Abstract
Zearalenone (ZEN) is converted to a nontoxic product by a lactonohydololase encoded by zhd101. An enhanced green fluorescent protein (EGFP) gene was fused to zhd101 (i.e., egfp::zhd101) and expressed in Escherichia coli. Both recombinant ZHD101 and EGFP::ZHD101 were purified to homogeneity and characterized. Maximal activity of ZHD101 toward ZEN was measured at approximately 37 to 45°C and pH 10.5 (kcat at 30°C, 0.51 s−1). The enzyme was irreversibly inactivated at pH values below 4.5 or by treatment with serine protease inhibitors. ZHD101 was also active against five ZEN cognates, although the efficiencies were generally low; e.g., the kcat was highest with zearalanone (1.5 s−1) and lowest with β-zearalenol (0.075 s−1). EGFP::ZHD101 had properties similar to those of the individual proteins with regard to the EGFP fluorescence and lactonohydrolase activity. Fortuitously, EGFP::ZHD101 exhibited a good correlation between the fluorescence intensity and reaction velocity under various pH conditions. We therefore used egfp::zhd101 to visually monitor the lactonohydrolase activity in genetically modified organisms and evaluated the usefulness of zhd101 for in vivo detoxification of ZEN. While recombinant E. coli and transgenic rice calluses exhibited strong EGFP fluorescence and completely degraded ZEN in liquid media, recombinant Saccharomyces cerevisiae gave poor fluorescence and did not eliminate all the toxicity of the mycotoxin in the medium; i.e., the rest of ZEN was transformed into an unfavorable substrate, β-zearalenol, by an as-yet-unidentified reductase and remained in the medium. Even so, as much as 75% of ZEN was detoxified by the yeast transformant, which is better than the detoxification system in which food-grade Lactobacillus strains are used (H. El-Nezami, N. Polychronaki, S. Salminen, and H. Mykkuäne, Appl. Environ. Microbiol. 68:3545-3549, 2002). An appropriate combination of a candidate host microbe and the codon-optimized synthetic gene may contribute significantly to establishing a mycotoxin detoxification system for food and feed.
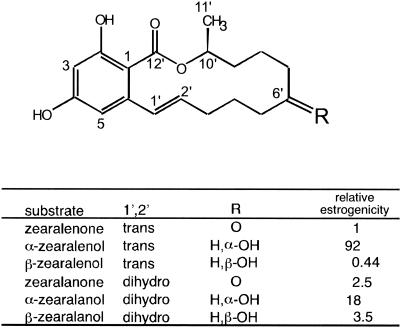
Zearalenone (ZEN), 6-(10-hydroxy-6-oxo-trans-1-undecenyl)-β-resorcylic acid lactone (Fig. 1), is a nonsteroid estrogenic mycotoxin that is produced by numerous Fusarium species in pre- or postharvest cereals (22). Under environmental conditions favorable for fungal growth, high levels of ZEN are frequently found in maize and other small grains, such as wheat and barley. The keto group at C-6′ is reduced to a more estrogenic compound, α-zearalenol, and/or its stereoisomeric cognate β-zearalenol (which has less estrogenicity than ZEN) by various microorganisms, including industrial yeast strains (4) and animal intestinal microbes (13). These mycotoxins have adverse health effects on various animals that ingest mold-infected cereals and cereal-derived food products (16).
FIG. 1.
Chemical structures of ZEN and cognate molecules. The values for relative estrogenicity are also shown (25).
ZEN causes severe morphological and functional disorders of reproductive organs in livestock, especially female swine (7). In feeding experiments with female swine, clear clinical symptoms (e.g., reduced feed efficiency, organ weight changes, reduced fertility) were observed when the diet contained 1 to 5 μg of ZEN/g (24). Consistent with the symptoms related to growth and reproduction, cell physiological studies demonstrated that ZEN binds to recombinant human estrogen receptors (17) and stimulates the growth of human breast cancer cells (25). Besides the hormonal effect, in long-term studies of carcinogenicity in mice significant increases in hepatocellular adenoma and pituitary tumors were observed at doses that were much greater than the ZEN concentrations that had the estrogenic effect (30).
To secure the safety of food and feed, regular monitoring of mycotoxin levels is necessary. Although a low-cost tool for monitoring ZEN levels with an engineered yeast strain has been developed, this method gives false-positive results because of other interfering estrogenic compounds, and it takes a long time to obtain results (18). In contrast, detoxification of mycotoxins appears to be a more attractive approach. Chemical treatments to reduce the ZEN levels in foods have had limited success, while thermal processing has turned out to be ineffective (for a review see reference 23). Several technologies based on adsorption to appropriate media are currently being developed. For example, food-grade Lactobacillus strains were useful for removing a certain amount of ZEN by binding to the cell surface (6). However, other tools are also needed for development of a more efficient and reliable method.
Enzymatic detoxification could be an efficient method for ZEN detoxification. Recently, we isolated a ZEN-detoxifying gene, zhd101, from Clonostachys rosea (28). ZHD101 converts ZEN to the nonestrogenic product 1-(3,5-dihydroxy-phenyl)-10′-hydroxy-1′-undecen-6′-one at an alkaline pH (12). However, the enzymatic properties of this lactonohydrolase were not examined previously. In this report, we describe in detail characterization of recombinant ZHD101 and EGFP::ZHD101, an N-terminal fusion of ZHD101 connected to an enhanced green fluorescence protein (EGFP) as an expression marker. Unexpectedly, we found that the fluorescence intensity of EGFP::ZHD101 was proportional to the ZEN-degrading activity at various pH values. This indicates that the detoxifying activity can be monitored by EGFP fluorescence under various physiological conditions. Guided by the fluorescence intensity, we obtained genetically modified (GM) organisms with strong ZEN detoxification activity. Below we discuss the prospect of the mycotoxin detoxification in food and feed by making use of these GM organisms.
