Abstract
Piscicolin 126 is a class IIa bacteriocin isolated from Carnobacterium piscicola JG126 that exhibits strong activity against Listeria monocytogenes. The gene encoding mature piscicolin 126 (m-pisA) was cloned into an Escherichia coli expression system and expressed as a thioredoxin-piscicolin 126 fusion protein that was purified by affinity chromatography. Purified recombinant piscicolin 126 was obtained after CNBr cleavage of the fusion protein followed by reversed-phase chromatography. Recombinant piscicolin 126 contained a single disulfide bond and had a mass identical to that of native piscicolin 126. This novel bacteriocin expression system generated approximately 26 mg of purified bacteriocin from 1 liter of E. coli culture. The purified recombinant piscicolin 126 acted by disruption of the bacterial cell membrane.
Bacteriocins produced by lactic acid bacteria (LAB) have been classified into four classes (23). The class II bacteriocins, which are small (<10 kDa), heat-stable, non-lanthionine-containing, and membrane-active amphipathic peptides, have been further divided into three groups on the basis of their sequence homology and mechanism of action (23, 29). The class IIa bacteriocins from the LAB are active against Listeria and contain an N-terminal consensus sequence of YGNGV. The antibacterial activity, mechanism of action, and structure-function relationships of class IIa bacteriocins have been investigated with a view to their development and application in the targeted prevention of growth of human pathogens in food. Class IIa bacteriocins are active at the cell membranes of gram-positive organisms, and their limited spectrum of activity is attributed to the requirement for a specific receptor interaction on the target cell (11, 12, 16, 17, 34, 38). Class IIa bacteriocins cause the efflux of accumulated compounds and ions (1, 5, 8, 25, 39), a dissipation of the membrane potential (1), and depletion of ATP (8), thereby preventing growth of the organism.
A requirement for the continued improvement of structure-function models of class IIa bacteriocins through biophysical studies is the production and purification of large quantities (up to 10 mg) of bacteriocin. Bacteriocin production systems currently utilized include expression in the native strain (19, 21), recombinant expression systems in LAB (3, 24), recombinant expression by use of Escherichia coli (27, 28), and chemical synthesis (15, 37). The utilization of recombinant expression systems permits the generation of fusion proteins or incorporation of affinity tags that improve purification. Chemical synthesis of class IIa bacteriocins produced large amounts (1 g) of biologically active bacteriocin (15, 37); however, the low purity of the chemically synthesized material resulted in a less-efficient bacteriocin production system. In vitro translation systems for the production of bacteriocins can also be applied. However, a problem with both chemical synthesis and in vitro translation systems is the requirement to form intramolecular disulfide bonds to produce a biologically active peptide.
A review of bacteriocin purification methods by Carolissen-Mackay et al. (7) highlighted the large variety and complexity of the methods used and the low yield of bacteriocin obtained with these procedures. For the class IIa bacteriocins, formation of the conserved disulfide bond(s) dictates an extracellular localization of the expressed bacteriocin. Therefore, the first step in most purification systems is the concentration of bacteriocins from the large volumes of culture supernatants (4, 9, 20, 21, 30, 32, 35). The second and subsequent steps in bacteriocin purification schemes often employ different types of column chromatography, and several methods have exploited the hydrophobic and cationic nature of bacteriocins (19, 35, 36).
An effective expression and purification system for bacteriocins requires (i) correct disulfide bond formation, (ii) a simple and rapid purification method, and (iii) large yields. This report describes a novel recombinant bacteriocin expression and purification procedure for piscicolin 126, a class IIa bacteriocin that is produced by Carnobacterium piscicola JG126 (21). The recombinant protein was purified by affinity chromatography from an E. coli cell extract. The active bacteriocin was obtained following chemical cleavage of the fusion protein and a final reversed-phase high-pressure liquid chromatography (HPLC) purification. This procedure could be applied to any antibacterial peptide that does not contain methionine.
MATERIALS AND METHODS
Chemicals and enzymes.
All chemicals used in this investigation were analytical reagent grade and were from Sigma Chemical Company (St. Louis, Mo.) or BDH (Poole, United Kingdom). Agarose was from Amresco (Solon, Ohio) or FMC BioProducts (Rockland, Maine). Enzymes were from Roche (Mannheim, Germany) or New England Biolabs (Beverly, Mass.). Media for bacterial growth were from Oxoid (Basingstoke, United Kingdom). Dye-deoxy terminator DNA sequencing reaction mixtures were from Applied Biosystems (Foster City, Calif.).
