic disease progression, worse pain and extra skeletal disease at study entry. After adjustment for these factors, the atrasentan versus placebo hazard ratio remained virtually unchanged (HR: 1.03 (95% CIs 0.89 –1.20, p=0.67).
Figure 3.
Kaplan Meier curves for overall survival in each arm.
We also evaluated treatment interactions with stratification factors and other risk factors to evaluate any trend in differential treatment with respect to OS within subsets of patients. Atrasentan interactions with PSA, performance status, Gleason score, race, type of progression at study entry, bisphosphonate use, bone pain, and disease involve lymph nodes or other visceral sites provided no evidence of differential treatment effect (all p > 0.10). The proportional hazards assumption was not violated for either the univariate or multivariable models with outcomes of OS or PFS (OS: atrasentan univariate p=0.88; multivariable atrasentan p=0.32).
370 patients completed chemotherapy or ceased chemotherapy because of adverse effects and were registered to continue their assigned blinded atrasentan or placebo through to a total of 52 weeks. There was no difference between arms for post-chemotherapy OS in the patients that continued blinded study drug after chemotherapy ceased (HR=1.08; 95% CI 0.83, 1.42, p=0.56).
Discussion
This intergroup placebo-controlled phase III study of atrasentan in addition to standard docetaxel chemotherapy and prednisone was negative for its two primary endpoints: composite PFS and OS. There were no major safety implications for the addition of atrasentan and in fact some adverse events were numerically more common in the placebo group. The study was designed to select patients with bone metastases to test the hypothesis that endothelin pathway inhibition predominantly in osteoblasts with atrasentan would slow disease progression and improve survival. In the context of a single agent phase III atrasentan study and 3 phase III studies with the newer endothelin antagonist zibotentan that have recently reported negative results in advanced prostate cancer, endothelin pathway inhibitors appear to be non-viable in this disease context. 10, 11, 18–20.
Is the osteoblast and its interaction with the prostate cancer cell in the bone metastasis milieu a valid target for prostate cancer therapeutics? In this study we saw that patients with markedly elevated markers of bone turnover did appear to benefit from atrasentan over placebo (Lara et al 2013). This group comprised only 6% of the patients accrued to the study. In addition, patients with only evidence of osseous metastases appeared to benefit from atrasentan where those with visceral involvement trended towards doing worse. This suggests that it may have been possible to design a more stringent study accruing only those with bone metastases, the worse serum bone turnover parameters and excluding any significant visceral involvement. The result would have been to limit accrual to less than 3% of the pool of CRPC patients leaving questions of practicality and generalizability.
As SWOG S0421 was being completed, several other events occurred in CRPC drug development contemporaneously. The first is the reporting of multiple negative studies in which a targeted agent had been partnered with docetaxel in a randomized phase II or III setting to try and improve outcomes in CRPC. These agents include high-dose calcitriol (DN-101), bevacizumab, aflibercept, vandetanib, zibotentan, lenalidomide, GVAX, imatinib, oblimersen, and AT101 11, 21–28. Second, a randomized trial reported the advent of an effective therapy to target the osteoblast and its milieu, Radium 223 29. In that phase III trial in symptomatic CRPC with osseous but not visceral metastases, who were unsuited for, declined or previously were exposed to docetaxel, Radium 223 produced that major palliative benefit and improved overall survival and suggested major surrogacy with bone turnover markers particularly serum alkaline phosphatase. This suggested that the target is real but, when taken with data from SWOG S0421 and others, that small molecule targeting of the endothelin receptor is either too selective or simply not sufficiently efficacious to change outcome. Third, we have several novel therapies that improve survival including novel immunotherapy with sipuleucel-T, androgen receptor pathway blockade with abiraterone acetate or enzalutamide, and chemotherapy with cabazitaxel for patients after docetaxel chemotherapy 30–32. It is likely that each of these new agents will be used earlier in the natural history of advanced prostate cancer 33. All of this progress means that clinicians and patients have broader options in metastatic prostate cancer, however, with docetaxel chemotherapy still seen as a key therapy for suitable men with CRPC.
