Abstract
Exotosin (EXT) proteins are involved in the chain elongation step of heparan sulfate (HS) biosynthesis, which is intricately involved in organ development. Loss of function mutations (LOF) in EXT1 and EXT2 result in hereditary exostoses (HME). Interestingly, HS plays a role in pancreas development and beta-cell function, and genetic variations in EXT2 are associated with an increased risk for type 2 diabetes mellitus. We hypothesized that loss of function of EXT1 or EXT2 in subjects with hereditary multiple exostoses (HME) affects pancreatic insulin secretion capacity and development. We performed an oral glucose tolerance test (OGTT) followed by hyperglycemic clamps to investigate first-phase glucose-stimulated insulin secretion (GSIS) in HME patients and age and gender matched non-affected relatives. Pancreas volume was assessed with magnetic resonance imaging (MRI). OGTT did not reveal significant differences in glucose disposal, but there was a markedly lower GSIS in HME subjects during hyperglycemic clamp (iAUC HME: 0.72 [0.46–1.16] vs. controls 1.53 [0.69–3.36] nmol·l−1·min−1, p<0.05). Maximal insulin response following arginine challenge was also significantly attenuated (iAUC HME: 7.14 [4.22–10.5] vs. controls 10.2 [7.91–12.70] nmol·l−1·min−1 p<0.05), indicative of an impaired beta-cell reserve. MRI revealed a significantly smaller pancreatic volume in HME subjects (HME: 72.0±15.8 vs. controls 96.5±26.0 cm3 p = 0.04). In conclusion, loss of function of EXT proteins may affect beta-cell mass and insulin secretion capacity in humans, and render subjects at a higher risk of developing type 2 diabetes when exposed to environmental risk factors.
Introduction
Heparan sulfate proteoglycans (HSPGs) play a role in many biological processes including fine-tuning most of the physiological and pathological processes related to fetal organ development, lipid metabolism and inflammation [1]. EXT1 and EXT2 genes encode for an endoplasmic reticulum-resident glycosyltransferase complex involved in chain elongation and possibly chain initiation of heparan sulfate biosynthesis [2], [3]. The EXT gene family consists of 5 genes, including EXTL1 (EXT-like 1), EXTL2 and EXTL3, which encode proteins that catalyze GlcNac transferase reactions. Whereas the function of EXT1 and EXT2 has been widely recognized, the precise role of EXTL3 and a related protein EXTL2 in heparan sulfate (HS) biosynthesis remains unclear [4]–[6]. Of note, Extl3 was reported to be involved in murine pancreatic beta-cell development [7]–[9].
Heterozygous loss of function (LOF) mutations in EXT1 and EXT2 are known to be involved in the development of hereditary multiple exostoses (HME) syndrome [10], a disorder with a reported prevalence of 1/50.000 individuals [11], and have been shown to lead to both locally (exostosis plate) [12] and systemically [13] altered heparan sulfate composition. Consequently, growth of multiple bony tumors (i.e. exostoses or osteochondromas) after birth and throughout childhood, lasting until closure of the growth plates, were observed, which can result in skeletal deformities and malignancies [14]. Main complications are a direct result of compression of neighbouring tissue or structures and involve pain, disturbance of blood circulation, and in rare cases spinal/cervical cord compression [15].
Interestingly, common single nucleotide polymorphisms (SNPs) in EXT2 were associated with increased risk for the development of type 2 diabetes mellitus (DM2) [16]. Despite conflicting results [17]–[21], a recent meta-analysis of Liu et al replicated the original observed significant association between common genetic variants in EXT2 and the risk of developing DM2 [22]. In line, SNPs in EXT2 have also been associated with impaired glucose clearance in DM2 as assessed by an oral glucose tolerance test [23]. Of note, both EXT1 and EXT2 genes are expressed in human pancreas (https://www.lsbm.org) pathophysiological role of EXT in pancreas function (insulin secretion) remains to be elucidated.
In the present study we designed a dedicated series of investigations to unravel the effect of disrupted heparan sulfate synthesis on beta-cell function and mass, as well as insulin secretion, in humans with heterozygous loss of function mutations in EXT1 or EXT2.
Methods
We enrolled Dutch HME subjects, based on established heterozygous mutations in either EXT1 or EXT2, and non-carrier relatives over 18 years of age, without pre-existent type 1 or 2 diabetes. We tested for alterations in glucose metabolism and beta-cell reserve. Written informed consent was obtained after explanation of the study. The study was approved by the institutional review board of the Academic Medical Center of the University of Amsterdam and carried out according to the Declaration of Helsinki.
Oral Glucose Tolerance Test (OGTT) and hyperglycemic clamp
After an overnight fast, a standardized OGTT was performed. After baseline venous sampling subjects were asked to ingest 75 g glucose. At t = 30, 60, 90 and 120 minutes a 4.5 ml blood sample was obtained for assessment of blood glucose, insulin and C-peptide.
