Abstract
Cancer awareness public campaigns aim to shorten the interval between symptom onset and presentation to a doctor (the ‘patient interval’). Appreciating variation in promptness of presentation can help to better target awareness campaigns. We explored variation in patient intervals recorded in consultations with general practitioners among 10,297 English patients subsequently diagnosed with one of 18 cancers (bladder, brain, breast, colorectal, endometrial, leukaemia, lung, lymphoma, melanoma, multiple myeloma, oesophageal, oro-pharyngeal, ovarian, pancreatic, prostate, renal, stomach, and unknown primary) using data from of the National Audit of Cancer Diagnosis in Primary Care (2009–2010). Proportions of patients with ‘prompt’/‘non-prompt’ presentation (0–14 or 15+ days from symptom onset, respectively) were described and respective odds ratios were calculated by multivariable logistic regression. The overall median recorded patient interval was 10 days (IQR 0–38). Of all patients, 56% presented promptly. Prompt presentation was more frequent among older or housebound patients (p < 0.001). Prompt presentation was most frequent for bladder and renal cancer (74% and 70%, respectively); and least frequent for oro-pharyngeal and oesophageal cancer (34% and 39%, respectively, p <.001). Using lung cancer as reference, the adjusted odds ratios of non-prompt presentation were 2.26 (95% confidence interval 1.57–3.25) and 0.42 (0.34–0.52) for oro-pharyngeal and bladder cancer, respectively. Sensitivity analyses produced similar findings. Routinely recorded patient interval data reveal considerable variation in the promptness of presentation. These findings can help to prioritise public awareness initiatives and research focusing on symptoms of cancers associated with greater risk of non-prompt presentation, such as oro-pharyngeal and oesophageal cancer.
What's new?
A critical aspect of cancer diagnosis is how promptly patients consult a doctor after they first notice initial symptoms. Here, the authors examine differences in this so-called patient interval in English patients subsequently diagnosed with one of 18 cancers. On average, patients with bladder and renal cancer as well as older and housebound patients consulted a doctor relatively promptly while patients with oro-pharyngeal and oesophageal cancer took the longest until first presenting to a general practitioner. The authors point out that cancer awareness campaigns should encompass symptoms of oro-pharyngeal and oesophageal cancer aiming to shorten the patient interval for these cancers.
Keywords: cancer, patient interval, promptness, presentation, delay, oro-pharyngeal, oesophageal, bladder, renal, variation
Diagnosing cancer promptly in symptomatic patients is a key aspect of contemporary cancer control policies in different countries.1–5 After symptom onset, delays in establishing the diagnosis may occur both before a patient presents to a doctor and post-presentation.6 In most cancer patients, initial symptoms have low specificity, as they are also associated with benign diseases.7 Appropriate appraisal and interpretation of symptoms that may be related to cancer by both patients (pre-presentation) and their doctor (post-presentation) are critical for timely diagnosis.6,8 There is large variation between different patient groups in the promptness with which general practitioners suspect the diagnosis of cancer and refer patients to specialists (i.e. in the ‘primary care interval’).9,10 It is also plausible that there is variation in the promptness with which cancer patients seek medical help (i.e. in the ‘patient interval’, defined as the period between first symptom onset and first relevant presentation to a doctor6). Variation in the patient interval may exist both between patients with different socio-demographic characteristics (since cancer awareness, beliefs and attitudes vary between socio-demographic groups or country populations11–16; and between patients with different cancers (given wide variability in the nature of symptoms of different tumours). Understanding variation in the patient interval can help to identify patient groups at higher risk of non-prompt presentation, enabling better targeting of public health awareness interventions.17
Evidence about patient interval variation is however limited, partly because accurate measurement is known to be challenging.6,18 Determining the date of onset of symptoms or bodily changes (the start of the patient interval) is difficult, given the potential for inaccurate or biased patient recall, and the gradual onset of many symptoms.6,18 The date of first presentation to a doctor with symptoms caused by cancer (the end of the patient interval) is often easy to identify, but there can be difficulties in determining the first relevant consultation in patients with multi-morbidity. Acknowledging these difficulties, broadly, patient interval information can be obtained either from the patients themselves (through interviews or questionnaire surveys19–22 or from their medical consultation records.23,24 Either approach has advantages and disadvantages (Box). Although elicitation of symptom duration typically forms a key part of medical consultations, medical records studies assume accurate elicitation and recording of this information. On the other hand, patient interview or questionnaire studies can provide detailed information but may lack representativeness (as, by their nature, cancer patients who die early or are too sick soon after diagnosis are typically not included in such studies).
Box.
Principal advantages and limitations of the two main approaches to measuring the patient interval
| Strengths | Limitations | |
|---|---|---|
| Patient interview (or questionnaire) studies | Potentially highly accurate and detailedCan allow for detailed (‘in-depth’) appreciation of relevant symptoms and their time of onset. | Limited representativeness (generalisability)Patients dying soon after symptom onset/diagnosis and those ‘too ill to take part’ are unlikely to be included. |
| Studies of medical consultation records | High representativeness (generalisability)Information about all cancer patients can be included, even for those with poor prognosis/only short-term survival. | Potential limitations in completeness and accuracyRely on doctors appropriately eliciting the timing of symptom onset as part of history taking and accurately interpreting and recording this information. Patient interval information may be missing. |
Appreciating both the strengths and the limitations of patient interval studies that are based on information from medical records, we conducted a secondary analysis of data from the National Audit of Cancer Diagnosis in Primary Care, 2009–2010.25 Our aim was to explore variation in the routinely recorded (i.e. during general practice consultations) patient interval of patients subsequently diagnosed with cancer. We were particularly interested in exploring likely variation by socio-demographic characteristic and cancer diagnosis.