MATERIALS AND METHODS
Plasmid construction and microbial transformation.
Plasmid pET12a-zhd101 contains the coding region of zhd101 between the NdeI and BamHI sites of the expression vector pET12a (Novagen, Darmstadt, Germany) (28). For construction of pET19b-egfp::zhd101, egfp::zhd101 was excised from pAct1-egfp::zhd101 (11) by digestion with NcoI (partial) and BamHI and inserted into another expression vector, pET19b. These plasmids were transformed into Escherichia coli BL21 star (DE3) (Invitrogen, Carlsbad, Calif.) for high-level expression of the recombinant proteins.
To construct an expression plasmid for yeast, the HindIII-NotI fragment of egfp::zhd101 excised from pEGFP-zhd101 (11) was cloned into the corresponding sites of pYC6/CT (Invitrogen) to generate pYC6/CT-egfp::zhd101. The GAL1 promoter on pYC6/CT is repressed in the presence of glucose and is derepressed by galactose added in place of glucose (8). The CEN6/ARSH4 sequence of pYC6/CT-egfp::zhd101 allows nonintegrative low-copy replication in yeast (26). Competent cells of the Saccharomyces cerevisiae INVSc1 strain (Invitrogen) were prepared by using an S.c. EasyComp kit (Invitrogen). Transformants were selected by using 50 μg of blasticidin S per ml on a YPD plate (1% yeast extract, 2% peptone, 2% glucose, 2% agar). For induction of recombinant proteins, yeast transformants were transferred to induction medium (2% raffinose, 2% galactose, 0.5% ammonium sulfate, 0.67% yeast nitrogen base, 0.115% Hollenberg supplement mixture from Bio 101 Inc., La Jolla, Calif.).
Expression and purification of recombinant proteins in E. coli.
E. coli transformants carrying pET12a-zhd101 and pET19b-egfp::zhd101 were incubated in 100 ml of liquid medium (CIRCLEGROW; Bio 101 Inc.) supplemented with ampicillin (100 μg/ml). Expression of the gene was induced by addition of 0.4 mM isopropyl β-d-thiogalactoside (IPTG). After 24 h of incubation at 25°C, the bacterial cells were collected by centrifugation (5,000 × g for 15 min) and suspended in 10 ml of buffer A (10 mM Tris-HCl, pH 7.5). Then each cell suspension was disrupted by sonication on ice, and the supernatant fraction was collected by centrifugation (5,000 × g for 15 min). For purification of EGFP::ZHD101, ammonium sulfate was added to the crude enzyme solution at 40 to 60% saturation, and the precipitate was recovered by centrifugation (10, 000 × g for 1 h at 4°C). After dialysis of the precipitated fraction against buffer A for 3 h, samples were applied to a 5-ml HiTrap Q HP column (Amersham Bioscience, Piscataway, N.J.) and eluted by using a 1 M NaCl linear gradient in 20 ml of buffer A. For purification of EGFP::ZHD101, the supernatant fractions were directly separated by HiTrap Q chromatography after passage through a 0.45-μm-pore-size filter (glass microfiber filter; Whatman, Clifton, N.J.). The active fractions were subsequently fractionated by gel permeation chromatography (Superdex 75 HR 10/30; Amersham Biosciences) and, if necessary, then by anion-exchange chromatography (Mono Q HR 5/5; Amersham Biosciences) as described previously (28). Since the amount of recombinant ZHD101 produced was much larger than the amount of EGFP::ZHD101 produced, ZHD101 was purified from the homogenized cell extracts by a two-step purification procedure with HiTrap Q and Superdex 75 columns. The purity of the recombinant proteins was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Biophysical parameters of purified enzymes.
The theoretical molar extinction coefficients (ɛ280) of purified enzymes were calculated from the numbers of Tyr (ɛ280 = 1,197 M−1 cm−1) and Trp (ɛ280 = 5,559 M−1 cm−1) residues as described previously (15); values of 38,568 and 58,491 M−1 cm−1 per subunit were obtained for ZHD101 and EGFP::ZHD101, respectively. The UV absorbance at 280 nm of purified enzymes was measured by using a DU7400 spectrophotometer (Beckman Coulter, Inc., Fullerton, Calif.), and the protein concentrations were determined based on the ɛ280 values. The fluorescence intensity of EGFP::ZHD101 (emission wavelength, 510 ± 10 nm; excitation wavelength, 490 ± 10 nm) was measured with a spectrofluorometer (VersaFluor cuvette fluorometer; Bio-Rad Laboratories, Hercules, Calif.). The relative fluorescence intensity of EGFP::ZHD101 was normalized to the molar extinction coefficient by using purified EGFP as the standard (21).
Kinetic analysis of enzymes.
Activities of recombinant enzymes were determined by monitoring both degradation of a substrate and production of the cleaved product. The incubation mixture (total volume, 100 μl) contained a substrate (ZEN, α-zearalenol, β-zearalenol, zearalanone, α-zearalanol, or β-zearalanol [Sigma, St. Louis, Mo.]) at a concentration of 5 to 100 μM and an enzyme (ZHD101 or EGFP::ZHD101) at a concentration of 5 to 50 nM in 100 mM Tris-HCl (pH 6.5 to 10.5), 100 mM acetate buffer (pH 4.5 to 6.2), or 100 mM citrate buffer (pH 3.0). The enzyme reaction was carried out at 30°C for 10 to 60 min and was terminated by adding an appropriate amount of 1 N HCl. Then 10 to 20 μl of the reaction mixture was analyzed by using a high-performance liquid chromatography (HPLC) system (SCL-10A; Shimadzu) equipped with a PEGASIL octyldecyl silane column (diameter, 4.6 mm; length, 250 mm; Senshu Scientific Co., Tokyo, Japan). Samples were eluted with 0.1% trifluoroacetic acid-acetonitrile (40:60) at a flow rate of 1.0 ml/min, and the results were monitored at 254 nm. The amounts of the substrate and product were calculated from their peak areas on the HPLC chromatogram, as described previously (27). The kinetic parameters kcat and Km were determined by fitting data to a Michaelis-Menten equation by using the program KALEIDAGRAPH (Synergy Software, Reading, Pa.).