On a separate study day, a hyperglycemic clamp was performed after an overnight fast. On the day of study antecubital veins of both arms were cannulated for blood sampling and infusion of fluids. All bedside glucose measurements were performed using a calibrated glucose sensor (YSI 2300 STAT S; YSI, Yellow Springs, OH). Based on the fasting plasma glucose level and the subject's bodyweight first phase insulin secretion was determined using a 20% glucose bolus (weight/70×10 – plasma glucose = ml required) given over 1 min, aiming to achieve a plasma glucose level of 14 mmol/l. Subsequently, blood glucose levels were maintained at 14 mmol/l by continuous glucose infusion. Pump settings (glucose infusion rate) were adapted based on blood glucose levels at t = 0, 2.5, 5, 7.5, 10 and 20 minutes. Simultaneously, blood samples were collected for insulin and C-peptide determination. After 120 minutes an arginine bolus (5 gram) was given, followed by measurement of plasma insulin levels at t = 125, 130,140 and 150 minutes. Basal fasting glucose, HbA1c, total cholesterol, HDL and LDL cholesterol, triglycerides and free fatty acids (FFAs) were assessed in fasting plasma using standard laboratory procedures. GLP-1 levels were determined using al RIALINCO assay [24]. Osteocalcin was measured using an immunoradiometric assay (Biosource/Medgenix Diagnostics, Fleuris, Belgium) as previously published [25]. Fecal elastase levels were assessed using a commercially available ELISA kit for Elastase 1 (ScheBo).
Glucose was determined by the hexokinase method (Hitachi), Insulin was determined on an Immulite 2000 system (Diagnostic Products, Los Angeles, CA). C-peptide was measured by RIA (RIA-coat C-peptide; Byk-Sangtec Diagnostica, Dietzenbach, Germany).
Using data from the OGTT and clamp, homeostasis model assessment (HOMA) indexes were calculated for insulin sensitivity (HOMA-ir = insulin (picomoles)/6.945*glucose (millimoles)/22.5) and insulin secretion (HOMA-β = 20* fasting insulin (picomoles)/6.954/glucose (millimoles-3.5). Under stable conditions of constant hyperglycemiathe amount of glucose infused (milligrams per kilogram) equals the amount of metabolized glucose (M). M was calculated as the average glucose infusion rate during the last 30 min of the clamp (t = 90 through t = 120). The M value divided by the average plasma insulin concentration (I) during the same interval, the M/I ratio, provides a measurement of tissue sensitivity to insulin (micrograms per kilogram per minute per picomole per liter) [26]. The disposition index is calculated as the product of the M/I ratio. Insulin sensitivity was estimated, using the metabolic clearance rate (MCR) of glucose and the insulin sensitivity index (ISI), as described previously [27]. Overall glucose-stimulated insulin secretion was calculated as AUCinsulin/AUCglucose ratio.
Magnetic resonance imaging
In a subset of previous participants (both HME-subjects and healthy controls) we performed abdominal imaging preceding the OGTT, using a 3-T MR scanner (Intera, Philips Healthcare, Best, The Netherlands). A T2-weighted two-dimensional transversal half-Fourier single-shot turbo spin-echo (HASTE) sequence was obtained in a breath hold to determine the volume of the pancreas. Scan parameters were: TR/TE 600/70 ms; FA 90 degrees; number of slices: 20, FOV450 mm×315 mm, voxel sizes: 1.4 mm×1.4 mm×4.00 mm; slice gap 1 mm. Images were analyzed by 2 independent, blinded investigators (ICC = 0.85, 95% CI 0.61–0.94) using ITK Snap software version 2.4 (University of Pennsylvania). Pancreatic area was delineated in each imaging slice and the number of voxels in this area was determined, subsequently this number was transcribed to volume in cubic millimetres. The mean area of separate measurements was used.
Power calculation and statistical analysis
Based on our previous findings in a oral glucose tolerance tests in carriers with a rare loss-of function mutation (ABCA1) [28], the difference between the mean incremental area under the curve (IAUC) for the carriers (245 [153–353]) and the controls (155 [84–198]), was 90 mmol l−1 min−1. Assuming a two group t-test with a 0,050 two-sided significance level and a power of 80%, we need to include 14 subjects in each group.
Data are presented as mean ± SD or medians with interquartile range [IQR] unless stated otherwise. Normally distributed baseline characteristics were compared using a student's t test (all but triglycerides). Differences in triglyceride and free fatty acid levels (FFA), known not to be normally distributed, and continues outcome variables were assessed using the nonparametric Mann-Whitney U test. All repeated measurements are reported by incremental AUC (area above baseline), computed by the trapezoidal rule. A p-value of less than 0.05 was used to indicate significant differences. All analyses were performed with SPSS software version 19.0.0.1.
