Abstract
Purpose
The role of spot sign on computed tomography angiography (CTA) for predicting hematoma expansion (HE) after primary intracerebral hemorrhage (ICH) has been the focus of many studies. Our study sought to evaluate the predictive accuracy of spot signs for HE in a meta-analytic approach.
Materials and Methods
The database of Pubmed, Embase, and the Cochrane Library were searched for eligible studies. Researches were included if they reported data on HE in primary ICH patients, assessed by spot sign on first-pass CTA. Studies with additional data of second-pass CTA, post-contrast CT (PCCT) and CT perfusion (CTP) were also included.
Results
18 studies were pooled into the meta-analysis, including 14 studies of first-pass CTA, and 7 studies of combined CT modalities. In evaluating the accuracy of spot sign for predicting HE, studies of first-pass CTA showed that the sensitivity was 53% (95% CI, 49%–57%) with a specificity of 88% (95% CI, 86%–89%). The pooled positive likelihood ratio (PLR) was 4.70 (95% CI, 3.28–6.74) and the negative likelihood ratio (NLR) was 0.44 (95% CI, 0.34–0.58). For studies of combined CT modalities, the sensitivity was 73% (95% CI, 67%–79%) with a specificity of 88% (95% CI, 86%–90%). The aggregated PLR was 6.76 (95% CI, 3.70–12.34) and the overall NLR was 0.17 (95% CI 0.06–0.48).
Conclusions
Spot signs appeared to be a reliable imaging biomarker for HE. The additional detection of delayed spot sign was helpful in improving the predictive accuracy of early spot signs. Awareness of our results may impact the primary ICH care by providing supportive evidence for the use of combined CT modalities in detecting spot signs.
Introduction
Intracerebral hemorrhage (ICH) is the cause of up to 15% of all strokes and carries a poor prognosis. It represents a considerable unmet medical need despite recent advances [1], [2]. Hematoma expansion (HE) has been recognized as an independent predictor for clinical deterioration, mortality, and poor outcome after ICH [3]–[5]. However, hemostatic drugs were not verified to restrict HE in previous trials [2], and they were criticized to include a great proportion of patients who may not have benefited from hemostatic treatment because their bleeding had already ceased [6].
It is not easy to determine which population is likely to develop HE. Recently, spot sign detected by CT angiography (CTA) has emerged as a potential predictor for HE after primary ICH (primary ICH). However, the underlying pathophysiology mechanisms remained unclear with a series of possible explanations, including Charcot-Bouchard microaneurysms, pseudoaneurysms, fibrin globes, and breakdown of blood-brain barrier [7]–[9].
Original studies on spot sign differed in population, imaging techniques, radiographic criteria, and definition of outcomes, with a wide range of predictive values. Two recent reviews have summarized the role of CTA spot sign in primary ICH [10], [11]. However, no published report has explored the predictive accuracy of spot sign by using a meta-analytic approach. Thus, we carried out this study aiming to evaluate the accuracy of spot sign in predicting HE after primary ICH.
Methods
Search Strategy
The supporting PRISMA checklist is available as supporting information; see S1 PRISMA Checklist. We conducted this meta-analysis according to the PRISMA statement [12]. We systematically searched Pubmed, Embase, and the Cochrane Library up to August 2014, restricting to English language. Search terms and keywords were grouped in the following search strategy: (“spot sign” OR “contrast extravasation” OR “postcontrast leakage” OR “computed tomography angiography” OR “CTA” OR “postcontrast CT”) AND (“intracerebral hemorrhage” OR “intracerebral hematoma” OR “intracranial hematoma” AND (“hematoma expansion” OR “hematoma growth” OR “hematoma enlargement” OR “recurrent bleeding”). Further, we searched the reference lists of relevant articles for additional studies.
Definition
Definitions of spot sign varied across studies [10]. Currently, spot sign was well-acknowledged as the foci of enhancement within the intracranial hematoma, detected on CTA source images [10]. We accepted a broad concept of spot sign that was detected by various CT modalities, including CTA, CTP and PCCT. Accordingly, we categorized spot signs into early spot signs and delayed spot signs. The early type was detected by the first-pass CTA. The delayed type was detected by the second-pass CTA, PCCT, or CTP. The first-pass CTA images were normally acquired in the arterial phase within 30 seconds after contrast injection. The second-pass CTA, namely the delayed CTA, was normally performed between 40 seconds to 3 minutes after contrast injection, which assessed the spot sign during the venous phase [10]. We accepted the definition of HE as an increase in ICH volume of >6 ml or >30% from the baseline ICH volume [8], [14].
Study Selection
Two reviewers (FZD and MG) screened titles and abstracts to identify eligible studies. Studies were included when meeting the following criteria: (1) original research; (2) investigated spot sign on CTA in patients with primary ICH; (3) reported data of HE in spot-sign negative and spot-sign positive groups; (4) reported clear definition of HE, which at least showed an increase in ICH volume of >6 ml or >30% from the baseline ICH volume. We excluded studies of secondary ICH resulting from trauma, tumor, intracranial aneurysm, arteriovenous malformation, or other causes. Studies examining spot signs by MRI were excluded.
Data Extraction and Quality Assessment
Two assessors (FZD and RJ) reviewed the full text of selected studies. Data were extracted independently in standardized forms. When duplicate cohorts were detected, the most informative cohort was included. The following items were extracted: author, year, study design, sample size, gender, CT modalities, CT type, definition of HE, time from onset to CTA, time from initial CT to HE assessment, and blinded assessment. Raw data were extracted into 2×2 contingency tables of positive and negative spot sign against clinical outcomes. Given the diagnostic feature of our research, selected papers were critically appraised through the QUADAS tool [13]. The reference standards in our study were clinical outcomes. So, we omitted one item on the time period from index test to reference standard, as the reference standard diagnoses are largely reached within a short period, thus eliminating the possible delayed verification bias [14].
