Abstract
Both preclinical and clinical data suggest that activation of the PI3K/AKT/mTOR pathway in response to hormonal therapy results in acquired endocrine therapy resistance. We evaluated differences in activation of the PI3K/AKT/mTOR pathway in estrogen receptor α (ERα) positive primary and corresponding metastatic breast cancer tissues using immunohistochemistry for downstream activated proteins, like phosphorylated mTOR (p-mTOR), phosphorylated 4E Binding Protein 1 (p-4EBP1) and phosphorylated p70S6K (p-p70S6K). For p-mTOR and p-4EBP1, the proportion of immunostained tumor cells (0–100%) was scored. Cytoplasmic intensity (0–3) was assessed for p-p70S6K. The difference between expression of these activated PI3K/AKT/mTOR proteins- in primary and metastatic tumor was calculated and tested for an association with adjuvant endocrine therapy. In patients who had received endocrine therapy (N = 34), p-mTOR expression increased in metastatic tumor lesions compared to the primary tumor (median difference 45%), while in patients who had not received adjuvant endocrine therapy (N = 37), no difference was found. Similar results were observed for p-4EBP1 and p-p70S6K expression. In multivariate analyses, adjuvant endocrine therapy was significantly associated with an increase in p-mTOR (p = 0.01), p-4EBP1 (p = 0.03) and p-p70S6K (p = 0.001), indicating that compensatory activation of the PI3K/AKT/mTOR pathway might indeed be a clinically relevant resistance mechanism resulting in acquired endocrine therapy resistance.
What's new?
Inhibitors of the PI3K/AKT/mTOR pathway can overcome the resistance to estrogen-depletion therapy that often develops in metastatic breast cancer. In this study, the authors compared primary and metastatic tumors; their results suggest that activation of the PI3K/AKT/mTOR pathway in patients who receive adjuvant endocrine therapy is a clinically relevant mechanism of acquired hormone resistance. For identification of companion diagnostics for PI3K/AKT/mTOR inhibitors, the authors conclude that analyzing primary tumor tissue may often fail to predict treatment response in metastatic breast cancer.
Keywords: PI3K/AKT/mTOR pathway; endocrine therapy, acquired hormone resistance
Until recently, interference with estrogen receptor alpha (ERα) and/or HER2 signaling were the only molecular targeted therapies clinically available for breast cancer patients. With the approval of everolimus for postmenopausal patients with ERα-positive metastatic breast cancer, targeting the PI3K/AKT/mTOR pathway has become a new therapeutic option in the disease. In breast cancer cell lines exposed to long term estrogen depletion, activation of the PI3K/AKT/mTOR pathway occurs as an adaptive change and results in hormone independent cell growth.1 This escape from hormone dependency can be overcome by exposure of cells to inhibitors of the PI3K/AKT/mTOR pathway, like mTOR inhibitors.1,2 Metastatic breast cancer patients with previous exposure to endocrine therapy do derive substantial benefit from the addition of an mTOR inhibitor to endocrine therapy compared to endocrine therapy alone.3,4 This suggests that mTOR activation in response to anti-estrogens is indeed a clinically relevant mechanism, resulting in acquired endocrine therapy resistance. Nevertheless, the occurrence of compensatory activation of the PI3K/AKT/mTOR pathway in response to anti-estrogens has not yet been well established in clinical samples. Tumor biopsies from patients that progressed after treatment with anti-estrogens are not routinely taken in clinical practice. In addition, in prospective randomized trials, fresh biopsies from metastatic tumors are hardly ever mandatory. In a small series of primary breast tumors and their distant metastases, Akcakanat et al. observed discordant expression of phosphorylated 4EBP1, a marker of mTOR activity.5 Most of these primary tumors were ERα negative and thereby an association with adjuvant endocrine therapy could not be explored. Our aim was to evaluate changes in PI3K/AKT/mTOR pathway activation between ERα positive primary tumors and corresponding metastatic tumors and to test whether potential differences are associated with adjuvant endocrine therapy.