Material and Methods
Data
We analysed data from the (English) National Audit of Cancer Diagnosis in Primary Care (2009–2010).25 Information from patient records on different aspects of the diagnostic process was collected by general practitioners or other primary care professionals in an estimated total of 1,170 general practices (∼14% of all practices in England).25,26 Audited patients were incident cases of cancer within the audit period and were representative of the age and diagnosis case-mix of English cancer patients.25 Although participation to the audit was voluntary, the organisational characteristics and care quality of participating and non-participating practices were similar.27 Screening-detected cases were excluded from the audit. The patient interval was defined as the number of days from first symptom onset to first presentation to a general practitioner with relevant symptoms based on information in the patients' records.6,25 Patients were categorised as housebound if primary care encounters usually occurred at home – we included information on housebound status in the analysis as a marker of severe co-morbidity, because of theoretical concerns that patients with higher levels of co-morbidity may be disadvantaged in respect of the timeliness of cancer diagnosis. The analysis was a priori restricted to patients who first presented to a general practitioner with any of 18 cancers for which variation in respect of the primary care interval was previously explored and were aged 15 or older.25,26 Analysis was restricted to records with patient interval values of up to two years, and complete information on outcome and exposure variables of interest (Fig. 1).
Figure 1.
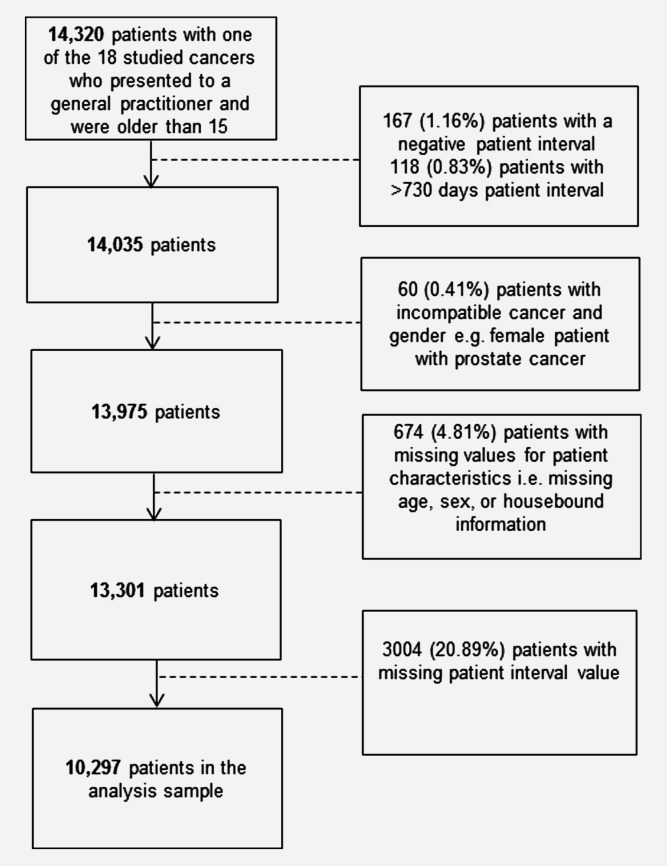
Derivation of the analysis sample. Percentage values relate to the initial sample of 14,320 patients with one of the 18 studied cancers.
Analysis
We aimed to profile variation in promptness of presentation to a general practitioner after symptom onset. There is no uniform approach to analysing patient interval data, which tend to be zero-inflated and right-skewed. Therefore, we first described the key patient interval statistics (median and other relevant centile values) by patient group. Subsequently, we analysed variation in respect of a binary form of the patient interval (0–14 vs 15 or more days – hereafter, we use the terms prompt/non-prompt to denote either category, respectively). Our choice of binary cut-off was pragmatic – choosing a short-term period during which it could be reasonably assumed that most patients who did decide to see their doctor would have been able to do so. Additional short-term binary cut-off values were explored in sensitivity analysis (see below).
In univariable analysis, we examined crude differences between different patient groups in respect of the median patient interval, the proportion of non-prompt presenters and respective crude odds ratios. Subsequently, multivariable logistic regression was used to explore independent associations between patient characteristics or cancer diagnosis and prompt/non-prompt presentation. Further, interactions between cancer diagnosis and age and cancer diagnosis and gender were explored. Robust estimation of standard errors was used to account for potential clustering of observations.
Sensitivity analysis
We first repeated the multivariable regression model using alternative binary categories of the patient interval (0–7 vs 8+, 0–21 vs 22+ and 0–30 vs 31+ days, respectively). Complete case analysis pre-supposes that missing information is Missing Completely At Random which is a strong assumption. We, therefore, used extreme-case scenario analysis (assuming data are Missing Not At Random) by repeating the multivariable analysis assuming patients with missing interval values were either ‘all non-prompt’ or ‘all prompt’ presenters – these analyses do not intend to represent a real situation but are useful to illustrate the largest possible bias that could be introduced by missing patient interval information. We used further sensitivity analyses to explore potential confounding by ethnic group among patients with known ethnicity and the impact of only including patients with interval values of up to a year. Analysis was undertaken using Stata 11 (Stata Corporation, Texas).
Results
Of an initial 14,320 patients with one of the 18 cancers examined, 10,297 (72%) were included in complete case analysis (Fig. 1). The main single source of sample attrition was missing patient interval (3,004 or 21% of initially eligible patients). Patients with missing patient interval were more likely to be older and men (p < 0.001 for both) without evidence for an association with housebound status (p = 0.342). Missing patient interval also varied by cancer (p < 0.001), being most common among patients with leukaemia, prostate cancer, melanoma and multiple myeloma (41%, 40%, 36% and 28%, respectively, Supporting Information Appendix 1). Hereafter, results related to complete case analysis except were otherwise noted. Characteristics of included patients are shown in Table2.