Statistical Analysis
The Meta-Disc software 1.4 (Clinical Biostatistics, Ramony Cajal Hospital, Madrid, Spain) was used to perform analyses of predictive accuracy [15]. The sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic OR (DOR) were calculated [16]. Although PLR above 10 or NLR below 0.1 represented the most conclusive predictive value, we accepted PLR above 5 or NLR below 0.2 as satisfactory predictive values [17], [18]. The DerSimonian and Laird's random-effects model was employed for pooling the results. The heterogeneity between studies was assessed qualitatively by Cochran's Q test, and quantitatively by I2 statistic. A P value of less than 0.05 by Cochran's test indicated significant heterogeneity. A study with an I2 greater than 50% suggested substantial heterogeneity. The threshold effect was indicated when a “shoulder arm” pattern was present, or when the Spearman correlation coefficient in the threshold analysis showing a strong positive correlation [15]. Summary receiver operating characteristic (SROC) curves were further constructed by using the Moses-Shapiro-Littenberg method [19]. The Q*index and area under the curve (AUC) were calculated [20]. Because likelihood ratios, DORs, and SROC curves had the advantage of considering both the sensitivity and specificity data, they are more valuable for evaluating the diagnostic accuracy than sensitivity or specificity.
The publication bias was visually inspected by Funnel plot and statistically calculated by Deek's test [21]. The STATA software (version 12.0; Stata Corporation, College Station, Texas) was employed to explore the publication bias. We inferred several potential sources of heterogeneity a priori: (1) study design (prospective or retrospective); (2) sample size (<100 or ≥100); (3) results interpretation (blinded assessment or non-blinded assessment of radiographic features); (4) time to CTA (<6 h or ≥6 h). Subgroup analyses and univariate meta-regression were conducted to explore heterogeneity. A threshold of P <0.1 was defined for publication bias or heterogeneity existed.
Results
Literature Search and Study Characteristics
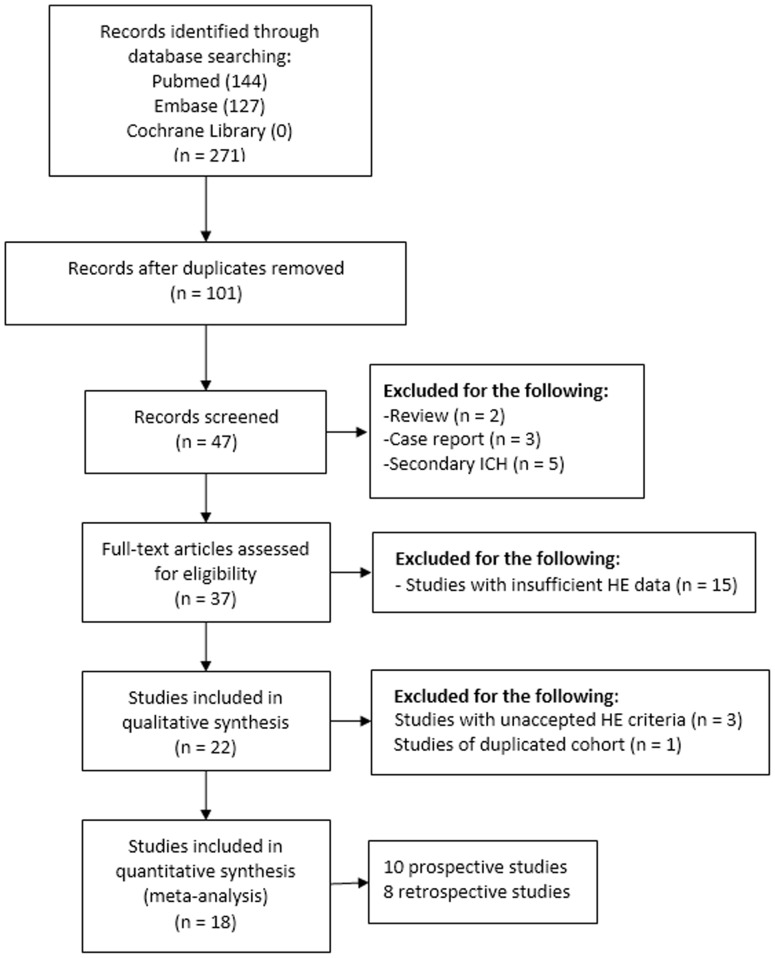
We initially retrieved 271 articles. Then we identified 47 relevant original studies after removal of duplications. Twenty-eight studies were further excluded, involving 5 studies of secondary ICH [22]–[26], 3 case reports [27]–[29], 2 review article [10], [11], 15 studies with insufficiently detailed records of HE data [7], [30]–[44], and 3 studies with definition of HE contradicting with our HE criteria [45]–[47]. Then we carefully assessed studies carried out by the same institutions [9], [48]–[53], and data from two identical cohorts were incorporated together [48], [49]. Thus, 18 studies were included into the meta-analysis, including 10 prospective studies, and 8 retrospective studies (Fig. 1). The study characteristics were summarized in Table 1. Rodriguez-Luna et al. performed post hoc analysis of the previous PREDICT study, with both early spot signs and delayed spot signs dynamically investigated . As the early spot signs were already included from the original PREDICT study [52], we only included the data of delayed spot signs in the post-hoc report [53]. The characteristics of studies were shown in Table 1. According to the modified 13 items QUADAS tool, most studies were of high quality (S1 Table). Notably, the criterion satisfied least was blinded assessment of spot sign and hematoma expansion.
Figure 1. The flowdiagram for selection of eligible studies.