Bacterial strains, culture conditions, plasmids, and oligonucleotides.
The E. coli strains used in this investigation were E. coli UT5600 (New England Biolabs) and E. coli AD494(DE3) (Novagen, Madison, Wis.). Enterococcus faecalis SF1 (no. 3901) and Listeria monocytogenes 4A (no. 2310) were obtained from the Food Science Australia collection. All bacterial strains were incubated under conditions recommended by the supplier. Solid medium was prepared by the addition of bacteriological agar to a final concentration of 1.5% (wt/vol), and when required, ampicillin and kanamycin were present at concentrations of 200 and 15 μg/ml, respectively. The oligonucleotides used were G12-1Met (TGGGTACCGACGACGACGACATGAAGTATTACGGAAATG), Mut 3′ (ATTCCGGACACGGATCCTTT), T7 promoter (TAATACGACTCACTATAGGG), and T7 terminator (GCTAGTTATTGCTCAGCGG). The gene encoding mature piscicolin 126 (m-pisA) was amplified from C. piscicola JG126 and cloned into the E. coli plasmid pPOW (31). The plasmid pPOW m-pisA was a gift from Kim Harmark and was used as the template for all subcloning procedures. The E. coli expression plasmid pET32a was obtained from Novagen. All plasmids used in this investigation are described in Table 1.
TABLE 1.
Plasmids used in this investigation
| Plasmid | Relevant properties | Source or reference |
|---|---|---|
| pPOW | Ampr, λ PL PR, λ cI857 thermosensitive repressor, pelB signal peptide | 31 |
| pPOW m-pisA | m-pisA cloned into pPOW to produce piscicolin 126 containing the PelB signal peptide, the signal peptide is removed upon membrane translocation | K. Harmark, unpublished data |
| pET32a m-pisA | Ampr, T7 promoter, m-pisA cloned into pET32a to produce a thioredoxin fusion protein called Trx-piscicolin 126, Trx-piscicolin 126 contained a His tag for affinity purification and an enterokinase site for cleavage of the fusion protein | This study |
| pET32aM m-pisA | Ampr, T7 promoter, m-pisA cloned into pET32a to produce a thioredoxin fusion protein called Trxm-piscicolin 126, Trxm-piscicolin 126 contained a His tag for affinity purification and a Met residue immediately preceding piscicolin 126 for cleavage of the fusion protein with CNBr | This study |
Expression and purification of recombinant piscicolin 126.
E. coli AD494(DE3) cultures containing pET32aM m-pisA were incubated in 2.4 liter of 2YT medium containing kanamycin, ampicillin, and 1% (vol/vol) antifoam A at 37°C until the optical density at 600 nm (OD600) of the culture was 1.5. Protein expression was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to 0.1 mM. Glucose (12 and 6 g) was added to the culture immediately following induction and at 2 h postinduction, respectively. Cells were harvested by centrifugation (4,000 × g for 20 min at 4°C) 4 h postinduction, and the cell pellets were stored at −70°C until required.
Cell pellets were resuspended in wash buffer (5 mM imidazole, 100 mM NaCl, 10% [vol/vol] glycerol, 8 M urea, 20 mM Tris-HCl [pH 8.0]) to 1/50 of the culture volume and lysed by sonication. The cell extract was obtained by centrifugation (18,000 × g for 30 min at 4°C), and soluble recombinant piscicolin 126 was bound to 12 ml of Talon affinity resin (Clontech, Palo Alto, Calif.) by gentle mixing at 25°C for 30 min. The resin was loaded onto a column and washed with 10 column volumes of wash buffer, and the bound protein was eluted with 4 column volumes of elution buffer (wash buffer containing 200 mM imidazole). Protein fractions were analyzed by 10% Tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stored at 4°C in elution buffer. Protein concentrations were determined by using the enhanced bicinchoninic acid assay (Pierce) with bovine serum albumin as the protein standard.
Protein cleavage by use of cyanogen bromide.
Cyanogen bromide cleavage of recombinant piscicolin 126 protein at Met residues was performed in 60% formic acid as described by Ausubel (2) by using a methionine-to-CNBr molar ratio of 1:20 and incubation in the dark for 18 h. The solution was applied to Sep-Pak Plus C18 reversed-phase cartridges (Waters, Milford, Mass.) washed with reverse-phase buffer A (0.1% [vol/vol] trifluoroacetic acid), and bound protein was eluted with reverse-phase buffer B (0.1% [vol/vo]) trifluoroacetic acid, 80% [vol/vol] acetonitrile). All fractions were analyzed for antibacterial activity. Active fractions were pooled, the acetonitrile was evaporated under a stream of N2 gas, and the samples were freeze dried.