Table 1.
Sample characteristics and descriptive statistics for patient interval by patient characteristic and cancer (n = 10,297)
| Patient interval binary category |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient interval (days) |
Prompt (0–14 days) |
Non-prompt (15+ days) |
||||||||||
| N | 25th Centile | Median | 75th Centile | 90th Centile | 95th Centile | p-Value1 | N | % | N | % | p-Value2 | |
| Age | ||||||||||||
| 15–44 | 784 | 1 | 13 | 42 | 120 | 242 | p < 0.0001 | 439 | 56.0 | 345 | 44.0 | p < 0.0001 |
| 45–54 | 1,220 | 1 | 14 | 45 | 108 | 187 | 649 | 53.2 | 571 | 46.8 | ||
| 55–64 | 2,170 | 0 | 12 | 41 | 102 | 191 | 1,186 | 54.7 | 984 | 45.3 | ||
| 65–74 | 2,807 | 0 | 11 | 43 | 112 | 185 | 1,526 | 54.4 | 1,281 | 45.6 | ||
| 75–84 | 2,459 | 0 | 7 | 31 | 92 | 183 | 1,463 | 59.5 | 996 | 40.5 | ||
| 85+ | 857 | 0 | 7 | 31 | 95 | 188 | 526 | 61.4 | 331 | 38.6 | ||
| Sex | ||||||||||||
| Male | 5,028 | 0 | 11 | 43 | 116 | 200 | p = 0.37 | 2,742 | 54.5 | 2,286 | 45.5 | p = 0.0008 |
| Female | 5,269 | 0 | 10 | 33 | 92 | 182 | 3,047 | 57.8 | 2,222 | 42.2 | ||
| Cancer | ||||||||||||
| Bladder | 601 | 0 | 2 | 16 | 67 | 141 | p < 0.0001 | 446 | 74.2 | 155 | 25.8 | p < 0.0001 |
| Renal | 209 | 0 | 3 | 19 | 74 | 184 | 146 | 69.9 | 63 | 30.1 | ||
| Brain | 125 | 1 | 7 | 26 | 96 | 154 | 81 | 64.8 | 44 | 35.2 | ||
| Breast | 2,124 | 1 | 7 | 27 | 77 | 164 | 1371 | 64.5 | 753 | 35.5 | ||
| Unknown primary | 110 | 0 | 7 | 23 | 64.5 | 104 | 69 | 62.7 | 41 | 37.3 | ||
| Leukaemia | 239 | 0 | 7 | 30 | 86 | 140 | 144 | 60.3 | 95 | 39.7 | ||
| Prostate | 1,386 | 0 | 6 | 42 | 151 | 283 | 813 | 58.7 | 573 | 41.3 | ||
| Pancreatic | 272 | 1 | 9.5 | 31 | 73 | 97 | 162 | 59.6 | 110 | 40.4 | ||
| Stomach | 187 | 0 | 9 | 33 | 125 | 205 | 104 | 55.6 | 83 | 44.4 | ||
| Lung | 1,126 | 0 | 12 | 33 | 87 | 138 | 622 | 55.2 | 504 | 44.8 | ||
| Myeloma | 127 | 0 | 14 | 40 | 95 | 193 | 69 | 54.3 | 58 | 45.7 | ||
| Endometrial | 311 | 1 | 14 | 57 | 152 | 259 | 165 | 53.1 | 146 | 46.9 | ||
| Ovarian | 270 | 2 | 14 | 51 | 113.5 | 172 | 144 | 53.3 | 126 | 46.7 | ||
| Lymphoma | 482 | 1 | 14 | 43 | 92 | 183 | 243 | 50.4 | 239 | 49.6 | ||
| Melanoma | 477 | 0 | 20 | 69 | 241 | 366 | 216 | 45.3 | 261 | 54.7 | ||
| Colorectal | 1,697 | 1 | 19 | 60 | 131 | 203 | 786 | 46.3 | 911 | 53.7 | ||
| Oesophageal | 407 | 7 | 22 | 46 | 99 | 152 | 158 | 38.8 | 249 | 61.2 | ||
| Oro-pharyngeal | 147 | 7 | 30 | 62 | 122 | 212 | 50 | 34.0 | 97 | 66.0 | ||
| Housebound status | ||||||||||||
| No | 9,707 | 0 | 11 | 39 | 103 | 188 | p < 0.0001 | 5399 | 55.6 | 4308 | 44.4 | p < 0.0001 |
| Yes | 590 | 0 | 5 | 28 | 91 | 200 | 390 | 66.1 | 200 | 33.9 | ||
| Total | 10,297 | 0 | 10 | 38 | 103 | 189 | 5,789 | 56.2 | 4,508 | 43.8 | ||
Kruskal–Wallis test.
Chi-squared test.
The overall median patient interval was 10 days (inter-quartile range 0–38 days); about half of all patients (5,789, or 56%) had an interval of up to 14 days, i.e. were prompt presenters by our definition (Table2). There was substantial variation in promptness of presentation by age, housebound status and cancer diagnosis (p < 0.001 for all). Prompt presentation was more frequent among older patients; and those who were housebound (66% vs 56% among those non-housebound). These differences were also apparent when examining various centiles of the patient interval which tended to be shorter for older and housebound patients (Table2). Prompt presentation was most frequent among patients with bladder and renal cancer (74% and 70%, respectively). Conversely, oro-pharyngeal and oesophageal cancer had the lowest proportions of prompt presenters (34% and 39%, respectively).
