Abstract
Prion protein (PrPc) has been previously reported to be involved in gastric cancer (GC) development and progression. However, the association between expression of PrPc and GC prognosis is yet poorly characterized. In the present study, the expressions of PrPc and MGr1-Ag/37LRP, a protein interacting with PrPc, were detected using the tissue microarray technique and immunohistochemical method to compare clinicopathological parameters of 238 GC patients. We found that the expressions of PrPc and MGr1-Ag/37LRP were upregulated in GC lesions compared with their expressions in adjacent noncancerous tissues (p < 0.01). High expression of PrPc was detected in 37.39% (89/238) of GC patients and positively correlated with the expression of MGr1-Ag/37LRP (r = 0.532, p < 0.001). PrPc expression was associated with a number of clinicopathological parameters including depth of invasion and lymph node metastasis of the tumor (p < 0.001). High expression of PrPc brought a poorer prognosis than low PrPc expression. Moreover, GC patients with high level of PrPc and high level of MGr1-Ag/37LRP had the poorest prognosis. Multivariate survival analysis suggested that, along with other parameters, combined expression of PrPc and MGr1-Ag/37LRP was independent prognostic factors for GC patients. These data indicates that overexpression of PrPc, combined with MGr1-Ag/37LRP, is predictive of poor prognosis in GC and thereby could be used to guide the clinical decision.
What's new?
Prion protein was originally exclusively associated with prion disease but has now been linked to other processes such as cancer and inflammation. Here the authors examined the role of prion protein and its receptor MGr1-Ag/37LRP in gastric cancer. They found that both factors were upregulated in gastric cancer, and not in neighboring healthy, tissues and established the combined expression of prion protein and MGr1-Ag/37LRP as an independent prognostic factor for gastric cancer patients. These studies support a new role of prion protein in cancer and identify a new biomarker for a more accurate prediction of prognosis in gastric cancer patients.
Keywords: PrPc, MGr1-Ag/37LRP, gastric cancer, prognosis
Cellular prion protein (PrPc) is a highly conserved glycoprotein present in all vertebrates and has the same protein sequence as the scrapie prion protein (PrPsc), a pathogenic factor in scrapie in sheep, bovine spongiform encephalopathy in cattle and kuru in human beings.1,2 In addition to the central involvement of PrPc in prion diseases, several lines of evidence has intriguingly implicated PrPc in human tumors including glioblastoma,3 breast,4,5 prostate6 and colorectal7,8 cancers. PrPc is also found to be related to prognosis in colorectal cancer8 and could be an great predictor in the chemotherapy of breast cancer.9 Our previous work demonstrated that PrPc was overexpressed in gastric cancer (GC), correlated with histopathological differentiation parameters and that forced expression of PrPc could inhibit apoptosis and promote proliferation, metastasis and multidrug resistance (MDR) in GC cells.10–13 All of these evidences indicate that PrPc plays a key role in GC carcinogenesis and progression. However, the prognostic value of PrPc hasn't been evaluated in GC.
MGr1-Ag/37LRP was previously reported by our lab as an upregulated protein in GC drug-resistant cell SGC7901/VCR and identified as the 37-kDa laminin receptor precursor (37LRP).14 Forced expression of MGr1-Ag/37LRP in GC cells could increase drug resistance toward a variety of chemotherapy drugs.15–17 Previous studies demonstrated that 37LRP was involved in tumor development and progression.18–20 Moreover, it has been shown that 37LRP acts as a receptor for PrPsc, as well as a receptor or coreceptor for PrPc in prion disease.21,22 PrPc is co-localized with 37LRP on the surface of mammalian cells.22 37LRP is essential for the propagation and accumulation of PrPsc in scrapieinfected cells and its expression is related to the degree of PrPsc propagation.23,24 These results led us to speculate that MGr1-Ag/37LRP may influence the expression of PrPc in GC by interacting with each other, which might better predict the prognosis.
Therefore, it is reasonable to address the hypothesis that PrPc and MGr1-Ag/37LRP act together to influence GC biology and that effect of PrPc expression on outcome will be modulated by expression of MGr1-Ag/37LRP. In this study, we assessed the expressions of PrPc and MGr1-Ag/37LRP in human GC tissues and their correlation with clinicopathologic features and patient survival. We also examined whether the combined expressions of PrPc and MGr1-Ag/37LRP could serve as predictive markers for patient prognosis.
Material and Methods
Patients and tissue samples
After being approved by the ethics committee of the Fourth Military Medical University and written informed consent for use of the resected samples, 238 patients who underwent GC surgery at the Xijing Hospital of Fourth Military Medical University (Xi'an, China) between January 2003 and December 2005 were included in this retrospective study. None of the patients had received preoperative chemotherapy or radiotherapy. Tissue specimens from 72 patients without malignancy undergoing endoscopic biopsy of normal-appearing gastric mucosa served as control. Histomorphology of all the primary tumors specimens were confirmed with hematoxylin-eosin (H&E) staining by Department of Pathology, Xijing Hospital. Patients' clinical information, such as sex, age, differentiation status, tumor location and TNM stage was collected and stored in a database. Follow-up information of all participants was updated every 3 month by telephone visit and questionnaire letters. Complete follow-up was updated until death or January 2013, whichever came first. Death of participants was ascertained by reporting from the family and verified by review of public records. Finally, the median follow-up period of the enrolled patients was 25 months. All tissues were formalin-fixed and paraffin-embedded samples.