Results
Beta-cell function and glucose metabolism in human EXT carriers versus controls
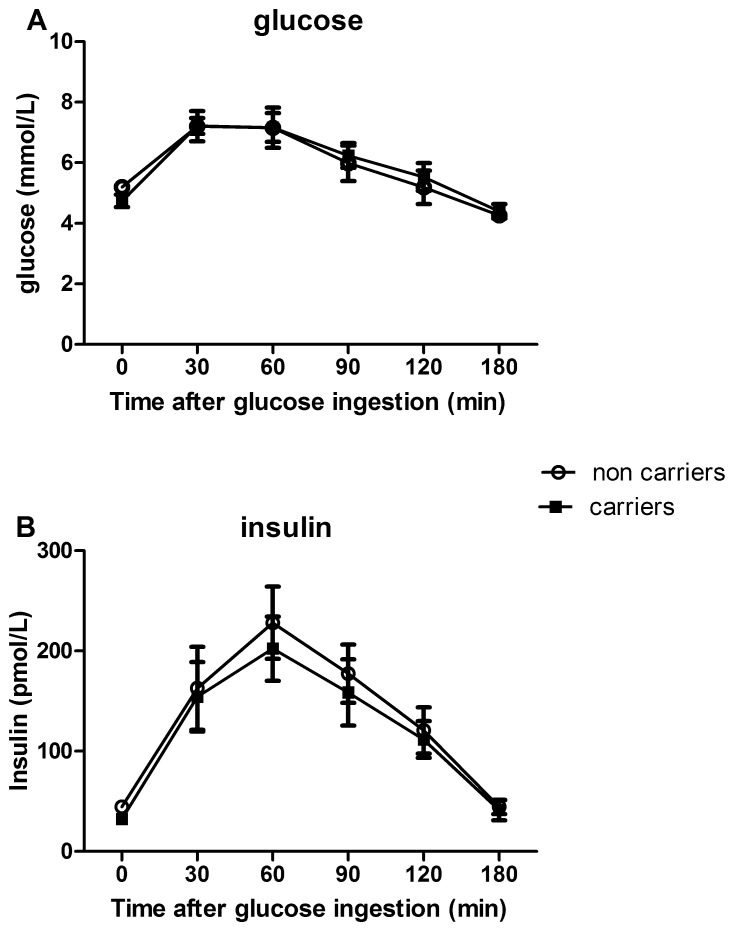
We included 16 EXT1 carriers and 6 EXT2 carriers (for a list of identified mutations see S1 and S2 Table) as well as 26 age and gender matched non carrier controls, whom participated in the OGTT, clamp or both (for baseline characteristics per study see S3 and S4 Tables). Age, BMI, fasting glucose, HbA1C and insulin levels, as well as basal lipid levels (including FFAs – only in OGTT group, see S3 Table), were all comparable between carriers and control subjects (Table 1). HME subjects are characterized by elevated osteocalcin levels (S3 Table), a protein recognized as a marker of bone formation [29]. Assessing exocrine pancreas function by fecal elastase [30], no differences were found (S3 Table). No difference was reported in family history for diabetes (Table 1). EXT carriers and controls displayed a similar response during OGTT with respect to plasma glucose (iAUC: carriers: 233 [157–286] vs controls: 160 [100–281] nmol·l−1·min−1 n.s.) and plasma insulin levels (iAUC: carriers; 17.4 [6.5–24.1] vs controls; 18.3 [12.6–23.0] nmol·l−1·min−1) (Figs. 1A and 1B). Markers of insulin resistance and beta-cell function were not significantly different between the HME subjects and controls (Table 2).
Table 1. Baseline characteristics of all study subjects.
| Noncarriers | Carriers | P-value | |
| (N = 25) | (N = 22) | ||
| Age (years) | 45±14 | 38±10 | 0.064 |
| Men | 11 (42) | 7 (31) | |
| BMI | 25.0±3.30 | 25.5±4.3 | 0.591 |
| BSA | 1.9±0.21 | 1.9±0.19 | 0.505 |
| Cholesterol (mmol/l) | |||
| Total | 5.26±1.25 | 4.87±1.16 | 0.295 |
| LDL | 3.23±1.14 | 3.03±1.06 | 0.537 |
| HDL | 1.51±0.41 | 1.34±0.41 | 0.153 |
| Triglycerides (mmol/l) | 0.90[0.67–1.19] | 0.87[0.55–1.34] | 1.00 |
| Fasting glucose (mmol/l) | 5.0±0.66 | 4.8±0.48 | 0.251 |
| Hba1c | |||
| mmol/mol | 36±3.9 | 34±3.6 | 0.403 |
| % | 5.4±0.34 | 5.3±0.32 | 0.391 |
| Fasting insulin (pmol/l) | 49±34 | 39±28 | 0.291 |
| Family history | |||
| Diabetes | 4 (15 | 3 (14) | |
| CVD | 3 (12) | 4 (29) |
Data are means ± SD, n (%), or median [IQR]. Abbreviations: BMI = Body Mass Index; BSA = Body Surface Area; LDL = Low Density Lipoprotein. HDL = High Density Lipoprotein. CVD = Cardiovascular Disease.
Figure 1. Plasma glucose (A) and insulin curves (B) after OGTT in HME subjects (closed squares) and controls (circles).
Table 2. Beta-cell function and insulin sensitivity parameters.
| Noncarriers | Carriers | P-value | |
| Baseline HOMA index (all) | |||
| HOMA-ir | 1.23[0.80–1.80] | 0.95[0.56–1.47] | 0.16 |
| HOMA-β | 79[48–128] | 78[49–147] | 0.89 |
| Insulinogenic index (ogtt) | 50.7[37.8–176.5] | 60.3[31.1–71.4] | 1.00 |
| (pmol/mmol) | |||
| AUCinsulin/AUCglucose ratio | 87.8[73.6–261.4] | 79.8[50.6–105.2] | 1.00 |
| (ogtt) (pmol/mmol) | |||
| ISIcomp (ogtt) | 23.9[20.9–41.5] | 33.5[18.5–46.9] | 0.07 |
| (µmol/(kg min pmol L)) | |||
| Disposition index (clamp) | 22.1[15.2–41.9] | 25.6[10.0–33.1] | 1.00 |
| MCR (ogtt) (ml/(min kg)) | 9.8[9.1–10.4] | 10.1[9.2–10.6] | 0.37 |
Values are presented as median [interquartile range]. Abbreviations: HOMA = homeostatis model assessment; AUC = area under the curve; ISIcomp = index of composite whole-body insulin sensitivity; MCR = metabolic clearance rate.