Table3 and Figures 2 and 3 describe odds ratios of non-prompt presentation derived by both univariable and multivariable logistic regression. Except for gender for which there was no evidence of variation in the multivariable analysis (p = 0.17), these analyses produced similar findings, indicating only a limited degree of confounding between exposure variables. The largest degree of variation (>5-fold) in the odds of prompt presentation is seen between patients with different cancers. Specifically, using patients with lung cancer as the reference group, the odds ratios of non-prompt presentation were 2.26 (95% confidence interval 1.57–3.25) and 0.42 (0.34–0.52) for patients with oro-pharyngeal and bladder cancer, respectively. There was no evidence of interaction between cancer diagnosis and either age or sex (p = 0.29 for both).
Table 2.
Proportion of patients with non-prompt presentation and respective unadjusted and adjusted odds ratios (n = 10,297)
| % Non-prompt (15+ days) presentation1 | Unadjusted odds ratios for non-prompt (15+ days) presentation | Adjusted odds ratios for non-prompt presentation (15+ days) by patient characteristic and cancer diagnosis | |||
|---|---|---|---|---|---|
| Age | |||||
| 15–44 (N = 784) | 44.0 | 0.94 (0.80–1.10) | p < 0.0001 | 0.99 (0.84–1.17) | p = 0.0003 |
| 45–54 (N = 1,220) | 46.8 | 1.05 (0.92–1.20) | 1.12 (0.98–1.30) | ||
| 55–64 (N = 2,170) | 45.3 | 0.99 (0.88–1.11) | 0.98 (0.87–1.10) | ||
| 65–74 (N = 2,807) | 45.6 | Baseline | Baseline | ||
| 75–84 (N = 2,459) | 40.5 | 0.81 (0.73–0.90) | 0.83 (0.74–0.93) | ||
| 85+ (N = 857) | 38.6 | 0.75 (0.64–0.88) | 0.83 (0.70–0.98) | ||
| Gender | |||||
| Male (N = 5,028) | 45.5 | Baseline | p = 0.0008 | Baseline | p = 0.17 |
| Female (N = 5,269) | 42.2 | 0.87 (0.81–0.95) | 0.93 (0.84–1.03) | ||
| Cancer type | |||||
| Bladder (N = 601) | 25.8 | 0.43 (0.35–0.53) | p < 0.0001 | 0.42 (0.34–0.52) | p < 0.0001 |
| Renal (N = 209) | 30.1 | 0.53 (0.39–0.73) | 0.51 (0.37–0.71) | ||
| Brain (N = 125) | 35.2 | 0.67 (0.46–0.99) | 0.66 (0.45–0.98) | ||
| Breast (N = 2,124) | 35.5 | 0.68 (0.58–0.79) | 0.67 (0.57–0.78) | ||
| Unknown primary (N = 110) | 37.3 | 0.73 (0.49–1.10) | 0.75 (0.50–1.12) | ||
| Leukaemia (N = 239) | 39.7 | 0.81 (0.61–1.08) | 0.78 (0.58–1.03) | ||
| Prostate (N = 1,386) | 41.3 | 0.87 (0.74–1.02) | 0.83 (0.70–0.98) | ||
| Pancreatic (N = 272) | 40.4 | 0.84 (0.64–1.10) | 0.85 (0.65–1.11) | ||
| Stomach (N = 187) | 44.4 | 0.98 (0.72–1.34) | 0.99 (0.73–1.35) | ||
| Lung (N = 1,126) | 44.8 | Baseline | Baseline | ||
| Myeloma (N = 127) | 45.7 | 1.04 (0.72–1.50) | 1.01 (0.70–1.47) | ||
| Endometrial (N = 311) | 46.9 | 1.09 (0.85–1.40) | 1.08 (0.84–1.40) | ||
| Ovarian (N = 270) | 46.7 | 1.08 (0.83–1.41) | 1.09 (0.83–1.43) | ||
| Lymphoma (N = 482) | 49.6 | 1.21 (0.98–1.50) | 1.15 (0.93 - 1.43) | ||
| Melanoma (N = 477) | 54.7 | 1.49 (1.20–1.85) | 1.41 (1.13–1.75) | ||
| Colorectal (N = 1,697) | 53.7 | 1.43 (1.23–1.66) | 1.43 (1.23–1.67) | ||
| Oesophageal (N = 407) | 61.2 | 1.94 (1.54–2.45) | 1.94 (1.54–2.45) | ||
| Oropharynheal (N = 147) | 66.0 | 2.39 (1.67–3.43) | 2.26 (1.57–3.25) | ||
| Housebound | |||||
| No (N = 9,707) | 44.4 | Baseline | p < 0.0001 | Baseline | p < 0.0001 |
| Yes (N = 590) | 33.9 | 0.64 (0.54–0.77) | 0.67 (0.56–0.81) | ||
This column repeats information presented in Table2, for ease of reference regarding crude proportions.
Figure 2.
