Abstract
This phase II/III, double-blind, randomized trial assessed the efficacy, immunogenicity and safety of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in young Chinese women (ClinicalTrials.gov registration NCT00779766). Women aged 18–25 years from Jiangsu province were randomized (1:1) to receive HPV vaccine (n = 3,026) or Al(OH)3 control (n = 3,025) at months 0, 1 and 6. The primary objective was vaccine efficacy (VE) against HPV-16/18 associated 6-month persistent infection (PI) and/or cervical intraepithelial neoplasia (CIN) 1+. Secondary objectives were VE against virological and clinical endpoints associated with HPV-16/18 and with high-risk HPV types, immunogenicity and safety. Mean follow-up for the according-to-protocol cohort for efficacy (ATP-E) was ∼15 months after the third dose. In the ATP-E (vaccine = 2,889; control = 2,894), for initially HPV DNA negative and seronegative subjects, HPV-16/18 related VE (95% CI) was 94.2% (62.7, 99.9) against 6-month PI and/or CIN1+ and 93.8% (60.2, 99.9) against cytological abnormalities. VE against HPV-16/18 associated CIN1+ and CIN2+ was 100% (−50.4, 100) and 100% (−140.2, 100), respectively (no cases in the vaccine group and 4 CIN1+ and 3 CIN2+ cases in the control group). At Month 7, at least 99.7% of initially seronegative vaccine recipients had seroconverted for HPV-16/18; geometric mean antibody titres (95% CI) were 6,996 (6,212 to 7,880) EU/mL for anti-HPV-16 and 3,309 (2,942 to 3,723) EU/mL for anti-HPV-18. Safety outcomes between groups were generally similar. The HPV-16/18 AS04-adjuvanted vaccine is effective, immunogenic and has a clinically acceptable safety profile in young Chinese women. Prophylactic HPV vaccination has the potential to substantially reduce the burden of cervical cancer in China.
What's New?
With an estimated 75,000 new cases and 34,000 women dying from the disease annually, cervical cancer is a major public-health concern in China. This is the first large scale randomised clinical trial of a human papillomavirus (HPV) vaccine in China. The vaccine was found to be effective, immunogenic, and to have a clinically acceptable safety profile in young Chinese women. Prophylactic HPV vaccination thus has the potential to substantially reduce the burden of cervical cancers and precancers in China.
Keywords: human papillomavirus vaccine, China, efficacy, safety, immunogenicity
With an estimated 75,000 new cases and ∼34,000 women dying from the disease annually, cervical cancer is a major public health concern in China.1 A pooled analysis of population-based studies in China revealed a high prevalence of high-risk human papillomavirus (HPV) infection and cervical intraepithelial neoplasia (CIN) 2+, indicating that the cervical cancer burden may be underestimated.2 China could face a significantly increased burden of this disease in the absence of implementation of comprehensive vaccination and effective screening programs.2
Consistent with observations worldwide, HPV-16 and HPV-18 are the genotypes most commonly associated with cervical cancer in China,3–5 and two prophylactic vaccines based on L1 proteins of HPV-16 and HPV-18 have been developed. In clinical trials, the HPV-16/18 AS04-adjuvanted vaccine (Cervarix®, GlaxoSmithKline Vaccines) was shown to be efficacious, immunogenic and to have a clinically acceptable safety profile in women from diverse geographic settings.6–9 The HPV-16/18 AS04-adjuvanted vaccine is licensed in more than 120 countries worldwide and is currently undergoing regulatory evaluation in China.
Following the completion of a Phase I trial in China,10 we now report data from the event-triggered final analysis of a Phase II/III trial of the vaccine in healthy young Chinese women. The primary objective was to assess vaccine efficacy (VE) for the prevention of 6-month persistent infection (PI) and/or histopathologically confirmed CIN1+ associated with HPV-16 and/or HPV-18 (HPV-16/18). Secondary objectives included efficacy against other virological and clinical endpoints associated with HPV-16/18 and other high-risk HPV types, immunogenicity and safety. An interim analysis of immunogenicity and safety was performed on a subset of subjects at month 7.11 The study is continuing in a blinded fashion and participants will be followed to month 48.
Material and Methods
Study design
This is an ongoing, double-blind, randomized placebo-controlled trial. Enrolment started in October 2008 and the last visit for the event-triggered analysis was in April 2011. The trial was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and the International Conference on Harmonisation Good Clinical Practice guidelines. This study is registered with ClinicalTrials.gov, number NCT00779766. The study protocol and informed consent were reviewed and approved by the ethics committees of the Center for Disease Control and Prevention (CDC) Jiangsu Province and the Cancer Foundation of China. An independent local Data Monitoring Committee was set up to provide advice and recommendation on statistical analysis and conduct of the study. Written informed consent was obtained from each participant prior to the performance of any study-specific procedures.
Participants
Healthy Chinese women aged 18–25 years at the time of first vaccination were enrolled at four sites in Jiangsu Province (Binhai, Jintan, Lianshui and Xuzhou CDCs). Women of childbearing potential were to be abstinent or to have used adequate contraceptive precautions for 30 days prior to first vaccination and agreed to continue such precautions for 2 months after completion of the vaccination series. Virgins were not enrolled in the study due to cultural and ethical considerations. Women had to have a single intact cervix. Women who were pregnant or breastfeeding, had an immunosuppressive or immunodeficient condition, a history of colposcopy, an allergic disease likely to be exacerbated by any component of the vaccine or previously received HPV vaccination or 3-O-desacyl-4′-monophosphoryl lipid A (MPL) or AS04 adjuvant were excluded.
Randomization and masking
Women were randomized 1:1 to receive HPV-16/18 AS04-adjuvanted vaccine or aluminum hydroxide as a control. Treatment allocation at the investigator site was performed using a central internet-based randomization system. All participants, investigators and study staff were blinded to individual subject treatment assignments and results.
Each vaccine dose contained HPV-16 and HPV-18 L1 virus-like particles (20 µg of each) adjuvanted with AS04 (50 µg MPL and 0.5 mg aluminum hydroxide). The vaccine and control were identical in appearance and were provided in prefilled syringes. 0.5 mL of vaccine or control was administered into the deltoid muscle at 0, 1 and 6 months.
Cytology and histopathology
Women were scheduled to attend a gynaecological examination with cervical samples collected at day 0 and at 6-month intervals until month 48. Cytological evaluation was conducted at the Cancer Institute of the Chinese Academy of Medical Sciences (CICAMS); results were reported according to the Bethesda 2001 classification system.12 Participants with abnormal results were managed according to prespecified algorithms, based on the American Society for Colposcopy and Cervical Pathology and European guidelines. The algorithms were consistent with those used in other global efficacy studies conducted with this vaccine.6,7 Histopathological analysis was performed by a panel of gynaecological pathologists at CICAMS. An independent committee reviewed blinded data for women assumed to meet criteria for efficacy endpoints, to make final case assignments.