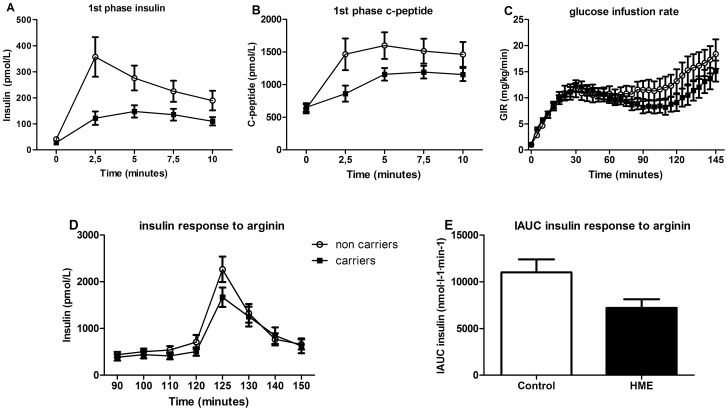
We noted a trend towards lower plasma insulin levels in HME subjects, thus we subsequently performed a hyperglycemic normoinsulinemic clamp followed by arginine infusion. During the hyperglycemic clamp, first phase insulin response to an intravenous glucose bolus (as determined by incremental AUC) was lower in carriers than control subjects (0.72 [0.46–1.16] vs. 1.53 [0.69–3.36] nmol·l−1·min−1, p = 0.017) (Fig. 2A). In addition, C-peptide responses were also significantly lower in carriers (3.57 [2.26–5.00] vs. 6.62 [4.48–9.84] nmol·l−1·min−1 p<0.008) (Fig. 2B). In line with the HOMA data, glucose infusion rates were comparable between groups (iAUC carriers vs. controls 11.1 [8.88–19.00] vs. 14.5 [11.98–23.98] mg·kg−1·min−1 n.s.) (Fig. 2C), as well as disposition indices (Table 2), suggesting that differences observed in insulin and C-peptide secretion cannot be attributed to differences in insulin tolerance. Following an intravenous arginine bolus to assess maximal insulin reserve, the peak response was significantly impaired in EXT carriers compared to controls (iAUC from t = 120: 7.14 [4.22–10.95] vs. 10.32 [7.91–12.70] nmol·l−1·min−1 p<0.028) (Figs. 2D and E).
Figure 2. Functional (GSIS) pancreas reserve in HME subjects (closed sq) versus controls (circles).
A and B: The first-phase insulin and C-peptide response to a hyperglycaemic clamp was lower in HME subjects compared to controls. C: The glucose infusion rate (GIR), an estimation of the amount of glucose being metabolized, was not different between groups. D and E: Insulin secretion after an intravenous bolus of arginine was lower in carriers vs controls. * p = 0.028.
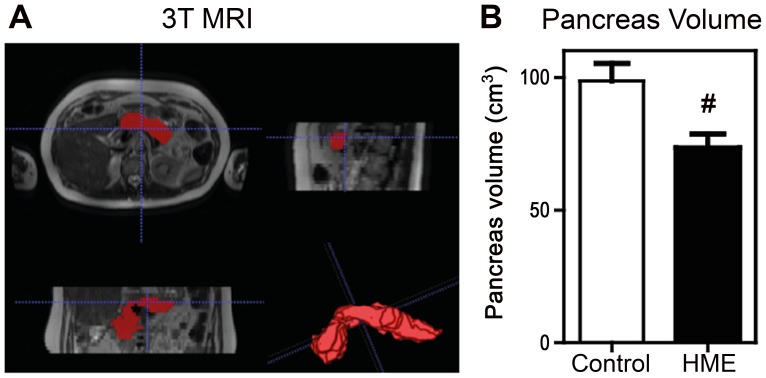
To further investigate the decreased beta-cell insulin secretory capacity we set out to detect potential differences in anatomical pancreatic volume in n = 8 EXT carriers and n = 8 non-carrier controls (Table 3). Abdominal MRI imaging revealed a significantly smaller pancreatic volume in EXT carriers compared to control subjects (72.0±15.8 vs. 96.5±26.0 cm3 p = 0.04) (Fig 3).
Table 3. Baseline characteristics of participants in MRI.
| Noncarriers | Carriers | P-value | |
| (N = 8) | (N = 8) | ||
| Age (years) | 40±13 | 39±9 | 0.85 |
| Men | 3 (37) | 3 (37) | |
| Length (m) | 1.72±0.10 | 1.72±0.10 | 0.89 |
| Weight | 74±9 | 78±13 | 0.60 |
| BMI | 24.6±1.4 | 25.6±4.4 | 0.54 |
| BSA | 1.8±0.15 | 1.9±0.18 | 0.38 |
Data are means ± SD, n (%), or median [IQR]. Abbreviations: BMI = body mass index; BSA = body surface area.
Figure 3. Pancreas volume, assessed with 3T MRI, was smaller in HME subjects than controls (A) Example of axial (top left), sagital (top right) and coronal (bottom left) view and 3D visualization (bottom right) of delineated pancreas.