Construction of tissue microarray (TMA)
TMA blocks containing the GCs and the non-cancerous gastric mucosae were prepared using the method described previously.25 Briefly, tissue cylinders with a diameter of 2 mm were punched out from the targeted area of each donor tissue block and transferred into a recipient block using a TMA instrument; each TMA block contained 9 to 10 noncancerous gastric mucosae as controls. Consecutive 4-μm thick sections were then cut from each of the TMA blocks and 1 section from each block was H&E stained for histological verification of the adequacy of the arrayed tumor tissues. Qualified samples were defined as those in which the tumor tissue occupied >10% of the core area. Sections were then placed on microscope slides for immunohistochemistry.
Immunohistochemistry
Immunohistochemistry was performed by avidin-biotinperoxidase method on all the TMA slides. All sections were deparaffinized in xylene and dehydrated through a graduated alcohol series before endogenous peroxidase activity was blocked with 0.5% H2O2 in methanol for 10 min. The antigen retrieval was done by microwaving sections in 0.01 M sodium citrate, pH 6.0. Nonspecific binding was blocked by incubating sections with 10% normal goat serum in PBS for 1 h at room temperature. Without washing, sections were incubated with anti-PrPc (1:200, Sigma, Swampscott, MA) or MGr1 antibody (1:50, developed in our laboratory)in PBS at 4°C overnight in a moist box. Negative controls were performed by replacing the primary antibody with preimmune rabbit serum. Biotinylated anti-IgG (1:400, Sigma) was incubated with the sections for 1 h at room temperature and detected with a streptavidin-peroxidase complex. The brown color indicative of peroxidase activity was obtained by incubating with 0.1% 3, 3-diaminobenzidine (Sigma) in PBS with 0.05% H2O2 for 5 min at room temperature.
Evaluation of immunostaining intensity
Multiple tissue arrays were scored independently by two pathologists blinded to the clinicopathologic characteristics and outcome of the patients. The quality of immunohistochemical staining (intensity and appropriate cellular localization of reaction) was also assessed and compared with verify the quality and representativeness of the TMAs. The staining was evaluated by scanning the entire tissue specimen under low magnification (×40) and then confirmed under high magnification (×200 and ×400). The protein expression was visualized and classified based on the percentage of positive cells and the intensity of staining. The percentage of positive cells was divided into five grades (percentage cores): <1% (0), 1–25% (1), 26–50% (2), 51–75% (3) and >75% (4). The intensity of staining was divided into four grades (intensity scores): negative (0), weak (1), moderate (2) and strong (3). The histological score (H-score) was determined using the following formula: overall scores = percentage score × intensity score. An overall score of 0–12 was calculated and graded as low (score: 0–4) or high (score: 5–12). All stained slides were interpreted by another pathologist unaware of other data. Specimens will be rescored if difference of scores from two pathologists was >3. The concordance of PrPc scoring between the two observers was 0.92 (p < 0.001) and that of MGr1-Ag/37LRP was 0.94 (p < 0.001), indicating substantial agreement.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 for Windows (SPSS, Chicago, IL). To assess the relationships between the expression of PrPc and MGr1-Ag/37LRP and the clinicopathological parameters of the patients with GC, the χ2 test or Fisher exact test was used. Spearman rank correlation test was employed to evaluate the relationship between PrPc and MGr1-Ag/37LRP expression in GC. The cumulative survival rate was calculated using a life table method. Univariate survival analysis was performed using the Kaplan-Meier method and the significance of difference between groups was analyzed with the log-rank test. Stepwise multivariate survival analysis was carried out using the Cox proportional hazards model. Variables that were significant in the univariate analysis were included in the model with the Backward Wald method. p < 0.05 was considered statistically significant and all p values were 2-sided.
Results
Expression of PrPc and MGr1-Ag/37LRP in GC and noncancerous gastric mucosa
To elucidate the biologic significance of PrPc in GC, we examined the immunohistochemical expression of PrPc in clinical GC tissue samples. High expression of PrPc protein was detected in 89 (37.39%) of the 238 patients with GC and 6(8.33%) in the 72 noncancerous gastric mucosae. The immunostaining for PrPc was mainly located in the membranes and cytoplasms of the tumor cells (Supporting Information Fig. S1). The PrPc expression in GC was significantly higher than that in the noncancerous gastric mucosae (p < 0.001). Similarly, high expression of MGr1-Ag/37LRP protein was detected in 76 (31.93%) of the 238 patients with GC and 11(15.28%) in the 72 noncancerous gastric mucosae. The immunostaining for MGr1-Ag/37LRP was dominantly distributed on the membranes and cytoplasms of the tumor cells (Supporting Information Fig. S2). The MGr1-Ag/37LRP expression in GC was significantly higher than that in non-cancerous gastric mucosae (p < 0.01).
Correlation between expression of PrPc and MGr1-Ag/37LRP in GC
A high coincidental expression of the PrPc and MGr1-Ag/37LRP proteins was observed in GC (Table1). Of the 149 patients with low expression of PrPc, 130 (87.25%) also had a low expression of MGr1-Ag/37LRP. Meanwhile, of the 89 patients with high expression of PrPc, 57 (64.04%) also had a high expression of MGr1-Ag/37LRP. The correlation between the expression of PrPc and MGr1-Ag/37LRP in patients with GC was statistically significant (r = 0.532, p < 0.001).
Table 1.