HPV DNA testing
Cervical and biopsy samples were tested for DNA from 14 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) using SPF10 PCR-DEIA-LiPA25 version 1 (manufactured by Labo Biomedial Product, Rijswijk, the Netherlands based on licensed Innogenetics technology). HPV positive samples were also tested using HPV-16 and HPV-18 type-specific polymerase chain reaction (PCR).13 Detection of HPV DNA in the tissue biopsies was regarded as associated with the lesion. If more than one HPV type was detected, causality was attributed on the basis of detection of the same HPV type in at least one of the two preceding cytological samples.6
Immunogenicity
Blood samples were scheduled for collection from a subset of women at the Binhai site at months 0, 7, 12, 24, 36 and 48. HPV-16 and HPV-18 antibodies were measured using enzyme-linked immunosorbent assay (ELISA)14 at the National Institutes for Food and Drug Control, China. Assay cut-offs were 8 and 7 ELISA units (EU)/mL for anti-HPV-16 and anti-HPV-18, respectively.
Safety
Solicited local (pain, swelling and redness) and general (fatigue, fever, gastrointestinal symptoms, headache, arthralgia, myalgia, rash and urticaria) symptoms were recorded in diary cards for 7 days after each vaccination. Unsolicited symptoms were recorded for 30 days after each vaccination. Pregnancies and outcomes, serious adverse events (SAEs) and medically significant conditions were reported throughout the study.
Statistical analysis
The sample size was based on the number of events needed to demonstrate VE for key efficacy endpoints in the according-to-protocol cohort for efficacy (ATP-E), for those women who were seronegative by ELISA at month 0 and DNA negative by PCR at months 0 and 6 for the HPV type considered in the analysis. Assuming VE of 85%,6 it was calculated that 17 cases of the primary endpoint (6-month PI and/or CIN1+ associated with HPV-16 and/or HPV-18) were needed to provide at least 90% power to obtain a significant result (defined as lower limit of the 95% confidence interval [CI] for VE above 0%). Thus, final analysis was to be triggered when at least 17 cases of the primary endpoint had accrued. Based on an estimated yearly event rate of 1% for 6-month PI and/or CIN1+ in the control group,6 it was estimated that ∼3,000 evaluable subjects (1,500 per group) would be needed to accumulate 17 events before all subjects had completed their month 24 visit. The target enrolment of 6,000 women was estimated to provide ∼4,800 evaluable subjects (2,400 per group) at month 24 assuming that 20% of enrolled subjects would be nonevaluable because of drop-out or baseline seropositivity or DNA positivity for HPV-16 or HPV-18.
Assuming VE of 90% for the secondary endpoint of CIN2+ associated with HPV-16 and/or HPV-18,6,7 it was calculated that 12 cases of CIN2+ would be needed in the ATP-E to have at least 80% power to obtain a significant result. Based on an estimated yearly rate of 0.16% for CIN2+ associated with HPV-16 and/or HPV-18 in the control group,6,7 it is expected that 12 cases of CIN2+ will have accrued by the time of the end-of-study analysis at month 48, assuming that 4,200 subjects (2,100 per group) will be evaluable at the time of that analysis.
The principal analysis of efficacy was performed on the fully vaccinated ATP-E, which included all evaluable women who met eligibility criteria and complied with protocol procedures who received three doses of vaccine or control and had normal or low-grade cytology at baseline. Analysis was also performed on the total vaccinated cohort for efficacy (TVC-E), which included all vaccinated women for whom efficacy data were available and who had normal or low-grade cytology at baseline. The principal analysis was conducted in women who were HPV DNA negative at month 0 (ATP-E and TVC-E) and month 6 (ATP-E) and were seronegative at month 0 for the HPV type considered in the analysis.
For virological endpoints, a subject was defined as having a persistent cervical infection for a specified HPV type if there was a sequence of positive HPV DNA samples for that HPV type, not interrupted by negative samples, such that the total range was more than 5 months (>150 days) apart for 6-month PI and more than 10 months (>300 days) apart for 12-month PI. The start of the persistent cervical infection was defined as the date of the first positive sample in the sequence. Subjects had to have at least 5 months of follow-up after Month 12 to be included in analyses of 6-month PI and at least 10 months of follow-up after Month 12 to be included in analyses of 12-month PI.
VE was calculated using a conditional exact method,15 which computes an exact CI around the ratio of event rates in vaccine versus control groups and takes into account the follow-up time of participants within each group. VE was defined as 1 minus the rate ratio. The follow-up for each woman started the day after third vaccination for the ATP-E and the day after first vaccination for the TVC-E. Follow-up ended either at the time of an event or the last date when data were available for the considered endpoint.
Safety analyses were performed on the total vaccinated cohort (TVC) of all vaccinated women for whom safety data were available and data are summarised using descriptive statistics. To prevent unblinding, the treatment assignment is not disclosed for events reported in one group only.
Immunogenicity analyses were performed on the ATP cohort for immunogenicity (subjects who received three doses, met eligibility criteria, complied with protocol procedures and had results for antibodies against at least one vaccine antigen). Subjects who acquired either HPV-16 or HPV-18 infection during the trial were excluded from this cohort. Seropositivity rates and geometric mean antibody titres (GMTs) for each antigen with 95% CI were calculated. To maintain blinding, all analyses were carried out by an external statistician. Statistical analyses were performed using SAS® 9.2 and PROC StatXact® 8.1.
Results
Study population
A total of 6,081 women were enrolled at four sites in the Jiangsu Province of China and 6,051 subjects were included in the TVC (Fig. 1). Mean age at time of first vaccination was 23.0 years in each group. Other demographic and baseline characteristics are shown in Table1. Further description of baseline HPV DNA prevalence and seroprevalence are reported in a companion article.16
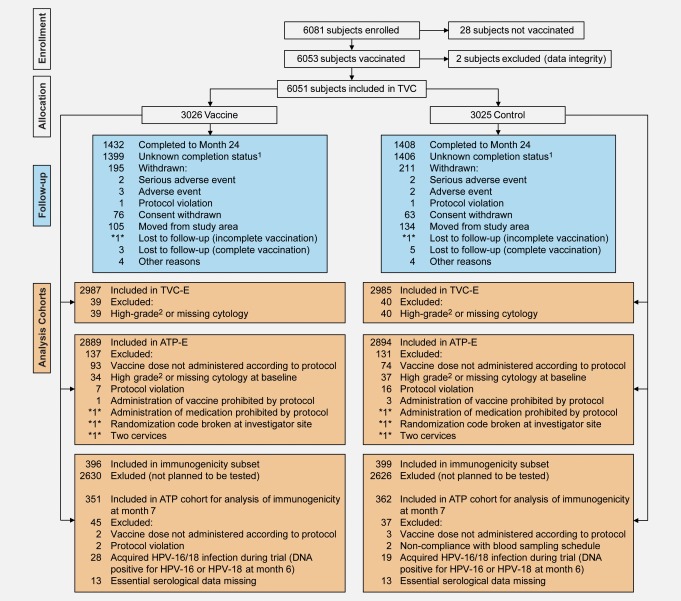
Figure 1.
