Abstract
Cutaneous melanoma is a relatively common cancer in adolescents and young adults in Australia, but detailed information about occurrence patterns and prognosis is limited. We evaluated incidence trends from 1982 to 2010 and recent survival rates in those aged 15–24 years in the state of Queensland. In situ and invasive melanoma cases were identified from the Queensland Cancer Registry. Incidence rates were age-standardised to the 2000 World population and trends calculated using joinpoint regression. Five-year relative survival was estimated by the period method and Poisson models were used to produce adjusted mortality hazard ratios. Average annual incidence rates for the 5-year period 2006–2010 were 6.3 per 100,000 [95% confidence interval (CI) 5.4, 7.2] for in situ and 10.1 per 100,000 (95% CI 9.0, 11.3) for invasive melanoma. Since the mid-1990s, incidence rates for in situ melanomas have been stabilizing while invasive melanoma has decreased in both sexes, mainly owing to declining rates of thin tumours (≤1 mm) (−5.4% per year, 95% CI −8.3%, −2.4%). Incidence rates of melanomas >1 mm in thickness have remained relatively unchanged since 1991 however. In the period 2006–2010, relative 5-year survival of 15–24 year olds with invasive melanoma was 95.7% (95% CI 92.9%, 97.5%). The subgroup with tumours >1 mm was nearly six times more likely to die within 5 years than those with thin tumours (adjusted hazard ratio = 5.53, 95% CI 1.72, 17.80). Incidence of thin melanoma in young people in Queensland is declining, suggesting benefits of primary prevention efforts are being realised.
What's new?
Although some of the highest known incidence rates ofcutaneous melanoma are found in Queensland, Australia, few studies have examined incidence specifically among 15- to 24-year-olds in the state. This evaluation shows that the incidence of invasive melanoma is relatively high for the 15- to 24-year-old age group. However, incidence rates were found to have declined generally among Queensland's adolescents and young adults since the mid- to late 1990s. The decline may be a reflection of successful primary prevention efforts in young people in recent decades.
Keywords: melanoma, incidence trends, survival rates, adolescents, young adults
Melanoma is one of the more common cancers in adolescence and young adulthood with incidence rates comparable to soft tissue sarcomas and central nervous system tumours, respectively, in England for example.1 In Australia between 2006 and 2010, melanoma accounted for 14 and 23% of all cancers diagnosed (excluding basal cell and squamous cell carcinomas) in 15–19 and 20–24 year olds, respectively.2 Studies reporting the specific incidence and mortality rates at these ages in white populations have been relatively few however.3,4 Even fewer studies have been conducted of trends in incidence of melanoma in adolescents and young adults in recent decades despite a background of steadily increasing incidence of cutaneous melanoma in general in the last 50 years in fair-skinned populations.5 One longitudinal study among a broader age group of adolescent girls and young women in California showed melanoma rates increasing from the early 1990s.6 Another more recent study of melanoma trends in the USA from 1992 to 2006 in children, teenagers and young adults showed that melanoma incidence rose significantly among females up to 30 years.7 On the other hand, after a similar rapid rise in teenage melanoma rates in Sweden in the 1980s, the incidence rate fell by 26% in the 1990s.8
The state of Queensland, Australia, has the highest known incidence rates of cutaneous melanoma.9,10 This is a reflection of its predominantly white population living in a subtropical environment with high levels of annual ultraviolet radiation exposure.11,12 There is no detailed information regarding melanoma incidence trends or survival rates in the 15- to 24-year-old age group in Queensland however, despite the importance of monitoring possible changes in rates at young ages of this largely preventable cancer. Therefore, we aimed to evaluate the recent trends in incidence of cutaneous melanoma among people aged 15–24 years old in Queensland, and also to investigate melanoma survival rates.
Material and Methods
De-identified data were obtained from the Queensland Cancer Registry, which is required by law to register all cancers (excluding basal and squamous cell carcinomas of the skin) that are malignant, in situ or of uncertain behaviour. For all residents of Queensland aged 15–24 years diagnosed with a first primary invasive or in situ melanoma (ICD-O code C44, morphology codes 8720–8799) in the period 1982–2010, information was collected on sex, age at diagnosis and year of diagnosis. Measured thickness of invasive melanomas was also available from 1991 onwards. If both an invasive and an in situ melanoma were recorded for the same person during the study period, only the invasive case was included, thus retaining a single melanoma diagnosis in the analysis for each person. Cases of in situ lentigo maligna melanoma (morphology code 87422) were excluded because of concerns about consistency of identification of these lesions over time.13 Those who had a history of cancer other than melanoma were included in the current analyses. Where applicable, details on the cause and year of death up to December 31, 2010, were obtained from routine record linkage between the Queensland Cancer Registry and the Australian National Death Index. The data extract also included a variable that specified the number of days between the exact date of diagnosis and either exact date of death or the end date of the study (December 31, 2010), whichever came first. This information enabled us to accurately assess survival.