Correlation between the expression of PrPc and MGr1-Ag/37LRP in GC
| PrPc |
Spearman's correlation |
||||
|---|---|---|---|---|---|
| MGr1-Ag/37LRP | Low | High | Total | r | p |
| Low | 130 | 32 | 162 | ||
| High | 19 | 57 | 76 | 0.532 | <0.001 |
| Total | 149 | 89 | 238 | ||
Association of PrPc and MGr1-Ag/37LRP expression with clinicopathological parameters
To further characterize the roles of PrPc and MGr1-Ag/37LRP in GC carcinogenesis, we analyzed the relationships between their expressions and the clinicopathological parameters of the GC patients. The expression of PrPc in GC was significantly related to age, depth of invasion, TNM stage, differentiation, lymph node metastasis and distant metastasis of tumor, while not related to gender, location and tumor size. GC patients with deep tumor invasion (T3 and T4), high TNM stage (stages III and IV), lymph node metastasis and distant metastasis had significantly higher expression of PrPc than those with superficial tumor invasion (T1 and T2), low TNM stage (stages I and II) and no lymph node and distant metastasis (Table2). Similarly, the expression of MGr1-Ag/37LRP in GC was significantly related to age, depth of invasion, TNM stage, differentiation, lymph node metastasis and distant metastasis of tumor and not related to gender, location and tumor size. Patients with deep tumor invasion (T3 and T4), high TNM stage (stages III and IV), lymph node and distant metastasis had significantly higher expression of MGr1-Ag/37LRP than those with superficial tumor invasion (T1 and T2), low TNM stage (stages I and II) and no lymph node and distant metastasis (Table2).
Table 2.
Association of PrPc and MGr1-Ag/37LRP expression with clinicopathological parameters of patients with GC
| Expression of PrPc (n) |
Expression of MGr1-Ag/37LRP(n) |
||||||
|---|---|---|---|---|---|---|---|
| Category | n | Low | High | p | Low | High | p |
| Gender | 0.683 | 0.522 | |||||
| Male | 151 | 96 | 55 | 105 | 46 | ||
| Female | 87 | 53 | 34 | 57 | 30 | ||
| Age | <0.01 | <0.05 | |||||
| <60 | 131 | 93 | 38 | 97 | 34 | ||
| ≥60 | 107 | 56 | 51 | 65 | 42 | ||
| Location of tumor | 0.201 | 0.546 | |||||
| Cardia | 13 | 9 | 4 | 9 | 4 | ||
| Body | 77 | 42 | 35 | 56 | 21 | ||
| Antrum | 148 | 98 | 50 | 97 | 51 | ||
| Tumor size (cm) | 0.176 | 0.501 | |||||
| <5 | 139 | 92 | 47 | 97 | 42 | ||
| ≥5 | 99 | 57 | 42 | 65 | 34 | ||
| Depth of invasion | <0.001 | <0.01 | |||||
| T1–T2 | 19 | 36 | 4 | 35 | 5 | ||
| T3–T4 | 198 | 113 | 85 | 127 | 71 | ||
| Stages | <0.001 | <0.01 | |||||
| I–II | 76 | 60 | 16 | 62 | 14 | ||
| III–IV | 162 | 89 | 73 | 100 | 62 | ||
| Differentiation | <0.05 | <0.05 | |||||
| Well and moderate | 121 | 85 | 36 | 91 | 30 | ||
| Poorly and not | 117 | 64 | 53 | 71 | 46 | ||
| Lymph node metastases | <0.001 | <0.001 | |||||
| 0 | 43 | 38 | 5 | 41 | 2 | ||
| ≥1 | 195 | 111 | 84 | 121 | 74 | ||
| Metastases to other organs | <0.01 | <0.01 | |||||
| Present | 15 | 2 | 13 | 3 | 12 | ||
| Absent | 223 | 147 | 76 | 159 | 64 | ||
Correlation of PrPc and MGr1-Ag/37LRP expression with prognosis
The protein expression of PrPc in GC was investigated for associations with overall survival using Kaplan–Meier analysis and log-rank test for significance estimates. Patients were divided into two groups those with high expression and those with low expression, as described in the evaluation of staining. A statistically significant difference of overall survival was found between GC patients with tumors having high PrPc expression and tumors with low PrPc expression. Kaplan–Meier survival curves showed that higher PrPc expression was associated with worse overall survival (log-rank test: p < 0.001; Fig. 1a). The 3- and 5-year cumulative survival rates were 53% and 32%, respectively, for patients with low PrPc expression and 12 and 6%, respectively, for those with high PrPc expression. The mean survival time for patients with low expression of PrPc was 46.28 months and 20.61 months for those with high expression of PrPc. Clearly, GC patients with high expression of PrPc have a poorer prognosis than those with low PrPc expression, with the unadjusted HR being 3.256 (95% CI: 2.080–5.098, p < 0.001; Table3). Likewise, Kaplan–Meier survival curves showed that higher MGr1-Ag/37LRP expression was associated with worse overall survival (log-rank test: p < 0.001; Fig. 1b). The 3- and 5-year cumulative survival rates were 49 and 31%, respectively, for GC patients with low expression of MGr1-Ag/37LRP and 13 and 4%, respectively, for those with high MGr1-Ag/37LRP expression. The mean survival time for patients with low expression of MGr1-Ag/37LRP in GC was 44.60 months and 19.79 months for those with high expression of MGr1-Ag/37LRP. Thus, GC patients with high expression of MGr1-Ag/37LRP have a poorer prognosis than those with low expression of MGr1-Ag/37LRP (p < 0.001). Univariate analysis indicated that the factors significantly associated with survival were age, tumor size, depth of invasion, TNM stage, differentiation and lymph node metastasis and distant metastasis, whereas gender and location of tumor were not related to the prognosis of the patients (Table3).