Flow of participants through the trial. TVC, total vaccinated cohort (all vaccinated subjects for whom data were available); TVC-E, total vaccinated cohort for efficacy (all vaccinated women for whom efficacy data were available and who had normal or low-grade cytology at baseline); ATP-E, according to protocol cohort for efficacy (all evaluable women who met eligibility criteria and complied with protocol procedures who received three doses of vaccine or control and had normal or low-grade cytology at baseline). *n*: number present in one group only and duplicated to avoid unblinding of ongoing study. 1Completion status at Month 24 was unknown for these subjects at the time of this event-triggered analysis. 2ASC-H, HSIL, AGC or malignancy. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table 1.
Demographic and baseline characteristics (TVC)
| Vaccine N = 3026 | Control N = 3025 | |
|---|---|---|
| Age at vaccination, years (SD) | 23.0 (1.70) | 23.0 (1.75) |
| Region within Jiangsu Province, n (%) | ||
| Xuzhou City | 842 (27.8) | 842 (27.8) |
| Jintan County | 771 (25.5) | 771 (25.5) |
| Binhai County | 601 (19.9) | 601 (19.9) |
| Lianshui County | 812 (26.8) | 811 (26.8) |
| Compliance with vaccination, n (%) | ||
| Received only one dose | 30 (1.0) | 33 (1.1) |
| Received only two doses | 62 (2.0) | 41 (1.4) |
| Received all three doses | 2,934 (97.0) | 2,951 (97.6) |
| Previous and prevalent infection at entry, n (%)1 | ||
| HPV-16 | ||
| Seronegative and HPV DNA negative | 2,085 (68.9) | 2,047 (67.7) |
| Seropositive and HPV DNA negative | 816 (27.0) | 865 (28.6) |
| Seronegative and HPV DNA positive | 26 (0.9) | 36 (1.2) |
| Seropositive and HPV DNA positive | 94 (3.1) | 66 (2.2) |
| Missing data | 5 (0.2) | 11 (0.4) |
| HPV-18 | ||
| Seronegative and HPV DNA negative | 2,514 (83.1) | 2,513 (83.1) |
| Seropositive and HPV DNA negative | 467 (15.4) | 465 (15.4) |
| Seronegative and HPV DNA positive | 15 (0.5) | 24 (0.8) |
| Seropositive and HPV DNA positive | 24 (0.8) | 11 (0.4) |
| Missing data | 6 (0.2) | 12 (0.4) |
| Cytological status at entry, n (%)2 | ||
| Normal cytology | 2,721 (89.9) | 2,695 (89.1) |
| With high-risk HPV DNA | 287 (10.6) | 271 (10.1) |
| With high-risk HPV DNA other than vaccine type | 226 (8.3) | 236 (8.8) |
| With vaccine type (HPV-16/18) DNA3 | 83 (3.1) | 58 (2.2) |
| ASC-US and LSIL | 266 (8.8) | 290 (9.6) |
| With high-risk HPV DNA | 157 (59.0) | 156 (53.8) |
| With high-risk HPV DNA other than vaccine type | 134 (50.4) | 130 (44.8) |
| With vaccine type (HPV-16/18) DNA3 | 45 (16.9) | 55 (19.0) |
| HSIL, ASC-H and AGC | 34 (1.1) | 29 (1.0) |
| With high-risk HPV DNA | 29 (85.3) | 24 (82.8) |
| With high-risk HPV DNA other than vaccine type | 17 (50.0) | 16 (55.2) |
| With vaccine type (HPV-16/18) DNA3 | 23 (67.7) | 15 (51.7) |
AGC: atypical glandular cells; ASC-H: atypical squamous cells cannot exclude HSIL; ASC-US: atypical squamous cells of undetermined significance; HSIL: high-grade squamous intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion; N: number of subjects; n (%): number (percentage) of subjects in a given category.
Serostatus determined by ELISA and HPV DNA in cervical samples determined by PCR.
Five women in the vaccine group and 11 women in the control group had missing cytology results.
Includes women with HPV-16, HPV-18 or both.
High-risk HPV includes HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68. High-risk HPV other than vaccine type includes HPV types 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68.
A total of 5,885 (97.3%) participants completed the full three-dose vaccination schedule (Table1). The proportion of participants who were known to have withdrawn from the study at the time of this event-triggered final analysis was balanced between the groups (vaccine: 195 [6.4%]; control: 211 [7.0%]). 5,972 (98.7%) and 5,783 (95.6%) participants were included in the TVC-E and ATP-E. Reasons for withdrawal and for exclusion from cohorts are shown in Figure 1.
At the time of analysis, the mean duration of follow-up was 15.3 months (standard deviation [SD] 3.8) for the ATP-E (counting started the day after the third dose) and 21.0 months (SD 4.7) for the TVC and TVC-E (counting started the day after the first dose). Follow-up will continue until Month 48.
Efficacy
In the ATP-E, for initially HPV DNA and seronegative subjects, high VE was shown for the primary endpoint of HPV-16/18 associated 6-month PI and/or histopathologically-confirmed CIN1+ (94.2% [62.7, 99.9]) (Table2). There were 17 cases in the control group (2 cases of 6-month PI plus CIN1+, 13 cases of 6-month PI alone and 2 cases of CIN1+ alone) and 1 case in the vaccine group (of 6-month PI). High VE for the primary endpoint was also shown in the TVC-E (88.9% [69.1, 97.1]; Table3).
Table 2.