Detoxification of ZEN and its derivatives by recombinant E. coli.
After IPTG induction of E. coli transformants carrying pET12a-zhd101 or pET19b-egfp::zhd101, mycotoxin was added to the culture medium in a siliconized flask at a final concentration of 2 μg/ml. Bacterial cells (initial density, 106 viable cells/ml) were collected at zero time, 10 min, and 1, 2, 4, 8, and 24 h by centrifugation at 5,000 × g for 10 min. An equal amount of methanol was added to each supernatant, and the diluted sample was filtered through a 0.2-μm-pore-size Millex filter (Millipore, Bedford, Mass.) before HPLC analysis. For extraction of the mycotoxin that may have been adsorbed to the bacterium, the cells were crushed with aluminum oxide by using a pestle and were extracted with 1 ml of methanol three times. The methanol fraction was evaporated under a constant flow of N2, dissolved in 1 ml of 50% methanol, and analyzed by HPLC as described above.
Metabolism of ZEN by strain INVSc1 of S. cerevisiae and transformants of this strain.
Strain INVSc1 and transformants of this strain were inoculated into induction medium. After 1 day of incubation at 28°C (approximately 1 × 108 cells/ml), 2 μg of ZEN per ml was added to each culture. Both the supernatant and the cell debris were analyzed to measure the amount of ZEN and its metabolites; the protocol described above for the detoxification experiment with E. coli was also used for the yeast experiment, except that the extracted samples were eluted from the HPLC column with trifluoroacetic acid-acetonitrile (55:45).
Particle bombardment of rice embryogenic scutellar tissues and selection of transgenic calluses.
For transformation of rice (Oryzae sativa subsp. japonica cv. Nipponbare) cells, mature surface-sterilized seeds were placed on D2 medium, which contained 4.57 g of Murashige minimal organics medium (Invitrogen) per liter, 30 g of sucrose per liter, 2 g of 2,4-dichlorophenoxyacetic acid per liter, and 2.5 g of gellan gum per liter, and were cultured for 7 days. The embryogenic scutellar tissues were bombarded with the ZEN-degrading plasmid pAct1-egfp::zhd101 (11) and a bialaphos resistance vector, pDM302 (31), by using the PDS-1000/He particle delivery system (Bio-Rad). The bombarded tissues were transferred to D2B5 medium (D2 medium containing 5 mg of bialaphos per liter) and cultured for 3 months by using serial 2-week subcultures on fresh D2B5 medium. The embryogenic callus cells were examined with a fluorescence stereomicroscope (MZ12; Leica Ltd., Cambridge, United Kingdom) with GFP-Plus filter sets (excitation wavelength, 460 to 500; emission wavelength, 505 nm; emission barrier, >510 nm) to select transgenic lines. After selection, transgenic calluses with different levels of fluorescence intensity were transferred to liquid D2 medium. Transferring portions of the cultures to fresh liquid D2 medium every 2 weeks established cultured cell suspensions of transgenic rice calluses.
Detoxification of ZEN by cultured suspension cells expressing egfp::zhd101.
The established transgenic rice cells were used for detoxification studies. The cultured cell suspensions were incubated in liquid D2 medium (pH 6.2) containing ZEN (1 μg/ml) in siliconized Erlenmeyer flasks with shaking (120 rpm) at 26°C for 7 days. Aliquots (100 μl) of the culture supernatant were collected on days 0, 1, 3, 5 and 7, and these samples were diluted twofold with methanol. After filtration through a 0.2-μm-pore-size Millex filter (Millipore), the samples were analyzed by HPLC as described above. The amount of ZEN remaining inside the cells was measured by an enzyme immunoassay at the end of the detoxification assay. ZEN was extracted with 70% methanol from powdered suspension cells that had been ground in liquid nitrogen and was quantified with a RIDASCREEN FAST Zearalenon kit (r-Biopharm AG, Darmstadt, Germany).
Fluorescence image acquisition and processing.
E. coli and S. cerevisiae transformants expressing egfp::zhd101 were observed with a fluorescence microscope (DM RA; Leica) equipped with a 460- to 500-nm excitation filter and a 512- to 542-emission filter. Fluorescent images were handled by using the QFlouoro (version 1.0) software (Leica). All photographs were taken under the same conditions (magnification, ×400; integration time, 200 ms) for E. coli and S. cerevisiae; image handling was also performed under the same conditions, including the threshold and the maximum level of intensity.
RESULTS
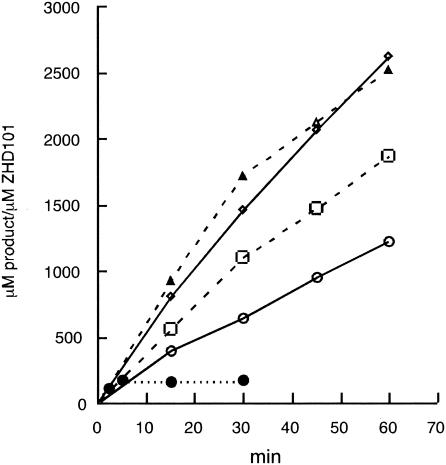
Characterization of recombinant ZHD101 protein. We measured the lactonohydrolase activity with ZEN at different temperatures (25, 30, 37, 45, and 50°C) in a time course experiment (Fig. 2). The optimum temperature of the enzyme was 37 to 45°C. Although ZHD101 was quite stable at 37°C and activity was not lost for at least 1 week, it was rapidly inactivated at 50°C.