Figure 1.
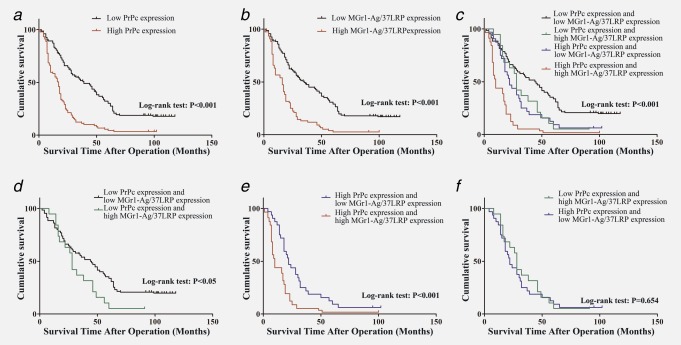
Kaplan-Meier analyses with a log rank test of survival. (a) Correlation of PrPc expression with overall survival (cum survival). (b) Correlation of MGr1-Ag/37LRP expression with overall survival. (c) Correlation of the combination of PrPc and MGr1-Ag/37LRP expression with overall survival. (d) Correlation of MGr1-Ag/37LRP expression with overall survival among patients with tumors of low PrPc expression. (e) Correlation of MGr1-Ag/37LRP expression with overall survival among patients with tumors of high PrPc expression. (f) Survival curves for patients with low PrPc/high MGr1-Ag/37LRP and high PrPc/low MGr1-Ag/37LRP. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table 3.
Univariate analysis of the correlation between clinicopathological parameters and survival of patients with GC
| Cumulative survival rates (%) |
||||||
|---|---|---|---|---|---|---|
| Clinicopathological parameters | 3-Years | 5-Years | Mean survival time (mo) | HR | 95% CI | p |
| Gender | 1.106 | 0.836–1.463 | 0.479 | |||
| Male | 38 | 22 | 37.49 | |||
| Female | 37 | 23 | 35.28 | |||
| Age | 1.91 | 1.458–2.523 | <0.001 | |||
| <60 | 50 | 29 | 45.15 | |||
| ≥60 | 23 | 14 | 26.32 | |||
| Location of tumor | 0.847 | 0.689–1.042 | 0.116 | |||
| Cardia | 69 | 15 | 48.23 | |||
| Body | 26 | 13 | 26.53 | |||
| Antrum | 41 | 28 | 40.95 | |||
| Tumor size, cm | 1.382 | 1.049–1.820 | <0.05 | |||
| <5 | 43 | 27 | 40.70 | |||
| ≥5 | 30 | 16 | 31.04 | |||
| Depth of invasion | 3.824 | 2.438–5.998 | <0.001 | |||
| T1–T2 | 80 | 65 | 70.03 | |||
| T3–T4 | 29 | 14 | 29.94 | |||
| Stages | 1.941 | 1.219–3.088 | <0.01 | |||
| I–II | 49 | 34 | 47.33 | |||
| III–IV | 33 | 17 | 31.69 | |||
| Differentiation | 0.736 | 0.560–0.968 | <0.05 | |||
| Well and moderate | 42 | 27 | 41.15 | |||
| Poorly and not | 33 | 17 | 32.06 | |||
| Lymph node metastases | 4.285 | 2.725–6.738 | <0.001 | |||
| 0 | 77 | 67 | 70.67 | |||
| ≥1 | 29 | 12 | 29.18 | |||
| Metastases to other organs | 2.245 | 1.297–3.884 | <0.01 | |||
| Present | 7 | 7 | 18.93 | |||
| Absent | 40 | 23 | 37.87 | |||
| Postoperative chemotherapy | 1.474 | 0.802–2.709 | 0.211 | |||
| Yes | 38 | 21 | 36.04 | |||
| No | 40 | 40 | 46.13 | |||
| PrPc expression | 2.642 | 1.980–3.524 | <0.001 | |||
| Low | 53 | 32 | 46.28 | |||
| High | 12 | 6 | 20.61 | |||
| MGr1-Ag/37LRP expression | 2.665 | 1.979–3.588 | <0.001 | |||
| Low | 49 | 31 | 44.60 | |||
| High | 13 | 4 | 19.79 | |||
mo: month; CI: confidence interval; HR: hazard ratio.
As statistical results of staining revealed a significant positive correlation between PrPc and MGr1-Ag/37LRP expression, we set out to detect whether prediction of GC prognosis was more accurate according to combined expression of PrPc and MGr1-Ag/37LRP than PrPc expression alone. Patients were subsequently divided into 4 groups: group 1, low expression of PrPc and low expression of MGr1-Ag/37LRP; group 2, low expression of PrPc and high expression of MGr1-Ag/37LRP; group 3, high expression of PrPc and low expression of MGr1-Ag/37LRP; group 4, high expression of PrPc and high expression of MGr1-Ag/37LRP. Kaplan-Meier analysis showed statistically distinct survival patterns among the four subgroups (Fig. 1c). GC patients with high level of PrPc and high level of MGr1-Ag/37LRP had the poorest prognosis. In both high PrPc level and low PrPc level tumors, patients with tumors of low MGr1-Ag/37LRP expression had a better survival compared with patients with tumors of high MGr1-Ag/37LRP expression (Figs. 1d and 1e), whereas the survival of patients with low PrPc/high MGr1-Ag/37LRP and high PrPc/low MGr1-Ag/37LRP was not statistically different (Fig. 1f).