VE against incident and persistent infection, cytological abnormalities and CIN associated with HPV-16 and/or HPV-18 in women who were HPV DNA negative and seronegative at baseline for the corresponding HPV type (according-to-protocol cohort for efficacy)
| Vaccine |
Control |
||||||
|---|---|---|---|---|---|---|---|
| Endpoint | N | n | Event rate (95% CI)1 | N | n | Event rate (95% CI)1 | VE (95% CI) |
| 6-Month PI and/or CIN1+ (primary endpoint) | |||||||
| HPV-16/182 | 2,497 | 1 | 0.03 (0.00, 0.18) | 2,502 | 17 | 0.56 (0.33, 0.90) | 94.2% (62.7, 99.9) |
| HPV-16 | 1,937 | 1 | 0.04 (0.00, 0.24) | 1,884 | 15 | 0.67 (0.37, 1.10) | 93.6% (58.1, 99.9) |
| HPV-18 | 2,320 | 0 | 0.00 (0.00, 0.13) | 2,314 | 3 | 0.11 (0.02, 0.31) | 100% (−140.3, 100) |
| Incident infection | |||||||
| HPV-16/182 | 2,497 | 15 | 0.49 (0.28, 0.82) | 2,502 | 49 | 1.62 (1.20, 2.15) | 69.6% (44.8, 84.2) |
| HPV-16 | 1,935 | 9 | 0.39 (0.18, 0.74) | 1,884 | 30 | 1.34 (0.90, 1.91) | 71.0% (37.3, 87.9) |
| HPV-18 | 2,321 | 7 | 0.25 (0.10, 0.51) | 2,314 | 21 | 0.75 (0.46, 1.14) | 66.9% (19.2, 88.1) |
| 6-Month PI | |||||||
| HPV-16/182 | 2,332 | 1 | 0.03 (0.00, 0.19) | 2,326 | 15 | 0.52 (0.29, 0.86) | 93.4% (57.1, 99.8) |
| HPV-16 | 1,810 | 1 | 0.04 (0.00, 0.25) | 1,747 | 13 | 0.61 (0.32, 1.04) | 92.6% (50.8, 99.8) |
| HPV-18 | 2,168 | 0 | 0.00 (0.00, 0.14) | 2,154 | 3 | 0.11 (0.02, 0.33) | 100% (−139.1, 100) |
| 12-Month PI | |||||||
| HPV-16/182 | 1,111 | 0 | 0.00 (0.00, 0.22) | 1,091 | 2 | 0.12 (0.01, 0.44) | 100% (−421.8, 100) |
| HPV-16 | 799 | 0 | 0.00 (0.00, 0.30) | 768 | 2 | 0.17 (0.02, 0.62) | 100% (−410.6, 100) |
| HPV-18 | 1,034 | 0 | 0.00 (0.00, 0.24) | 1,014 | 0 | 0.00 (0.00, 0.24) | – |
| Any cytological abnormality (ASC-US+) | |||||||
| HPV-16/182 | 2,494 | 1 | 0.04 (0.00, 0.22) | 2,502 | 16 | 0.65 (0.37, 1.05) | 93.8% (60.2, 99.9) |
| HPV-16 | 1,935 | 1 | 0.05 (0.00, 0.30) | 1,884 | 12 | 0.67 (0.34, 1.17) | 91.9% (45.5, 99.8) |
| HPV-18 | 2,318 | 0 | 0.00 (0.00, 0.16) | 2,314 | 5 | 0.22 (0.07, 0.51) | 100% (−7.8, 100) |
| CIN1+ | |||||||
| HPV-16/182 | 2,497 | 0 | 0.00 (0.00, 0.15) | 2,502 | 4 | 0.16 (0.04, 0.41) | 100% (−50.4, 100) |
| HPV-16 | 1,937 | 0 | 0.00 (0.00, 0.20) | 1,884 | 4 | 0.22 (0.06, 0.57) | 100% (−46.9, 100) |
| HPV-18 | 2,320 | 0 | 0.00 (0.00, 0.16) | 2,314 | 0 | 0.00 (0.00, 0.16) | – |
| CIN2+ | |||||||
| HPV-16/182 | 2,497 | 0 | 0.00 (0.00, 0.15) | 2,502 | 3 | 0.12 (0.02, 0.35) | 100% (−140.2, 100) |
| HPV-16 | 1,937 | 0 | 0.00 (0.00, 0.20) | 1,884 | 3 | 0.17 (0.03, 0.48) | 100% (−134.7, 100.0) |
| HPV-18 | 2,320 | 0 | 0.00 (0.00, 0.16) | 2,314 | 0 | 0.00 (0.00, 0.16) | – |
ASC-US+: atypical squamous cells of undetermined significance or higher; CIN1+: cervical intraepithelial neoplasia grade 1 or higher; CIN2+: cervical intraepithelial neoplasia grade 2 or higher; N: number of evaluable women in each group (for single HPV types women had to be DNA negative at months 0 and 6 and seronegative at month 0 for the corresponding HPV type; for combined HPV types women had to be DNA negative at months 0 and 6 and seronegative at month 0 for at least one HPV type); n: number of evaluable women reporting at least one event in each group; CI, confidence interval; PI: persistent infection.
Number of cases divided by sum of follow-up period (per 100 woman years); follow-up period started on day after third vaccine dose.
Women could be infected with one or both HPV types (thus, number of women with a HPV-16-associated lesion and number with a HPV-18-associated lesion might not equal number of women with a HPV-16/18-associated lesion).
Table 3.