Reversed-phase HPLC.
Freeze-dried piscicolin 126 was resuspended in buffer (50 mM acetate, 100 mM NaCl, 5% [vol/vol] glycerol [pH 4.0]) to the original volume and applied to a Resource reversed-phase column (AP Biotech, Uppsala, Sweden) with a 3-ml bed volume. Protein was eluted by using a linear elution gradient of 0 to 60% reversed-phase buffer B at 1 ml/min. Fractions were collected at 1-min intervals for the antibacterial assay.
Alkylation of sulfhydryl groups.
Proteins were alkylated by using 4-vinylpyridine under reducing and nonreducing conditions as described previously (21) with some minor modifications. Proteins were reduced by incubation with β-mercaptoethanol at 37°C for 1 h and subsequently alkylated at 37°C for 2 h with frequent emulsification. Alkylated protein was analyzed by mass spectrometry without further purification.
Mass spectrometry.
Mass spectrometry was performed by using the PE Biosystems Voyager DE-STR System 4180 matrix-assisted laser desorption ionization-time of flight mass spectrometer (Perkin-Elmer Applied Biosystems) in reflector mode. A default calibration was used with an accuracy of 0.01% or better. A nitrogen laser at a 337-nm wavelength with a 3-ns pulse was used to charge the sample, which was present in a matrix of α-cyano-4-hydroxycinnamic acid for samples less than 10 kDa and sinapinic acid for samples greater than 10 kDa. All data were corrected for background and smoothed postacquisition. Each mass determination was the average of the results from 100 mass acquisitions.
Determination of antibacterial activity.
Bacteriocin activity (in arbitrary units [AU] per milliliter) was determined by using the critical dilution method of Coventry et al. (10). The MIC of piscicolin at which growth of the culture was inhibited by 50% (MIC50) was determined by measuring growth inhibition of 2-ml cultures containing serially diluted piscicolin 126, essentially as described by Nieto Lozano et al. (30). All determinations were done in triplicate, and noninhibited controls were repeated six times. Inhibition (I) was determined by the equation I = 1 − (Am/Ao) (6), where Am is the OD600 of the sample and Ao is the OD600 of the uninhibited control. Data were fitted to a four-paramater sigmoidal equation with SigmaPlot, version 4 (Jandel Scientific). Constants derived from the regression analysis describing the asymptote values were used to determine the midpoint on the inhibition curve (Imid) and subsequently the MIC50.
K+ efflux assay.
K+ concentrations were measured at 5-s intervals by using a K+ ion-selective electrode with an internal reference (Orion Research, Boston, Mass.). Cells of L. monocytogenes 4A from an overnight culture were collected by centrifugation (4,000 × g, 15 min, 4°C), washed twice in a 10% volume of the standard K+ buffer (10 mM KCl, 0.2% glucose, 50 mM NaH2PO4 · Na2HPO4 [pH 6.70]), resuspended to a final OD600 of 18 (approximately 2.4 × 1010 cells/ml), and stored on ice for periods no longer than 4 h. Where appropriate, cells were resuspended to 2 ml in buffers of defined pH (pH 5.74, 6.40, 6.70, 7.02, and 7.66) or NaCl concentration (25, 50, 150, and 300 mM). Cells were equilibrated to 25°C, and K+ concentrations in the buffer were determined. A 10-μl sample of piscicolin 126 was added to the cells once the buffer K+ concentrations had been equilibrated. The data presented were a ΔK+ value, where zero was determined at the lowest buffer K+ concentration prior to the addition of piscicolin 126. The initial rate of K+ efflux was determined from the gradient of the tangent of the efflux curve, which was measured 1 min after the addition of piscicolin 126 for a period of 1 min. When performing assays in 300 mM NaCl, the maximum rate of K+ efflux was determined 2 min after the addition of piscicolin 126.
RESULTS
Synthesis and purification of recombinant Trxm-piscicolin 126.