(B) Pancreatic volumes (cm3) in HME subjects and controls. # p = 0.04.
Discussion
Here we provide the first evidence that carriers of loss-of-function mutations in EXT have a distinct perturbation in glucose-insulin homeostasis characterized by an impaired glucose stimulated insulin secretion response as well as a decreased peak-insulin secretory capacity. The latter is accompanied by a significant reduction in total pancreas volume, implying a structural beta-cell defect in carriers of loss-of-function mutations in EXT.
EXT mutation carriers displayed normal insulin sensitivity during OGTT. However, a trend towards reduction in plasma insulin response during OGTT was observed in these subjects. Using hyperglycemic clamps, we observed that EXT carriers were characterized by a reduced first-phase insulin response to hyperglycemia (GSIS) compared to noncarriers matched for age, sex, and BMI. Interestingly, in a cohort of Pima Indians, who are marked by high levels of insulin resistance and obesity, an association between SNPs in EXT2 and incidence of DM2 was found [23]. Together these findings suggest that HME subjects, being carriers of heterozygous EXT mutations might be at increased risk of developing DM2 when becoming obese, due to their underlying beta-cell defect.
Functional beta-cell defects in heterozygous carriers of EXT mutations
Our findings elute to either a functional, signalling defect of the beta-cell or a structural diminished β-cell mass. It has been recognized that GSIS reflects the available previously synthesized and stored insulin that can be secreted upon glucose stimulation [31]. After entering the β-cell via GLUT transporters, glucose is modified by glucokinase, the rate-limiting step in glucose sensing. Glycolysis results in ATP production and subsequently the ATP-sensitive potassium channel is closed, followed by membrane depolarization, increased calcium influx via the L-type calcium channel and finally, exocytosis of insulin-containing granules. This first-phase secretory response is augmented by a potassium channel-independent pathway, which is largely responsible for the second-phase insulin response [32].
It has been previously shown that specific inhibition of heparan sulfate synthesis in a mouse model by Extl3 knock-down results in impaired GSIS [7]. Inline, Takahashi et all showed that treatment of isolated islets with heparinase resulted in decreased insulin secretion upon glucose stimulation, together with decreased expression of Glut2, Sur1 and Stx1A. These data underline the important role for intact heparan sulfate in GIIS [7]. However, the expression of GLUT2 in human beta-cells is very low compared to that in mice pancreas [33]. Therefore, the proposed explanation for the reduction in insulin secretory capacity in these models through reduced GLUT2 expression with subsequent attenuated FGF signaling [34] can't be translated 1∶1 to the human situation.
Beta-cell function may also be affected by impaired Hedgehog (Hh) signalling, which has been proposed to play a role in insulin production [35], [36] and beta-cell function [37] throughout life. It was shown that tout-velu (ttv), an EXT1 analog in Drosophilia, is required for Hh diffusion [38], thus linking disturbances in heparan sulfate to beta-cell function. In this regard, it has also been reported that Wnt proteins are involved in GSIS in adult mouse islets [39].
Structural beta-cell defects in heterozygous carriers of EXT mutations
Both the first-phase insulin responses as well as the secretory responses to arginine were significantly impaired in EXT carriers. These findings contrast our previous results in subjects carrying a heterozygous mutation in ABCA1, showing decreased GSIS with an intact maximal insulin release capacity following arginine [28]. Arginine stimulates insulin secretion by directly inducing membrane depolarization independent of potassium channels and thus largely independent of glucose sensing and glucose metabolism pathways [40], [41]. Our findings suggest that in HME a structural, rather than a functional defect, may lead to decreased GSIS and arginine-insulin responses. MRI based pancreatic volume measurements in our HME subjects indeed lend further support to a structural defect in EXT carriers.
Beta-cell survival
In a recent study it was implicated that HSPG was involved in beta-cell survival, providing a buffer mechanism against reactive oxygen species (ROS) in the murine pancreas [42]. Indeed, it has been previously noted that beta-cell failure precedes the development of impaired glucose tolerance (IGT) in insulin resistant subjects [43] due to ROS induced exhaustion of the normal beta-cell capacity to adjust for increased insulin demand [44]. Thus HSPGs may have several roles in beta-cell homeostasis via either regulation of postnatal islet and pancreas development [7] or protection of the beta-cell against destruction later in life [45]. The inadvertent depletion of pancreas heparan sulfates in EXT carriers might render (the already decreased amount of) beta-cells vulnerable for exogenous pathogenic stimuli including obesity and older age. Unfortunately however, at this moment data on development of DM2 in HME patients cohorts of older age are not available, as increased morbidity and mortality due to malignant bone tumours resulted in loss of follow up.
Insulin signalling in HME carriers
The development of type 2 diabetes is a complex interplay of declining beta-cell function and subsequent development of insulin resistance, influenced by both genetic and environmental factors. In this respect, the elevated levels of osteocalcin found in our patients may play an interesting role. The elevated levels of osteocalcin might reflect altered bone formation in HME subjects, whom are characterized by the development of bony tumors [29]. Although in mouse models, osteocalcin improves insulin sensitivity [46] and beta-cell proliferation [47], translation of these data to our subjects with HME should be done with caution. For example, most epidemiological studies (reviewed in ref [29]) show an inverse correlation between plasma osteocalcin levels and the presence of insulin resistance. Murine studies have suggested that one of the mechanisms by which osteocalcin improves beta-cell function is through increased GLP-1 secretion [48], [49]. As we did not find differences in fasting GLP-1 levels in our subjects, this mechanism does not seem to be responsible for the found increase in beta cell function in our study. Nevertheless, further study on the relation between altered bone metabolism and glucose metabolism in HME subjects is warranted.