VE against incident and persistent infection, cytological abnormalities and CIN associated with HPV-16 and/or HPV-18 in women who were HPV DNA negative and seronegative at baseline for the corresponding HPV type (TVC for efficacy)
| Vaccine |
Control |
||||||
|---|---|---|---|---|---|---|---|
| Endpoint | N | n | Event rate (95% CI)1 | N | n | Event rate (95% CI)1 | VE (95% CI) |
| 6-Month PI and/or CIN1+ (primary endpoint) | |||||||
| HPV-16/182 | 2,543 | 4 | 0.09 (0.02, 0.23) | 2,554 | 36 | 0.83 (0.58, 1.15) | 88.9% (69.1, 97.1) |
| HPV-16 | 1,975 | 3 | 0.09 (0.02, 0.26) | 1,930 | 24 | 0.74 (0.47, 1.10) | 87.8% (60.0, 97.7) |
| HPV-18 | 2,362 | 1 | 0.02 (0.00, 0.14) | 2,366 | 13 | 0.32 (0.17, 0.55) | 92.3% (48.9, 99.8) |
| Incident infection | |||||||
| HPV-16/182 | 2,609 | 30 | 0.69 (0.46, 0.98) | 2,637 | 78 | 1.79 (1.41, 2.23) | 61.6% (40.9, 75.7) |
| HPV-16 | 2,021 | 19 | 0.57 (0.34, 0.88) | 1,995 | 48 | 1.47 (1.08, 1.94) | 61.4% (33.1, 78.6) |
| HPV-18 | 2,423 | 13 | 0.32 (0.17, 0.55) | 2,441 | 35 | 0.86 (0.60, 1.20) | 62.9% (28.2, 82.0) |
| 6-Month PI | |||||||
| HPV-16/182 | 2,517 | 3 | 0.07 (0.01, 0.20) | 2,531 | 33 | 0.76 (0.53, 1.07) | 91.0% (71.2, 98.2) |
| HPV-16 | 1,959 | 2 | 0.06 (0.01, 0.22) | 1,915 | 21 | 0.65 (0.40, 0.99) | 90.7% (62.1, 99.0) |
| HPV-18 | 2,340 | 1 | 0.02 (0.00, 0.14) | 2,346 | 13 | 0.32 (0.17, 0.55) | 92.3% (49.0, 99.8) |
| 12-month PI | |||||||
| HPV-16/182 | 2,411 | 2 | 0.05 (0.01, 0.17) | 2,434 | 12 | 0.28 (0.15, 0.49) | 83.3% (24.9, 98.2) |
| HPV-16 | 1,877 | 1 | 0.03 (0.00, 0.17) | 1,839 | 7 | 0.22 (0.09, 0.45) | 86.0% (−8.7, 99.7) |
| HPV-18 | 2,242 | 1 | 0.03 (0.00, 0.14) | 2,260 | 5 | 0.13 (0.04, 0.30) | 80.0% (−79.2, 99.6) |
| Any cytological abnormality (ASC-US+) | |||||||
| HPV-16/182 | 2,539 | 3 | 0.08 (0.02, 0.23) | 2,552 | 26 | 0.69 (0.45, 1.01) | 88.5% (62.5, 97.8) |
| HPV-16 | 1,972 | 2 | 0.07 (0.01, 0.25) | 1,929 | 19 | 0.68 (0.41, 1.06) | 89.7% (57.5, 98.8) |
| HPV-18 | 2,358 | 1 | 0.03 (0.00, 0.16) | 2,365 | 9 | 0.26 (0.12, 0.49) | 88.9% (20.1, 99.8) |
| CIN1+ | |||||||
| HPV-16/182 | 2,543 | 1 | 0.03 (0.00, 0.15) | 2,554 | 6 | 0.16 (0.06, 0.34) | 83.3% (−37.3, 99.6) |
| HPV-16 | 1,975 | 1 | 0.03 (0.00, 0.19) | 1,930 | 6 | 0.21 (0.08, 0.46) | 83.7% (−34.3, 99.7) |
| HPV-18 | 2,362 | 0 | 0.00 (0.00, 0.10) | 2,366 | 0 | 0.00 (0.00, 0.10) | – |
| CIN2+ | |||||||
| HPV-16/182 | 2,543 | 0 | 0.00 (0.00, 0.10) | 2,554 | 4 | 0.11 (0.03, 0.27) | 100% (−51.4, 100) |
| HPV-16 | 1,975 | 0 | 0.00 (0.00, 0.13) | 1,930 | 4 | 0.14 (0.04, 0.36) | 100% (−48.0, 100) |
| HPV-18 | 2,362 | 0 | 0.00 (0.00, 0.10) | 2,366 | 0 | 0.00 (0.00, 0.10) | – |
ASC-US+: atypical squamous cells of undetermined significance or higher; CIN1+: cervical intraepithelial neoplasia grade 1 or higher; CIN2+: cervical intraepithelial neoplasia grade 2 or higher; N: number of evaluable women in each group (for single HPV type women had to be DNA negative and seronegative for the corresponding HPV type at month 0; for combined HPV types women had to be DNA negative and seronegative for at least one HPV type at month 0); n: number of evaluable women reporting at least one event in each group; CI: confidence interval; PI: persistent infection.
Number of cases divided by sum of follow-up period (per 100 woman years); follow-up period started on day after first vaccine dose.
Women could be infected with one or both HPV types (thus, number of women with a HPV-16-associated lesion and number with a HPV-18-associated lesion might not equal number of women with a HPV-16/18-associated lesion).
In the ATP-E, VE was 93.4% (57.1, 99.8) against HPV-16/18 associated 6-month PI, 69.6% (44.8, 84.2) against incident cervical infection and 93.8% (60.2, 99.9) against any cytological abnormalities (Table2). VE against HPV-16 and/or HPV-18 associated 12-month PI was not statistically significant in the ATP-E (100% [-421.8, 100]), but was statistically significant in the TVC-E (83.3% [24.9, 98.2]) due to the additional cases accumulated in this larger cohort (Table3).
At the time of this analysis only a small number of cases of CIN were observed (Tables2 and 3). In the ATP-E, VE was 100% (−50.4, 100) against HPV-16/18 associated CIN1+ and 100% (−140.2, 100) against CIN2+, with 4 and 3 cases, respectively. All these cases were in the control group and were associated with HPV-16.
Immunogenicity
In the ATP cohort for immunogenicity, the seroconversion rate at month 7 in initially seronegative women was 100% for anti-HPV-16 and 99.7% for anti-HPV-18 and GMTs (95% CI) were 6,996 (6,212 to 7,880) and 3,309 (2,942 to 3,723) EU/mL, respectively (Fig. 2). All initially seropositive women in the vaccine group remained seropositive for anti-HPV-16 and anti-HPV-18 antibodies at month 7 and GMTs were 5,698 (4,703 and 6,904) and 3,242 (2,736 and 3,842) EU/mL, respectively.
Figure 2.