In conclusion, in this Intergroup phase III trial in patients with metastatic castrate resistant prostate cancer atrasentan did not improve progression-free or overall survival compared to docetaxel with prednisone chemotherapy. The osteoblast-prostate cancer interface remains a valid target based on bone marker studies, subset analyses and, most importantly, the success of Radium 223 in this setting. In the face of this and more than 10 other randomized trials examining the addition of targeted agents to standard of care treatment, single agent docetaxel remains one of the standard options for CRPC. Endothelin inhibitors do not have an established role in advanced prostate cancer.
Table 3.
Multivariable analysis of stratification and other risk factors and Atrasentan with Overall Survival and Progression-free Survival.
| Overall Survival | Progression-Free Survival | |||
|---|---|---|---|---|
| Factor | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Log(PSA) | 1.21 (1.15, 1.28) | <0.0001 | 1.07 (1.03, 1.12) | 0.001 |
| PS 2–3 vs. 0–1 | 2.06 (1.57, 2.70) | <00001 | 1.59 (1.22, 2.07) | 0.0006 |
| Gleason Score 7 vs. 5–6 8–10 vs. 5–6 |
0.97 (0.74, 1.26) 1.10 (0.86, 1.41) |
0.80 0.44 |
0.87 (0.68, 1.11) 1.04 (0.83, 1.31) |
0.26 0.73 |
| African American vs. Other Race | 0.76 (0.61, 0.95) | 0.017 | 0.87 (0.71, 1.07) | 0.18 |
| Measurable/Evaluable vs. PSA only Progression | 1.23 (1.02, 1.49) | 0.032 | 1.20 (1.01, 1.44) | 0.043 |
| Bisphosphonate Usage at Study entry (Y vs. N) | 1.02 (0.88, 1.19) | 0.79 | 1.09 (0.95, 1.26) | 0.23 |
| Worst Pain > 4 at Study Entry (vs. <= 4) 15 | 1.76 (1.51, 2.04) | <0.0001 | 1.43 (1.24, 1.65) | < 0.0001 |
| Positive Lymph Nodes | 1.09 (0.89, 1.34) | 0.39 | 1.09 (0.90, 1.32) | 0.37 |
| Extra-skeletal Disease (vs. none) | 1.26 (1.03, 1.53) | 0.024 | 1.30 (1.08, 1.57) | 0.005 |
| Prior Radical Prostatectomy | 0.93 (0.78, 1.10) | 0.37 | 0.89 ((0.77, 1.04) | 0.14 |
| Atrasentan vs. Placebo | 1.03 (0.89 1.20) | 0.67 | 0.99 (0.86, 1.13) | 0.87 |
Interaction of each risk factor with atrasentan for OS and PFS, p > 0.10
Panel: Research in context.
Systematic Review
A PubMed, American Society of Clinical Oncology and European Society for Medical Oncology website search was conducted on two separate occasions, February 28, 2005 and December 30, 2012 with the search terms “Phase III trials”, “docetaxel” and “prostate cancer”, limiting the search to clinical trials (i.e., reviews were excluded). In the first assessment 2 phase III trials showed an overall survival advantage for docetaxel alone or estramustine compared to the palliative agent, mitoxantrone. Phase II trials of target-directed agents suggested anti-prostate cancer activity in certain cohorts of patients with metastatic prostate cancer. Atrasentan showed evidence of disease stabilisation in patients with bone metastases and phase I data showed that it could be combined with docetaxel using full doses of each agent. After completion of our trial, the later systematic review with the same parameters found three trials identified on PubMed (with bevacizumab, high dose vitamin D and estramustine) and five trials on ASCO/ESMO sites (aflibercept, Gvax, lenalidomide, zibotentan, dasatinib) reported negative results for their primary endpoint of overall survival, while two trials with curtisen and strontium-89 are completed but are as yet unreported. None of the eight identified randomised, placebo-controlled phase III studies resulted in an overall survival benefit for the addition of any therapy to docetaxel. The SWOG S0421 trial assessed the addition of atrasentan to docetaxel in CRPC patients with skeletal metastasis compared to docetaxel with a matched placebo using double-blinded methodology.