Material and Methods
From a previously described series of 233 breast cancer patients from whom both primary tumor tissue as well as metachronous nonbone distant metastatic tumor tissue was collected,6 we selected patients based on adjuvant treatment data, which were obtained from The Netherlands cancer registry (IKNL, The Netherlands (http://www.ikcnet.nl/)). In The Netherlands, adjuvant hormonal therapy became standard of care for high-risk postmenopausal women around the year 1995 and for high-risk premenopausal women around 1999. From the total of 233 breast cancer patients, 174 primary breast tumors were ERα positive and a total of 60 of these ERα positive patients were treated with adjuvant endocrine therapy. Sufficient tumor material was available for 42 ERα positive patients who had received adjuvant endocrine therapy (original diagnosis 1985–2005). A comparable amount of control patients (n = 42) who had not received adjuvant endocrine therapy was selected (original diagnosis 1985–2007). The association between these two groups and known prognostics factors was calculated using Mann Whitney U or Fisher exact tests. Immunohistochemical analysis was carried out on 4-μm sections. ERα, progesterone receptor (PgR) and HER2 status were determined as previously described.6 Samples with 1% or more immunopositive ERα or PgR malignant cells were classified as hormone receptor-positive according to the new ASCO guidelines.7 Primary tumor and corresponding metastatic tumor tissue were stained for the expression of activated proteins downstream in the PI3K pathway using standardized protocols on the Ventana Benchmark® Ultra system automatic immunostainer with monoclonal antibodies raised against p-mTOR(Ser2448) (cell signaling No. 2976), p-4EBP1 (cell signaling 9456) and p-p70S6K (cell signaling 9206) (Supporting Information Table S1). For p-mTOR and p-4EBP1, the percentage of immunostained tumor cells was scored by one observer (J.W. and J.S., respectively). Cytoplasmic intensity (0–3) was assessed for p-p70S6K (scored by J.S.). Scoring of tumor slides was performed blinded to other data in the paired samples. The difference in expression of these activated proteins between primary and metastatic tumor was calculated. We assessed whether this difference between primary and metastatic tumor was associated with known clinico-pathological factors (age, location of metastasis, lymph node status, T-stage, grade, HER2 status and PgR status) or varied between patients who did and did not receive endocrine therapy, using Mann-Whitney tests. In addition, we performed a multivariate linear regression model including the same clinico-pathological factors.
Results
Of the 84 selected patients, a total of 71 (34 from patients who had received adjuvant endocrine therapy and 37 from patients who had not received endocrine therapy) could be used for analysis after staining with p-mTOR. For p-4EBP1 and p-p70S6K changes, a total of 67 and 68 tumor pairs, respectively, could be adequately assessed (Supporting Information Fig. S1). Location of metastasis was predominantly skin (N = 26) and liver (N = 21). In addition, metastases were localized in brain (N = 13), lung (N = 7) or gastro-intestinal (N = 4). Median time to metastasis was 54 months. Patient characteristics of both endocrine-treated patients and patients who had not received endocrine therapy are shown in Table1. Patients who had received endocrine therapy were older and had more often lymph node positive compared to patients who had not received adjuvant endocrine therapy.
Table 1.
Characteristics of patients who had not received endocrine therapy and who had received endocrine therapy
| Adjuvant endocrine therapy |
|||||
|---|---|---|---|---|---|
| Total | No (37) | Yes (34) | |||
| N (%) | N (%) | N (%) | p-value | ||
| Median time to metastasis (months) | 54 | 53 | 54 | 0.461 | |
| Median age (range) | (28–88) | 47 (28–74) | 55 (37–88) | 0.011 | |
| Location | Skin | 26 (37) | 15 (41) | 11 (32) | 0.622 |
| Other | 45 (63) | 22 (59) | 23 (68) | ||
| Grade | Grades 1–2 | 31 (44) | 18 (49) | 13 (38) | 0.472 |
| Grade 3 | 40 (56) | 19 (51) | 21 (62) | ||
| T-stage | T 1-2 | 51 (72) | 28 (76) | 23 (68) | 0.292 |
| T 3-4 | 9 (13) | 3 (8) | 6 (18) | ||
| Missing | 11 (15) | 6 (16) | 5 (15) | ||
| Lymph node status | Negative | 18 (25) | 15 (41) | 3 (9) | 0.0022 |
| Positive | 43 (61) | 17 (46) | 26 (76) | ||
| Missing | 10 (14) | 5 (14) | 5 (15) | ||
| Progesterone receptor | Negative | 9 (13) | 3 (8) | 6 (18) | 0.262 |
| Positive | 62 (87) | 34 (92) | 28 (82) | ||
| HER2 | Negative | 64 (90) | 36 (97) | 28 (82) | 0.052 |
| Positive | 7 (10) | 1 (3) | 6 (18) | ||
| Chemotherapy | No | 42 (59) | 19 (51) | 23 (68) | 0.232 |
| Yes | 29 (41) | 18 (49) | 11 (32) | ||
| Trastuzumab | No | 70 | 36 (97) | 34 (100) | 1.002 |
| Yes | 1 | 1 (3) | 0 (0) | ||
Mann Whitney U tests.