Table 1. Characteristics of included studies.
| Author, year | Study design | Location | Sample size | Male, % | Imaging modality | CT type | Definition of HE | Time from onset to CTA (h) | Time from initial CT to HE assessment (h) | Blinded assessment |
| Wada (2007) | Prospective | Canada | 39 | 67 | First-pass CTA; PCCT | 4-slice; 64-slice | >6 ml or>30% | <3 | <48 | NA |
| Goldstein (2007) | Retrospective | USA | 104 | 53 | First-pass CTA | Helical | >33% | <48 | <48 | Yes |
| Delgado (2009) | Retrospective | USA | 367 | 58 | First-pass CTA; second-pass CTA | 16-slice helical; 64-slice helical | >6 ml or>30% | Mean: 7.4 | <48 | Yes |
| Ederies (2009) | Retrospective | Canada | 61 | 67 | First-pass CTA; PCCT | 4-slice; 64-slice | >6 ml or >30% | <6 | <24 | Yes |
| Delgado (2010) | Retrospective | USA | 573 | 54 | First-pass CTA; second-pass CTA | 16-slice helical; 64-slice helical | >6 ml or >30% | Mean: 7.4 | <48 | Yes |
| Evans (2010) | Retrospective | Canada | 59 | 59 | First-pass CTA; PCCT | 4-slice; 64-slice | >6 ml or >30% | Mean: 4.5 | <24 | NA |
| Park (2010) | Prospective | Korea | 110 | 63 | First-pass CTA | 64-slice helical | >6 ml or >30% | <24 | <48 | NA |
| Wang (2011) | Retrospective | China | 312 | 61 | First-pass CTA | 64-slice helical | >6 ml or >30% | <3 | <24 | NA |
| Li (2011) | Prospective | China | 139 | 68 | First-pass CTA | 16-slice | >12.5 ml or >30% | <6 | <24 | Yes |
| Demchuk (2012) | Prospective | Multicenter* | 228 | 57 | First-pass CTA | No specifications | >6 ml or >33% | <6 | <24 | Yes |
| Brouwers (2012) | Retrospective | USA | 391 | 55 | First-pass CTA | NA | >6 ml or >33% | Median: 6 | <48 | Yes |
| Junior (2013) | Prospective | Brazil | 65 | 62 | Dynamic CTA | 64-slice | >6 ml or >33% | Mean: 10.9 | NA | NA |
| Rizos (2013) | Prospective | Germany | 101 | 61 | First-pass CTA | 16-slice | >6 ml or >33% | <6 | Median: 19 | Yes |
| Romero (2013) | Prospective | USA | 131 | 61 | First-pass CTA | 64-slice helical | >6 ml or >33% | <24 | <24 | NA |
| Sun (2013) | Prospective | China | 112 | 64 | First-pass CTA; CTP | 16-slice multidetector | >6 ml or >30% | <6 | <24 | Yes |
| Brouwers (2014) | Prospective | USA | 817 | 56 | First-pass CTA | NA | >6 ml or >33% | Median: 5.0 | Median: 18 | Yes |
| Hotta (2014) | Retrospective | Japan | 323 | 58 | First-pass CTA | 40-slice | >12.5 ml or >33% | <24 | <24 | NA |
| Rodriguez-Luna (2014) | Prospective | Multicenter* | 371 | NA | Dynamic CTA | NA | >6 ml or >33% | <6 | <24 | Yes |
CTP, CT perfusion; HE, hematoma expansion; NA, not available; PCCT, post-contrast CT; SS, spot sign.
*Multicenter included Canada, Spain, Germany, Poland, India, and USA.
First-pass CTA
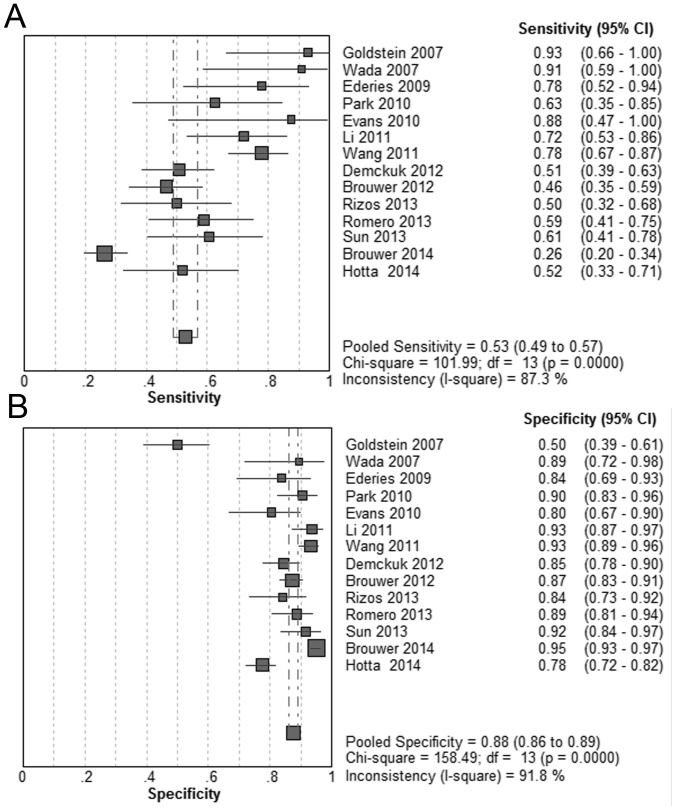
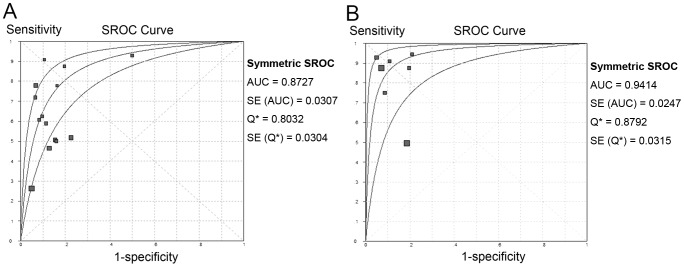
Fourteen relevant studies were identified. Spot sign was significantly associated with increased risk of HE (DOR = 11.84; 95% CI, 7.35–19.05; P<0.05; I2 = 71.6). The pooled sensitivity was 53% (95% CI, 49%–57%) with a specificity of 88% (95% CI, 86%–89%) (Fig. 2). The summary PLR was 4.70 (95% CI, 3.28–6.74) and the overall NLR was 0.44 (95% CI, 0.34–0.58). Significant heterogeneity was revealed for all results (P<0.05). The SROC curve yielded an AUC of 0.87 (Fig. 3A). (Table 2).