To further assess the predictive roles of PrPc and MGr1-Ag/37LRP in GC prognosis, their expressions and the clinicopathological parameters found to be significant on univariate analysis were included in the multivariate analysis with the Backward Wald method. Covariates included in the Cox regression model were age, tumor size, depth of invasion, differentiation, TNM stage, lymph node metastasis, distant metastasis, PrPc expression and MGr1-Ag/37LRP expression. The results of first step indicated that tumor location, size, differentiation and TNM stage were not independent predictors of GC prognosis (Supporting Information Table S2). After stepwise multivariate survival analysis, age, depth of invasion, lymph node metastasis, distant metastasis, PrPc expression and MGr1-Ag/37LRP expression were found to be independent prognostic factors for patients with GC (Table4). However, when combined expression of PrPc and MGr-1Ag/37LRP was included into the multivariable Cox regression model, only combined expression of PrPc and MGr-1Ag/37LRP, age, depth of invasion, lymph node metastasis and distant metastasis were independent prognostic factors, but not PrPc expression and MGr1-Ag/37LRP expression (Table5). These results indicated that combined expression of PrPc and MGr-1Ag/37LRP was a more significant predictor of GC prognosis, compared with PrPc expression and MGr1-Ag/37LRP expression separately.
Table 4.
Multivariate analysis of the correlation between clinicopathological parameters and survival time of patients with GC
| Multivariate analysis |
|||||
|---|---|---|---|---|---|
| Variables | Coefficient | Standard Error | HR | 95% CI for HR | p |
| Age (<60 vs. ≥60) 1.410 | 0.502 | 0.143 | 1.652 | 1.248–2.187 | <0.001 |
| Depth of invasion (T1–T2 vs. T3–T4) | 0.630 | 0.257 | 1.878 | 1.134–3.110 | <0.05 |
| Lymph node metastases (positive vs. negative) | 0.835 | 0.265 | 2.306 | 1.373–3.873 | <0.01 |
| Distant metastasis (positive vs. negative) | 0.625 | 0.297 | 1.869 | 1.044–3.345 | <0.05 |
| PrPc expression (low vs. high) | 0.553 | 0.164 | 1.738 | 1.260–2.396 | <0.001 |
| MGr1-Ag/37LRP expression (low vs. high) | 0.422 | 0.167 | 1.526 | 1.100–2.115 | <0.05 |
CI: confidence interval; HR: hazard ratio.
Table 5.
Multivariate analysis of the correlation between clinicopathological parameters and survival time of patients with GC (including combined expression of PrPc and MGr-1Ag/37LRP)
| Multivariate analysis |
|||||
|---|---|---|---|---|---|
| Variables | Coefficient | Standard Error | HR | 95% CI for HR | p |
| Age (<60 vs. ≥60) 1.410 | 0.509 | 0.143 | 1.663 | 1.256–2.203 | <0.001 |
| Depth of invasion (T1–T2 vs. T3–T4) | 0.595 | 0.262 | 1.814 | 1.085–3.032 | <0.05 |
| Lymph node metastases (positive vs. negative) | 0.872 | 0.269 | 2.391 | 1.412–4.048 | <0.001 |
| Distant metastasis (positive vs. negative) | 0.577 | 0.299 | 1.780 | 1.027–3.186 | <0.05 |
| Combined expression of PrPc and MGr-1Ag/37LRP | |||||
| Low PrPc and low MGr1-Ag/37LRP expression | 1 | reference | |||
| Low PrPc and high MGr1-Ag/37LRP expression | 0.174 | 0.261 | 1.190 | 0.713–1.986 | 0.505 |
| High PrPc and low MGr1-Ag/37LRP expression | 0.386 | 0.213 | 1.470 | 0.969–2.231 | 0.070 |
| High PrPc and high MGr1-Ag/37LRP expression | 1.017 | 0.187 | 2.765 | 1.916–3.989 | <0.001 |
CI: confidence interval; HR: hazard ratio.
Discussion
GC is one of the largest threats to human health. A major challenge in GC management now is to accurately predict outcome for each patient so that we can determine who will benefit from adjuvant therapy. To achieve this, people rely heavily on traditional pathologic variables. Currently the only prognostic system routinely employed for GC management is based on the International Union Against Cancer tumor-node metastasis (TNM) staging system.26 However, we cannot predict the clinical outcome of patients after surgery if only applying clinical parameters alone, because the outcome is not only to the high disease stage, but also to the biologic aggressiveness of the individual disease, which is characterized by high potential for metastasis and resistance to anticancer therapy.27 The discovery of molecular biological prognostic factors may aid in a more accurate prediction of clinical outcome and may also reveal novel predictive factors and therapeutic targets.28 Therefore, identification of a postoperative useful indicator to better understand the biological basis for the survival of GC patients may provide important clinically relevant insights into disease management.