In initial experiments, the gene encoding mature piscicolin 126 (m-pisA) was amplified from pPOW m-pisA (Table 1) and subcloned into pET32a to generate the vector pET32a m-pisA (Table 1). This piscicolin 126 fusion protein was expressed and purified with a good yield; however, the efficiency of enterokinase cleavage to release active piscicolin 126 was poor. This expression system was redesigned to facilitate chemical cleavage of the fusion protein by CNBr.
m-pisA was amplified from pPOW m-pisA with oligonucleotides G12-1Met and Mut3′. The amplicon was digested with KpnI and BamHI, cloned into pET32a, and used to transform E. coli AD494(DE3). The trxB knockout in this strain allows disulfide bonds in proteins to be formed in the cytoplasm (13). The nucleotide sequence of the resulting plasmid, pET32aM m-pisA (Table 1), confirmed that induction of cells containing pET32aM m-pisA would produce a TrxA-piscicolin 126 fusion protein designated Trxm-piscicolin 126 (Fig. 1). A cell extract was prepared from 2.4 liter of induced culture, and approximately 480 mg of Trxm-piscicolin 126 was obtained after purification of the fusion protein with Talon resin. As piscicolin 126 is 20.6% of the total mass of Trxm-piscicolin 126, this represents approximately 41 mg of piscicolin 126 per liter of culture. The Trxm-piscicolin 126 was estimated to be 90% pure by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown) and did not have activity against L. monocytogenes 4A.
FIG. 1.
pET32aM m-pisA sequence and translation. The synthesized protein, Trxm-piscicolin 126, is shown with all Met residues in bold to indicate the CNBr cleavage sites. The locations of the thioredoxin A tag, His tag, mature piscicolin 126 peptide, and relevant restriction sites are shown.
Approximately 135 mg of purified Trxm-piscicolin 126 in 60% formic acid buffer was digested with CNBr. CNBr cleaves proteins on the C-terminal side of Met residues, and these amino acids are spontaneously modified to homoserine lactone or homoserine (18). Based on the Met content of Trxm-piscicolin 126 (Fig. 1), six peptide fragments, one of which is piscicolin 126, were expected to be generated by CNBr cleavage. The CNBr-cleaved protein was bound to SepPak C18 cartridges and washed with reverse-phase buffer A to remove CNBr and formic acid. The bound proteins were eluted from the cartridges with reverse-phase buffer B, and 4 × 107 AU of bacteriocin activity (against L. monocytogenes 4a) was detected in the eluate. Evaporation of the acetonitrile from the eluate under a stream of N2 gas resulted in an approximately fivefold concentration of the eluate and significant protein precipitation. Both soluble and insoluble fractions contained anti-Listeria activity. The soluble fraction (23 ml total) of the eluate contained 12,800 and 819,200 AU of activity/ml against E. faecalis SF1 and L. monocytogenes 4A, respectively.
Identification of disulfide bonds in recombinant piscicolin 126.
Piscicolin 126 contains two cysteine residues that are conserved among the class IIa bacteriocins, and native piscicolin 126 contains a disulfide bond between these two residues (21). To confirm that the correct disulfide bond was formed in recombinant piscicolin 126, the peptide was alkylated with 4-vinylpyridine under reducing and nonreducing conditions. The mass of piscicolin 126 which had been alkylated under nonreducing conditions was 4,416.42 ± 0.75 Da and was identical to the previously reported mass of piscicolin 126 of 4,416.6 ± 1.9 Da (21). The mass of piscicolin 126 which had been alkylated under reducing conditions was 4,626.77 Da, representing an increase of 210.35 Da and demonstrating that a disulfide bond had formed between the two cysteine residues in piscicolin 126.
Purification of recombinant piscicolin 126.
The peptides present after CNBr cleavage of Trxm-piscicolin 126 were resolved by reversed-phase HPLC (data not shown). Eluted proteins were detected at 214 nm, and each fraction was assayed for activity against L. monocytogenes 4A. Activity was identified in a single peak, and the protein present in this peak was confirmed to be piscicolin 126 by mass spectrometry (data not shown). Using this procedure, 2.34 mg of purified piscicolin 126 was isolated from 2 ml of the soluble CNBr cleavage products.
Antibacterial activity of recombinant piscicolin 126.
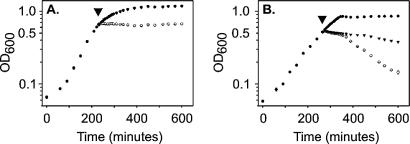
The specific activity of purified recombinant piscicolin 126 was determined (by the serial dilution method) to be 684 and 247,584 AU/mg against E. faecalis SF1 and L. monocytogenes 4A, respectively. Recombinant piscicolin 126 was added to mid-log-phase cultures of E. faecalis SF1 and L. monocytogenes 4A to determine the effect on bacterial growth. The addition of 2.6 AU of piscicolin 126/ml to E. faecalis SF1 had no effect on growth; however, 128 AU/ml caused a sustained inhibition of cell growth without any observed cell lysis (Fig. 2A). The addition of either 164 or 8,192 AU of piscicolin 126/ml to L. monocytogenes 4A caused growth inhibition within 10 min and a subsequent reduction in OD600 (Fig. 2B).