Fisher exact test (only cases without missing data were analyzed).
In the total group of 71 patients evaluable for p-mTOR changes, median p-mTOR expression in primary tumor tissue was 40% (41% mean), compared to 80% (65% mean) in tumor biopsies from metastatic sites. Median p-4EBP1 expression in primary tumor tissue was 20% (31% mean), compared to 60% (48% mean) in tumor biopsies from metastatic sites. The majority of tumors were negative for p-p70S6K. Mean cytoplasmic p-p70S6K intensity score in primary tumor tissue was 0.16 compared to 0.38 in tumor biopsies from metastatic tumors.
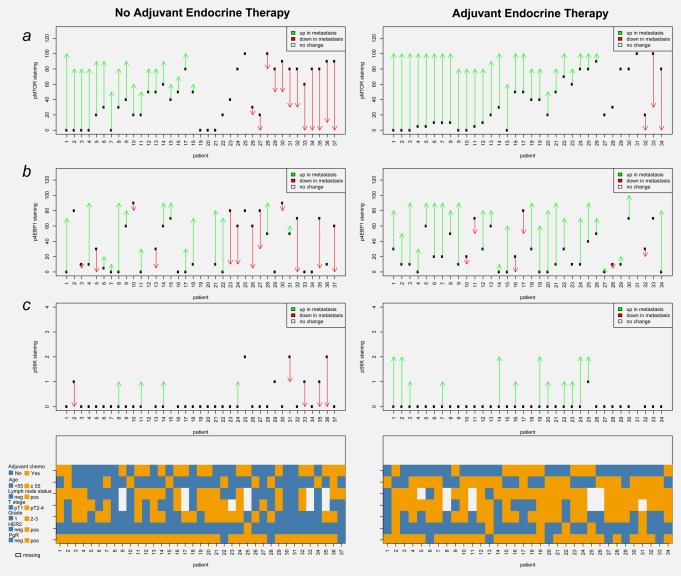
In univariate analyses, none of the evaluated clinico-pathological factors was significantly associated with a differential p-mTOR change between primary and metastatic tumor tissue (Table2). A high grade primary tumor was associated with increased p-4EBP1 expression in metastatic tumor tissue compared to the primary tumor (Table2). Positive HER2 status was associated with increased p-p70S6K expression in metastatic tumor tissue compared to the primary tumor (Table2). In patients who had received endocrine therapy we observed an increase in both p-mTOR and p-4EBP1 expression in metastatic tumor tissue compared to the primary tumor (median difference 45 and 30%, respectively) (Figs. 1a and 1b). This was significantly different from the change observed in patients who did not receive endocrine therapy (median p-mTOR difference 0% and median p-4EBP1 difference 0%) (Table2 and Figs. 1a and 1b) p = 0.003 and p = 0.02, respectively). Comparable results were observed for p-p70S6K, with a mean increase of 0.52 in patients who had received adjuvant endocrine therapy, compared to a –0.06 in patients who did not receive adjuvant endocrine therapy (p = 0.002) (Table2 and Fig. 1c).
Table 2.