Figure 2. Summary of sensitivity and specificity of first-pass CTA spot signs in predicting hematoma expansion.
Figure 3. Summary of SROCs of spot signs on first-pass CTA or combined CT modalities for predicting hematoma expansion.
The curve is the regression line that summarizes the overall predictive accuracy. The upper and lower curves represent confidence intervals. Squares indicate individual study estimates of sensitivity and 1-specificity. The size of each square is proportional to the sample size of the corresponding study. Q*, the maximum joint sensitivity and specificity on a symmetric ROC curve; SE (AUC), standard error of AUC; SE (Q*), standard error of the Q* value. (A) first-pass CTA; (B) combined CT modalities.
Table 2. Pooled results of spot signs detected by different CT modalities for predicting hematoma expansion.
| Imaging modality | Study, n | SEN (95% CI) | SPE (95% CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (SE) | Q (SE) |
| First-pass CTA | 14 | 53% (49%–57%) | 88% (86%–89%) | 4.70 (3.28–6.74) | 0.44 (0.34–0.58) | 11.84 (7.35–19.05) | 0.87 (0.03) | 0.80 (0.03) |
| First-pass CTA & Delayed CTA | 4 | 65% (57%–73%) | 88% (85%–91%) | 6.43 (2.02–20.50) | 0.29 (0.08–1.02) | 22.40 (2.47–203.26) | 0.99 (0) | 0.97 (0.01) |
| Post-contrast CT | 2 | 41% (24%–61%) | 93% (84%–98%) | 5.38 (2.05–14.13) | 0.64 (0.47–0.87) | 8.74 (2.64–28.98) | 0.50 (0) | 0.50 (0) |
| First-pass CTA & Post-contrast CT | 3 | 92% (78%–98%) | 82% (74%–88%) | 4.89 (3.29–7.27) | 0.10 (0.04–0.31) | 52.62 (14.46–191.51) | 0.94 (0.05) | 0.88 (0.06) |
| Combined CT modalities | 7 | 73% (67%–79%) | 88% (86%–90%) | 6.76 (3.70–12.34) | 0.17 (0.06–0.48) | 43.51 (10.03–188.81) | 0.94 (0.02) | 0.88 (0.03) |
AUC, area under curve; DOR, diagnostic odds ratio; NLR, negative likelihood ratio; PLR, positive likelihood ratio; SE, standard error; SEN, sensitivity; SPN, specificity.
The publication bias was represented by examining studies of first-pass CTA. No publication bias was revealed by visual inspection of the funnel or by the Deek's test (P = 0.21). The source of heterogeneity was explored by subgroup analyses. We could not establish the subgroup of time to CTA due to heterogeneous data. Subgroup analyses were performed in terms of the stratification of study design, sample size, and blinded assessment (Table 3). Notably, studies without blinded assessment of spot signs produced DOR estimates about twofold higher than studies of blinded assessment. Studies with sample size below 100 produced DOR estimates that were about 2.6 times higher than studies with sample size over 100. The univariate meta-regression was further carried out, whereas indicating no statistical significance for study design, sample size, or blinded assessment.
Table 3. Subgroup analyses relating to the diagnostic odds ratio (DOR) of first-pass CTA for hematoma expansion.
| Subgroups | Hematoma expansion | ||
| Studies, n | DOR (95% CI) | I2, % | |
| Study design | |||
| Prospective | 8 | 10.94 (6.58–18.19) | 58.0 |
| Retrospective | 6 | 12.79 (4.70–34.79) | 82.6 |
| Sample size | |||
| ≥100 | 11 | 10.41 (6.28–17.27) | 75.2 |
| <100 | 3 | 26.92 (9.44–76.74) | 0 |
| Blinded assessment | |||
| Yes | 8 | 8.91 (5.76–13.80) | 51.1 |
| None/Unknown | 6 | 17.56 (6.25–49.36) | 79.8 |
Delayed CTA
Four articles were pertinent, including two articles of second-pass CTA [48], [49], and two studies of venous phase CTA [53], [54]. Data of the second-pass CTA were combined together due to duplicate cohort. The predictive accuracy of spot signs detected on delayed CTA images alone could not be calculated because of insufficient subgroup data. All studies reported the pooled results of spot signs jointly detected by the early and delayed CTA. Spot sign was significantly associated with increasing risk of HE (OR, 22.4; 95% CI, 2.47–203.26; P<0.05; I2 = 94.3). The pooled sensitivity was 65% (95% CI, 57%–73%) with a specificity of 88% (95% CI, 85%–91%). The summary PLR was 6.43 (95% CI, 2.02–20.50) and the overall NLR was 0.29 (95% CI, 0.08–1.02). The SROC curve yielded an AUC of 0.99. (Table 2)
Post-contrast CT
Three studies additionally reported extravasation data on PCCT [7], [8], [55]. Data of extravasation detected by PCCT alone were available in 2 studies [8], [55]. The PCCT extravasation was significantly associated with increased risk of HE (OR, 8.74; 95% CI, 2.64–28.98; P<0.05; I2 = 0). The pooled sensitivity was 41% (95% CI, 24%–61%) and the pooled sensitivity was 93% (95% CI, 84%–98%). (Table 2)
We further assessed the combined use of CTA and PCCT. Spot signs detected by either method were included, and they were significantly associated with increased risk of HE (OR, 52.62; 95% CI, 14.46–191.51; P<0.05; I2 = 0). The pooled sensitivity was 92% (95% CI, 78%–98%) and the pooled specificity was 82% (95% CI, 74%–88%). The summary PLR was 4.89 (95% CI, 3.29–7.27) and the summary NLR was 0.10 (95% CI, 0.04–0.31). The SROC curve yielded an AUC of 0.94. (Table 2)
CT Perfusion
Only one study compared CTP with CTA, which precluded meta-analysis [56]. The sensitivity, specificity, PPV, and NPV for CTP spot-sign predicting hematoma expansion were 89.3%, 94.0%, 83.3% and 96.3%, respectively.