FIG. 2.
Inhibitory activity of piscicolin 126 against sensitive indicator organisms E. faecalis SF1 (A) and L. monocytogenes 4A (B). ○, piscicolin 126 corresponding to 128 and 8,192 AU/ml for E. faecalis SF1 and L. monocytogenes 4A, respectively; ▾, piscicolin 126 corresponding to 2.6 and 164 AU/ml for E. faecalis SF1 and L. monocytogenes 4A, respectively; •, no added piscicolin. Piscicolin 126 was added at the time points marked by the arrowheads.
Following a regression analysis of sigmoidal inhibition curves, the MIC50 of piscicolin 126 was determined for L. monocytogenes 4A across the log phase of growth (4, 5, and 6 h), and these data show an increase in the MIC50 with time. At 4, 5, and 6 h, the MIC50 was determined to be 11.63 ± 1.92, 11.99 ± 1.03, and 12.88 ± 0.92 nM, respectively.
Kinetics of recombinant piscicolin 126 function.
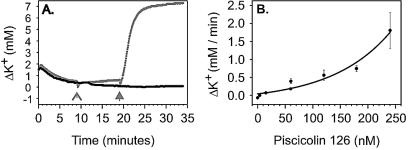
Recombinant piscicolin 126 activity was investigated by monitoring K+ permeability from L. monocytogenes 4A after the addition of a range of piscicolin 126 concentrations and at increasing ionic strength and pH. L. monocytogenes 4A accumulated and retained K+ after a 10-min incubation in the standard K+ buffer at room temperature. The addition of piscicolin 126 (240 nM) to K+ loaded cells of L. monocytogenes 4A caused the rapid efflux of accumulated K+ (Fig. 3A), whereas the addition of water had no effect. The initial rate of efflux of K+ from L. monocytogenes 4A after the addition of increasing concentrations of piscicolin 126 demonstrated that the rate of release of preaccumulated K+ was piscicolin 126 concentration dependent with an exponential relationship (Fig. 3B). A delay in the observed K+ efflux of between 10 and 15 s from the time of addition of piscicolin 126 was observed at all piscicolin 126 concentrations in the standard buffer.
FIG. 3.
Effect of piscicolin 126 on K+ efflux from L. monocytogenes 4A. (A) Cells were able to accumulate and hold K+ over the course of the assay (•). The addition of water had no effect on cells that had accumulated K+ ( , ⩚); however, the addition of piscicolin 126 caused a rapid efflux of accumulated K+ (
, ⩚); however, the addition of piscicolin 126 caused a rapid efflux of accumulated K+ ( ,
,  ). (B) The initial rate of K+ efflux from L. monocytogenes 4A was piscicolin 126 concentration dependent.
). (B) The initial rate of K+ efflux from L. monocytogenes 4A was piscicolin 126 concentration dependent.
A concentration of piscicolin 126 (120.1 nM) that gave an intermediate response in the concentration-dependent analyses was used in an investigation of the effect of pH and ionic strength on the rate of K+ efflux from L. monocytogenes 4A. Over the pH range investigated, a significantly greater rate (P < 0.05) of K+ efflux was observed at pH 6.4, with a marked reduction at both higher and lower pH (Fig. 4). At these pH values there was no change in the time required before efflux was observed. Control assays (no piscicolin 126) indicated that as the pH was lowered the K+ leakage over the assay period was increased (results not shown) and was potentially caused by acidic damaging of the cell. Increasing salt concentrations, from 25 to 300 mM NaCl, did not affect the rate of piscicolin 126-mediated K+ efflux, but the onset of K+ efflux was delayed up to 45 s at 300 mM NaCl.
FIG. 4.

Effect of buffer pH on rate of K+ efflux from L. monocytogenes 4A upon addition of piscicolin 126. A significant difference (P < 0.05) in the rate of K+ efflux is indicated by data points with the same letter (a to e).