Statistical analysis
Average annual incidence rates by age group, sex, tumour type and thickness [classified as ≤1 mm (thin) or >1 mm14] were calculated for the most recent 5-year period (2006–2010). Data obtained from the Australian Bureau of Statistics for the corresponding estimated resident population, stratified by age group and sex,15 were used as denominators. Rates were directly age-standardised to the 2000 World Standard Population.16
Incidence trends by sex and 5-year age group between 1982 and 2010, and by tumour thickness between 1991 and 2010, were investigated by calculating standardised incidence rates in yearly increments and applying joinpoint regression models.17 Joinpoint regression initially assumes a constant trend (no joinpoints) across the entire study period and then systematically determines whether there is any evidence of a significant change to the direction or magnitude of the trend using Monte Carlo permutation tests.17 The selected model was the option with the fewest joinpoints that provided the best fit to the observed data. In our analysis we used a maximum of two joinpoints for each model with a minimum of 5 years between joinpoints or between a joinpoint and either end of the time series. These specifications reduced the potential for the model to detect spurious changes in trends. Trends were expressed in terms of the annual percentage change. Statistical significance (p < 0.05) was determined by a two-sided t-test.
Five-year relative survival was estimated using the period method,18 which considers the survival experience of patients within a recent calendar period. For the purposes of our study, persons who were diagnosed with an invasive melanoma between the ages of 15 and 24 contributed to the survival estimates if they were a prevalent case at some time from January 1, 2006 to December 31, 2010. Survival was censored for those who were still alive at the end date. The Ederer II actuarial method19 was used to calculate expected survival by comparing the observed survival probability against the underlying mortality rates from all causes within the Queensland population,20 stratified by age group, sex and year.
Differences in survival for persons with invasive melanoma were formally assessed by calculating mortality hazard ratios. These were obtained by applying a generalised linear model with a Poisson error structure to examine excess mortality up to 5 years after diagnosis.21 The model was adjusted for age group, sex and tumour thickness.
All results are presented with corresponding 95% confidence intervals (CIs). Ethics committee approval was not required as routinely collected de-identified data were used.
Results
Between 1982 and 2010 there were a total of 2,986 first primary invasive or in situ melanomas diagnosed in people aged 15–24 years in Queensland. Of these, 16 (0.5%) with in situ lentigo maligna melanoma were excluded, leaving a total of 2,970 cases for analysis.
In the most recent 5-year period, 2006–2010, there were 503 incident cutaneous melanomas (45% male, 55% female) in those aged 15–24 years (Table1). Invasive melanomas (n = 311, 62%) were more common than in situ (n = 192, 38%), with average annual incidence rates of 10.1 per 100,000 and 6.3 per 100,000, respectively. Incidence rates of both in situ and invasive melanoma were somewhat higher among females (though differences were not statistically significant) and were twofold to threefold higher in 20–24 year olds (termed “young adults”) compared with 15–19 year olds (termed “adolescents”). Young adult females had the highest rates of both invasive (16.5 per 100,000) and in situ melanomas (9.8 per 100,000) (Table1).
Table 1.
Average annual incidence rates1 of in situ and invasive melanomas among adolescents and young adults (15–24 years old at diagnosis) by sex in Queensland, 2006–2010
| Age at melanoma diagnosis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15–19 years |
20–24 years |
15–24 years |
|||||||
| Tumour type/Sex | N | ASRa | (95% CI) | N | ASRa | (95% CI) | N | ASRa | (95% CI) |
| In situ | |||||||||
| Total | 51 | 3.4 | (2.6–4.5) | 141 | 9.2 | (7.7–10.8) | 192 | 6.3 | (5.4–7.2) |
| Males | 22 | 2.9 | (1.8–4.4) | 67 | 8.6 | (6.6–10.9) | 89 | 5.7 | (4.6–7.0) |
| Females | 29 | 4.0 | (2.7–5.8) | 74 | 9.8 | (7.7–12.3) | 103 | 6.8 | (5.6–8.3) |
| Invasive | |||||||||
| Total | 89 | 6.0 | (4.8–7.4) | 222 | 14.4 | (12.6–16.4) | 311 | 10.1 | (9.0–11.3) |
| Males | 40 | 5.3 | (3.8–7.2) | 97 | 12.4 | (10.1–15.1) | 137 | 8.8 | (7.4–10.4) |
| Females | 49 | 6.8 | (5.0–9.0) | 125 | 16.5 | (13.7–19.6) | 174 | 11.5 | (9.9–13.4) |
Rates are per 100,000 population and are directly age-standardised to the 2000 World Standard Population.