FIG. 2.
Effects of temperature on ZHD101 activity. The enzyme reaction was carried on at 25°C (○), 30°C (□), 37°C (◊), 45°C (▵), and 50°C (•).
Under acidic conditions, the enzyme was very unstable. When ZHD101 was incubated at pH 4.5 for 30 min, more than 95% of the activity was irreversibly lost. The activity could not be restored by dialysis against buffer A. Incubation of ZHD101 at pH 3.0 for 30 min completely inactivated the enzyme.
Treatment of ZHD101 with β-mercaptoethanol in buffer A for 1 h did not result in loss of the enzyme activity. However, reduction by β-mercaptoethanol caused ZHD101 to precipitate in 2 days, and the lactonohydrolase activity significantly decreased. Compared with the activity of untreated ZHD101, the activity of reduced ZHD101 was 0, 24, 73, and 99% less on days 1, 2, 3, and 7, respectively. Thus, although the disulfide bridge in ZHD101 (28) does not have any effect on the catalytic activity, it may play some role in maintaining the structural integrity and stability of the molecule.
Addition of 2 M urea inactivated ZHD101 completely, and the activity could not be restored by dialysis against buffer A. ZHD101 was also incubated in the presence of protease inhibitors, such as EDTA, E-64 (oxiranecarboxylic acid), 4-(2-aminoethyl)-benzenesulfonyl fluoride, phenylmethylsulfonyl fluoride, and pepstatin A. The activity was inhibited in a dose-dependent manner by serine protease inhibitors [15, 50, and 100% inhibition by 1, 10, and 100 μM 4-(2-aminoethyl)-benzenesulfonyl fluoride, respectively, and 46, 87, and 100% inhibition by 50, 500, and 5,000 μM phenylmethylsulfonyl fluoride, respectively]. However, other inhibitors did not affect the catalytic activity.
The kinetic parameters of ZHD101 with ZEN were determined at various pH values (Table 1). The activity of the enzyme was generally low (kcat < 1 s−1) in view of its use for detoxification of ZEN. As suggested by previous preliminary results (28), the maximal activity of ZHD101 occurred at pH 10.5; the kcat at pH 10.5 (0.51 s−1 at 30°C) was almost fivefold higher than that at pH 5.5. We also examined the kinetic parameters of the enzyme with α-zearalenol, β-zearalenol, zearalanone, α-zearalanol, and β-zearalanol (Fig. 1) at pH 7.5 and 10.5. Similar values were obtained with all of these ZEN cognates except β-zearalenol, which was the most unfavorable substrate for ZHD101 (Table 1); i.e., the kcat of the enzyme with β-zearalenol was much lower than that with ZEN (less than 20% of the value with ZEN at both pH 7.5 and 10.5).
TABLE 1.
Kinetic constants for ZHD101
| Substrate | pH | kcat (s−1) | Km (μM) | kcat/Km (s−1 M−1) |
|---|---|---|---|---|
| ZEN | 10.5 | 0.506 ± 0.051 | 34.3 ± 8.0 | 1.48 × 104 |
| 9.5 | 0.354 ± 0.030 | 16.7 ± 4.2 | 2.12 × 104 | |
| 8.5 | 0.230 ± 0.014 | 8.5 ± 2.1 | 2.72 × 104 | |
| 7.5 | 0.173 ± 0.011 | 5.1 ± 1.5 | 3.41 × 104 | |
| 6.5 | 0.173 ± 0.009 | 4.4 ± 0.8 | 3.96 × 104 | |
| 5.5 | 0.111 ± 0.005 | 44.5 ± 4.6 | 2.50 × 103 | |
| α-Zearalenol | 10.5 | 0.622 ± 0.063 | 111.3 ± 22.0 | 5.59 × 103 |
| 7.5 | 0.187 ± 0.005 | 13.8 ± 1.3 | 1.36 × 104 | |
| β-Zearalenol | 10.5 | 0.075 ± 0.004 | 15.1 ± 2.3 | 4.97 × 103 |
| 7.5 | 0.036 ± 0.004 | 2.2 ± 1.1 | 1.65 × 104 | |
| Zearalanone | 10.5 | 1.502 ± 0.126 | 118.2 ± 15.2 | 1.27 × 104 |
| 7.5 | 0.516 ± 0.039 | 21.0 ± 3.3 | 2.46 × 104 | |
| α-Zearalanol | 10.5 | 0.264 ± 0.016 | 45.2 ± 5.6 | 5.85 × 103 |
| 7.5 | 0.087 ± 0.010 | 19.3 ± 5.6 | 4.52 × 103 | |
| β-Zearalanol | 10.5 | 0.497 ± 0.036 | 63.5 ± 7.6 | 7.83 × 103 |
| 7.5 | 0.212 ± 0.010 | 25.4 ± 3.0 | 8.35 × 103 |
Characterization of recombinant EGFP::ZHD101 protein.
The kinetic parameters of recombinant protein EGFP::ZHD101 were also investigated, as shown in Table 2. When ZEN, α-zearalenol, and β-zearalenol were used as substrates, EGFP::ZHD101 had kcat and Km values similar to those of ZHD101. The results indicate that fusion of EGFP to the N terminus of ZHD101 did not affect the catalytic function of this enzyme.
TABLE 2.