Interpretation
Our findings provide definitive evidence for the lack of benefit from small molecule endothelin receptor A blockade with atrasentan in castration resistant prostate cancer patients with bone metastases. This negative result emphasizes the challenges of adding novel agents to standard therapies in CRPC, particularly docetaxel, even in the context of good biological rationale and supportive early phase clinical data. Docetaxel as a single agent remains as a standard of care in metastatic castration resistant prostate cancer.
Acknowledgments
Support: This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA46368, CA46441, CA58882, CA58861, CA12644, CA22433, CA46282, CA27057, CA58416, CA45807, CA45808, CA45450, CA42777, CA35281, CA20319, CA35090, CA76429, CA14028, CA67575, CA45377, CA68183, CA63848, CA74647, CA16385, CA35192, CA63844, CA11083, CA63845, CA76447, CA35128, CA13612, CA35431, CA76448, CA35178, CA35176, CA35119, CA35421, CA128567, CA04919, CA68183, CA45560, CA37981, CA58723, CA21115, CA16116, CA31949, and in part by Sanofi-Aventis and Abbott Laboratories.
References
- 1.Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321(7):419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 5.Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995;1(9):944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 6.Mundy GR, Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, et al. Endothelin-1 and osteoblastic metastasis. Proc Natl Acad Sci U S A. 2003;100(19):10588–10589. doi: 10.1073/pnas.2035063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, Wessale JL, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci U S A. 2003;100(19):10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson JB, Nabulsi AA, Vogelzang NJ, Breul J, Zonnenberg BA, Daliani DD, et al. Suppression of prostate cancer induced bone remodeling by the endothelin receptor A antagonist atrasentan. J Urol. 2003;169(3):1143–1149. doi: 10.1097/01.ju.0000042162.08938.27. [DOI] [PubMed] [Google Scholar]
- 9.Carducci MA, Padley RJ, Breul J, Vogelzang NJ, Zonnenberg BA, Daliani DD, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol. 2003;21(4):679–689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 10.Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110(9):1959–1966. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 11.James ND, Caty A, Payne H, Borre M, Zonnenberg BA, Beuzeboc P, et al. Final safety and efficacy analysis of the specific endothelin A receptor antagonist zibotentan (ZD4054) in patients with metastatic castration-resistant prostate cancer and bone metastases who were pain-free or mildly symptomatic for pain: a double-blind, placebo-controlled, randomized Phase II trial. BJU Int. 2010;106(7):966–973. doi: 10.1111/j.1464-410X.2010.09638.x. [DOI] [PubMed] [Google Scholar]
- 12.Akhavan A, McHugh KH, Guruli G, Bies RR, Zamboni WC, Strychor SA, et al. Endothelin receptor A blockade enhances taxane effects in prostate cancer. Neoplasia. 2006;8(9):725–732. doi: 10.1593/neo.06388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong AJ, Creel P, Turnbull J, Moore C, Jaffe TA, Haley S, et al. A phase I-II study of docetaxel and atrasentan in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2008;14(19):6270–6276. doi: 10.1158/1078-0432.CCR-08-1085. [DOI] [PubMed] [Google Scholar]
- 14.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 15.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 16.Berry DL, Moinpour CM, Jiang CS, Ankerst DP, Petrylak DP, Vinson LV, et al. Quality of life and pain in advanced stage prostate cancer: results of a Southwest Oncology Group randomized trial comparing docetaxel and estramustine to mitoxantrone and prednisone. J Clin Oncol. 2006;24(18):2828–2835. doi: 10.1200/JCO.2005.04.8207. [DOI] [PubMed] [Google Scholar]