Abbreviations: N: number; ASR: age-standardised rate; CI: confidence interval.
From 1982 until the mid- to late 1990s, incidence rates of in situ melanomas rose significantly in 15–24 year olds of both sexes (Fig. 1). Since then, rates have fluctuated in males and stabilised in females, with no significant trends observed. For invasive melanomas, incidence rates also peaked in the mid-1990s and then declined significantly. From 1994 onwards, incidence rates in males aged 15–24 have decreased by an average of 4.2% per year (95% CI −6.2%, −2.1%) and from 1997 in females aged 15–24 by an average of 4.9% per year (95% CI −7.6%, −2.2%). This significant downward trend in incidence rates of invasive melanoma was observed in both 15−19 and 20−24 year olds (Fig. 2).
Figure 1.
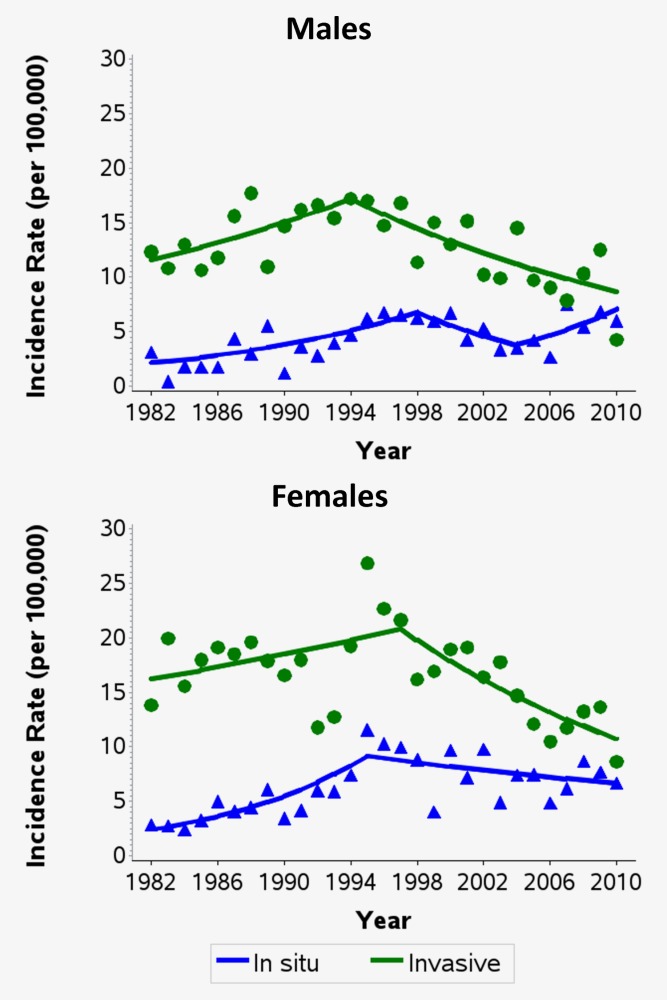
Trends in the age-standardised incidence rates of in situ and invasive melanomas among persons aged 15–24 by sex in Queensland, 1982–2010. Excludes in situ lentigo maligna melanoma. Rates were directly age-standardised to the 2000 World Standard Population. Trends calculated using joinpoint regression. Estimated annual percentage change (95% confidence intervals): Males, in situ: 1982–1998: +7.4% (+3.1%, +11.9%); 1998–2004: −9.2% (−24.9%, +9.7%); 2004–2010: +10.9% (−2.6%, +26.4%). Males, invasive: 1982–1994: +3.3% (+0.0%, +6.8%); 1994–2010: −4.2% (−6.2%, −2.1%). Females, in situ: 1982–1995: +10.8% (+5.9%, +15.9%); 1995–2010: −2.1% (−4.3%, +0.2%). Females, invasive: 1982–1997: +1.7% (−0.5%, +3.9%); 1997–2010: −4.9% (−7.6%, −2.2%). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 2.