Overall Combined CT Modalities
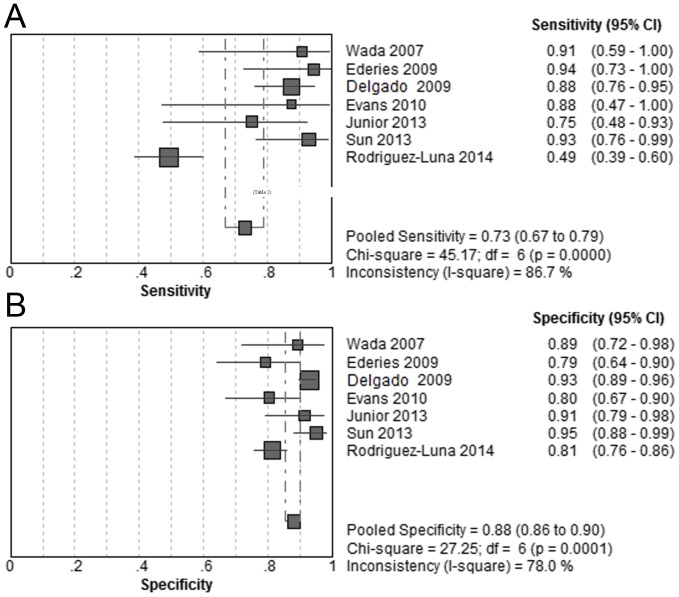
To examine the strength of combined CT modalities in detecting spot sign, we pooled the results of CTA combined with any additional CT modality, namely the joint CT modalities. Accordingly, 7 studies were included. Spot sign was significantly associated with increased risk of HE (OR, 43.51; 95% CI, 10.03–188.81; P<0.05; I2 = 88.5). The pooled sensitivity was 73% (95% CI, 67%–79%) and the pooled specificity was 88% (95% CI, 86%–90%) (Fig. 4). The summary PLR was 6.76 (95% CI, 3.70–12.34) and the summary NLR was 0.17 (95% CI, 0.06–0.48). The SROC curve yielded an AUC of 0.94 (Fig. 3B). (Table 2) To explore the source of heterogeneity, we further conducted sensitivity analysis by excluding the studies one by one. When excluding study by Rodriguez-Luna et al., the pooled diagnostic OR was 76.81 (95% CI, 41.21–143.15), without evidence of heterogeneity (I2 = 0, P 0.54).
Figure 4. Summary of sensitivity and specificity of spot signs on combined CT modalities in predicting hematoma expansion.
Discussion
We are aware of one recent systematic review regarding spot sign [11]. Notably, the included criteria was quite strict, as only studies reporting clinical outcomes (prognostic scale scores or death) of patients over 18 years were included. Thus, only 6 studies were reviewed. The author failed to conduct meta-analysis to pool the predictive accuracy of spot sign. Besides, the authors only focused on first-pass CTA spot signs. In comparison, firstly, our research included more updated and comprehensive studies, with those reporting HE data rather than clinical outcome data included. We did not constrict the age criteria since most related studies reported a wide range of ages. Secondly, a meta-analysis was performed to pool the predictive accuracy of spot sign, not only with SROC curves depicted, but also with different combination of imaging modalities compared. Thirdly, we systematically explored potential biases and sources of heterogeneity, which were scant in the previous review.
Spot sign is a dynamic radiological parameter with different sensitivity and specificity, depending on the imaging delay after contrast administration [48], [55], [57]. In general, the delayed time ranges from 5 s to 40 s for first-pass CTA [8], [9], has a median of 2 to 3 minutes for second-pass CTA [48], [49], and has a range of 3 to 5 minutes for PCCT [31]. Delayed imaging (>2 min) may help detect spot sign with increased time interval during which contrast is circulating and permeating into the hematoma. The acquisition of first-pass CTA may be too quick relative to bolus injection and not permit sufficient time for spot opacification, and thus miss delayed spot sign [10].
Our pooled results revealed that the early spot sign on first-pass CTA had a moderate sensitivity and a high specificity for predicting HE. In comparison, the spot sign detected by combined CT modalities had a higher sensitivity and similar specificity for predicting HE. Especially, the combined use of first-pass CTAand PCCT showed the highest sensitivity of 92%. However, the result of this combined modality was based on only three studies and future investigations are needed to verify this finding. When calculating likelihood ratios, only the combined modalities showed a PLR above 5 as well as a NLR below 0.2, which indicated satisfactory predictive values. Our study highlighted the strength of additionally performing dynamic CTA, PCCT, or CTP, which would be beneficial for improving the predictive accuracy of first-pass CTA spot sign.
When used for patient selection, a predictive imaging biomarker needs to have a high sensitivity to minimize the risk of excluding hemorrhage patients who might benefit from timely hemostatic interventions. In fact, the low sensitivity of early spot sign has been a major concern in its application in clinical trials [10]. It has been proposed that decreased sensitivity of the spot sign may be secondary to differences in scanner speed. Most studies scanned their patients on 4-, 16-, and 64-slice scanners, and not on the new faster scanners with 128- and 320-slice scanners [58]. However, as the advanced scanner was not available in many hospitals, the combined CT modalities were worthy of consideration to improve the sensitivity.