Association between change in activated PI3K/AKT/mTOR protein expression and clinico-pathological factors
| Number | Difference in expression of activated PI3K/AKT/mTOR protein between metastatic and primary tumor tissue | P value1 | ||
|---|---|---|---|---|
| Association between median change in p-mTOR expression and clinico-pathological factors | ||||
| age | < 55 year | 41 | 20% | 0.52 |
| ≥ 55 year | 30 | 30% | ||
| location | skin | 26 | 25% | 0.762 |
| liver | 21 | 20% | ||
| brain | 13 | 0% | ||
| lung | 7 | 60% | ||
| gastro-intestinal | 4 | 40% | ||
| grade | grade 1-2 | 31 | 20% | 0.34 |
| grade 3 | 40 | 30% | ||
| T-stage | T 1-2 | 51 | 30% | 0.77 |
| T 3-4 | 9 | 20% | ||
| missing | 11 | 10% | ||
| lymph node status | negative | 18 | 15% | 0.94 |
| positive | 43 | 30% | ||
| missing | 10 | 20% | ||
| Progesterone receptor | negative | 9 | 0% | 0.16 |
| positive | 62 | 30% | ||
| HER2 | negative | 64 | 25% | 0.56 |
| positive | 7 | 30% | ||
| endocrine therapy | no | 37 | 0% | 0.003 |
| yes | 34 | 45% | ||
| chemotherapy | no | 42 | 30% | 0.25 |
| yes | 29 | 20% | ||
| all | 71 | 40% | ||
| Association between median change in p-4EBP1 expression and clinico-pathological factors | ||||
| age | < 55 year | 38 | 18% | 0.81 |
| ≥ 55 year | 29 | 20% | ||
| location | skin | 25 | 10% | 0.532 |
| liver | 21 | 40% | ||
| brain | 12 | 0% | ||
| lung | 7 | 30% | ||
| gastro-intestinal | 2 | -15% | ||
| grade | grade 1-2 | 29 | 0% | 0.03 |
| grade 3 | 38 | 30% | ||
| T-stage | T 1-2 | 48 | 18% | 0.37 |
| T 3-4 | 9 | 30% | ||
| missing | 10 | 10% | ||
| lymph node status | negative | 18 | 13% | 0.85 |
| positive | 39 | 30% | ||
| missing | 10 | 20% | ||
| Progesterone receptor | negative | 9 | 20% | 0.99 |
| positive | 58 | 18% | ||
| HER2 | negative | 61 | 10% | 0.48 |
| positive | 6 | 40% | ||
| endocrine therapy | no | 34 | 0% | 0.02 |
| yes | 33 | 30% | ||
| chemotherapy | no | 40 | 20% | 0.84 |
| yes | 27 | 0% | ||
| all | 67 | 30% | ||
| Association between mean change in p-p706K expression and clinico-pathological factors | ||||
| age | < 55 year | 40 | 0.28 | 0.53 |
| ≥ 55 year | 28 | 0.14 | ||
| location | skin | 26 | -0.50 | 0.072 |
| liver | 20 | 0.38 | ||
| brain | 13 | -0.04 | ||
| lung | 7 | 0.40 | ||
| gastro-intestinal | 2 | 0.57 | ||
| grade | grade 1-2 | 29 | 0.17 | 0.48 |
| grade 3 | 39 | 0.26 | ||
| T-stage | T 1-2 | 49 | 0.24 | 0.33 |
| T 3-4 | 9 | 0.44 | ||
| missing | 10 | -0.10 | ||
| lymph node status | negative | 17 | 0.12 | 0.21 |
| positive | 41 | 0.34 | ||
| missing | 10 | -0.10 | ||
| Progesterone receptor | negative | 9 | 0.11 | 0.60 |
| positive | 59 | 0.24 | ||
| HER2 | negative | 62 | 0.15 | 0.03 |
| positive | 6 | 1.00 | ||
| endocrine therapy | no | 35 | -0.06 | 0.002 |
| yes | 33 | 0.52 | ||
| chemotherapy | no | 40 | 0.20 | 0.67 |
| yes | 28 | 0.25 | ||
| all | 68 | |||
Mann-Whitney test (only cases without missing values were analyzed), except for
Kruskall Wallis test.
Figure 1.
Changes in activated PI3K/AKT/mTOR protein expression between primary and corresponding metastatic tumor tissue from patients who had not received adjuvant endocrine therapy (left panels) and who had received adjuvant endocrine therapy (right panels). (a) Change in p-mTOR expression between primary and corresponding metastatic tumor. (b) Change in p-4EBP1 expression between primary and corresponding metastatic tumor. (c) Change in p-p70S6K expression between primary and corresponding metastatic tumor. pos, positive; neg, negative.
In multivariate regression models, adjuvant endocrine therapy was significantly associated with an increase in p-mTOR (p = 0.01), p-4EBP1 (p = 0.03) and p-p70S6K (p = 0.001) (Supporting Information Tables S2–S4). In addition, in multivariate analysis, a positive PgR status was associated with an increase in p-mTOR (p = 0.01). The results of multivariate regression analysis did not substantially change when a cutoff of 10% was applied for ERα positivity. A sensitivity analysis performed in patients who did not receive chemotherapy showed no significant association between adjuvant endocrine therapy and change in p-4EBP1 (p = 0.38) or p-p70S6K (p = 0.12) (Supporting Information Tables S5 and S6). However adjuvant endocrine therapy remained significantly associated with an increase in p-mTOR expression in metastatic tumor tissue compared to primary tumors (p = 0.005) (Supporting Information Table S7).