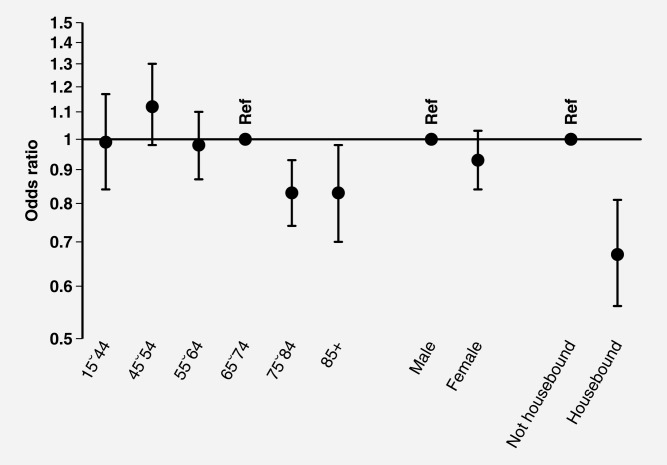
Multivariable logistic regression outputs (adjusted odds ratios and 95% confidence intervals) for non-prompt presentation by sociodemographic characteristic (n = 10,297).
Figure 3.
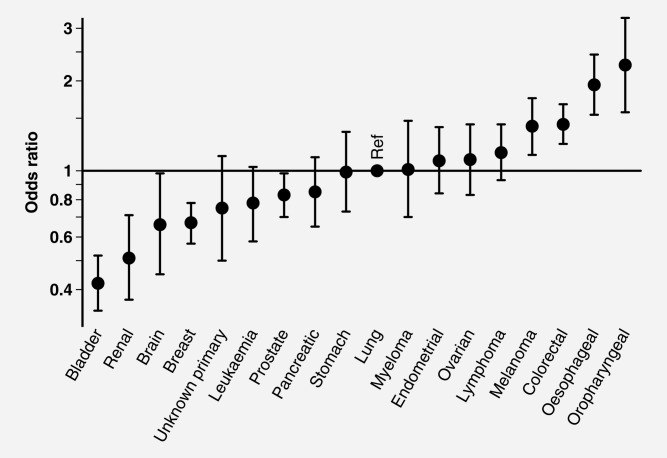
Multivariable logistic regression outputs (adjusted odds ratios and 95% confidence intervals) for non-prompt presentation by cancer diagnosis (n = 10,297).
Sensitivity analysis
Using different binary categories of patient interval produced similar findings (Supporting Information Appendix 2). Assuming patients with missing data were either ‘all non-prompt’ or ‘all prompt’ presenters did either attenuate or accentuate patterns of variation observed in the main analysis, respectively, particularly by age and the four cancers with relatively high proportions of missing interval data (leukaemia, prostate, melanoma and myeloma) (Supporting Information Appendix 3). The degree of confounding by ethnicity was very limited (Supporting Information Appendix 4). Only including patients with interval values of up to a year produced highly concordant findings (results not shown).
Discussion
In this study, among patients with any of the 18 cancers prompt presentation was most frequent among those with bladder and renal cancer, and least frequent among patients with oro-pharyngeal and oesophageal cancer. Prompt presentation was also more frequent in older and housebound patients.
Of the 18 cancers included in our study, 12 were also examined previously by an audit (medical record) study of Scottish patients23; and 10 by a similar Danish study.24 Among Scottish cancer patients, those with ‘head and neck’ (including oro-pharyngeal) cancer presented least promptly, whilst those with bladder and ‘other urological’ (including renal) cancers did so most promptly.23 In general, median reported patient interval values for Scottish patients were similar to those reported here; however, those reported for Danish patients were longer – potentially reflecting differences in patient populations or in methods of data recording and collection (Supporting Information Appendix 5).23,24 However, the Spearman rank correlation coefficient for pair-wise comparisons of median patient interval values by cancer is 0.87 (p = 0.0002) and 0.59 (p = 0.071) between the present and the Scottish or the Danish study, respectively – indicating an overall high degree of rank concordance.23,24 Similarly, most Finnish patients with pharyngeal cancer have patient intervals longer than a month.28
Prior evidence is inconsistent regarding the presence and direction of associations between age and patient interval for different cancers.22,28–32 We are unaware of previous descriptions of variation in patient interval by housebound status. Housebound patients may be more prone to seeking help promptly, or are monitored more frequently and, therefore, assessed more promptly. Disability may confer paradoxical benefits in respect of stage at diagnosis of cancer.33
One of the strengths of our study is that it included patients with many different cancers. The representativeness of the patient population and of participating practices was good.25,27 Because of continuous sampling, the study population can be assumed to be a relatively unbiased sample of incident cases first presenting to general practitioners, also including patients with poor prognosis. The robustness of the findings was explored by a range of sensitivity analyses, which generally provided similar findings. Although patient interval data were missing for between a fifth and a quarter of patients, sensitivity analyses using extreme-case assumptions indicated that this factor might have biased the findings regarding patients with four cancers with a relatively high proportion of missing data (leukaemia, prostate cancer, melanoma and myeloma); in contrast, patterns of variation for patients with all other cancers, and particularly for those with bladder, renal, oesophageal and oro-pharyngeal cancer, remained similar. Two of the cancers with higher than average proportion of missing patient interval data were prostate cancer and leukaemia. For those cancers, diagnostic suspicion is sometimes first raised at an asymptomatic stage or incidentally, based on the findings of relatively simple-to-perform blood tests (such as Prostate Specific Antigen testing or Full Blood Count). In such circumstances, the diagnosis is not symptom-driven and, therefore, measurement of the patient interval is a priori not applicable. These factors may explain the higher proportion of missing interval information for those cancers.