DISCUSSION
The expression system developed for piscicolin 126 provides ease of purification and a high yield of bacteriocin with correct disulfide bond formation and can be applied to any class IIa bacteriocin that does not contain methionine. Unique features of this expression system are (i) the use of E. coli AD494(DE3) to facilitate the formation of disulfide bonds in the cytoplasm, (ii) the incorporation of a thioredoxin A fusion protein to improve protein solubility and to enhance disulfide bond formation, (iii) purification of recombinant piscicolin 126 from the small volume of the cell extract rather than the culture medium, (iv) His tag affinity purification in buffers containing 8 M urea to decrease protease activity and prevent aggregation, and (v) incorporation of a Met residue immediately preceding mature piscicolin 126 to facilitate high-efficiency (∼100%) and low-cost cleavage of the fusion protein by using CNBr. A final HPLC separation of the chemically cleaved material yielded purified recombinant piscicolin 126.
Approximately 2.3 mg of piscicolin 126 was purified from 2 ml of the soluble fraction obtained after CNBr cleavage. Therefore, without considering the substantial activity that was present in the insoluble fraction, this represents approximately 26 mg of purified piscicolin 126/liter of culture. The physical characteristics of recombinant piscicolin 126, such as the mass and the presence of the disulfide bond, were confirmed to be identical to those of native piscicolin 126 (21).
This method produced bacteriocin at higher yields than other methods and thus represents an important development for biophysical and structure-function investigations of class IIa bacteriocins. Several expression systems that offer fusion tags for increased solubility and E. coli expression strains that enable the formation of disulfide bonds in the cytoplasm are available commercially, and it is expected that each of these systems would behave in a manner similar to the one described here. A survey of 20 class IIa bacteriocins indicated that 70% did not contain Met, and therefore, this procedure would be suitable for their synthesis and purification. The removal of Met from the other 30% of bacteriocins would permit the use of this procedure and may also improve the stability of the purified peptide, as has been demonstrated for pediocin PA-1 (22).
The pET32a m-pisA expression system initially developed for piscicolin 126 required enterokinase to cleave the fusion protein. Using this system, piscicolin 126 activity declined over the course of the proteolytic reaction, conditions were difficult to optimize, and less than 50% of the fusion protein was digested. The most comparable expression system developed to date for class IIa bacteriocins was that of Quadri et al. (33). They also required proteolytic cleavage to release the active bacteriocin and obtained an efficiency between 12 and 37%. The efficiency of chemical cleavage is significantly greater than proteolytic cleavage and significantly improves bacteriocin production methods.
Functional data from this investigation suggest that recombinant bacteriocins produced with this system would be suitable for investigations focusing on biophysical characterizations. Recombinant piscicolin 126 produced using this expression system had an antibacterial activity similar to those of bacteriocins purified from their native hosts. The MIC50 of piscicolin 126 against L. monocytogenes 4A increased over time, as has been reported earlier (6), and was within an order of magnitude of other class IIa bacteriocins tested against the same indicator organism (14).
Piscicolin 126 caused a rapid K+ efflux from L. monocytogenes 4A, demonstrating a biological function at the cell membrane (25, 26) consistent with other class IIa bacteriocins. The rate of K+ efflux was piscicolin 126 concentration dependent and significantly greater at pH 6.4; however, it was not affected by increasing NaCl concentration. The delay time between the addition of piscicolin 126 and the observation of K+ efflux was not affected by the piscicolin 126 concentration or pH; however, it was affected by increasing salt concentration as has also been observed for lactacin F (1). These data indicate that at low ionic strength the rate of pore formation is constant, and increasing the concentration of piscicolin 126 creates a greater number of pores in the bacterial cell membrane. At increased ionic strength, the attraction of piscicolin 126 to the membrane is reduced; however, the ability to form a pore is not.
Acknowledgments
We gratefully acknowledge the gift of pPOW from Barbara Power and pPOW m-pisA from Kim Harmark.
G.G. acknowledges the support of the CRC for International Food Manufacture and Packaging Science and the Australian Postgraduate Award for financial assistance.
REFERENCES
- 1.Abee, T., T. R. Klaenhammer, and L. Letellier. 1994. Kinetic studies of the action of lactacin F, a bacteriocin produced by Lactobacillus johnsonii that forms poration complexes in the cytoplasmic membrane. Appl. Environ. Microbiol. 60:1006-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M. 1991. Current protocols in molecular biology. Wiley Interscience, New York, N.Y.