PrPc has been found to regulate a wide variety of cellular processes during development and play a crucial role in human malignant diseases.29,30 Overexpression of PrPc is correlated to the acquisition by tumor cells of malignant phenotypes. Upon temozolomide treatment in gliomas, PrPc exerted its antiapoptotic activity by inhibiting PKA-mediated par-4 phosphorylation at the T155 residue that were important for par-4 activation, nuclear entry and initiation of apoptosis.31 It was found in recent study that there was a physical and functional interaction between PrPc and CD44 in breast cancer32 and colorectal cancer,8 thereby conferring high metastatic capacity. Our previous studies showed that PrPc significantly promoted the adhesive, invasive and in vivo metastatic capacities of the GC cells and overexpression of PrPc accelerated the proliferation of GC cells through transcription activation of cyclin D3 to facilitate G1/S-phase transition.10–13 Moreover, Meslin et al. demonstrated that adjuvant chemotherapy of breast cancer could be highly effective in patients with ER-negative/PrPc-negative tumors, while its efficacy seems to be reduced in patients with ER-negative/PrPc-positive tumors.9 Not long ago it was proved that PrPc could be candidate biomarkers for colorectal adenoma-to-carcinoma progression.33 Du et al. recently reported that the level of PrPc is inversely correlated with patient survival in colorectal cancer.8 The above findings suggest that PrPc might be a great predictor of cancer prognosis, to guide the postoperative adjuvant therapy.
In this study, we evaluated the expression of PrPc in GC and its prognostic implications. The expression of PrPc was upregulated in GC tissues as compared with that in noncancerous gastric mucosae and elevated PrPc expression was significantly associated with age, TNM stage, depth of invasion, differentiation and lymph node and distant metastasis. Kaplan-Meier analysis of the survival curves showed a significantly poor survival for patients with tumors of high PrPc levels. Cox proportional hazards model adjusted for age, gender, differentiation status and TNM stage showed the same trend as Kaplan-Meier survival analysis. Moreover, multivariate analysis showed PrPc expression was a marker of poor survival independent of the known clinical prognostic indicators. Our results are in agreement with Meslin9 and Du8 et al. reports that correlated elevated expression of PrPc with the poor progression and prognosis in many tumors. These findings would contribute to identify high risk patients who should receive more aggressive adjuvant chemotherapy and thus low risk patients receive less after potentially curative surgery. It also suggested that PrPc could be a potential therapeutic target in the molecular pathways determining the behavior of GC.
MGr1-Ag was an upregulated protein in drug-resistant cell SGC7901/VCR, which was derived from the human gastric adenocarcinoma cell line SGC7901 by stepwise selection in vitro, with vincristine used as an inducing reagent.34,35 Sequence analysis revealed that the MGr1-Ag was identical to human 37-kDa laminin receptor precursor protein (37LRP).14 Further studies suggested that MGr1-Ag/37LRP could promote MDR of GC cells via enhancing cell adhesion and then inhibiting drug-induced apoptosis.15–17 Several studies indicated that 37LRP was involved in tumor development and progression.18–20 Moreover, an interaction between 37LRP and PrPc was first established in a two-hybrid screening of a HeLa cDNA expression library for PrPc-interacting proteins.22,36,37 The pathogenic relevance of 37LRP/67LR and PrPc interaction was revealed in tissues and cells of scrapie compared with nonscrapie-infected mice.36 Tissues with high levels of PrPsc accumulation displayed correspondingly high levels of 37LRP, in particular in the brain tissue.36 Recent studies showed that 37LRP is not only involved in the PrPc metabolism, but fulfills also a crucial role in prion propagation. The important role of the 37LRP in mediating binding and internalization of the PrPc and its involvement in pathological mechanisms has been clearly demonstrated.21,23,38,39 However, the specific role of the cross-talk between PrPc and 37LRP in cancer has not yet been addressed.
We found in our study that patients with high expression of PrPc also had a high rate of upregulated expression of MGr1-Ag/37LRP in GC and the expression of PrPc positively correlated with that of MGr1-Ag/37LRP. The expression of MGr1-Ag/37LRP was significantly correlated with tumor size, depth of invasion, differentiation, TNM stage, distant metastasis and lymph node metastasis, all of which are related to tumor progression. Furthermore, patients with high expression of MGr1-Ag/37LRP had a significantly shorter overall survival time than those with low expression and the prognostic effect of MGr1-Ag/37LRP expression was independent of conventional prognostic factors. Moreover, Kaplan–Meier analysis showed GC patients with high level of PrPc and high level of MGr1-Ag/37LRP had the poorest prognosis. The predictive role of PrPc for prognosis might be modulated by MGr1-Ag/37LRP status. The results provided the first evidence for PrPc to determine the prognosis of GC patients and positively correlated with MGr1-Ag/37LRP. It could contribute to a more accurate prediction of the prognosis of patients following surgery and consequently to make tailored treatment of each patient, thus preventing patients from receiving excessive or insufficient adjuvant treatment, both of which are harmful. These results also indicate that the cross-talk between PrPc and MGr1-Ag/37LRP is likely to play a role in the mechanisms underlying carcinogenesis, development and progression of GC.
Several limitations need to be considered and clarified, when interpreting our results. First, it was conducted in a retrospective manner and in a limited number of patients. A prospective study in a large population is warranted to clarify the prognostic role of Prpc and MGr1-Ag/37LRP expression in GC. Second, Because of the subjective nature of scoring method used in our study, further study is needed to reevaluate the immunoreactivity of both proteins by another team of two pathologists independently, thereby certifying the reproducibility of immunohistochemical scores among evaluators. Third, the interaction between PrPc and MGr1-Ag/37LRP has not been illustrated and more molecular biology studies are need to explore the mechanism.
In conclusion, to our knowledge, this is the first study to describe the relation between PrPc and MGr1-Ag/37LRP as well as the predictive roles of them in GC. Our retrospective study provided convincing evidence that PrPc could be an independent predictor of prognosis for patients with GC, which might be modulated by MGr1-Ag/37LRP expression. On the basis of the TNM stage of the tumor, the expression of PrPc and MGr1-Ag/37LRP in GC will help identify patients with high potential for poor outcomes, thereby guiding the clinical decision if adjuvant therapy would be used. It also suggested a possible mechanism for the cross-talk between PrPc and MGr1-Ag/37LRP to modulate GC carcinogenesis and progression. Therefore, targeting PrPc and MGr1-Ag/37LRP could be a promising therapeutic strategy for future therapies of GC.