In subgroup analysis, it seemed that studies assessed without blindness had a higher DOR than those with blinded assessment. However, results from non-blinded assessment may not be reliable. Considering possible awareness of clinical outcomes, CT readers may be more cautious when assessing patients with HE, whereas may be more unwary when assessing those without HE. In prospective trials, clinicians who were aware of imaging results may encourage interventions for arresting HE in patients with spot signs. The increased exposure suspicion bias and therapy dilution bias may weaken the genuine predictive accuracy [59], [60]. Thus, the double-blinded assessment of radiographic features as well as clinical features are pivotal to minimize bias in future trials.
It constituted a major limitation that the baseline ICH volume and time from onset to CTA varied greatly across the included studies. Subgroup analyses were not performed due to overly heterogeneous data. However, as we failed to identify significant confounding factor in meta-regression analyses, the variations in baseline ICH volume or time to CTA may contribute to the heterogeneity. Spot sign may be more easily detected in large hematoma, which also marks those patients with more severe underlying vasculopathy or coagulopathy [9]. Patients with larger baseline ICH volume might have already bled more with more poor condition [52], and small ICHs have been suggested to be associated with less hematoma expansion and better outcome [61]. Some studies showed a declined accuracy of spot sign for predicting HE as time interval prolongs, suggesting that HE occurred mainly during the first few hours following ictus [62]. However, some studies opposed, arguing that a substantial number of patients destined to suffer from HE present either late or with an unknown symptom onset time. Spot sign may accurately identify those patients irrespective of time to CTA [9], [43], [51]. In light of these controversies, interactions among these factors are worthy of further investigations. Of interest, the ultra-early hematoma growth, which represented the adjusted baseline ICH volume by onset-to-imaging time, was shown to be faster in spot-sign positive patients and better predict HE [44]. Recently, a 9-point prediction score comprised of baseline ICH volume, time to CT, CTA spot sign, and warfarin use was developed, which correlated well with HE and other outcomes [50].
Several other limitations of our study should be acknowledged. The number of included studies was still limited with small sample sizes, especially those regarding spot signs on delayed CT modalities. Few studies have implemented a joint modality to detect spot sign. For retrospective studies, the decision to perform CTA was made by the clinicians, rather than a standard protocol, and thus increased the risk of selection bias. Few studies stated that the neuro-radiologists who evaluated spot signs were blinded to the results of clinical data or non-contrast CT. The result of combined modalities was based on relatively small number of studies. Given the difficulty in sorting heterogeneous confounding factors, such as timing, scanner type, and patient population, meta-regression analyses or subgroup analyses were not performed for combined modalities. Thus, we could not preclude the possibility that the higher predictive value was in fact an artifact of these characteristics. Some other crucial clinical variables, including age, consciousness level, blood pressure level, hypertension or anticoagulation history, and coagulation parameters, were mostly unknown and not well compared between groups, and thus preclude their incorporation into subgroup analyses. Besides, it is difficult to balance the potential imaging variables, including leukoaraiosis, brain atrophy, previous stroke lesions, and complex imaging parameters [6]. One important confounding radiological factor is the kinetics of the contrast bolus, which depend on patientrelated and injectionrelated variables, including cardiac output, concentration of the contrast medium, and injection rate. Although these factors are crucial to the spot sign appearance and magnitude, they were generally unclear and variable [1]. Finally, refinements in imaging techniques and validation of multi-itemed predictive scales, such as the spot sign score, are expected to further increase the predictive accuracy for patients who may develop HE after hemorrhage [49], [63].
Despite these limitations, our results demonstrated that spot sign appeared to be a useful imaging biomarker for predicting HE among patients with Primary ICH. Especially, the combined CT modalities showed satisfactory predictive accuracy of spot signs for hematoma enlargement. As most previous studies focused on early spot signs, we highlighted the additional value of delayed spot signs. Further studies are warranted, not only to investigate the mechanism of CE, but also to assess its power of selecting patients in clinical trials.
Supporting Information
Results of quality assessment by the QUADAS tool.