- 3.Axelsson, L., T. Katla, M. Bjornslett, V. G. Eijsink, and A. Holck. 1998. A system for heterologous expression of bacteriocins in Lactobacillus sake. FEMS Microbiol Lett. 168:137-143. [DOI] [PubMed] [Google Scholar]
- 4.Bhugaloo-Vial, P., J. P. Douliez, D. Moll, X. Dousset, P. Boyaval, and D. Marion. 1999. Delineation of key amino acid side chains and peptide domains for antimicrobial properties of divercin V41, a pediocin-like bacteriocin secreted by Carnobacterium divergens V41. Appl Environ Microbiol. 65:2895-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhunia, A. K., M. C. Johnson, B. Ray, and N. Kalchayanand. 1991. Mode of action of pediocin AcH from Pediococcus acidilactici H on sensitive bacterial stains. J. Appl. Bacteriol. 70:25-33. [Google Scholar]
- 6.Cabo, M. L., M. A. Murado, M. P. Gonzalez, and L. Pastoriza. 1999. A method for bacteriocin quantification. J. Appl. Microbiol. 87:907-914. [DOI] [PubMed] [Google Scholar]
- 7.Carolissen-Mackay, V., G. Arendse, and J. W. Hastings. 1997. Purification of bacteriocins of lactic acid bacteria: problems and pointers. Int. J. Food Microbiol. 34:1-16. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y., and T. J. Montville. 1995. Efflux of ions and ATP depletion induced by pediocin PA-1 are concomitant with cell death in Listeria monocytogenes Scott A. J. Appl. Bacteriol. 79:684-690. [Google Scholar]
- 9.Coventry, M. J., J. B. Gordon, M. Alexander, M. W. Hickey, and J. Wan. 1996. A food-grade process for isolation and partial purification of bacteriocins of lactic acid bacteria that uses diatomite calcium silicate. Appl. Environ. Microbiol. 62:1764-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coventry, M. J., J. Wan, J. B. Gordon, R. F. Mawson, and M. W. Hickey. 1996. Production of brevicin 286 by Lactobacillus brevis VB286 and partial characterization. J. Appl. Bacteriol. 80:91-98. [DOI] [PubMed] [Google Scholar]
- 11.Dalet, K., C. Briand, Y. Cenatiempo, and Y. Hechard. 2000. The rpoN gene of Enterococcus faecalis directs sensitivity to subclass IIa bacteriocins. Curr. Microbiol. 41:441-443. [DOI] [PubMed] [Google Scholar]
- 12.Dalet, K., Y. Cenatiempo, P. Cossart, and Y. Hechard. 2001. A sigma(54)-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiology 147:3263-3269. [DOI] [PubMed] [Google Scholar]
- 13.Derman, A. I., W. A. Prinz, D. Belin, and J. Beckwith. 1993. Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science 262:1744-1747. [DOI] [PubMed] [Google Scholar]
- 14.Eijsink, V. G., M. Skeie, P. H. Middelhoven, M. B. Brurberg, and I. F. Nes. 1998. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl. Environ. Microbiol. 64:3275-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fimland, G., O. R. Blingsmo, K. Sletten, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1996. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: the C-terminal region is important for determining specificity. Appl. Environ. Microbiol. 62:3313-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fimland, G., R. Jack, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1998. The bactericidal activity of pediocin PA-1 is specifically inhibited by a 15-mer fragment that spans the bacteriocin from the center toward the C terminus. Appl. Environ. Microbiol. 64:5057-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gravesen, A., P. Warthoe, S. Knochel, and K. Thirstrup. 2000. Restriction fragment differential display of pediocin-resistant Listeria monocytogenes 412 mutants shows consistent overexpression of a putative beta-glucoside-specific PTS system. Microbiology 146:1381-1389. [DOI] [PubMed] [Google Scholar]
- 18.Gross, E. 1967. The cyanogen bromide reaction. Methods Enzymol. 11:238-255. [Google Scholar]
- 19.Guyonnet, D., C. Fremaux, Y. Cenatiempo, and J. M. Berjeaud. 2000. Method for rapid purification of class IIa bacteriocins and comparison of their activities. Appl. Environ. Microbiol. 66:1744-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastings, J. W., M. Sailer, K. Johnson, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1991. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J. Bacteriol. 173:7491-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jack, R. W., J. Wan, J. Gordon, K. Harmark, B. E. Davidson, A. J. Hillier, R. E. Wettenhall, M. W. Hickey, and M. J. Coventry. 1996. Characterization of the chemical and antimicrobial properties of piscicolin 126, a bacteriocin produced by Carnobacterium piscicola JG126. Appl. Environ. Microbiol. 62:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnsen, L., G. Fimland, V. Eijsink, and J. Nissen-Meyer. 2000. Engineering increased stability in the antimicrobial peptide pediocin PA-1. Appl. Environ. Microbiol. 66:4798-4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 24.Kleerebezem, M., M. M. Beerthuyzen, E. E. Vaughan, W. M. de Vos, and O. P. Kuipers. 1997. Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl. Environ. Microbiol. 63:4581-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuzaki, K., M. Fukui, N. Fujii, and K. Miyajima. 1991. Interactions of an antimicrobial peptide, tachyplesin I, with lipid membranes. Biochim. Biophys. Acta 1070:259-264. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzaki, K., K. Sugishita, M. Harada, N. Fujii, and K. Miyajima. 1997. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim. Biophys. Acta 1327:119-130. [DOI] [PubMed] [Google Scholar]
- 27.Miller, K. W., R. Schamber, Y. Chen, and B. Ray. 1998. Production of active chimeric pediocin AcH in Escherichia coli in the absence of processing and secretion genes from the Pediococcus pap operon. Appl Environ Microbiol. 64:14-20. (Erratum, 64:1587.) [DOI] [PMC free article] [PubMed]
- 28.Miller, K. W., R. Schamber, O. Osmanagaoglu, and B. Ray. 1998. Isolation and characterization of pediocin AcH chimeric protein mutants with altered bactericidal activity. Appl. Environ. Microbiol. 64:1997-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nes, I. F., and H. Holo. 2000. Class II antimicrobial peptides from lactic acid bacteria. Biopolymers 55:50-61. [DOI] [PubMed] [Google Scholar]
- 30.Nieto Lozano, J. C., J. Nissen-Meyer, K. Sletten, C. Pelaz, and I. F. Nes. 1992. Purification and amino acid sequence of a bacteriocin produced by Pediococcus acidilactici. J. Gen. Microbiol. 138:1985-1990. [DOI] [PubMed] [Google Scholar]
- 31.Power, B. E., N. Ivancic, V. R. Harley, R. G. Webster, A. A. Kortt, R. A. Irving, and P. J. Hudson. 1992. High-level temperature-induced synthesis of an antibody VH-domain in Escherichia coli using the PelB secretion signal. Gene 113:95-99. [DOI] [PubMed] [Google Scholar]
- 32.Quadri, L. E., M. Sailer, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1994. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J. Biol. Chem. 269:12204-12211. [PubMed] [Google Scholar]
- 33.Quadri, L. E., L. Z. Yan, M. E. Stiles, and J. C. Vederas. 1997. Effect of amino acid substitutions on the activity of carnobacteriocin B2. Overproduction of the antimicrobial peptide, its engineered variants, and its precursor in Escherichia coli. J. Biol. Chem. 272:3384-3388. [DOI] [PubMed] [Google Scholar]
- 34.Ramnath, M., M. Beukes, K. Tamura, and J. W. Hastings. 2000. Absence of a putative mannose-specific phosphotransferase system enzyme IIAB component in a leucocin A-resistant strain of Listeria monocytogenes, as shown by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Appl. Environ. Microbiol. 66:3098-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uteng, M., H. H. Hauge, I. Brondz, J. Nissen-Meyer, and G. Fimland. 2002. Rapid two-step procedure for large-scale purification of pediocin-like bacteriocins and other cationic antimicrobial peptides from complex culture medium. Appl. Environ. Microbiol. 68:952-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venema, K., M. L. Chikindas, J. F. M. L. Seegers, A. J. Haandrikman, K. J. Leenhouts, G. Venema, and J. Kok. 1997. Rapid and efficient purification method for small, hydrophobic, cationic bacteriocins: purification of lactococcin B and pediocin PA-1. Appl. Environ. Microbiol. 63:305-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, Y., M. E. Henz, N. L. Gallagher, S. Chai, A. C. Gibbs, L. Z. Yan, M. E. Stiles, D. S. Wishart, and J. C. Vederas. 1999. Solution structure of carnobacteriocin B2 and implications for structure-activity relationships among type IIa bacteriocins from lactic acid bacteria. Biochemistry 38:15438-15447. [DOI] [PubMed] [Google Scholar]
- 38.Yan, L. Z., A. C. Gibbs, M. E. Stiles, D. S. Wishart, and J. C. Vederas. 2000. Analogues of bacteriocins: antimicrobial specificity and interactions of leucocin A with its enantiomer, carnobacteriocin B2 and truncated derivatives. J. Med. Chem. 43:4579-4581. [DOI] [PubMed] [Google Scholar]
- 39.Yildirim, Z., D. K. Winters, and M. G. Johnson. 1999. Purification, amino acid sequence and mode of action of bifidocin B produced by Bifidobacterium bifidum NCFB 1454. J. Appl. Microbiol. 86:45-54. [DOI] [PubMed] [Google Scholar]