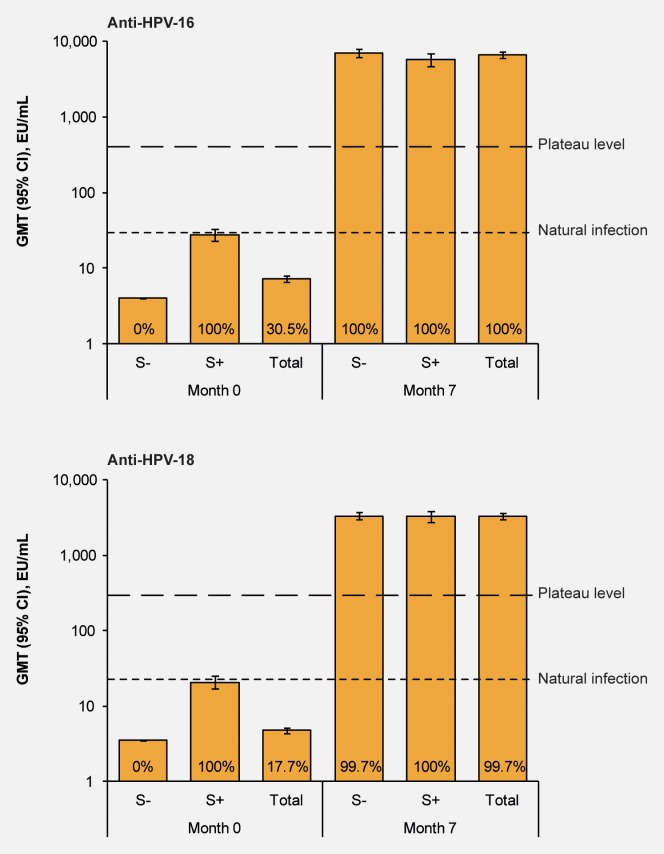
GMTs at Month 0 and Month 7 for women in the vaccine group by baseline serostatus (according-to-protocol cohort for immunogenicity). CI: confidence interval; GMT: geometric mean antibody titre; S-: seronegative prior to vaccination (n = 244 for anti-HPV-16 and n = 289 for anti-HPV-18); S+, seropositive (titre ≥8 EU/mL for anti-HPV-16 and ≥7 EU/mL for anti-HPV-18) prior to vaccination (n = 107 for anti-HPV-16 and n = 62 for anti-HPV-18); Total, all women irrespective of serostatus (n = 351). Natural infection, GMT in women who had cleared a natural infection.6 Plateau level, GMT at the plateau level (Month 45–50) in a previous study in women aged 15–25 years in which sustained protection with the HPV-16/18 AS04-adjuvanted vaccine was shown up to 6.4 years after first vaccination.9 Numbers at the base of each bar are the percentage of seropositive women. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Safety
Solicited symptoms were generally mild, self-limiting and of short duration (Supporting Information 1 and 2). Injection site symptoms (pain, redness and swelling) were reported in a numerically higher percentage of subjects in the vaccine group than the control group.
There were no apparent differences in the incidence of unsolicited symptoms, SAEs, medically significant conditions, new onset chronic diseases, new onset autoimmune diseases or pregnancy outcomes between groups (Table4).
Table 4.
Safety and pregnancy outcomes at the time of event-triggered final analysis (TVC)
| Vaccine N = 3026 | Control N = 3025 | |
|---|---|---|
| Unsolicited symptoms within 30 days postvaccination, n (%) | ||
| Any | 793 (26.2) | 775 (25.6) |
| Grade 3 | 18 (0.6) | 18 (0.6) |
| Related to vaccination1 | 35 (1.2) | 32 (1.1) |
| Serious and other significant adverse events, n (%) | ||
| Serious adverse events | 29 (1.0) | 55 (1.8) |
| Number of serious adverse events | 36 | 57 |
| Number of serious adverse events related to vaccination1 | *1* | *1* |
| Fatal adverse events | *1* | *1* |
| Adverse events leading to premature discontinuation | 5 (0.2) | 4 (0.1) |
| Medically significant conditions | 158 (5.2) | 156 (5.2) |
| New onset chronic diseases | 8 (0.3) | 11 (0.4) |
| New onset autoimmune diseases | 2 (0.1) | 2 (0.1) |
| Pregnancy outcomes, n (%) | ||
| Live infant: no apparent congenital anomaly | 106 (56.4) | 124 (54.1) |
| Live infant: congenital anomaly2 | *1* | *1* |
| Elective termination: no apparent congenital anomaly | 41 (21.8) | 65 (28.4) |
| Elective termination: congenital anomaly2 | *1* | *1* |
| Spontaneous abortion: no apparent congenital anomaly | 7 (3.7) | 9 (3.9) |
| Still birth: no apparent congenital anomaly | *1* | *1* |
| Ectopic pregnancy | 4 (2.1) | 6 (2.6) |
| Pregnancy ongoing | 27 (14.4) | 23 (10.0) |
N: number of evaluable women in each group; n (%): number (percentage) of subjects with the event.
*n*: number present in one group only and duplicated to avoid unblinding of ongoing study.
Assessed as causally related by the investigator.
The congenital anomalies for the live births were acleistocardia, congenital muscular torticollis and cleft lip and palate. The congenital anomaly for the elective termination was cyst on head and hydrocephalus.
At the time of this analysis, one fatal SAE (suicide) was reported, which was assessed by the investigator as unrelated to vaccination. One SAE (gastrointestinal tract infection) was assessed by the investigator as possibly related to vaccination. Treatment allocation for these events has not been disclosed to avoid unblinding.
Discussion
China has a population of over 500 million women aged 15 years and older17 who are at risk of developing cervical cancer, but at present there are no vaccines licensed in mainland China for the prevention of this disease. In previous global studies with HPV vaccines, a relatively small proportion of subjects were of Chinese ethnicity.6,18,19 This trial of the HPV-16/18 AS04-adjuvanted vaccine is the first large, controlled clinical study of an HPV vaccine in mainland China. The current prospectively defined, event-triggered analysis showed that the vaccine has an acceptable safety profile and good immunogenicity in Chinese women and provides preliminary evidence regarding VE in this population. This timely information will be useful to policy makers in China and other Asian countries to support decisions regarding HPV vaccination for the prevention of cervical cancer in addition to cervical screening programs. Moreover, our data add to the body of evidence on HPV vaccines across different regions and ethnicities.
Our trial had a largely similar design to other large trials evaluating the efficacy of the HPV-16/18 AS04-adjuvanted vaccine, including the global PATRICIA (PApilloma TRIal against Cancer In young Adults) trial6,7 and the community-based trial in Guanacaste, Costa Rica,20 with regard to age of study subjects, vaccination schedule, clinical management for abnormal cytology and colposcopy referral and HPV DNA testing. A major difference between the current trial and these other studies is that the primary endpoint was prospectively defined as prevention of 6-month PI and/or histopathologically confirmed CIN1+ associated with HPV-16/18, with CIN2+ as a secondary endpoint, whereas the PATRICIA and Guanacaste trials defined CIN2+ as the primary endpoint.6,7,20
Although CIN2+ is the traditional surrogate to cervical cancer for evaluation of VE,21 it is limited by the large sample size and/or extensive follow-up required to obtain enough cases for assessment. PI is a reliably determined and objective clinical marker, independent of HPV type multiplicity, which shows good predictive value to CIN3 and cervical cancer.22–28 Global bodies such as the World Health Organization have recognized the utility of virological endpoints for the evaluation of HPV vaccines,21 although there is some debate on how persistent HPV infection should be defined when used as a surrogate. A meta-analysis conducted by Koshiol et al. suggested that association between HPV persistence and high-grade disease appeared strongest for longer duration of HPV infection (>12 months) and wider HPV testing intervals (>6 months or >12 months), but even the shortest HPV duration and testing intervals (≤6 months) were associated with an increased relative risk for development of high-grade disease.22 Thus, to gain an early insight into VE in the Chinese setting over the first 2 years of our study, in addition to immunogenicity and safety, the current event-triggered analysis used a composite of virological and clinical endpoints, incorporating a 6-month definition of PI. The trial is ongoing and efficacy data from a 4-year end-of-study analysis will allow further evaluation of efficacy, including longer term persistence and CIN2+, when more endpoints have accrued.
