Abstract
Previous observations that aquaporin overexpression increases the freeze tolerance of baker's yeast (Saccharomyces cerevisiae) without negatively affecting the growth or fermentation characteristics held promise for the development of commercial baker's yeast strains used in frozen dough applications. In this study we found that overexpression of the aquaporin-encoding genes AQY1-1 and AQY2-1 improves the freeze tolerance of industrial strain AT25, but only in small doughs under laboratory conditions and not in large doughs under industrial conditions. We found that the difference in the freezing rate is apparently responsible for the difference in the results. We tested six different cooling rates and found that at high cooling rates aquaporin overexpression significantly improved the survival of yeast cells, while at low cooling rates there was no significant effect. Differences in the cultivation conditions and in the thawing rate did not influence the freeze tolerance under the conditions tested. Survival after freezing is determined mainly by two factors, cellular dehydration and intracellular ice crystal formation, which depend in an inverse manner on the cooling velocity. In accordance with this so-called two-factor hypothesis of freezing injury, we suggest that water permeability is limiting, and therefore that aquaporin function is advantageous, only under rapid freezing conditions. If this hypothesis is correct, then aquaporin overexpression is not expected to affect the leavening capacity of yeast cells in large, industrial frozen doughs, which do not freeze rapidly. Our results imply that aquaporin-overexpressing strains have less potential for use in frozen doughs than originally thought.
Although much correlative evidence is available, the determinants of freeze resistance in Saccharomyces cerevisiae are largely unknown (for a review see reference 34). The injury sustained during freezing and thawing is caused by a combination of multiple types of stress imposed on the cells, such as changes in temperature, water content, water state, pH, and free radical, ion, and solute concentrations. The concomitant loss of survival is not attributable to any one form of injury and depends on the cooling and warming rates, the final temperature, the duration of freezing, and the suspending medium (23, 24).
When a cell suspension is cooled to a temperature of 0°C or less, both the suspending medium and the cells initially supercool. Extracellular ice crystal formation precedes intracellular freezing and is determined by the freezing point of the suspending medium and the presence of ice-nucleating agents. Based only on osmolarity, the freezing point of the cytoplasm is predicted to be approximately −1°C. Nevertheless, the cell interior typically remains unfrozen until the temperature is −10 to −15°C. This intracellular supercooling may be due to prevention of growth of ice into the cell interior by the cell membrane and due to the lack of nucleators of supercooled water within the cell (23, 24).
Following external freezing and internal supercooling, a chemical gradient for free water is established between the extracellular ice-containing water and the intracellular supercooled water. This thermodynamically unstable situation can be resolved either by water outflow or by internal freezing. At low cooling rates, ice crystal formation remains mainly extracellular, and water outflow from the cell is sufficient to restore the chemical equilibrium and minimize the intracellular supercooling. If cells are cooled rapidly, extensive intracellular supercooling occurs, and the chemical gradient is eliminated by freezing cellular water. The critical cooling rate at which intracellular ice crystals are formed is determined by the cell type, particularly the surface-to-volume ratio of the cell and the water permeability; larger, spherical cells and cells that are less permeable to water have a lower critical cooling rate (23, 24). Although the characteristics of primary lesions resulting from slow and rapid freezing are different, both processes are detrimental to the cell. This is the so-called two-factor hypothesis of freezing, which postulates that survival after freezing is mainly affected by two factors, cellular dehydration and intracellular ice crystal formation, which depend in opposite ways on the cooling velocity (23, 24).
Several cellular factors affect survival of yeast cells following freezing and thawing (for a review see reference 34). High trehalose levels (1, 6, 12, 13, 36) and high levels of heat shock protein expression (17) largely explain the freeze resistance acquired following exposure to mild forms of other stresses (22) and the influence of growth conditions on yeast freeze tolerance (10, 27, 30, 36). Data on the importance of cold shock proteins (18, 19) for freeze resistance are contradictory (16, 30). Given the oxidative and osmotic stress component of freeze stress, superoxide dismutases (29) and compatible solutes (28, 31, 32) also seem to protect yeast cells upon freezing. The importance of plasma membrane characteristics, such as fluidity and permeability, in relation to yeast freeze resistance has not been resolved (4, 20, 21). Finally, anti-freeze proteins (25) and hydrophylins (14, 15) are known to increase freeze tolerance.
Freeze tolerance and expression of the aquaporin-encoding genes AQY1 and AQY2 have been correlated based on a genome-wide gene expression analysis of the freeze-resistant industrial mutant strain AT25 (33). Overexpression of AQY1-1 or AQY2-1 in this strain increased freeze tolerance, both in cell suspensions and in small doughs, without negatively affecting growth or fermentation characteristics. Thus, the modification of aquaporin expression held promise for improving the freeze tolerance of commercial baker's yeast strains used in frozen dough applications. Unfortunately, aquaporin-overexpressing strains in industrial conditions were no more freeze tolerant than the controls were.