There are several limitations. The validity of patient interval data is contingent on several factors: patients need to have been able to accurately appreciate the onset of their symptoms and recall relevant dates; their doctors need to have been able to elicit and appropriately interpret information about the patient interval during consultations, and to have accurately entered it in the patient records. Although elicitation of information on symptom duration is a key aspect of a medical consultation, inaccuracies and omissions may occur in any of the above steps. However, previous research indicates that inaccurate patient recall of diagnostic intervals is unlikely to be systematic (e.g. biased towards either over- or under-estimation of patient interval).34 It is also unreasonable to assume that recall inaccuracies will be grossly differential between patients with different cancers – for example, between patients with bladder and oro-pharyngeal cancer, given the large size of the observed variation between these cancers (∼5-fold difference in odds ratios). Although we believe this assertion to be reasonable, there is no direct evidence for it, and future evidence from relevant clinical psychology studies would be useful. It is important to also consider that non-systematic errors of this kind would result in under-estimation of true variation; therefore, our reported estimates of socio-demographic or cancer diagnosis differences may be conservative. We were not able to examine variation in the patient interval of patients with cancer whose first presentation did not involve previous contact with their general practitioner. Some patients have long or very long patient intervals, e.g. 90 or 180 days (Table2) and the predictors of very long patient intervals may be different to the predictors of delay in respect of shorter intervals. We plan to explore variation in the patient interval amongst these patients in the future. Our findings relate to a population of English cancer patients, and extrapolations to other populations should therefore be cautious. Although at least some of the observed findings may be relevant, research questions about variation in the patient interval in other country populations are best addressed by new empirical evidence.
Promptness of presentation is a concept also applicable to a larger group of patients who experience symptoms but do not necessarily have cancer (or any other formal diagnosis). For example, even among patients with ‘alarm’ symptoms mandating specialist referral for investigation of suspected cancer, only one in nine are found to have cancer, whereas eight in nine patients with relevant symptoms will have another diagnosis.35 Therefore, future research should also explore variation in the timeliness of presentation among the broader population of patients with symptoms likely to be related to cancer, and not only among cancer cases. Although clearly important, exploring variation in patient interval among patients with symptoms (not simply among cancer patients) was impossible given our data.14
Measuring patient interval is challenging as it cannot be ‘objectively’ measured.6,18,36 Additional difficulties arise in the context of co-morbidity. Both patient interviews/surveys and medical record studies have strengths and limitations (Box). Patient interview/questionnaire studies can be subject to survivorship bias (differential attrition) as those who die early do not contribute information and their intervals may be different to those of survivors. In contrast, studies based on medical records information can provide for relatively large samples (including patients with rarer cancers) whilst limiting the potential for survivorship bias (as continuous sampling of all incident cases is possible). Ideally studies should encompass both approaches, and also measure patient intervals of patients with relevant symptoms who do not necessarily have cancer, as in the DISCOVERY programme's SYMPTOM study, due to report 2014 (http://discovery-programme.org/symptom_study.php).
Variation in the promptness of presentation by cancer is likely to reflect differences in how patients appreciate and appraise typical symptoms of different cancers. Symptoms with abrupt and unexplained onset such as bleeding are associated with shorter patient intervals.22,29–31 As patients with bladder and renal cancer often present with haematuria, this may explain why patients with these two cancers seem to present more promptly than patients with any other examined cancer.23 Other factors, such as symptom frequency, duration and intensity may also matter. Familiarity with signs and symptoms in the context of previous self-limiting illness (e.g. oral ulcerations) has been judged responsible for non-prompt presentation of patients with oro-pharyngeal cancer.37,38
Whilst there is very strong evidence of variation in prompt presentation between patients with different cancers we cannot reliably distinguish between all individual cancers, particularly for cancers in the middle of the spectrum. We suggest that interpretation considers the general pattern of variation, particularly focusing on comparisons of the extremes (e.g. oro-pharyngeal or oesophageal vs bladder or renal cancer). We specifically draw attention to oro-pharyngeal and oesophageal cancer – the two cancers with the highest proportions of non-prompt presenters and longest median patient intervals. Oro-pharyngeal cancer has relatively poor 5-year relative survival (typically <50% for most sub-sites except lip). Together with our own findings, these considerations can support the development of awareness campaigns for oro-pharyngeal cancer.39 Oesophageal cancer also has poor prognosis (5-year relative survival <20%). Although dysphagia is a common cardinal symptom of oesophageal cancer (and one with relatively high specificity40, the findings indicate that patients with oesophageal cancer do not present promptly. These findings concord with prior evidence indicating that awareness of ‘difficulty swallowing’ as a potential sign of cancer is particularly poor among members of the British public (lowest compared with other eight cancer symptoms).11 Specifically, only about 1 in 20 respondents would immediately recall dysphagia as a symptom of cancer – in contrast lump/swelling (the symptom with the highest spontaneous recall) would be recalled by two thirds of respondents.11 These findings would therefore support the development of awareness campaigns about the importance of dysphagia. The clinical and population health outcomes of awareness campaigns nevertheless need to be evaluated, ideally using controlled designs.41
As the aim of public awareness interventions is to decrease patient intervals,17 we strongly advocate the conduct of regular surveys of patient interval in representative samples of cancer patients to help monitor the impact of such interventions and progress towards improving the timeliness of presentation in the population. Appreciating variation in promptness of presentation can help to better target and tailor such interventions. Given the findings, prioritising public awareness interventions for symptoms of oro-pharyngeal and oesophageal cancer is particularly justified.
Acknowledgments
We are grateful to all primary care professionals in participating practices for collecting, collating and submitting anonymous data; and respective Cancer Networks, the Royal College of General Practitioners, the National Cancer Action Team and the National Clinical Intelligence Network (NCIN) of Public Health England (PHE) for supporting the audit. Aspects of this paper formed the material for the MPhil in Public Health dissertation by SK (University of Cambridge).