This event-triggered final analysis demonstrated high prophylactic efficacy of the HPV-16/18 AS04-adjuvanted vaccine against the composite primary endpoint of 6-month PI and/or CIN1+ associated with HPV-16 and/or HPV-18. High VE against incident infection, 6-month PI and cytological abnormalities associated with HPV-16 and/or HPV-18 was also shown. Due to the relatively short duration of follow-up at the time of this event-triggered analysis, the effect size for the primary composite endpoint was principally driven by cases of 6-month PI. The number of cases of CIN was limited, although all observed cases in the ATP-E were in the control group. There was also an insufficient number of HPV-18 infections to robustly assess VE against 6-month PI and/or CIN1+ associated with HPV-18 alone. It is expected that by the time of the end-of-study analysis, more data will be available for these endpoints.
Our results are in line with observations from the global PATRICIA trial, which evaluated the efficacy of the HPV-16/18 AS04-adjuvanted vaccine in women aged 15–25 years from a broad range of nationalities and ethnicities.6,7 In PATRICIA, high VE was shown against 6-month and 12-month PI and CIN2+, associated with HPV-16/18.6,7 In a 4-year end-of-study analysis of PATRICIA high VE was also shown against CIN2+ and CIN3+ irrespective of HPV type in the lesion29 and consistent cross-protective efficacy was shown against HPV-31, -33, -45 and -51.30 Results from the current trial are also consistent with a Phase II trial of the HPV-16/18-AS04 adjuvanted vaccine conducted in Japan, in which statistically significant VE was shown against 6-month and 12-month PI and cytological abnormalities associated with HPV-16/18.31
The follow-up time for our analysis is closer to that of the interim analysis of the global PATRICIA trial (∼15 months after the first vaccine dose)6 than that of the event-triggered final analysis,7 or the end-of-study analysis.29 This is an important consideration when comparing results across studies, because the observed efficacy estimates appeared to increase with longer duration of follow-up in PATRICIA. The planned 4-year follow-up of more than 6,000 subjects in the current trial should contribute to a better understanding of VE in the Chinese population, including cross-protection against non-vaccine HPV types.
Strong antibody responses were elicited by the vaccine against both HPV-16 and HPV-18 in the population of young Chinese women. Antibody levels one month after the last vaccine dose were in the same range as those observed in women of a similar age participating in global studies.6,32 It has previously been shown that total and neutralising antibody titres elicited by the HPV-16/18 AS04-adjuvanted vaccine are sustained at levels several-fold above those observed for natural infection for up to 9.4 years after vaccination.33 We are continuing to evaluate immunogenicity in the current study and the kinetics of the immune response in Chinese women will be reported more fully in due course.
Safety outcomes between groups were generally similar, except injection site symptoms were more common following administration of vaccine than control, in line with global findings.8 Symptoms did not affect overall compliance with the vaccination schedule. Incidence of solicited symptoms was generally lower than in a previous pooled analysis evaluating the safety of the HPV-16/18 AS04-adjuvanted vaccine in a multiethnic and geographically diverse cohort of almost 30,000 girls and women aged ≥10 years, which showed that the vaccine had an acceptable safety profile in women of all ages.8 The only notable difference compared with the established reactogenicity profile is that the incidence of fever was higher in the current study than reported previously (∼10% vs. 5%).8 This is due to the threshold for fever, which was defined as oral or axillary temperature of >37.0°C in the current study (as per Chinese clinical trial regulation) and >37.5°C in global studies. The incidence of fever was ∼2% in this study using the definition of 37.5°C. The incidence of grade 3 fever, defined using a universal threshold of >39.0°C, was similarly low in this study and the previous pooled analysis (<0.5%).8
Since the current HPV vaccines are prophylactic and do not alter the course of prevalent HPV infection or pre-existing lesions,34 vaccination of women before sexual debut is a cost-effective and pragmatic strategy to prevent cervical cancer, especially in the absence of effective screening programs. In a cross-sectional epidemiologic survey across urban and rural areas of China, there was a trend towards earlier sexual debut (median age of 17 years) and riskier sexual behaviours in younger cohorts of Chinese women.35 Furthermore, 15% of women in this study were DNA positive for high-risk HPV at baseline.16 These findings suggest that HPV vaccination of girls at an early age, before they leave compulsory education, is likely to contribute to the prevention of HPV infection and cervical cancer in China.
In conclusion, the study results show that the HPV-16/18 AS04-adjuvanted vaccine is effective, immunogenic and has a clinically acceptable safety profile in young Chinese women. Prophylactic HPV vaccination has the potential to substantially reduce the burden of cervical cancers and precancers in China.
Cervarix is a registered trade mark of the GlaxoSmithKline group of companies. SAS is a registered trade mark of SAS Institute Inc. StatXact is a registered trade mark of Cytel Inc.
Acknowledgments
The authors would like to thank the study participants and the staff members of the different sites for their contribution to the study. In addition, the authors would like to thank the following contributors: Endpoint committee: Nancy Kiviat (University of Washington, School of Medicine, Seattle, WA, USA), Keith Klugman (The Rollins School of Public Health, Emory University, Atlanta, GA, USA), Pekka Nieminen (Helsinki University Central Hospital, Helsinki, Finland). Data monitoring committee (DMC): Yao Chen (DMC Chairman, Peking University, Beijing, China) and Qinping Liao (Peking University, Beijing, China), Wen Di and Binghua Su (Shanghai Jiaotong University, Shanghai, China), Hongtu Liu (Chinese Center for Disease Control and Prevention, Beijing, China). Clinical study and laboratory support: Anco Molijn (DDL Diagnostic Laboratory, Rijswijk, The Netherlands). Christophe De Jaegher, Gary Edwards, Yunkun He, Nathalie Houard, Jenny Jiang, Edwin Kolp, Jean-Louis Maroye, Ariane Meurée, Nicholas Perombelon, Kristina Sennvik, Olivier Thiange, Christine Van Hoof, Rebecca Zhang, Richard Zhao, Julia Zima (all from the GlaxoSmithKline group of companies). Data management and statistical support: Christelle Durand, Issam Jamiai, Philippe Marius, Sibel Tokgoz (all from the GlaxoSmithKline group of companies). Publication writing assistance: Julie Taylor (Peak Biomedical Ltd, UK) on behalf of GlaxoSmithKline Vaccines. Editorial assistance and manuscript coordination: Jean-Michel Heine (Keyrus Biopharma, Belgium) on behalf of GlaxoSmithKline Vaccines.