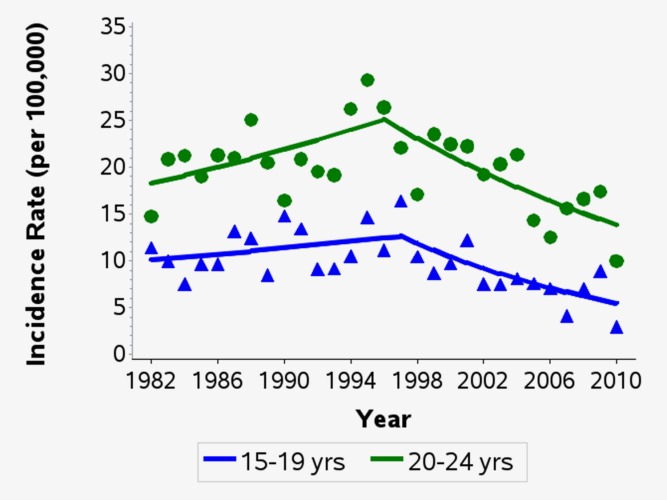
Age-specific trends in the incidence rates of invasive melanomas among persons aged 15–24 in Queensland, 1982–2010. Rates were directly age-standardised to the 2000 World Standard Population. Trends calculated using joinpoint regression. Estimated annual percentage change (95% confidence intervals): Age 15–19: 1982–1997: +1.5% (−1.2%, +4.3%); 1997–2010: −6.2% (−9.6%, −2.6%). Age 20–24: 1982–1996: +2.3% (+0.1%, +4.5%); 1996–2010: −4.1% (−6.3%, −2.0%). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For cases of invasive melanoma between 2006 and 2010 with tumour thickness available (n = 294, 95%), 82% (n = 240) were thin tumours (≤1 mm) (Table2). The distribution was similar within the 15–19 (80% thin tumours) and 20–24 year olds (82% thin tumours). Trends by thickness showed a significant decrease in the incidence of thin invasive melanomas by an average of 5.4% per year (95% CI −8.3%, −2.4%) since 1997. Since 1991, incidence of melanomas >1 mm has remained relatively unchanged (a nonsignificant decrease of 1.0% per year) and incidence of invasive melanomas with unknown thickness has declined significantly (Fig. 3).
Table 2.
Average annual incidence rates1 of invasive melanomas among adolescents and young adults (15–24 years old at diagnosis) by sex and tumour thickness2 in Queensland, 2006–2010
| Age at melanoma diagnosis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15–19 years |
20–24 years |
15–24 years |
|||||||
| Tumour thickness/Sex | N | ASR1 | (95% CI) | N | ASR1 | (95% CI) | N | ASR1 | (95% CI) |
| ≤1 mm | |||||||||
| Total | 68 | 4.6 | (3.6–5.8) | 172 | 11.2 | (9.6–13.0) | 240 | 7.8 | (6.9–8.9) |
| Males | 31 | 4.1 | (2.8–5.8) | 73 | 9.3 | (7.3–11.8) | 104 | 6.7 | (5.5–8.1) |
| Females | 37 | 5.1 | (3.6–7.1) | 99 | 13.1 | (10.6–15.9) | 136 | 9.0 | (7.6–10.7) |
| >1 mm | |||||||||
| Total | 17 | 1.1 | (0.7–1.8) | 37 | 2.4 | (1.7–3.3) | 54 | 1.8 | (1.3–2.3) |
| Males | 7 | 0.9 | (0.4–1.9) | 18 | 2.3 | (1.4–3.6) | 25 | 1.6 | (1.0–2.4) |
| Females | 10 | 1.4 | (0.7–2.5) | 19 | 2.5 | (1.5–3.9) | 29 | 1.9 | (1.3–2.8) |
Rates are per 100,000 population and are directly age-standardised to the 2000 World Standard Population
Information on thickness was missing for a total of 17 invasive melanoma cases (5.5%) in the 15–24 age group between 2006 and 2010.
Abbreviations: N: number; ASR: age-standardised rate; CI: confidence interval.
Figure 3.