Acknowledgments
The authors acknowledge Zhen Chen, Guangbo Tang and Jianhua Dou from the State Key Laboratory of Cancer Biology for the assistance in tissue sample collection.
Glossary
- 37LRP
37-kDa laminin receptor precursor
- ECM
extracellular matrix
- GC
gastric cancer
- H&E
hematoxylin-eosin
- MDR
multidrug resistance
- PrPc
prion protein
- PrPsc
scrapie prion protein
- TMA
tissue microarray
- TNM
tumor-node metastasis
- VCR
vincristine
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
References
- 1.Bueler H, Aguzzi A, Sailer A, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–47. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Klein MA, Aguzzi A. The neuroimmune interface in prion diseases. News Physiol Sci. 2000;15:250–255. doi: 10.1152/physiologyonline.2000.15.5.250. [DOI] [PubMed] [Google Scholar]
- 3.Kikuchi Y, Kakeya T, Yamazaki T, et al. G1-dependent prion protein expression in human glioblastoma cell line T98G. Biol Pharm Bull. 2002;25:728–33. doi: 10.1248/bpb.25.728. [DOI] [PubMed] [Google Scholar]
- 4.Meslin F, Hamai A, Gao P, et al. Silencing of prion protein sensitizes breast adriamycin-resistant carcinoma cells to TRAIL-mediated cell death. Cancer Res. 2007;67:10910–9. doi: 10.1158/0008-5472.CAN-07-0512. [DOI] [PubMed] [Google Scholar]
- 5.Roucou X, Giannopoulos PN, Zhang Y, et al. Cellular prion protein inhibits proapoptotic Bax conformational change in human neurons and in breast carcinoma MCF-7 cells. Cell Death Differ. 2005;12:783–95. doi: 10.1038/sj.cdd.4401629. [DOI] [PubMed] [Google Scholar]
- 6.Sauer H, Dagdanova A, Hescheler J, et al. Redox-regulation of intrinsic prion expression in multicellular prostate tumor spheroids. Free Radic Biol Med. 1999;27:1276–83. doi: 10.1016/s0891-5849(99)00164-1. [DOI] [PubMed] [Google Scholar]
- 7.Antonacopoulou AG, Palli M, Marousi S, et al. Prion protein expression and the M129V polymorphism of the PRNP gene in patients with colorectal cancer. Mol Carcinog. 2010;49:693–9. doi: 10.1002/mc.20642. [DOI] [PubMed] [Google Scholar]
- 8.Du L, Rao G, Wang H, et al. CD44-positive cancer stem cells expressing cellular prion protein contribute to metastatic capacity in colorectal cancer. Cancer Res. 2013;73:2682–94. doi: 10.1158/0008-5472.CAN-12-3759. [DOI] [PubMed] [Google Scholar]
- 9.Meslin F, Conforti R, Mazouni C, et al. Efficacy of adjuvant chemotherapy according to Prion protein expression in patients with estrogen receptor-negative breast cancer. Ann Oncol. 2007;18:1793–8. doi: 10.1093/annonc/mdm406. [DOI] [PubMed] [Google Scholar]
- 10.Du J, Pan Y, Shi Y, et al. Overexpression and significance of prion protein in gastric cancer and multidrug-resistant gastric carcinoma cell line SGC7901/ADR. Int J Cancer. 2005;113:213–20. doi: 10.1002/ijc.20570. [DOI] [PubMed] [Google Scholar]
- 11.Pan Y, Zhao L, Liang J, et al. Cellular prion protein promotes invasion and metastasis of gastric cancer. FASEB J. 2006;20:1886–8. doi: 10.1096/fj.06-6138fje. [DOI] [PubMed] [Google Scholar]
- 12.Liang J, Pan Y, Zhang D, et al. Cellular prion protein promotes proliferation and G1/S transition of human gastric cancer cells SGC7901 and AGS. FASEB J. 2007;21:2247–56. doi: 10.1096/fj.06-7799com. [DOI] [PubMed] [Google Scholar]
- 13.Liang J, Wang J, Luo G, et al. Function of PrPC (1-OPRD) in biological activities of gastric cancer cell lines. J Cell Mol Med. 2009;13:4453–64. doi: 10.1111/j.1582-4934.2009.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Zhai H, Wang X, et al. Multidrug-resistance-associated protein MGr1-Ag is identical to the human 37-kDa laminin receptor precursor. Cell Mol Life Sci. 2002;59:1577–83. doi: 10.1007/s00018-002-8531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Han Y, Wang X, et al. MGr1-Ag is associated with multidrug-resistant phenotype of gastric cancer cells. Gastric Cancer. 2002;5:154–9. doi: 10.1007/s101200200027. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Sun L, Zhang H, et al. Hypoxia-mediated up-regulation of MGr1-Ag/37LRP in gastric cancers occurs via hypoxia-inducible-factor 1-dependent mechanism and contributes to drug resistance. Int J Cancer. 2009;124:1707–15. doi: 10.1002/ijc.24135. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Zhang H, Sun L, et al. ERK/MAPK activation involves hypoxia-induced MGr1-Ag/37LRP expression and contributes to apoptosis resistance in gastric cancer. Int J Cancer. 2010;127:820–9. doi: 10.1002/ijc.25098. [DOI] [PubMed] [Google Scholar]
- 18.Jackers P, Minoletti F, Belotti D, et al. Isolation from a multigene family of the active human gene of the metastasis-associated multifunctional protein 37LRP/p40 at chromosome 3p21.3. Oncogene. 1996;13:495–503. [PubMed] [Google Scholar]