Study limitations
Several issues in our study deserve closer attention. First, based on the small number of available mutation carriers in The Netherlands this precludes analysis regarding individual effects of EXT1 or EXT2 on beta-cell function and size. However, although mutations in either EXT1 or EXT2 can result in development of Hereditary Multiple Exostoses, mutations in EXT1 are associated with a higher disease burden [50], [51]. This is most likely due to an more pronounced biological function for EXT1 albeit that EXT2 is required to allow the proper function of EXT1 [52].
Second, a reduced pancreas volume could be accompanied by an initial protective increase in islet proliferation. In the present study, we cannot rule out this possibility, however, our functional data regarding the impaired maximum insulin release upon the arginine bolus in HME subjects do not support this hypothesis.
Finally, as large clinical cohorts of well genotyped HME subjects are currently not available, further studies are needed to address the question whether a similar mechanism of decreased pancreas volume might partially underly the genetic association between EXT and defective insulin secretion in HME subjects with differences in BMI.
Nevertheless, we now provide the first evidence on the relation between genetic defects in heparan sulfate synthesis and decreased pancreas anatomic volume with ensuing impaired beta-cell reserve capacity in carriers of loss-of-function mutations in EXT.
Supporting Information
Mutations in the EXT1 gene in our cohort.
(DOC)
Mutations in the EXT2 gene in our cohort.
(DOC)
Baseline characteristics of study subjects in OGTT.
(DOC)
Baseline characteristics of study subjects in clamp.
(DOC)
Supporting information file containing data for baseline characteristics.
(SAV)
Supporting information file containing data for OGTT.
(SAV)
Supporting information file containing data for Clamp.
(SAV)
Supporting information file containing data for MRI.
(SAV)
Acknowledgments
We are grateful to all participating HME subjects and Mr. Jan de Lange from the Dutch HME Foundation (www.hme-mo.nl) for their help with inclusion and being able to perform this study.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data are from the MARE study whose authors may be contacted at the department of Vascular Medicine, Academic Medical Center, Amsterdam, The Netherlands.
Funding Statement
M. Nieuwdorp is supported by Nederlandse Organisatie voor Wetenschappelijk Onderzoek(NWO)-VENI grant 2008 (016.096.044); http://www.nwo.nl/. J.K. Kruit is supported by a grant from the Dutch Diabetes foundation (2009.80.114); http://www.diabetesfonds.nl. J.D. Esko is supported by National Institutes of Health (NIH GM33063); http://www.nih.gov/. S.J. Bernelot Moens and E.S.G. Stroes received a grant from CardioVasculair Onderzoek Nederland (CVON-GENIUS)number CVON2011-19; http://www.cvon.eu/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bishop JR, Schuksz M, Esko JD (2007) Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446:1030–1037. [DOI] [PubMed] [Google Scholar]
- 2. McCormick C, Leduc Y, Martindale D, Mattison K, Esford LE, et al. (1998) The putative tumour suppressor EXT1 alters the expression of cell-surface heparan sulfate. Nat Genet 19:158–161. [DOI] [PubMed] [Google Scholar]
- 3. Lind T, Tufaro F, McCormick C, Lindahl U, Lidholt K (1998) The putative tumor suppressors EXT1 and EXT2 are glycosyltransferases required for the biosynthesis of heparan sulfate. J Biol Chem 273:26265–26268. [DOI] [PubMed] [Google Scholar]
- 4. Nadanaka S, Kitagawa H (2008) Heparan sulphate biosynthesis and disease. J Biochem 144:7–14. [DOI] [PubMed] [Google Scholar]
- 5. Okada M, Nadanaka S, Shoji N, Tamura J-I, Kitagawa H (2010) Biosynthesis of heparan sulfate in EXT1-deficient cells. Biochem J 428:463–471. [DOI] [PubMed] [Google Scholar]
- 6. Nadanaka S, Zhou S, Kagiyama S, Shoji N, Sugahara K, et al. (2013) EXTL2, a member of the EXT family of tumor suppressors, controls glycosaminoglycan biosynthesis in a xylose kinase-dependent manner. J Biol Chem 288:9321–9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takahashi I, Noguchi N, Nata K, Yamada S, Kaneiwa T, et al. (2009) Important role of heparan sulfate in postnatal islet growth and insulin secretion. Biochem Biophys Res Commun 383:113–118. [DOI] [PubMed] [Google Scholar]
- 8. Zertal-Zidani S, Bounacer A, Scharfmann R (2007) Regulation of pancreatic endocrine cell differentiation by sulphated proteoglycans. Diabetologia 50:585–595. [DOI] [PubMed] [Google Scholar]
- 9. Osman NMS, Kagohashi Y, Udagawa J, Otani H (2003) Alpha1,4-N-acetylglucosaminyltransferase encoding gene EXTL3 expression pattern in mouse adult and developing tissues with special attention to the pancreas. Anat Embryol (Berl) 207:333–341. [DOI] [PubMed] [Google Scholar]