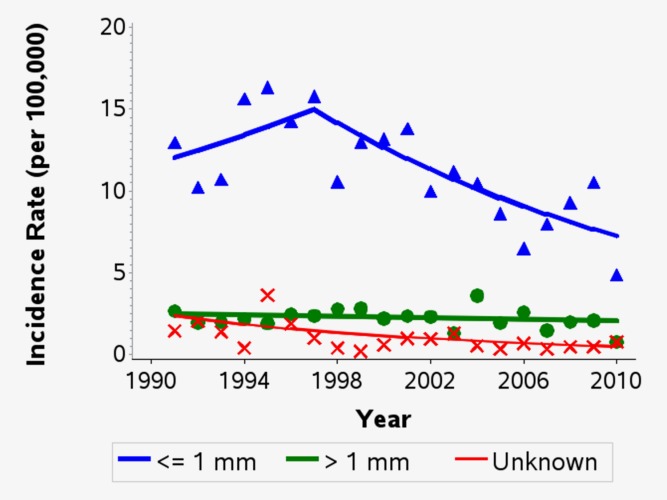
Trends in the age-standardised incidence rates of invasive melanomas by thickness among persons aged 15–24 in Queensland, 1991–2010. Rates were directly age-standardised to the 2000 World Standard Population. Trends calculated using joinpoint regression. Estimated annual percentage change (95% confidence intervals): ≤1 mm thick: 1991–1997: +3.7% (−5.1%, +13.4%); 1997–2010: −5.4% (−8.3%, −2.4%). >1 mm thick: 1991–2010: −1.0% (−3.3%, +1.4%). Unknown thickness: 1991–2010: −7.7% (−11.8%, −3.4%). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Regarding melanoma mortality in these young age groups in Queensland, the 5-year relative survival between 2006 and 2010 was 95.7% (95% CI 92.9%, 97.5%). There was no significant variation in the survival rate by sex or age at diagnosis. However, young persons with tumours >1 mm were significantly more likely to die from their melanoma within 5 years of diagnosis (adjusted hazard ratio = 5.53; 95% CI 1.72, 17.80) than those with thin tumours (Table3).
Table 3.
Five-year relative survival and adjusted hazard ratios for invasive melanomas among adolescents and young adults (15–24 years old at diagnosis) by sex, age group and tumour thickness at diagnosis in Queensland, 2006–2010
| Five-year relative survival2 |
Adjusted hazard ratio |
|||||
|---|---|---|---|---|---|---|
| Characteristic | N1 | % | (95% CI) | HR3 | (95% CI) | p-Value |
| Total | 674 | 95.7 | (92.9–97.5) | |||
| Sex | ||||||
| Females | 381 | 96.9 | (93.0–98.6) | 1.004 | 0.3075 | |
| Males | 293 | 94.2 | (89.0–97.1) | 1.76 | (0.59–5.23) | 0.307 |
| Age group | ||||||
| 15–19 | 201 | 97.2 | (91.2–99.2) | 1.004 | 0.4095 | |
| 20–24 | 473 | 95.1 | (91.4–97.3) | 1.73 | (0.47–6.37) | 0.409 |
| Tumour thickness at diagnosis3 | ||||||
| ≤1 mm | 528 | 97.9 | (95.1–99.2) | 1.004 | 0.0105 | |
| >1 mm | 111 | 89.0 | (78.2–94.7) | 5.53 | (1.72–17.80) | 0.004 |
| Unknown | 35 | 87.3 | (57.6–96.8) | 5.96 | (1.11–31.90) | 0.037 |
The number of persons who were eligible to contribute to the survival calculations
Survival calculated using the period method for persons who were at risk of mortality due to invasive melanoma between January 1, 2006 and December 31, 2010
Hazard ratios are for mortality within 5 years of diagnosis and are adjusted for all the other characteristics in the table
Reference group
p-Value corresponding to the overall effect for that variable.
Abbreviations: N: number; CI: confidence interval; HR: hazard ratio.
Discussion
From 1994 to 2010 in males and from 1997 to 2010 in females, the incidence rates of melanoma in young people aged between 15 and 24 years in Queensland have been in substantial decline by an average 4–5% per year. Most of this decrease has occurred in thin invasive melanomas, with no significant change in incidence rates for melanomas measuring >1 mm in thickness over the study period. The incidence of in situ melanoma was somewhat lower than the incidence of invasive melanoma in Queensland youth and has shown no particular trend for the last 15 years or so to 2010. With regard to survival, Queensland melanoma patients in this age group generally had a good outcome with a 5-year survival rate of around 96% between 2006 and 2010.