- 19.Formisano P, Ragno P, Pesapane A, et al. PED/PEA-15 interacts with the 67 kD laminin receptor and regulates cell adhesion, migration, proliferation and apoptosis. J Cell Mol Med. 2012;16:1435–46. doi: 10.1111/j.1582-4934.2011.01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DG, Choi JW, Lee JY, et al. Interaction of two translational components, lysyl-tRNA synthetase and p40/37LRP, in plasma membrane promotes laminin-dependent cell migration. FASEB J. 2012;26:4142–59. doi: 10.1096/fj.12-207639. [DOI] [PubMed] [Google Scholar]
- 21.Gauczynski S, Nikles D, El-Gogo S, et al. The 37-kDa/67-kDa laminin receptor acts as a receptor for infectious prions and is inhibited by polysulfated glycanes. J Infect Dis. 2006;194:702–9. doi: 10.1086/505914. [DOI] [PubMed] [Google Scholar]
- 22.Gauczynski S, Peyrin JM, Haik S, et al. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J. 2001;20:5863–75. doi: 10.1093/emboj/20.21.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leucht C, Simoneau S, Rey C, et al. The 37 kDa/67 kDa laminin receptor is required for PrP(Sc) propagation in scrapie-infected neuronal cells. EMBO Rep. 2003;4:290–5. doi: 10.1038/sj.embor.embor768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao JW, Su XO, Li YX, et al. Variable levels of 37-kDa/67-kDa laminin receptor (RPSA) mRNA in ovine tissues: potential contribution to the regulatory processes of PrPSc propagation? Anim Biotechnol. 2009;20:151–5. doi: 10.1080/10495390902996863. [DOI] [PubMed] [Google Scholar]
- 25.Bremnes RM, Veve R, Gabrielson E, et al. High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J Clin Oncol. 2002;20:2417–28. doi: 10.1200/JCO.2002.08.159. [DOI] [PubMed] [Google Scholar]
- 26.Greene FL, Sobin LH. The TNM system: our language for cancer care. J Surg Oncol. 2002;80:119–20. doi: 10.1002/jso.10114. [DOI] [PubMed] [Google Scholar]
- 27.Ahn HS, Lee HJ, Hahn S, et al. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116:5592–8. doi: 10.1002/cncr.25550. [DOI] [PubMed] [Google Scholar]
- 28.Oldenhuis CN, Oosting SF, Gietema JA, et al. Prognostic versus predictive value of biomarkers in oncology. Eur J Cancer. 2008;44:946–53. doi: 10.1016/j.ejca.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Antony H, Wiegmans AP, Wei MQ, et al. Potential roles for prions and protein-only inheritance in cancer. Cancer Metastasis Rev. 2012;31:1–19. doi: 10.1007/s10555-011-9325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehrpour M, Codogno P. Prion protein: from physiology to cancer biology. Cancer Lett. 2010;290:1–23. doi: 10.1016/j.canlet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Zhuang D, Liu Y, Mao Y, et al. TMZ-induced PrPc/par-4 interaction promotes the survival of human glioma cells. Int J Cancer. 2012;130:309–18. doi: 10.1002/ijc.25985. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Y, Tao L, Xu J, et al. Mol Carcinog. CD44/cellular prion protein interact in multidrug resistant breast cancer cells and correlate with responses to neoadjuvant chemotherapy in breast cancer patients. in press. [DOI] [PubMed] [Google Scholar]
- 33.de Wit M, Jimenez CR, Carvalho B, et al. Cell surface proteomics identifies glucose transporter type 1 and prion protein as candidate biomarkers for colorectal adenoma-to-carcinoma progression. Gut. 2012;61:855–64. doi: 10.1136/gutjnl-2011-300511. [DOI] [PubMed] [Google Scholar]
- 34.Fan D, Zhang X, Chen X, et al. Bird's-eye view on gastric cancer research of the past 25 years. J Gastroenterol Hepatol. 2005;20:360–5. doi: 10.1111/j.1440-1746.2005.03797.x. [DOI] [PubMed] [Google Scholar]
- 35.Fan K, Fan D, Cheng LF, et al. Expression of multidrug resistance-related markers in gastric cancer. Anticancer Res. 2000;20:4809–14. [PubMed] [Google Scholar]
- 36.Rieger R, Edenhofer F, Lasmezas CI, et al. The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat Med. 1997;3:1383–8. doi: 10.1038/nm1297-1383. [DOI] [PubMed] [Google Scholar]
- 37.Hundt C, Peyrin JM, Haik S, et al. Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J. 2001;20:5876–86. doi: 10.1093/emboj/20.21.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morel E, Andrieu T, Casagrande F, et al. Bovine prion is endocytosed by human enterocytes via the 37 kDa/67 kDa laminin receptor. Am J Pathol. 2005;167:1033–42. doi: 10.1016/S0002-9440(10)61192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolodziejczak D, Da Costa Dias B, Zuber C, et al. Prion interaction with the 37-kDa/67-kDa laminin receptor on enterocytes as a cellular model for intestinal uptake of prions. J Mol Biol. 2010;402:293–300. doi: 10.1016/j.jmb.2010.06.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information