Kinetic constants for EGFP::ZHD101
| Substrate | pH | kcat (s−1) | Km (μM) | kcat/Km (s−1 M−1) |
|---|---|---|---|---|
| ZEN | 10.5 | 0.443 ± 0.026 | 11.5 ± 2.5 | 3.84 × 104 |
| 9.5 | 0.528 ± 0.030 | 10.3 ± 2.1 | 5.13 × 104 | |
| 8.5 | 0.307 ± 0.012 | 6.3 ± 1.2 | 4.84 × 104 | |
| 7.5 | 0.267 ± 0.011 | 5.1 ± 1.1 | 5.25 × 104 | |
| 6.5 | 0.245 ± 0.015 | 8.3 ± 1.8 | 2.96 × 104 | |
| 5.5 | 0.154 ± 0.013 | 21.0 ± 5.0 | 7.33 × 103 | |
| α-Zearalenol | 10.5 | 0.763 ± 0.133 | 136.8 ± 35.6 | 5.58 × 103 |
| 7.5 | 0.185 ± 0.007 | 4.9 ± 0.9 | 3.74 × 104 | |
| β-Zearalenol | 10.5 | 0.146 ± 0.006 | 14.1 ± 2.0 | 1.04 × 104 |
| 7.5 | 0.055 ± 0.003 | 1.9 ± 0.6 | 2.90 × 104 |
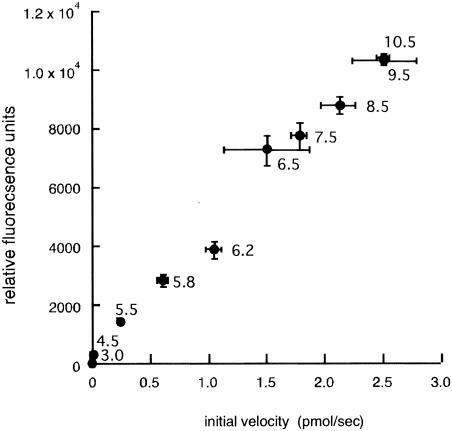
The molar fluorescence coefficients of the EGFP fusion enzyme and the original EGFP were similar (57,207 and 53,000, respectively), indicating that the fluorescence intensity was not affected by the C-terminally fused ZHD101 polypeptide in the fusion enzyme. The relationship between enzyme activity and the fluorescence intensity of the fusion enzyme was also examined at various pH values. The fluorescence of EGFP::ZHD101 decreased as the pH decreased, as did the lactonohydrolase activity of the fusion enzyme with ZEN as the substrate. Both the fluorescence intensity and the enzyme activity were sensitive to a change in pH, especially at pH values below 6.5. Fortuitously, the fluorescence intensity and the reaction velocity exhibited a good correlation (r = 0.9898) over the pH range from 3.0 to 10.5 (Fig. 3).
FIG. 3.
Relationship between the lactonohydrolase activity and the fluorescence intensity of EGFP::ZHD101 at various pH values. Four picomoles of EGFP::ZHD101 was used for each reaction.
Detoxification of ZEN and its derivatives by recombinant E. coli.
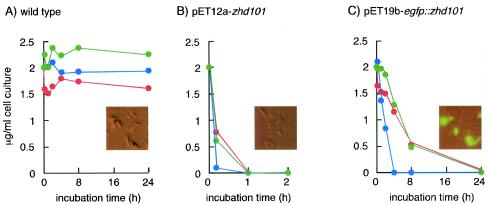
We next investigated whether recombinant E. coli strains carrying the detoxifying gene are able to remove ZEN and its derivatives in liquid medium. Wild-type or recombinant E. coli BL21 star (DE3) cells grown in the presence of IPTG were supplemented with 2 μg of ZEN per ml, 2 μg of α-zearalenol per ml, or 2 μg of β-zearalenol per ml. The wild-type bacteria metabolized neither ZEN nor its derivatives, and the mycotoxins were fully recovered as nontransformed forms from the media (Fig. 4A); they were not absorbed or adsorbed to the bacterial cells. On the other hand, an IPTG-induced culture of the recombinant bacteria carrying pET12a-zhd101 completely removed ZEN and its derivatives from the media (Fig. 4B). From the HPLC elution profile of the medium (diluted with an equal amount of methanol), we were able to detect the degradation product of ZEN (12), the structure of which was confirmed by gas chromatography-mass spectrometry analysis (data not shown).
FIG. 4.
Detoxification of ZEN and its derivatives by recombinant E. coli. Wild-type E. coli BL21 star (DE3) (A) and transformants of this strain carrying pET12a-zhd101 (B) and pET19b-egfp::zhd101 (C) were incubated with 2 μg of ZEN per ml (blue), 2 μg of α-zearalenol per ml (red), or 2 μg of β-zearalenol per ml (green). The amounts of ZEN and its derivatives were measured at appropriate times. (Insets) Fluorescent images of bacterial cells.
E. coli cells transformed with pET19b-egfp::zhd101 also degraded all of the ZEN and the ZEN derivatives α-zearalenol and β-zearalenol in the media (Fig. 4C). The degradation of α-zearalenol and β-zearalenol by recombinant E. coli was quite inefficient compared to the degradation of ZEN (Fig. 4B and C). For complete detoxification of the ZEN derivatives with E. coli (pET12a-zhd101) and E. coli (pET19b-egfp::zhd101), the incubation periods necessary were 1 and 24 h, respectively.
Detoxification of ZEN by recombinant yeast.
We first examined the fate of ZEN in the liquid medium by incubation with the wild-type yeast strain. As shown in Fig. 5A, approximately 60% of the ZEN added to the culture was stereoselectively reduced to β-zearalenol by day 4 and recovered from the medium. The total amount of mycotoxin (i.e., the sum of the amount of ZEN and the amount of β-zearalenol) was almost equal to the initial amount of ZEN, and trace amounts of other metabolites (e.g., α-zearalenol, which exhibits more potent estrogenicity) were not detected in either the supernatant or the cell debris.