(DOCX)
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Romero JM (2012) Stroke: is spot sign the answer for intracerebral haemorrhage? Nat Rev Neurol 8:300–301. [DOI] [PubMed] [Google Scholar]
- 2. Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, et al. (2008) Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 358:2127–2137. [DOI] [PubMed] [Google Scholar]
- 3. Delcourt C, Huang Y, Arima H, Chalmers J, Davis SM, et al. (2012) Hematoma growth and outcomes in intracerebral hemorrhage: the INTERACT1 study. Neurology 79:314–319. [DOI] [PubMed] [Google Scholar]
- 4. Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, et al. (2006) Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 66:1175–1181. [DOI] [PubMed] [Google Scholar]
- 5. Morgenstern LB, Hemphill JC 3rd, Anderson C, Becker K, Broderick JP, et al. (2010) Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 41:2108–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wardlaw JM (2012) Prediction of haematoma expansion with the CTA spot sign: a useful biomarker? Lancet Neurol 11:294–295. [DOI] [PubMed] [Google Scholar]
- 7. Evans A, Demchuk A, Symons SP, Dowlatshahi D, Gladstone DJ, et al. (2010) The spot sign is more common in the absence of multiple prior microbleeds. Stroke 41:2210–2217. [DOI] [PubMed] [Google Scholar]
- 8. Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, et al. (2007) CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 38:1257–1262. [DOI] [PubMed] [Google Scholar]
- 9. Goldstein JN, Fazen LE, Snider R, Schwab K, Greenberg SM, et al. (2007) Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology 68:889–894. [DOI] [PubMed] [Google Scholar]
- 10. Brouwers HB, Goldstein JN, Romero JM, Rosand J (2012) Clinical applications of the computed tomography angiography spot sign in acute intracerebral hemorrhage: a review. Stroke 43:3427–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Del Giudice A, D'Amico D, Sobesky J, Wellwood I (2014) Accuracy of the spot sign on computed tomography angiography as a predictor of haematoma enlargement after acute spontaneous intracerebral haemorrhage: a systematic review. Cerebrovasc Dis 37:268–276. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verhaegen J, Gallos ID, van Mello NM, Abdel-Aziz M, Takwoingi Y, et al. (2012) Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: meta-analysis of cohort studies. BMJ 345:e6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A (2006) Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. [DOI] [PubMed] [Google Scholar]
- 17. Jaeschke R, Guyatt GH, Sackett DL (1994) Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 271:703–707. [DOI] [PubMed] [Google Scholar]
- 18. Van den Bruel A, Haj-Hassan T, Thompson M, Buntinx F, Mant D (2010) Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet 375:834–845. [DOI] [PubMed] [Google Scholar]
- 19. Moses LE, Shapiro D, Littenberg B (1993) Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 12:1293–1316. [DOI] [PubMed] [Google Scholar]
- 20. Swets JA (1988) Measuring the accuracy of diagnostic systems. Science 240:1285–1293. [DOI] [PubMed] [Google Scholar]
- 21. Deeks JJ, Macaskill P, Irwig L (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58:882–893. [DOI] [PubMed] [Google Scholar]
- 22. Huang AP, Lee CW, Hsieh HJ, Yang CC, Tsai YH, et al. (2011) Early parenchymal contrast extravasation predicts subsequent hemorrhage progression, clinical deterioration, and need for surgery in patients with traumatic cerebral contusion. J Trauma 71:1593–1599. [DOI] [PubMed] [Google Scholar]
- 23. Delgado Almandoz JE, Kelly HR, Schaefer PW, Brouwers HB, Yoo AJ, et al. (2012) CT angiography spot sign predicts in-hospital mortality in patients with secondary intracerebral hemorrhage. J Neurointerv Surg 4:442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letourneau-Guillon L, Huynh T, Jakobovic R, Milwid R, Symons SP, et al. (2012) Traumatic Intracranial Hematomas: Prognostic Value of Contrast Extravasation. AJNR Am J Neuroradiol. [DOI] [PMC free article] [PubMed]
- 25. Kobata H, Sugie A, Yoritsune E, Miyata T, Toho T (2012) Intracranial extravasation of contrast medium during diagnostic CT angiography in the initial evaluation of subarachnoid hemorrhage: report of 16 cases and review of the literature. SpringerPlus 2013:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brouwers HB, Backes D, Kimberly WT, Schwab K, Romero JM, et al. (2013) Computed tomography angiography spot sign does not predict case fatality in aneurysmal subarachnoid hemorrhage with intraparenchymal extension. Stroke 44:1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chakraborty S, Blacquiere D, Lum C, Stotts G (2010) Dynamic nature of the CT angiographic “spot sign”. Br J Radiol 83:e216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bermejo PG, Garcia JA, Perez-Fernandez S, Arenillas JF (2010) Spot sign and live-imaged dramatic intracerebral hematoma expansion. Neurology 75:834. [DOI] [PubMed] [Google Scholar]
- 29. Tan LA, Kasliwal MK, Johnson AK, Lopes DK (2014) The “Spot Sign” secondary to a ruptured lenticulostriate artery aneurysm. Clin Imaging 38:508–509. [DOI] [PubMed] [Google Scholar]
- 30. Gazzola S, Aviv RI, Gladstone DJ, Mallia G, Li V, et al. (2008) Vascular and nonvascular mimics of the CT angiography “spot sign” in patients with secondary intracerebral hemorrhage. Stroke 39:1177–1183. [DOI] [PubMed] [Google Scholar]
- 31. d'Esterre CD, Chia TL, Jairath A, Lee TY, Symons SP, et al. (2011) Early rate of contrast extravasation in patients with intracerebral hemorrhage. AJNR Am J Neuroradiol 32:1879–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huynh TJ, Keith J, Aviv RI (2012) Histopathological Characteristics of the “Spot Sign” in Spontaneous Intracerebral Hemorrhage. Arch Neurol: 1–2. [DOI] [PubMed]
- 33.Koculym A, Huynh TJ, Jakubovic R, Zhang L, Aviv RI (2013) CT perfusion spot sign improves sensitivity for prediction of outcome compared with CTA and postcontrast CT. AJNR Am J Neuroradiol 34:: 965–970, S961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sorimachi T, Osada T, Baba T, Inoue G, Atsumi H, et al. (2013) The striate artery, hematoma, and spot sign on coronal images of computed tomography angiography in putaminal intracerebral hemorrhage. Stroke 44:1830–1832. [DOI] [PubMed] [Google Scholar]