- 10. Duncan G, McCormick C, Tufaro F (2001) The link between heparan sulfate and hereditary bone disease: finding a function for the EXT family of putative tumor suppressor proteins. J Clin Invest 108:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmale GA, Conrad EU, Raskind WH (1994) The natural history of hereditary multiple exostoses. J Bone Joint Surg Am 76:986–992. [DOI] [PubMed] [Google Scholar]
- 12. Hecht JT, Hall CR, Snuggs M, Hayes E, Haynes R, et al. (2002) Heparan sulfate abnormalities in exostosis growth plates. Bone 31:199–204. [DOI] [PubMed] [Google Scholar]
- 13. Anower-E-Khuda MF, Matsumoto K, Habuchi H, Morita H, Yokochi T, et al. (2013) Glycosaminoglycans in the blood of hereditary multiple exostoses patients: Half reduction of heparan sulfate to chondroitin sulfate ratio and the possible diagnostic application. Glycobiology 23:865–876. [DOI] [PubMed] [Google Scholar]
- 14. Solomon L (1964) Herditary Multiple Exostosis. Am J Hum Genet 16:351–363. [PMC free article] [PubMed] [Google Scholar]
- 15. Bari MS, Jahangir Alam MM, Chowdhury FR, Dhar PB, Begum A (2012) Hereditary multiple exostoses causing cord compression. J Coll Physicians Surg Pak 22:797–799. [PubMed] [Google Scholar]
- 16. Sladek R, Rocheleau G, Rung J, Dina C, Shen L, et al. (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885. [DOI] [PubMed] [Google Scholar]
- 17. Herder C, Rathmann W, Strassburger K, Finner H, Grallert H, et al. (2008) Variants of the PPARG, IGF2BP2, CDKAL1, HHEX, and TCF7L2 genes confer risk of type 2 diabetes independently of BMI in the German KORA studies. Horm Metab Res 40:722–726. [DOI] [PubMed] [Google Scholar]
- 18. Omori S, Tanaka Y, Takahashi A, Hirose H, Kashiwagi A, et al. (2008) Association of CDKAL1, IGF2BP2, CDKN2A/B, HHEX, SLC30A8, and KCNJ11 with susceptibility to type 2 diabetes in a Japanese population. Diabetes 57:791–795. [DOI] [PubMed] [Google Scholar]
- 19. Kifagi C, Makni K, Boudawara M, Mnif F, Hamza N, et al. (2011) Association of genetic variations in TCF7L2, SLC30A8, HHEX, LOC387761, and EXT2 with Type 2 diabetes mellitus in Tunisia. Genet Test Mol Biomarkers 15:399–405. [DOI] [PubMed] [Google Scholar]
- 20. Horikoshi M, Hara K, Ito C, Shojima N, Nagai R, et al. (2007) Variations in the HHEX gene are associated with increased risk of type 2 diabetes in the Japanese population. Diabetologia 50:2461–2466. [DOI] [PubMed] [Google Scholar]
- 21. Lewis JP, Palmer ND, Hicks PJ, Sale MM, Langefeld CD, et al. (2008) Association analysis in african americans of European-derived type 2 diabetes single nucleotide polymorphisms from whole-genome association studies. Diabetes 57:2220–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu L, Yang X, Wang H, Cui G, Xu Y, et al. (2013) Association between variants of EXT2 and type 2 diabetes: a replication and meta-analysis. Hum Genet 132:139–145. [DOI] [PubMed] [Google Scholar]
- 23. Rong R, Hanson RL, Ortiz D, Wiedrich C, Kobes S, et al. (2009) Association analysis of variation in/near FTO, CDKAL1, SLC30A8, HHEX, EXT2, IGF2BP2, LOC387761, and CDKN2B with type 2 diabetes and related quantitative traits in Pima Indians. Diabetes 58:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heijboer AC, Frans A, Lomecky M, Blankenstein MA (2011) Analysis of glucagon-like peptide 1; what to measure? Clin Chim Acta 412:1191–1194. [DOI] [PubMed] [Google Scholar]
- 25. Kuchuk NO, Pluijm SMF, van Schoor NM, Looman CWN, Smit JH, et al. (2009) Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab 94:1244–1250. [DOI] [PubMed] [Google Scholar]
- 26. Nijpels G, Boorsma W, Dekker JM, Hoeksema F, Kostense PJ, et al. (2008) Absence of an acute insulin response predicts onset of type 2 diabetes in a Caucasian population with impaired glucose tolerance. J Clin Endocrinol Metab 93:2633–2638. [DOI] [PubMed] [Google Scholar]
- 27. Tripathy D, Almgren P, Tuomi T, Groop L (2004) Contribution of insulin-stimulated glucose uptake and basal hepatic insulin sensitivity to surrogate measures of insulin sensitivity. Diabetes Care 27:2204–2210. [DOI] [PubMed] [Google Scholar]
- 28. Vergeer M, Brunham LR, Koetsveld J, Kruit JK, Verchere CB, et al. (2010) Carriers of loss-of-function mutations in ABCA1 display pancreatic beta-cell dysfunction. Diabetes Care 33:869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Booth SL, Centi A, Smith SR, Gundberg C (2012) The role of osteocalcin in human glucose metabolism: marker or mediator? Nat Rev Endocrinol 9:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park JW, Jang J-Y, Kim E-J, Kang MJ, Kwon W, et al. (2013) Effects of pancreatectomy on nutritional state, pancreatic function and quality of life. Br J Surg 100:1064–1070. [DOI] [PubMed] [Google Scholar]
- 31. Straub SG, Sharp GWG (2002) Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev 18:451–463. [DOI] [PubMed] [Google Scholar]