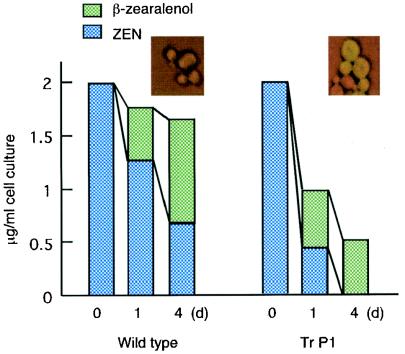
FIG. 5.
Detoxification of ZEN by recombinant yeast. ZEN (2 μg/ml) was added to an induced yeast culture. Yeast cells were collected on days 0, 1, and 4 for the wild-type and Tr P1. The bars indicate the amounts of ZEN and β-zearalenol. (Insets) Fluorescent images of yeast cells.
One of the yeast transformants, Tr P1, was incubated with ZEN, and the metabolites were analyzed with the reversed-phase column in the HPLC system. Tr P1 stably maintained intact copies of the plasmid outside the host genome in the absence of blasticidin S during the assay. As shown in Fig. 5, Tr P1 metabolized a significant amount of ZEN by day 1, and as much as 75% of the ZEN (all the ZEN that was not subjected to β-reduction [see below]) in the medium was completely removed by day 4. Again, we were able to detect the degradation product of ZEN (confirmed by gas chromatography-mass spectrometry) from the medium by HPLC (data not shown). Approximately 25% of ZEN remained in the medium as β-zearalenol, which is an unfavorable substrate for ZHD101 or EGFP::ZHD101 (Tables 1 and 2).
Detoxification of ZEN by transgenic rice cultured suspension cells.
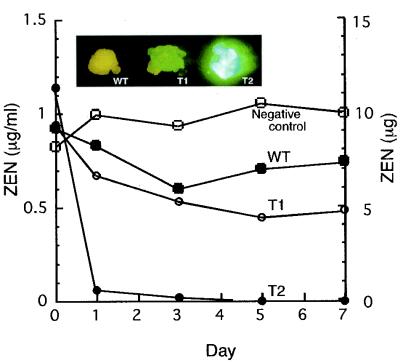
For the ZEN degradation assay, we used transgenic lines that exhibited low (Tr T1) and high (Tr T2) levels of EGFP fluorescence (Fig. 6). The fluorescent images of the two transgenic lines (Tr T1 and Tr T2) supported the results of the detoxification experiment; while cultured cell suspensions of Tr T2 rapidly detoxified almost all the ZEN in the liquid medium on day 1, a significant amount of ZEN remained in the Tr T1 culture supernatant on day 7. Compared to the fluorescence intensities of yeast transformants carrying egfp::zhd101, the fluorescence intensity of Tr T2 was very high. The amounts of ZEN within the cells were negligible (0.316 and 0.016 μg for Tr T1 and Tr T2, respectively) at the end of the assay.
FIG. 6.
Detoxification of ZEN by transgenic rice cultured suspension cells. ZEN (1 μg/ml) was added to cultured suspension cells of the wild type, Tr T1, and Tr T2. The media were collected on days 0, 1, 3, 5, and 7. The y axes on the left and right indicate the concentrations and total amounts of ZEN in the culture media, respectively. (Insets) Fluorescent images of rice calluses.WT, wild type; T1, Tr T1; T2, Tr T2.
Analysis of codon usage.
Analysis of the sequence of zhd101 with the Graphical Codon Usage Analyzer (http://gcua.schoedl.de/index.html) revealed that there were significant differences between the codon use of zhd101and that of S. cerevisiae; 13 and 59 of 265 codons of zhd101 were classified as “very few used codons” (i.e., less than 10% of the total codons for the corresponding amino acids were used) and “few used codons” (i.e., less than 20% of total codons for the corresponding amino acids were used), respectively. These codons were scattered throughout the coding region of the gene. The coding region of egfp similarly contained as many as 9 “very few used codons” and 74 “few used codons” in its coding region, which is obviously a significant drawback for expression of egfp::zhd101 in yeast strains. The codon usages were also unsuitable for expression in other microbes, such as Lactobacillus plantarum and Aspergillus oryzae. In contrast, the codon usage of egfp::zhd101 was suitable for expression in plants.
DISCUSSION
Purification of ZHD101 and cloning of the encoding gene from C. rosea have been described previously. Although the recombinant fission yeast Schizosaccharomyces pombe transformed with zhd101 produced a trace amount of the enzyme, detoxification of the mycotoxin did not occur in vivo (28). The insufficient expression of zhd101 was most likely due to the instability of the fission yeast plasmid pcDSP21 (14). In this study, we examined the biochemical properties of recombinant ZHD101 and EGFP::ZHD101 and evaluated the usefulness of zhd101 in the development of ZEN detoxification systems with various GM organisms. To facilitate selection of transformants with strong ZEN detoxification activity, we temporarily used egfp::zhd101 (which visually indicates the lactonohydrolase activity at various pH values) for transformation. While E. coli transformants showed strong EGFP fluorescence and detoxification activity, yeast transformants showed less fluorescence and weaker activity.
Recently, El-Nezami et al. (6) described detoxification of ZEN and α-zearalenol (2 μg/ml each) in media with food-grade Lactobacillus strains. In the system of these workers, these mycotoxins are removed from the media by adsorption to the bacterial cell surface. However, as much as 1010 viable cells/ml (nearly the limit of cell suspension) was required to remove approximately 50% of the mycotoxins in the media. In comparison, the initial inocula used in our experiment were much smaller (106 cells of E. coli per ml and 108 cells of S. cerevisiae per ml), but the detoxification of ZEN (2 μg/ml) was more efficient. Almost all ZEN and zearalenol (both α and β isomers) were completely inactivated by recombinant E. coli expressing zhd101 within 1 h, and as much as 75% of ZEN in the media was inactivated by the recombinant S. cerevisiae within 4 days. These results suggest that zhd101 could be used in developing a detoxification system for ZEN regardless of the apparently problematic features of the enzyme (i.e., the low kcat and high optimal pH) in biotechnological applications.