- 17.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557–572. [Google Scholar]
- 18.Nelson JB, Love W, Chin JL, Saad F, Schulman CC, Sleep DJ, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008 doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson JB, Fizazi K, Miller K, Higano C, Moul JW, Akaza H, et al. Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer. 2012 doi: 10.1002/cncr.27674. [DOI] [PubMed] [Google Scholar]
- 20.Yu EY, Miller K, Nelson J, Gleave M, Fizazi K, Moul JW, et al. Detection of previously unidentified metastatic disease as a leading cause of screening failure in a phase III trial of zibotentan versus placebo in patients with nonmetastatic, castration resistant prostate cancer. J Urol. 2012;188(1):103–109. doi: 10.1016/j.juro.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horti J, Widmark A, Stenzl A, Federico MH, Abratt RP, Sanders N, et al. A randomized, double-blind, placebo-controlled phase II study of vandetanib plus docetaxel/prednisolone in patients with hormone-refractory prostate cancer. Cancer Biother Radiopharm. 2009;24(2):175–180. doi: 10.1089/cbr.2008.0588. [DOI] [PubMed] [Google Scholar]
- 22.Small EJ, Demkow T, Gerritsen WR, Rolland F, Hoskin P, Smith DC, et al. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC). 2009 Genitourinary Cancers Symposium; 2009; Orlando, FL. 2009. p. A 7. [Google Scholar]
- 23.Mathew P, Thall PF, Bucana CD, Oh WK, Morris MJ, Jones DM, et al. Platelet-derived growth factor receptor inhibition and chemotherapy for castration-resistant prostate cancer with bone metastases. Clin Cancer Res. 2007;13(19):5816–5824. doi: 10.1158/1078-0432.CCR-07-1269. [DOI] [PubMed] [Google Scholar]
- 24.Sternberg CN, Dumez H, Van Poppel H, Skoneczna I, Sella A, Daugaard G, et al. Docetaxel plus oblimersen sodium (Bcl-2 antisense oligonucleotide): an EORTC multicenter, randomized phase II study in patients with castration-resistant prostate cancer. Ann Oncol. 2009;20(7):1264–1269. doi: 10.1093/annonc/mdn784. [DOI] [PubMed] [Google Scholar]
- 25.Scher HI, Jia X, Chi K, de Wit R, Berry WR, Albers P, et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol. 2011;29(16):2191–2198. doi: 10.1200/JCO.2010.32.8815. [DOI] [PubMed] [Google Scholar]
- 26.Kelly WK, Halabi S, Carducci M, George D, Mahoney JF, Stadler WM, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012;30(13):1534–1540. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrylak DP, Fizazi K, Sternberg CN, Budnik B, De Wit R, Wiechno PJ, et al. A Phase 3 study to evaluate the efficacy and safety of docetaxel and prednisone with or without lenalidomide in patients with castrate-resistant prostate cancer (CRPC): The MAINSAIL trial. ESMO Congress; 2012 September 30, 2012; Vienna, Austria. 2012. p. Abstract LBA24. [Google Scholar]
- 28.Sonpavde G, Matveev V, Burke JM, Caton JR, Fleming MT, Hutson TE, et al. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Ann Oncol. 2012;23(7):1803–1808. doi: 10.1093/annonc/mdr555. [DOI] [PubMed] [Google Scholar]
- 29.Parker C, Nilsson S, Heinrich D, O'Sullivan JM, Fossa SD, Chodacki A, et al. Updated analysis of the phase III, double-blind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer (CRPC) patients with bone metastases (ALSYMPCA) ASCO Meeting Abstracts. 2012;30(15_suppl):LBA4512. [Google Scholar]
- 30.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 31.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller MD, et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N Engl J Med. 2012 doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 33.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]