In general, incidence rates of invasive melanoma between the ages of 15 and 24 years in Queensland are some three to five times higher than the corresponding rates in the USA or Europe. A US study showed that from 1999 to 2006 the average annual incidence rates of invasive melanoma in males aged 15–19 years were 1.4 per 100,000 and 2.2 per 100,000 in females.4 In those aged 20–24 years the corresponding rates were 2.8 and 6.4 per 100,000 in males and females, respectively.4 Average incidence rates in the Netherlands from 1989 to 2009 appeared similar to the USA in those aged 15−19 years old (1.6 per 100,000 for males and 3.0 per 100,000 for females) and in the 20−24 age group, at 3.9 (males) and 8.7 (females) per 100,000.3 Similar incidence rates of melanoma in these young age groups are currently seen in other European populations, as shown by the latest available rates in 2011 for England [15−19 years: 1.2 (males) and 1.5 (females) per 100,000; 20−24 years: 2.2 (males) and 4.8 (females) per 100,000]22; Finland [15−19 years: 1.2 (males) and 3.7 (females) per 100,000; 20−24 years: 1.2 (males) and 5.0 (females) per 100,000]23,24 and Sweden [15−19 years: 2.6 (males) and 2.0 (females) per 100,000; 20−24 years: 3.0 (males) and 5.1 (females) per 100,000].23,24
With regard to survival, Queensland melanoma patients in this young age group had virtually the same survival rate at around 96% as all Australians aged 0–19, 20–29 and 30–39 years from 1998 to 200425 and young adults aged 20–24 years in the USA,26 and better survival at 5 years than seen in Australians aged 60–69 (91%).25 As seen across all ages27,28 those diagnosed with lesions >1 mm were at higher risk of death of those with thin melanoma. The generally favourable outcome in Queensland is likely due in part to early detection resulting from increased skin cancer awareness.29
Ultraviolet (UV) radiation is the most important environmental risk factor for cutaneous melanoma.11 The higher rates of melanoma in Queensland than elsewhere, among not only youth but also overall, are consistent with the high ambient solar levels year-round in Queensland12 interacting with the susceptible, predominantly white population. Melanoma and other skin cancers are a major public health problem in this state13 and coordinated efforts to control skin cancer have been in place since the early 1980s.29 Sun-protection programs have aimed to raise public awareness of the harmful effects of UV radiation exposure and to promote sun-safe behaviours, with advice to limit time outdoors, to seek shade, wear-protective clothing and apply sunscreen when exposed to the sun for extended periods of time.30 Parts of these programs have focussed on children's sun protection and in particular, policies in child-care centres and schools have been introduced to strengthen and reinforce sun-protective behaviours while outdoors.29 The observed decline in thin invasive melanoma in those aged 15–24 years is likely due in large part to their being exposed to primary prevention programs from birth. Cross-sectional surveys conducted in the Queensland population in the late 1980s and early 1990s showed substantial improvements in attitudes towards sun protection and in corresponding behaviours across the population including among teenagers.31 Specifically, those aged 14–19 years in 1991–1992 showed significantly reduced desire for a suntan and significant improvements in hat wearing, use of sunscreen, time spent outdoors on the weekend and overall skin protection compared to those surveyed in 1988–1989.31 Corresponding declines in melanoma incidence in children in Queensland have been similarly attributed to sun awareness campaigns,32 given that many interventions targeting parents and teachers, as well as children themselves, have been shown to be effective in increasing children's sun protection.33–36
The declines in melanoma incidence rates seen in Queensland youth have also been observed in Sweden8 where public health campaigns aimed at reducing sun exposure began slightly later (in the mid-1980s). Such declines have been ascribed by some to the effects of increasing numbers of young people at low risk of melanoma in the general population due to increased migration.8,32 While this dilution of baseline risk may have contributed to some degree, it is unlikely to explain the substantial decrease observed in Queensland at least; in countries such as England, where some regional populations have high proportions who are dark-skinned, inclusion of low-risk ethnic groups in the denominator has been found to not distort regional incidence rates to any great degree.37
The results in relation to tumour thickness over time were restricted to recent years in order to minimise biases introduced by improvements in recording, but nonetheless they serve to underscore that there remains a small subgroup of young patients who are being diagnosed with melanomas measuring >1 mm in thickness at much the same rate as they were 20 years ago. For some reason, this group has not benefited from the prevention or early detection programs in place for more than four decades in Queensland. Possible explanations are that their disease developed along a different aetiological pathway whereby reduction in sun exposure was not sufficient to reduce incidence and that their melanomas were rapidly growing and therefore less likely to be detected when thin.
One of the major strengths of this study is that the observed rates of incident melanoma in young people have high precision, being based on a relatively large study cohort because of the high-incidence source population. Moreover, melanoma registration in Queensland is believed to be virtually complete.38 On the other hand, data regarding mortality were more limited in quantity because of the high survival rates. We also lacked information on clinical stage at diagnosis, which is not collected by the Queensland Cancer Registry, and for the same reason we were unable to account for skin cancer risk factors such as skin type and sun exposure behaviour. Although data on body site and histological subtype were available, numbers of cases after stratification by site or subtype of melanoma were too small for meaningful analysis of their possible influence on observed trends.
In conclusion, our study shows that the incidence rates of invasive melanoma are relatively high in adolescents and young adults in Queensland compared with other white populations as expected due to the state's sunny climate. However, the risk of death due to melanoma in this age group is generally low except among a small proportion of cases whose melanomas are already >1 mm thick when diagnosed. We have also shown that the incidence of thin invasive melanoma has declined since the mid- to late 1990s among young people who have been exposed to Queensland's primary prevention and early detection programs since birth.