Glossary
- AGC
atypical glandular cells
- ASC-H
atypical squamous cells cannot exclude high-grade squamous intraepithelial lesions
- ASC-US+
atypical squamous cells of undetermined significance or higher
- ASC-US
atypical squamous cells of undetermined significance
- ATP-E
according-to-protocol cohort for efficacy
- CDC
Center for Disease Control and Prevention
- CI
confidence interval
- CICAMS
Cancer Institute and Hospital, Chinese Academy of Medical Sciences
- CIN
cervical intraepithelial neoplasia
- ELISA
enzyme-linked immunosorbent assay
- EU
ELISA units
- GMT
geometric mean antibody titre
- HPV
human papillomavirus
- HSIL
high-grade squamous intraepithelial lesions
- LSIL
low-grade squamous intraepithelial lesions
- MPL
3-O-desacyl-4′-monophosphoryl lipid A
- PCR
polymerase chain reaction
- PI
persistent infection
- SAE
serious adverse event
- SD
standard deviation
- TVC-E
total vaccinated cohort for efficacy
- TVC
total vaccinated cohort
- VE
vaccine efficacy
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Information
Supplementary Information
References
- 1.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; 2010. Available at http://globocan.iarc.fr. Accessed on: 7 August 2013.
- 2.Zhao FH, Lewkowitz AK, Hu SY, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies. Int J Cancer. 2012;131:2929–38. doi: 10.1002/ijc.27571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao YP, Li N, Smith JS, et al. Human papillomavirus type-distribution in the cervix of Chinese women: a meta-analysis. Int J STD AIDS. 2008;19:106–11. doi: 10.1258/ijsa.2007.007113. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zhang X, Molijn A, et al. Human papillomavirus type-distribution in cervical cancer in China: the importance of HPV 16 and 18. Cancer Causes Control. 2009;20:1705–13. doi: 10.1007/s10552-009-9422-z. [DOI] [PubMed] [Google Scholar]
- 5.Li N, Franceschi S, Howell-Jones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–35. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 6.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 7.Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)−16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 8.Descamps D, Hardt K, Spiessens B, et al. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin. 2009;5:332–40. doi: 10.4161/hv.5.5.7211. [DOI] [PubMed] [Google Scholar]
- 9.The GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)−16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374:1975–85. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhu FC, Li CG, Pan HX, et al. Safety and immunogenicity of human papillomavirus-16/18 AS04-adjuvanted vaccine in healthy Chinese females aged 15 to 45 years: a phase I trial. Chin J Cancer. 2011;30:559–64. doi: 10.5732/cjc.010.10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu FC, Li CG, Gu SK, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18–25 years. Abstract presented at the AOGIN (Asia-Oceania Research Organisation in Genital Infection and Neoplasia) Interim Meeting, Bali, Indonesia, 2011.
- 12.Apgar BS, Zoschnick L, Wright TC., Jr The 2001 Bethesda System terminology. Am Fam Physician. 2003;68:1992–8. [PubMed] [Google Scholar]
- 13.van Doorn LJ, Molijn A, Kleter B, et al. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44:3292–8. doi: 10.1128/JCM.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dessy FJ, Giannini SL, Bougelet CA, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4:425–34. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 15.Dragalin V, Fedorov V, Cheuvart B. Statistical approaches to establishing vaccine safety. Stat Med. 2002;21:877–93. doi: 10.1002/sim.1039. [DOI] [PubMed] [Google Scholar]
- 16.Zhao FH, Zhu FC, Chen W, et al. Baseline prevalence and type distribution of human papillomavirus in healthy Chinese women aged 18–25 years enrolled in a clinical trial. Int J Cancer. doi: 10.1002/ijc.28896. 2014 Apr 17. doi: 10.1002/ijc.28896. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2012 Revision. Available at: http://esa.un.org/wpp/Excel-Data/population.htm. Accessed on 20 November 2013.
- 18.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 19.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 20.Herrero R, Hildesheim A, Rodriguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26:4795–808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization Expert Committee on Biological Standardization. Guidelines to assure the quality, safety and efficacy of recombinant human papillomavirus virus-like particle vaccines. WHO, Geneva, Oct 23–27, 2006. Available at http://www.who.int/biologicals/publications/trs/areas/vaccines/human_papillomavirus/HPVg%20Final%20BS%202050%20.pdf. Accessed on 7 August 2013.
- 22.Koshiol J, Lindsay L, Pimenta JM, et al. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:123–37. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810–9. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 24.Renshaw AA, Davey DD, Birdsong GG, et al. Precision in gynecologic cytologic interpretation: a study from the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2003;127:1413–20. doi: 10.5858/2003-127-1413-PIGCIA. [DOI] [PubMed] [Google Scholar]
- 25.Stoler MH, Schiffman M. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500–5. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- 26.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–9. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 27.Castle PE, Solomon D, Schiffman M, et al. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst. 2005;97:1066–71. doi: 10.1093/jnci/dji186. [DOI] [PubMed] [Google Scholar]
- 28.Castle PE, Rodriguez AC, Burk RD, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;339:b2569. doi: 10.1136/bmj.b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler CM, Castellsague X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–10. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 31.Konno R, Tamura S, Dobbelaere K, et al. Efficacy of human papillomavirus type 16/18 AS04-adjuvanted vaccine in Japanese women aged 20 to 25 years: final analysis of a phase 2 double-blind, randomized controlled trial. Int J Gynecol Cancer. 2010;20:847–55. doi: 10.1111/IGC.0b013e3181da2128. [DOI] [PubMed] [Google Scholar]
- 32.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 33.Naud P, Roteli-Martins CM, De Carvalho N, et al. HPV-16/18 vaccine: sustained immunogenicity and efficacy up to 9.4 years. Abstract presented at 27th International Papillomavirus Conference and Clinical Workshop, Berlin, Germany, 2011. Available at http://www.hpv2011.org/pics/1/4/Abstract%20Book%202%20APSC%20WEBB%20110922.pdf. Accessed on 7 August 2013.
- 34.Hildesheim A, Herrero R, Wacholder S, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 35.Zhao FH, Tiggelaar SM, Hu SY, et al. A multi-center survey of age of sexual debut and sexual behavior in Chinese women: Suggestions for optimal age of human papillomavirus vaccination in China. Cancer Epidemiol. 2012;36:384–90. doi: 10.1016/j.canep.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information