Discussion
In this study, we showed that adjuvant endocrine therapy is significantly associated with an increase in expression of downstream activated proteins in the PI3K pathway in biopsies from subsequent metastatic tumor tissue compared to corresponding primary tumor tissue. This suggests that PI3K pathway activation in response to anti-estrogens results in acquired hormone resistance.
The observed increase in p-mTOR expression in metastatic tumor tissue was not only associated with adjuvant endocrine therapy, but in our multivariate analysis we did also observe an association with a positive progesterone receptor status. It is well known that PgR expression is driven by estrogen receptor signaling.9 We hypothesize that tumors that are highly dependent on ER-signaling are more likely to acquire activation of additional growth factor pathways compared to tumors that are not selectively dependent on ER-signaling.
A limitation of our study is the lack of a control group of patients that did not relapse. The analysis of PI3K/AKT/mTOR pathway activation in a control group of adjuvant endocrine treated patients who did not relapse is however unfeasible. To analyze differences in PI3K/AKT/mTOR pathway activation between responders and nonresponders, serial biopsies from patients treated with neo-adjuvant endocrine therapy would be suitable. Cavazonni et al.2 observed an increase in PI3K/AKT/mTOR related gene and protein expression in a small series of breast tumor biopsies from patients who progressed after neo-adjuvant endocrine therapy (N = 9). Unfortunately, tumor biopsies from patients who did not progress were not analyzed. Nevertheless, these and our results suggest that adaptive activation of the PI3K/AKT/mTOR pathway in response to adjuvant endocrine therapy is a clinically relevant mechanism resulting in acquired endocrine therapy resistance. An alternative explanation for the observed increase in PI3K/AKT/mTOR pathway activity could be that endocrine therapy results in clonal selection of tumor cells that are not selectively dependent on estrogen signaling. Although it is conceivable that small biopsies might have had better fixation and therefore would be more likely to stain for phospho-proteins, the observed increase in expression of phosphorylated PI3K/AKT/mTOR pathway proteins in the group of endocrine treated patients was significantly different from the group of patients who did not receive endocrine therapy, and is therefore not likely to be simply explained by difference in fixation.
Apart from acquired adjuvant endocrine therapy resistance, activation of the PI3K/AKT/mTOR pathway has previously been associated with intrinsic resistance. Retrospective analysis of p-mTOR expression in primary tumor tissue from breast cancer patients randomized between adjuvant tamoxifen versus no systemic therapy indicated that those patients whose tumor exhibit high p-mTOR expression do not benefit from adjuvant tamoxifen.10,11 Similar results were observed for p-p70S6K expression.10
Activation of the PI3K/AKT/mTOR pathway does not only result in endocrine therapy resistance, but may also cause trastuzumab resistance in HER2 positive breast cancer.12 Similar to our results, Chandarlapaty et al. observed a higher rate of PI3K/AKT/mTOR pathway activation in metastatic breast tumors from patients with previous exposure to trastuzumab compared to trastuzumab naïve controls.13 The number of patients treated with trastuzumab in our series was too small to analyze the association between trastuzumab and differences in activation of the PI3K/AKT/mTOR pathway. Considering these adaptive molecular changes in response to various treatments, it seems clear that the biological behavior of a metastatic tumor cannot always be predicted by the molecular make-up of the primary tumor. Consequently, for identification of companion diagnostics for molecular targeted drugs, analysis of primary tumor tissue is likely to fail to predict treatment response in the metastatic setting. As an example, biomarker analysis in metastatic breast cancer patients who participated in the Cleopatra trial (randomizing between Pertuzumab and placebo) failed to identify a subgroup of patients particularly benefitting from dual HER2 blockade.14 The majority of analyzed tumor tissue in this study originated from primary tumors. Translational studies within the TAMRAD phase II trial were selectively performed in primary tumor tissues from a subset of 51 patients. In this small subset, only a trend towards increased benefit from the addition of everolimus to tamoxifen was observed for patients with high expression of p-4EBP1).15 Biomarker analysis in primary tumor tissue from 227 patients who participated in the Bolero-2 trial showed that the benefit from everolimus added to exemestane was regardless of genetic alterations in the PI3K pathway.16 Since inhibitors of the PI3K/AKT/mTOR are not without side effects, one would like to specifically identify those patients who will substantially benefit from these drugs. For identification of potential companion diagnostics for these drugs, it is of utmost importance to mandate biopsies from metastatic tumor lesions in future clinical trials. This will improve our knowledge about drug sensitivity and escape mechanisms and thereby accelerate developments that may eventually result in managing hormone receptor positive breast cancer as a chronic disease.