Acknowledgments
The authors thank the staff of the Queensland Cancer Registry who provided the data extract used in this study. Michelle R. Iannacone was supported by the National Health and Medical Research Council of Australia (NHMRC) Program Grant (No. 552429). Peter D. Baade was supported by an NHMRC Career Development Fellowship (ID1005334).
References
- 1.Alston RD, Geraci M, Eden TO, et al. Changes in cancer incidence in teenagers and young adults (ages 13 to 24 years) in England 1979-2003. Cancer. 2008;113:2807–15. doi: 10.1002/cncr.23901. [DOI] [PubMed] [Google Scholar]
- 2. .Australian Institute of Health and Welfare. Australian Cancer Incidence and Mortality (ACIM) books. Available at: http://www.aihw.gov.au/acim-books/ (Accessed 8 April 2014)
- 3.Aben KK, van Gaal C, van Gils NA, et al. Cancer in adolescents and young adults (15–29 years): a population-based study in the Netherlands 1989-2009. Acta Oncol. 2012;51:922–33. doi: 10.3109/0284186X.2012.705891. [DOI] [PubMed] [Google Scholar]
- 4.Weir HK, Marrett LD, Cokkinides V, et al. Melanoma in adolescents and young adults (ages 15-39 years): United States, 1999–2006. J Am Acad Dermatol. 2011;65:S38–S49. doi: 10.1016/j.jaad.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erdmann F, Lortet-Tieulent J, Schuz J, et al. International trends in the incidence of malignant melanoma 1953–2008—are recent generations at higher or lower risk? Int J Cancer. 2013;132:385–400. doi: 10.1002/ijc.27616. [DOI] [PubMed] [Google Scholar]
- 6.Hausauer AK, Swetter SM, Cockburn MG, et al. Increases in melanoma among adolescent girls and young women in California: trends by socioeconomic status and UV radiation exposure. Arch Dermatol. 2011;147:783–9. doi: 10.1001/archdermatol.2011.44. [DOI] [PubMed] [Google Scholar]
- 7.Senerchia AA, Ribeiro KB, Rodriguez-Galindo C. Trends in incidence of primary cutaneous malignancies in children, adolescents, and young adults: a population-based study. Pediatr Blood Cancer. 2014;61:211–16. doi: 10.1002/pbc.24639. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson PM, Fredrikson M. Cutaneous malignant melanoma in children and adolescents in Sweden, 1993–2002: the increasing trend is broken. Int J Cancer. 2007;121:323–8. doi: 10.1002/ijc.22692. [DOI] [PubMed] [Google Scholar]
- 9.Australian Institute of Health & Welfare. Cancer in Australia, 2001. AIHW Cat No CAN18. Canberra: AIHW, 2004.
- 10.Parkin DM, Ferlay J, Curado MP, et al. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer. 2010;127:2918–27. doi: 10.1002/ijc.25517. [DOI] [PubMed] [Google Scholar]
- 11.El Ghissassi F, Baan R, Straif K, et al. A review of human carcinogens—part D: radiation. Lancet Oncol. 2009;10:751–2. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- 12.Neale RE, Hamilton AR, Janda M, et al. Seasonal variation in measured solar ultraviolet radiation exposure of adults in subtropical Australia. Photochem Photobiol. 2010;86:445–8. doi: 10.1111/j.1751-1097.2009.00686.x. [DOI] [PubMed] [Google Scholar]
- 13.Coory M, Baade P, Aitken J, et al. Trends for in situ and invasive melanoma in Queensland, Australia, 1982–2002. Cancer Causes Control. 2006;17:21–7. doi: 10.1007/s10552-005-3637-4. [DOI] [PubMed] [Google Scholar]
- 14.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Australian Bureau of Statistics. Australia: ABS; 2012. Australian Demographic Statistics, Dec 2011: 2011 census edition—preliminary. [Google Scholar]
- 16.Ahmad OB, Boschi-Pinto C, Lopez AD, et al. Age standardization of rates: a new WHO standard. GPE Discussion Paper Series No 31. Geneva: World Health Organization; 2001. [Google Scholar]
- 17.Kim H, Fay M, Feuer E, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Brenner H, Gefeller O, Hakulinen T. Period analysis for 'up-to-date' cancer survival data: theory, empirical evaluation, computation realisation and applications. Eur J Cancer. 2004;40:326–35. doi: 10.1016/j.ejca.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. NCI Monogr. 1961;6:101–21. [PubMed] [Google Scholar]
- 20.Australian Bureau of Statistics. Australia: ABS; 2012. Deaths, Australia, 2011. [Google Scholar]