Contributors
The study was initiated by SK and GL in the context of SK's MPhil in Public Health studies (University of Cambridge) supervised by GL. Material and Methods were further developed and modified in discussions with GAA and all other authors. CLS and SK performed most analyses. GPR and SMcP led different aspects of the National Audit project and data collation. RDN and FMW commented on the methods and findings alongside all other authors. All authors commented on initial drafts and saw and approved the final draft of the paper.
Ethical Approval
Not required. An integral part of the National Audit of Cancer Diagnosis in Primary Care project was that anonymous data may subsequently be used for purposes of early diagnosis research, and access to these data was granted by the Audit oversight group.
Funding statement
The paper was materially supported by the NHS Public Health Training Scheme in the East of England, sponsoring SK for this Master's studies and by the National Institute for Health Research (PDF-2011-04-047), supporting GL through a Post-Doctoral Fellowship award. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
References
- 1.Department of Health. 2011. Improving outcomes: a strategy for cancer http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_123371 (last accessed December 2013)
- 2.Prades J, Espinas JA, Font R, Argimon JM, Borras JM. Implementing a cancer fast-track programme between primary and secondary care in Catalonia (Spain): a mixed methods study. Br J Cancer. 2011;105(6):753–9. doi: 10.1038/bjc.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.New South Wales Government. 2011. Cancer Plan 2011–15 http://www.cancerplan.cancerinstitute.org.au/ (last accessed December 2013)
- 4.Olesen F, Hansen RP, Vedsted P. Delay in diagnosis: the experience in Denmark. Br J Cancer. 2009;101(Suppl. 2):S5–8. doi: 10.1038/sj.bjc.6605383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Care Ontario. 2009. Diagnostic assessment programs: an environmental scan https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=64068 (last accessed December 2013)
- 6.Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, Campbell C, Andersen RS, Hamilton W, Olesen F, Rose P, Nafees S, van Rijswijk E, Hiom S, Muth C, Beyer M, Neal RD. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106(7):1262–7. doi: 10.1038/bjc.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones R, Charlton J, Latinovic R, Gulliford MC. Alarm symptoms and identification of non-cancer diagnoses in primary care: cohort study. BMJ. 2009;339:b3094. doi: 10.1136/bmj.b3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter F, Webster A, Scott S, Emery J. The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy. 2012;17(2):110–8. doi: 10.1258/jhsrp.2011.010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neal RD, Allgar VL. Sociodemographic factors and delays in the diagnosis of six cancers: analysis of data from the “National Survey of NHS Patients: Cancer”. Br J Cancer. 2005;92(11):1971–5. doi: 10.1038/sj.bjc.6602623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyratzopoulos G, Neal RD, Barbiere JM, Rubin GP, Abel GA. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012;13(4):353–65. doi: 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- 11.Robb K, Stubbings S, Ramirez A, Macleod U, Austoker J, Waller J, Hiom S, Wardle J. Public awareness of cancer in Britain: a population-based survey of adults. Br J Cancer. 2009;101(Suppl. 2):S18–23. doi: 10.1038/sj.bjc.6605386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waller J, Robb K, Stubbings S, Ramirez A, Macleod U, Austoker J, Hiom S, Wardle J. Awareness of cancer symptoms and anticipated help seeking among ethnic minority groups in England. Br J Cancer. 2009;101(Suppl. 2):S24–30. doi: 10.1038/sj.bjc.6605387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon AE, Waller J, Robb K, Wardle J. Patient delay in presentation of possible cancer symptoms: the contribution of knowledge and attitudes in a population sample from the United Kingdom. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2272–7. doi: 10.1158/1055-9965.EPI-10-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svendsen RP, Jarbol DE, Larsen PV, Støvring H, Hansen BL, Soendergaard J. Associations between health care seeking and socioeconomic and demographic determinants among people reporting alarm symptoms of cancer: a population-based cross-sectional study. Fam Pract. 2013 doi: 10.1093/fampra/cmt036. Jul 17 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Forbes LJ, Atkins L, Thurnham A, Layburn J, Haste F, Ramirez AJ. Breast cancer awareness and barriers to symptomatic presentation among women from different ethnic groups in East London. Br J Cancer. 2011;105(10):1474–9. doi: 10.1038/bjc.2011.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forbes LJ, Simon AE, Warburton F, Boniface D, Brain KE, Dessaix A, Donnelly C, Haynes K, Hvidberg L, Lagerlund M, Lockwood G, Tishelman C, Vedsted P, Vigmostad MN, Ramirez AJ, Wardle J International Cancer Benchmarking Partnership Module 2 Working Group. Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): do they contribute to differences in cancer survival? Br J Cancer. 2013;108(2):292–300. doi: 10.1038/bjc.2012.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer research UK. 2013. About Be Clear on Cancer [cited 2013 08/07/2013]. Available from: http://www.cancerresearchuk.org/cancer-info/spotcancerearly/naedi/beclearoncancer/background (last accessed January 2013)