- 35. Boulouis G, Dumas A, Betensky RA, Brouwers HB, Fotiadis P, et al. (2014) Anatomic pattern of intracerebral hemorrhage expansion: relation to CT angiography spot sign and hematoma center. Stroke 45:1154–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Radmanesh F, Falcone GJ, Anderson CD, Battey TW, Ayres AM, et al. (2014) Risk factors for computed tomography angiography spot sign in deep and lobar intracerebral hemorrhage are shared. Stroke 45:1833–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dowlatshahi D, Wasserman JK, Momoli F, Petrcich W, Stotts G, et al. (2014) Evolution of computed tomography angiography spot sign is consistent with a site of active hemorrhage in acute intracerebral hemorrhage. Stroke 45:277–280. [DOI] [PubMed] [Google Scholar]
- 38. Brouwers HB, Raffeld MR, van Nieuwenhuizen KM, Falcone GJ, Ayres AM, et al. (2014) CT angiography spot sign in intracerebral hemorrhage predicts active bleeding during surgery. Neurology 83:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huynh TJ, Demchuk AM, Dowlatshahi D, Gladstone DJ, Krischek O, et al. (2013) Spot sign number is the most important spot sign characteristic for predicting hematoma expansion using first-pass computed tomography angiography: analysis from the PREDICT study. Stroke 44:972–977. [DOI] [PubMed] [Google Scholar]
- 40. Huynh TJ, Flaherty ML, Gladstone DJ, Broderick JP, Demchuk AM, et al. (2014) Multicenter accuracy and interobserver agreement of spot sign identification in acute intracerebral hemorrhage. Stroke 45:107–112. [DOI] [PubMed] [Google Scholar]
- 41. Havsteen I, Ovesen C, Christensen AF, Hansen CK, Nielsen JK, et al. (2014) Showing no spot sign is a strong predictor of independent living after intracerebral haemorrhage. Cerebrovasc Dis 37:164–170. [DOI] [PubMed] [Google Scholar]
- 42. Becker KJ, Baxter AB, Bybee HM, Tirschwell DL, Abouelsaad T, et al. (1999) Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke 30:2025–2032. [DOI] [PubMed] [Google Scholar]
- 43. Kim J, Smith A, Hemphill JC 3rd, Smith WS, Lu Y, et al. (2008) Contrast extravasation on CT predicts mortality in primary intracerebral hemorrhage. AJNR Am J Neuroradiol 29:520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rodriguez-Luna D, Rubiera M, Ribo M, Coscojuela P, Pineiro S, et al. (2011) Ultraearly hematoma growth predicts poor outcome after acute intracerebral hemorrhage. Neurology 77:1599–1604. [DOI] [PubMed] [Google Scholar]
- 45. Murai Y, Takagi R, Ikeda Y, Yamamoto Y, Teramoto A (1999) Three-dimensional computerized tomography angiography in patients with hyperacute intracerebral hemorrhage. J Neurosurg 91:424–431. [DOI] [PubMed] [Google Scholar]
- 46. Hallevi H, Abraham AT, Barreto AD, Grotta JC, Savitz SI (2010) The spot sign in intracerebral hemorrhage: the importance of looking for contrast extravasation. Cerebrovasc Dis 29:217–220. [DOI] [PubMed] [Google Scholar]
- 47. Ovesen C, Christensen AF, Krieger DW, Rosenbaum S, Havsteen I, et al. (2014) Time Course of Early Postadmission Hematoma Expansion in Spontaneous Intracerebral Hemorrhage. Stroke 45:994–999. [DOI] [PubMed] [Google Scholar]
- 48. Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, et al. (2009) Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke 40:2994–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Oleinik A, et al. (2010) The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke 41:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brouwers HB, Chang Y, Falcone GJ, Cai X, Ayres AM, et al. (2014) Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol 71:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brouwers HB, Falcone GJ, McNamara KA, Ayres AM, Oleinik A, et al. (2012) CTA spot sign predicts hematoma expansion in patients with delayed presentation after intracerebral hemorrhage. Neurocrit Care 17:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, et al. (2012) Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol 11:307–314. [DOI] [PubMed] [Google Scholar]
- 53. Rodriguez-Luna D, Dowlatshahi D, Aviv RI, Molina CA, Silva Y, et al. (2014) Venous phase of computed tomography angiography increases spot sign detection, but intracerebral hemorrhage expansion is greater in spot signs detected in arterial phase. Stroke 45:734–739. [DOI] [PubMed] [Google Scholar]
- 54. Rosa Junior M, Rocha AJ, Saade N, Maia Junior AC, Gagliardi RJ (2013) Active extravasation of contrast within the hemorrhage (spot sign): a multidetector computed tomography finding that predicts growth and a worse prognosis in non-traumatic intracerebral hemorrhage. Arq Neuropsiquiatr 71:791–797. [DOI] [PubMed] [Google Scholar]
- 55. Ederies A, Demchuk A, Chia T, Gladstone DJ, Dowlatshahi D, et al. (2009) Postcontrast CT extravasation is associated with hematoma expansion in CTA spot negative patients. Stroke 40:1672–1676. [DOI] [PubMed] [Google Scholar]
- 56. Sun SJ, Gao PY, Sui BB, Hou XY, Lin Y, et al. (2013) “Dynamic spot sign” on CT perfusion source images predicts haematoma expansion in acute intracerebral haemorrhage. Eur Radiol 23:1846–1854. [DOI] [PubMed] [Google Scholar]
- 57. Chakraborty S, Alhazzaa M, Wasserman JK, Sun YY, Stotts G, et al. (2014) Dynamic characterization of the CT angiographic ‘spot sign’. PLoS One 9:e90431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Romero JM, Brouwers HB, Lu J, Delgado Almandoz JE, Kelly H, et al. (2013) Prospective validation of the computed tomographic angiography spot sign score for intracerebral hemorrhage. Stroke 44:3097–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lindholm L, Rosen M (2000) What is the “golden standard” for assessing population-based interventions?–problems of dilution bias. J Epidemiol Community Health 54:617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sackett DL (1979) Bias in analytic research. J Chronic Dis 32:51–63. [DOI] [PubMed] [Google Scholar]
- 61. Dowlatshahi D, Smith EE, Flaherty ML, Ali M, Lyden P, et al. (2011) Small intracerebral haemorrhages are associated with less haematoma expansion and better outcomes. Int J Stroke 6:201–206. [DOI] [PubMed] [Google Scholar]
- 62. Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, et al. (1997) Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 28:1–5. [DOI] [PubMed] [Google Scholar]
- 63. Romero JM, Heit JJ, Delgado Almandoz JE, Goldstein JN, Lu J, et al. (2012) Spot sign score predicts rapid bleeding in spontaneous intracerebral hemorrhage. Emerg Radiol 19:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of quality assessment by the QUADAS tool.
(DOCX)
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.