- 21.Dickman PW, Sloggett A, Hills M, et al. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 22. ONS (2013) Cancer Registration Statistics, England, 2011. Contains public sector information licensed under the Open Government Licence v2.0. Available at: http://www.ons.gov.uk/ons/datasets-and-tables/index.html?pageSize=50&sortBy=none&sortDirection=none&newquery=melanoma&content-type=Reference+table&content-type=Dataset (Accessed 8 April 2014)
- 23.Engholm G, Ferlay J, Christensen N, et al. NORDCAN: cancer incidence, mortality, prevalence and survival in the Nordic Countries, version 6.0 (04.12.2013). Association of the Nordic Cancer Registries, Danish Cancer Society. Available at: http://www.ancr.nu (Accessed 8 April 2014)
- 24.Engholm G, Ferlay J, Christensen N, et al. NORDCAN—a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49:725–36. doi: 10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
- 25.Australian Institute of Health and Welfare. 2008. , Cancer Australia & Australasian Association of CancerRegistries. Cancer survival and prevalence in Australia: cancers diagnosed from 1982 to 2004.Cancer Series no. 42. Cat. no. CAN 38. Canberra: AIHW,
- 26. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gove). Cancer Statistics Review 1975–2010. 5-Year relative survival for the top 5 cancer sites by age, all races, both sexes. Available at: http://seer.cancer.gov/csr/1975_2010/browse_csr.php?sectionSEL=32&pageSEL=sect_32_table.20.html (Accessed 8 April 2014)
- 27.Luke CG, Coventry BJ, Foster-Smith EJ, et al. A critical analysis of reasons for improved survival from invasive cutaneous melanoma. Cancer Causes Control. 2003;14:871–8. doi: 10.1023/b:caco.0000003838.48507.8b. [DOI] [PubMed] [Google Scholar]
- 28.Marashi-Pour S, Morrell S, Cooke-Yarborough C, et al. Competing risk analysis of mortality from invasive cutaneous melanoma in New South Wales: a population-based study, 1988–2007. Aust New Zealand J Public Health. 2012;36:441–5. doi: 10.1111/j.1753-6405.2012.00912.x. [DOI] [PubMed] [Google Scholar]
- 29.Iannacone MR, Green AC. Melanoma Manage. Towards skin cancer prevention and early detection: evolution of skin cancer awareness campaigns in Australia. , in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marks R. Skin cancer control in the 1990's, from slip! Slop! Slap! To sun smart. Australas J Dermatol. 1990;31:1–4. doi: 10.1111/j.1440-0960.1990.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 31.Baade PD, Balanda KP, Lowe JB. Changes in skin protection behaviors, attitudes, and sunburn: in a population with the highest incidence of skin cancer in the world. Cancer Detect Prev. 1996;20:566–75. [PubMed] [Google Scholar]
- 32.Baade PD, Green AC, Smithers BM, et al. Trends in melanoma incidence among children: possible influence of sun-protection programs. Expert Rev Anticancer Ther. 2011;11:661–4. doi: 10.1586/era.11.28. [DOI] [PubMed] [Google Scholar]
- 33.Aulbert W, Parpart C, Schulz-Hornbostel R, et al. Certification of sun protection practices in a German child day-care centre improves children's sun protection—the 'SunPass' pilot study. Br J Dermatol. 2009;161 (Suppl 3):5–12. doi: 10.1111/j.1365-2133.2009.09443.x. [DOI] [PubMed] [Google Scholar]
- 34.Berneburg M, Surber C. Children and sun protection. Br J Dermatol. 2009;161 (Suppl 3):33–9. doi: 10.1111/j.1365-2133.2009.09447.x. [DOI] [PubMed] [Google Scholar]
- 35.Hunter S, Love-Jackson K, Abdulla R, et al. Sun protection at elementary schools: a cluster randomized trial. J Natl Cancer Inst. 2010;102:484–92. doi: 10.1093/jnci/djq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones SB, Beckmann K, Rayner J. Australian primary schools' sun protection policy and practice: evaluating the impact of the National SunSmart Schools Program. Health Promot J Austr. 2008;19:86–90. doi: 10.1071/he08086. [DOI] [PubMed] [Google Scholar]
- 37.Wallingford SC, Alston RD, Birch JM, et al. Regional melanoma incidence in England, 1996–2006: reversal of north-south latitude trends among young females. Br J Dermatol. 2013;169:880–8. doi: 10.1111/bjd.12460. [DOI] [PubMed] [Google Scholar]
- 38.Queensland Caner Registry, Cancer Council Queensland. Brisbane: CCQ; 2013. , Queensland Health. Cancer in Queensland, 1982–2011. [Google Scholar]