- 32. Straub SG, Sharp GWG (2004) Hypothesis: one rate-limiting step controls the magnitude of both phases of glucose-stimulated insulin secretion. Am J Physiol Cell Physiol 287:C565–71. [DOI] [PubMed] [Google Scholar]
- 33. McCulloch LJ, van de Bunt M, Braun M, Frayn KN, Clark A, et al. (2011) GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: implications for understanding genetic association signals at this locus. Mol Genet Metab 104:648–653. [DOI] [PubMed] [Google Scholar]
- 34. Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM (1991) Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 64:841–848. [DOI] [PubMed] [Google Scholar]
- 35. Thomas MK, Rastalsky N, Lee JH, Habener JF (2000) Hedgehog signaling regulation of insulin production by pancreatic beta-cells. Diabetes 49:2039–2047. [DOI] [PubMed] [Google Scholar]
- 36. Kayed H, Kleeff J, Osman T, Keleg S, Büchler MW, et al. (2006) Hedgehog signaling in the normal and diseased pancreas. Pancreas 32:119–129. [DOI] [PubMed] [Google Scholar]
- 37. Nybakken K, Perrimon N (2002) Heparan sulfate proteoglycan modulation of developmental signaling in Drosophila. Biochim Biophys Acta 1573:280–291. [DOI] [PubMed] [Google Scholar]
- 38. Bellaiche Y, The I, Perrimon N (1998) Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature 394:85–88. [DOI] [PubMed] [Google Scholar]
- 39. Fujino T, Asaba H, Kang M-J, Ikeda Y, Sone H, et al. (2003) Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci U S A 100:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fajans SS, Floyd JC, Knopf RF, Guntsche EM, Rull JA, et al. (1967) A difference in mechanism by which leucine and other amino acids induce insulin release. J Clin Endocrinol Metab 27:1600–1606. [DOI] [PubMed] [Google Scholar]
- 41. Thams P, Capito K (1999) L-arginine stimulation of glucose-induced insulin secretion through membrane depolarization and independent of nitric oxide. Eur J Endocrinol 140:87–93. [DOI] [PubMed] [Google Scholar]
- 42. Ziolkowski AF, Popp SK, Freeman C, Parish CR, Simeonovic CJ (2012) Heparan sulfate and heparanase play key roles in mouse β cell survival and autoimmune diabetes. J Clin Invest 122:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, et al. (1993) Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42:1663–1672. [DOI] [PubMed] [Google Scholar]
- 44. Fridlyand LE, Philipson LH (2006) Reactive species and early manifestation of insulin resistance in type 2 diabetes. Diabetes Obes Metab 8:136–145. [DOI] [PubMed] [Google Scholar]
- 45. Raats CJ, Bakker MA, van den Born J, Berden JH (1997) Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem 272:26734–26741. [DOI] [PubMed] [Google Scholar]
- 46. Wei J, Ferron M, Clarke CJ, Hannun YA, Jiang H, et al. (2014) Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest 124:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wei J, Hanna T, Suda N, Karsenty G, Ducy P (2014) Osteocalcin promotes β-cell proliferation during development and adulthood through Gprc6a. Diabetes 63:1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mizokami A, Yasutake Y, Gao J, Matsuda M, Takahashi I, et al. (2013) Osteocalcin induces release of glucagon-like peptide-1 and thereby stimulates insulin secretion in mice. PLoS One 8:e57375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mizokami A, Yasutake Y, Higashi S, Kawakubo-Yasukochi T, Chishaki S, et al. (2014) Oral administration of osteocalcin improves glucose utilization by stimulating glucagon-like peptide-1 secretion. Bone 69C:68–79. [DOI] [PubMed] [Google Scholar]
- 50. Jäger M, Westhoff B, Portier S, Leube B, Hardt K, et al. (2007) Clinical outcome and genotype in patients with hereditary multiple exostoses. J Orthop Res 25:1541–1551. [DOI] [PubMed] [Google Scholar]
- 51. Pedrini E, Jennes I, Tremosini M, Milanesi A, Mordenti M, et al. (2011) Genotype-phenotype correlation study in 529 patients with multiple hereditary exostoses: identification of “protective” and “risk” factors. J Bone Joint Surg Am 93:2294–2302. [DOI] [PubMed] [Google Scholar]
- 52. Busse M, Feta A, Presto J, Wilén M, Grønning M, et al. (2007) Contribution of EXT1, EXT2, and EXTL3 to heparan sulfate chain elongation. J Biol Chem 282:32802–32810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mutations in the EXT1 gene in our cohort.
(DOC)
Mutations in the EXT2 gene in our cohort.
(DOC)
Baseline characteristics of study subjects in OGTT.
(DOC)
Baseline characteristics of study subjects in clamp.
(DOC)
Supporting information file containing data for baseline characteristics.
(SAV)
Supporting information file containing data for OGTT.
(SAV)
Supporting information file containing data for Clamp.
(SAV)
Supporting information file containing data for MRI.
(SAV)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data are from the MARE study whose authors may be contacted at the department of Vascular Medicine, Academic Medical Center, Amsterdam, The Netherlands.