Previous studies revealed that beer and wine yeast strains converted ZEN to both α- and β-zearalenols and that the β isomer predominated in all experiments with various strains but that the levels were different; other possible transformation products (e.g., ZEN β-glucosides conjugated by filamentous fungi) were not detected even at trace levels (4). Consistent with these observations, wild-type strain INVSc1 of S. cerevisiae stereoselectively reduced the keto group at C-6′ of ZEN by an as-yet-unidentified reductase; approximately 60% of ZEN (2 μg/ml) was transformed to β-zearalenol, which was not a good substrate for the enzyme (Table 2). While E. coli transformants expressing egfp::zhd101 (driven by a very powerful T7 promoter on a high-copy-number plasmid) completely degraded 2 μg of β-zearalenol per ml in media, the transformed yeast strains could not detoxify this mycotoxin. This was likely due to the much lower level of expression of the gene (although it was driven by a strong GAL1 promoter) in yeast, as indicated by the marked difference in fluorescence intensity between the bacterial and yeast transformants (Fig. 4 and 5). To improve detoxification by the yeast transformants, it is necessary to increase expression of zhd101 to a level comparable to that in the E. coli transformants (Fig. 4C). By using a synthetic gene codon optimized for expression in suitable microbes (e.g., food-grade S. cerevisiae or Lactobacillus strains), the detoxification system may be exploited to develop GM food and feed in the future.
For cost-effective detoxification of ZEN, molecular breeding of crops with an ability to degrade the mycotoxin offers an alternative solution. A similar trial has been performed for detoxification of another mycotoxin; a gene encoding an enzyme that degrades fumonisin was cloned, and transgenic maize was tested for the ability to degrade the mycotoxin (5). Therefore, we evaluated the usefulness of zhd101 using cultured suspension cells of model monocotyledonous rice plants. In spite of the lower pH of the liquid medium, integrative transformants of cultured suspension cells transformed by egfp::zhd101 showed sufficient levels of fluorescence and ZEN-degrading activity. This encouraging result may indicate that it is feasible to produce transgenic cereals with sufficient ZEN detoxification activity in contaminated grains. However, food- and feed-related applications of zhd101 require further research and examination due to public and scientific concerns about GM technology.
So far, crops with beneficial traits, such as virus, insect, or herbicide resistance, have received official approval from regulatory agencies in the United States, the People's Republic of China, and other countries, and they are commercially available on a worldwide scale (19). On the other hand, there are only a few examples of authorized use of GM microbes, all including yeasts, in the United Kingdom and Japan, and such microbes have not been commercialized yet in food and feed (1, 2, 10). The major concern with GM microbes is the greater possibility of horizontal DNA transfer to members of other gut microbiota (20). Over the years, it has been generally agreed that such potential problems are avoidable and much exaggerated (29). Instead, there are increasing numbers of reports that describe potential benefits of live GM microbes in food and feed. For example, a feeding experiment demonstrated that GM ruminal bacteria are useful for protecting sheep from fluoroacetate poisoning (9). There is also a recent report that recombinant yeast may be exploited as a live vehicle in the human digestive environment (3). Since detoxification of ZEN is of great importance for stockbreeding and food safety, possible benefits related to the use of GM microbes (i.e., adding microbial cells that produce a ZEN-detoxifying enzyme to food and feed) should also be considered.
Acknowledgments
This research was supported in part by a grant for integrated research on the safety and physiological function of food to M.K. from the Ministry of Agriculture, Fishery, and Forestry of Japan.
We thank K. Mimori and T. Ochiai-Fukuda for transformation of rice calluses.
REFERENCES
- 1.Akada, R. 2002. Genetically modified industrial yeast ready for application. J. Biosci. Bioeng. 94:536-544. [DOI] [PubMed] [Google Scholar]
- 2.Aldhous, P. 1990. Genetic engineering. Modified yeast fine for food. Nature 344:186. [DOI] [PubMed] [Google Scholar]
- 3.Blanquet, S., J. P. Meunier, M. Minekus, S. Marol-Bonnin, and M. Alric. 2003. Recombinant Saccharomyces cerevisiae expressing P450 in artificial digestive systems: a model for biodetoxication in the human digestive environment. Appl. Environ. Microbiol. 69:2884-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böswald, C., G. Engelhardt, H. Vogel, and P. R. Wallnofer. 1995. Metabolism of the Fusarium mycotoxins zearalenone and deoxynivalenol by yeast strains of technological relevance. Nat. Toxins 3:138-144. [DOI] [PubMed] [Google Scholar]
- 5.Duvick, J. 2001. Prospects for reducing fumonisin contamination of maize through genetic modification. Environ. Health Perspect. 109(Suppl. 2):337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Nezami, H., N. Polychronaki, S. Salminen, and H. Mykkänen. 2002. Binding rather than metabolism may explain the interaction of two food-grade Lactobacillus strains with zearalenone and its derivative ά-zearalenol. Appl. Environ. Microbiol. 68:3545-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etienne, M., and M. Jemmali. 1982. Effects of zearalenone (F2) on estrous activity and reproduction in gilts. J. Anim. Sci. 55:1-10. [DOI] [PubMed] [Google Scholar]