- 18.Andersen RS, Vedsted P, Olesen F, Bro F, Søndergaard J. Patient delay in cancer studies: a discussion of methods and measures. BMC Health Serv Res. 2009;9:189. doi: 10.1186/1472-6963-9-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korsgaard M, Pedersen L, Laurberg S. Delay of diagnosis and treatment of colorectal cancer – a population-based Danish study. Cancer Detect Prev. 2008;32(1):45–51. doi: 10.1016/j.cdp.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen AF, Olesen F, Hansen RP, Zachariae R, Vedsted P. Social support, gender and patient delay. Br J Cancer. 2011;104(8):1249–55. doi: 10.1038/bjc.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corner J, Hopkinson J, Roffe L. Experience of health changes and reasons for delay in seeking care: a UK study of the months prior to the diagnosis of lung cancer. Soc Sci Med. 2006;62(6):1381–91. doi: 10.1016/j.socscimed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM, Campbell NC, MacLeod U, Lee AJ, Raja A, Wyke S, Ziebland SB, Duff EM, Ritchie LD, Nicolson MC. Factors contributing to the time taken to consult with symptoms of lung cancer: a cross-sectional study. Thorax. 2009;64(6):523–31. doi: 10.1136/thx.2008.096560. [DOI] [PubMed] [Google Scholar]
- 23.Baughan P, O'Neill B, Fletcher E. Auditing the diagnosis of cancer in primary care: the experience in Scotland. Br J Cancer. 2009;101(Suppl. 2):S87–91. doi: 10.1038/sj.bjc.6605397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen RP, Vedsted P, Sokolowski I, Søndergaard J, Olesen F. Time intervals from first symptom to treatment of cancer: a cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv Res. 2011;11:284. doi: 10.1186/1472-6963-11-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin G, Elliott K, McPhail S. 2011. Royal College of General Practitioners. National audit of cancer diagnosis in primary care https://www.dur.ac.uk/resources/school.health/erdu/NationalAuditofCancerDiagnosisinPrimaryCare.pdf (last accessed January 2013)
- 26.Lyratzopoulos G, Abel GA, McPhail S, Neal RD, Rubin GP. Measures of promptness of cancer diagnosis in primary care: secondary analysis of national audit data on patients with 18 common and rarer cancers. Br J Cancer. 2013;108(7):1550–1. doi: 10.1038/bjc.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyratzopoulos G, Abel GA, McPhail S, Neal RD, Rubin GP. Gender inequalities in the promptness of diagnosis of bladder and renal cancer after symptomatic presentation: evidence from secondary analysis of an English primary care audit survey. BMJ Open. 2013;3(6):e002861. doi: 10.1136/bmjopen-2013-002861. ). pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koivunen P, Rantala N, Hyrynkangas K, Jokinen K, Alho OP. The impact of patient and professional diagnostic delays on survival in pharyngeal cancer. Cancer. 2001;92(11):2885–91. doi: 10.1002/1097-0142(20011201)92:11<2885::aid-cncr10119>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 29.Macdonald S, Macleod U, Campbell NC, Weller D, Mitchell E. Systematic review of factors influencing patient and practitioner delay in diagnosis of upper gastrointestinal cancer. Br J Cancer. 2006;94(9):1272–80. doi: 10.1038/sj.bjc.6603089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macleod U, Mitchell ED, Burgess C, Macdonald S, Ramirez AJ. Risk factors for delayed presentation and referral of symptomatic cancer: evidence for common cancers. Br J Cancer. 2009;101(Suppl. 2):S92–101. doi: 10.1038/sj.bjc.6605398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giroux Leprieur E, Labrune S, Giraud V, Gendry T, Cobarzan D, Chinet T. Delay between the initial symptoms, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer. 2012;13(5):363–8. doi: 10.1016/j.cllc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Brochez L, Verhaeghe E, Bleyen L, Naeyaert JM. Time delays and related factors in the diagnosis of cutaneous melanoma. Eur J Cancer. 2001;37(7):843–8. doi: 10.1016/s0959-8049(00)00418-4. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy EP, Ngo LH, Chirikos TN, Roetzheim RG, Li D, Drews RE, Iezzoni LI. Cancer stage at diagnosis and survival among persons with Social Security Disability Insurance on Medicare. Health Serv Res. 2007;42(2):611–28. doi: 10.1111/j.1475-6773.2006.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch BM, Youlden D, Fritschi L, Newman B, Pakenham KI, Leggett B, Owen N, Aitken JF. Self-reported information on the diagnosis of colorectal cancer was reliable but not necessarily valid. J Clin Epidemiol. 2008;61(5):498–504. doi: 10.1016/j.jclinepi.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Meechan D, Gildea C, Hollingworth L, Richards MA, Riley D, Rubin G. Variation in use of the 2-week referral pathway for suspected cancer: a cross-sectional analysis. Br J Gen Pract. 2012;62(602):e590–7. doi: 10.3399/bjgp12X654551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott S, Walter F. Studying help-seeking for symptoms: the challenges of methods and models. Social Personal Psychol Compass. 2010;4:531–47. [Google Scholar]
- 37.Rogers SN, Vedpathak SV, Lowe D. Reasons for delayed presentation in oral and oropharyngeal cancer: the patients perspective. Br J Oral Maxillofac Surg. 2011;49(5):349–53. doi: 10.1016/j.bjoms.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 38.Scott SE, Grunfeld EA, Main J, McGurk M. Patient delay in oral cancer: a qualitative study of patients' experiences. Psychooncology. 2006;15(6):474–85. doi: 10.1002/pon.976. [DOI] [PubMed] [Google Scholar]
- 39.Rafiq R, Brocklehurst P, Rogers SN. Effect of mouth cancer awareness week on urgent suspected head and neck cancer referrals. Br J Oral Maxillofac Surg. 2013;51(7):e183–5. doi: 10.1016/j.bjoms.2012.04.270. [DOI] [PubMed] [Google Scholar]
- 40.Stapley S, Peters TJ, Neal RD, Rose PW, Walter FM, Hamilton W. The risk of oesophago-gastric cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2013;108(1):25–31. doi: 10.1038/bjc.2012.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Austoker J, Bankhead C, Forbes LJ, Atkins L, Martin F, Robb K, Wardle J, Ramirez AJ. Interventions to promote cancer awareness and early presentation: systematic review. Br J Cancer. 2009;101(Suppl. 2):S31–9. doi: 10.1038/sj.bjc.6605388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