In conclusion, we observed a significant association between adjuvant endocrine therapy and PI3K/AKT/mTOR pathway activation in metastatic breast tumor tissue, suggesting that compensatory activation of the PI3K/AKT/mTOR pathway is a clinically relevant mechanism resulting in acquired hormone resistance. This stresses the importance of performing biopsies from metastatic tumor lesions, not only for predictive biomarker identification in clinical trials but also in general clinical practice.
Acknowledgments
We thank all pathology departments throughout the Netherlands for submission of FFPE tumor blocks.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
References
- 1.Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, Higham C, Garcia-Echeverria C, Shyr Y, Arteaga CL. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–13. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavazzoni A, Bonelli MA, Fumarola C, et al. Overcoming acquired resistance to letrozole by targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones. Cancer Lett. 2012;323:77–87. doi: 10.1016/j.canlet.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30:2718–24. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 4.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akcakanat A, Sahin A, Shaye AN, Velasco MA, Meric-Bernstam F. Comparison of Akt/mTOR signaling in primary breast tumors and matched distant metastases. Cancer. 2008;112:2352–8. doi: 10.1002/cncr.23456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoefnagel LD, van d V, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12:R75. doi: 10.1186/bcr2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Diest PJ, Baak JP, Matze-Cok P, et al. Reproducibility of mitosis counting in 2,469 breast cancer specimens: results from the multicenter morphometric mammary carcinoma project. Hum Pathol. 1992;23:603–7. doi: 10.1016/0046-8177(92)90313-r. [DOI] [PubMed] [Google Scholar]
- 9.Osborne CK, Schiff R, Arpino G, Lee AS, Hilsenbeck VG. Endocrine responsiveness: understanding how progesterone receptor can be used to select endocrine therapy. Breast. 2005;14:458–65. doi: 10.1016/j.breast.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Beelen K, Opdam M, Severson TM, Koornstra RHT, Vincent AD, Wesseling J, Muris JJ, Berns EMJJ, Vermorken JB, van Diest PJ, Linn SC. Phosphorylated p-70S6K predicts tamoxifen resistance in postmenopausal breast cancer patients randomized between adjuvant tamoxifen versus no systemic treatment. Breast Cancer Res. 2014;16:R6. doi: 10.1186/bcr3598. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bostner J, Karlsson E, Pandiyan MJ, Westman H, Skoog L, Fornander T, Nordenskjold B, Stal O. Activation of Akt, mTOR, and the estrogen receptor as a signature to predict tamoxifen treatment benefit. Breast Cancer Res Treat. 2013;137:397–406. doi: 10.1007/s10549-012-2376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Chandarlapaty S, Sakr RA, Giri D, Patil S, Heguy A, Morrow M, Modi S, Norton L, Rosen N, Hudis C, King TA. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin Cancer Res. 2012;18:6784–91. doi: 10.1158/1078-0432.CCR-12-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baselga J, Cortes J, Im S-A, Clark E, Kiermaier A, Ross G, Swain SM. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in HER2-positive, first-line metastatic breast cancer (MBC) Cancer Res. 2012;72 doi: 10.1200/JCO.2013.54.5384. doi: 10.1158/0008-5472.SABCS12-S5-1. [DOI] [PubMed] [Google Scholar]
- 15.Treilleux I, Arnedos M, Cropet C, Ferrero JM, Lacourtoisie SA, Spaeth D, Levy C, Legouffe E, Pujade-Lauraine E, Wang Q, Bachelot T. Predictive markers of everolimus efficacy in hormone receptor positive (HR+) metastatic breast cancer (MBC): final results of the TAMRAD trial translational study. J Clin Oncol. 2013;31 abstract 510. [Google Scholar]
- 16.Hortobagyi GN, Piccart-Gebhart MJ, Rugo HS, et al. Correlation of molecular alterations with efficacy of everolimus in hormone receptor-positive, HER2-negative advanced breast cancer: results from BOLERO-2. J Clin Oncol. 2013;31 abstract 509. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information