- 8.Giniger, E., S. M. Varnum, and M. Ptashne. 1985. Specific DNA binding of GAL4, a positive regulatory protein of yeast. Cell 40:767-774. [DOI] [PubMed] [Google Scholar]
- 9.Gregg, K., B. Hamdorf, K. Henderson, J. Kopecny, and C. Wong. 1998. Genetically modified ruminal bacteria protect sheep from fluoroacetate poisoning. Appl. Environ. Microbiol. 64:3496-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond, J. R. 1995. Genetically-modified brewing yeasts for the 21st century. Progress to date. Yeast 11:1613-1627. [DOI] [PubMed] [Google Scholar]
- 11.Higa, A., M. Kimura, K. Mimori, T. Ochiai-Fukuda, T. Tokai, N. Takahashi-Ando, T. Nishiuchi, T. Igawa, M. Fujimura, H. Hamamoto, R. Usami, and I. Yamaguchi. 2003. Expression in cereal plants of genes that inactivate Fusarium mycotoxins. Biosci. Biotechnol. Biochem. 67:914-918. [DOI] [PubMed] [Google Scholar]
- 12.Kakeya, H., N. Takahashi-Ando, M. Kimura, R. Onose, I. Yamaguchi, and H. Osada. 2002. Biotransformation of the mycotoxin, zearalenone, to a non-estrogenic compound by a fungal strain of Clonostachys sp. Biosci. Biotechnol. Biochem. 66:2723-2726. [DOI] [PubMed] [Google Scholar]
- 13.Kiessling, K. H., H. Pettersson, K. Sandholm, and M. Olsen. 1984. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Appl. Environ. Microbiol. 47:1070-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura, M., T. Kamakura, Q. Z. Tao, I. Kaneko, and I. Yamaguchi. 1994. Cloning of the blasticidin S deaminase gene (BSD) from Aspergillus terreus and its use as a selectable marker for Schizosaccharomyces pombe and Pyricularia oryzae. Mol. Gen. Genet. 242:121-129. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, M., S. Sekido, Y. Isogai, and I. Yamaguchi. 2000. Expression, purification, and characterization of blasticidin S deaminase (BSD) from Aspergillus terreus: the role of catalytic zinc in enzyme structure. J. Biochem. 127:955-963. [DOI] [PubMed] [Google Scholar]
- 16.Kuiper-Goodman, T., P. M. Scott, and H. Watanabe. 1987. Risk assessment of the mycotoxin zearalenone. Reg. Tox. Pharmacol. 7:253-306. [DOI] [PubMed] [Google Scholar]
- 17.Miksicek, R. J. 1994. Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J. Steroid Biochem. Mol. Biol. 49:153-160. [DOI] [PubMed] [Google Scholar]
- 18.Mitterbauer, R., H. Weindorfer, N. Safaie, R. Krska, M. Lemmens, P. Ruckenbauer, K. Kuchler, and G. Adam. 2003. A sensitive and inexpensive yeast bioassay for the mycotoxin zearalenone and other compounds with estrogenic activity. Appl. Environ. Microbiol. 69:805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nap, J. P., P. L. Metz, M. Escaler, and A. J. Conner. 2003. The release of genetically modified crops into the environment. Part I. Overview of current status and regulations. Plant J. 33:1-18. [DOI] [PubMed] [Google Scholar]
- 20.Netherwood, T., R. Bowden, P. Harrison, A. G. O'Donnell, D. S. Parker, and H. J. Gilbert. 1999. Gene transfer in the gastrointestinal tract. Appl. Environ. Microbiol. 65:5139-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson, G. H., S. M. Knobel, W. D. Sharif, S. R. Kain, and D. W. Piston. 1997. Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys. J. 73:2782-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pittet, A. 1998. Natural occurrence of mycotoxins in foods and feeds—an updated review. Rev. Med. Vet. 149:479-492. [Google Scholar]
- 23.Ryu, D., L. S. Jackson, and L. B. Bullerman. 2002. Effects of processing on zearalenone. Adv. Exp. Med. Biol. 504:205-216. [DOI] [PubMed] [Google Scholar]
- 24.Scott, P. M., H. L. Trenholm, and M. D. Sutton. 1985. Mycotoxins: a Canadian perspective. National Research Council of Canada, Ottawa, Canada.
- 25.Shier, W. T., A. C. Shier, W. Xie, and C. J. Mirocha. 2001. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon 39:1435-1438. [DOI] [PubMed] [Google Scholar]
- 26.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi, N., H. Kakinuma, K. Hamada, K. Shimazaki, K. Takahashi, S. Niihata, Y. Aoki, H. Matsushita, and Y. Nishi. 1999. Efficient screening for catalytic antibodies using a short transition-state analog and detailed characterization of selected antibodies. Eur. J. Biochem. 261:108-114. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi-Ando, N., M. Kimura, H. Kakeya, H. Osada, and I. Yamaguchi. 2002. A novel lactonohydrolase responsible for the detoxification of zearalenone: enzyme purification and gene cloning. Biochem. J. 365:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuohy, K., M. Davies, P. Rumsby, C. Rumney, M. R. Adams, and I. R. Rowland. 2002. Monitoring transfer of recombinant and nonrecombinant plasmids between Lactococcus lactis strains and members of the human gastrointestinal microbiota in vivo—impact of donor cell number and diet. J. Appl. Microbiol. 93:954-964. [DOI] [PubMed] [Google Scholar]
- 30.U.S. National Toxicology Program. 1982. Carcinogenesis bioassay of zearalenone (CAS no. 17924-92-4) in F344/N rats and B6C3F1 mice (feed study). Natl. Toxicol. Program Tech. Rep. Ser. 235:1-155. [PubMed] [Google Scholar]
- 31.Wakita, Y., M. Otani, K. Iba, and T. Shimada. 1998. Co-integration, co-expression and co-segregation of an unlinked selectable marker gene and NtFAD3 gene in transgenic rice plants produced by particle bombardment. Genes Genet. Syst. 73:219-226. [DOI] [PubMed] [Google Scholar]