The goal of this work was to identify the cause of the difference between the results obtained with small doughs under laboratory conditions and the results obtained with large doughs under industrial conditions for freeze protection by aquaporin overexpression. We evaluated the cultivation conditions, freezing rate, and thawing rate as possible contributing factors. The results are important for understanding the molecular basis of freeze damage and the protective effect of aquaporin overexpression, as well as for improving industrial baker's yeast strains used in the preparation of frozen doughs.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
The strains used in this study were the laboratory strain BY4743 (MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0) (3) and the industrial strain AT25 (± diploid, probably aneuploid) (35). In these strains, pYX012 KanMX (vector), pYX012 KanMX/AQY1-1 (pAQY1-1), and pYX012 KanMX/AQY2-1 (pAQY2-1) have previously been integrated at the TPI locus, resulting in geneticin-resistant strains (33). In laboratory conditions, cells were routinely grown in YP (1% [wt/vol] yeast extract, 2% [wt/vol] Bacto Peptone [Difco]) with 2% glucose (YPD) at 30°C in an orbital shaker at 200 rpm. In addition, production under pilot-scale conditions was performed in fed-batch mode (20-liter fermentor containing 10 liters of molasses medium) (36).
RGC after freezing.
The residual glucose consumption (RGC) after fermentation and freezing was determined by comparing the average glucose consumption of two frozen samples (FGC) to the average glucose consumption of two control samples (IGC), as described previously [RGC = (FGC/IGC) × 100] (33). Briefly, equal amounts of stationary-phase cells (40-μl cell suspensions at an optical density at 600 nm of 20 for laboratory strains and at an optical density at 600 nm of 15 for industrial strains) were frozen 30 min after the addition of glucose in order to mimic the production of frozen dough, in which the dough is frozen after mixing, kneading, and eventually a short prefermentation period. After thawing, 400 μl of YP containing 33 mM glucose was added to both the control cells and the stressed cells, and after incubation at 30°C for 3 h (laboratory strains) or for 2.5 h (industrial strains) the amount of glucose taken up was measured.
Routinely, cells were frozen in standard 1.5-ml Eppendorf microcentrifuge tubes (VWR International, Leuven, Belgium) (referred to as small tubes or single plastic layer below) in an ethanol bath at −30°C for 1 h, stored in a freezer at −30°C for 1 day, and thawed at room temperature (between 18 and 22°C). To evaluate the effect of several successive rapid freezing treatments, the tubes were frozen in an ethanol bath at −30°C for 1 h and thawed at room temperature for 30 min, and this procedure was repeated up to 10 times.
Occasionally, cells were frozen slowly by putting the small tubes in a standard 50-ml plastic centrifuge tube (Falcon; Becton Dickinson, Erembodegem, Belgium) (referred to as large tube or double plastic layer below) stuffed with paper tissue (to create an insulating layer) or by putting the small tubes in a freezer at −30°C, as individual tubes or in a large tube. To evaluate the effect of several successive slow freezing treatments, the tubes were frozen in a freezer at −30°C for 1 h and thawed at room temperature for 30 min, and this procedure was repeated up to 30 times. Occasionally, cells were frozen very rapidly (by immersion of the small tubes in liquid nitrogen, as individual tubes or in a large tube). Unless indicated otherwise, cells were thawed at room temperature. Occasionally, cells were thawed more slowly (on ice) or more rapidly (in a water bath at room temperature or at 30°C). During freezing, temperature changes for four of these freezing conditions were monitored by using a thermocouple (type PT; diameter, 3 mm; Fisher Bioblock Scientific, Illkirch, France) connected to a data collector (Quick; Bioblock).
Survival in frozen dough. (i) Laboratory conditions.
The level of survival (expressed as a percentage of CFU) after fermentation and freezing was determined by comparing the average number of CFU isolated from two frozen doughs to the average number of CFU isolated from two nonfrozen control doughs, as described previously (33). Most doughs were frozen rapidly at −30°C in an ethanol bath or slowly in a freezer at −30°C; the exceptions were two nonfrozen controls that were analyzed immediately. After 1 h, the samples that were frozen in the ethanol bath were stored in the freezer at −30°C.
(ii) Industrial conditions.
At Lesaffre Développement, lean doughs were prepared by using the following formulation (3,000 g of flour per batch): 100 g of flour (14.5 to 15.0% protein on a 14% moisture basis), 63 g of water (with crushed ice), 2 g of salt (NaCl), 6 g (1.6% on a dry weight basis) of compressed yeast (from pilot-scale production), and 1 g of improver (Ibis Bleu; Lesaffre International Ingrédients, Marquette Lez Lille, France). For sugar-containing doughs, 10% saccharose was added. The target dough temperature was 19°C at all stages of preparation. The dough was kneaded mechanically for 15 min with a spiral mixer (FPI 50; VMI, Montaigu, France) and subsequently divided and molded. After prefermentation for 30 min in an air-conditioned room at 19°C, dough portions were frozen at −35°C with ventilation until the core temperature was −20°C. The temperature profiles in the cores of the dough portions were measured with a thermocouple (type K; diameter, 1.3 mm; Bioblock) connected to a data collector (HotBox; Bioblock). The doughs were subsequently stored at −20°C. At different times a dough portion was thawed at 0°C, the yeast gassing power was measured with a Zymotachigraph at 27°C for 2 h, and the dough proofing time was determined at 35°C.
RESULTS
Effect of aquaporin overexpression on freeze tolerance in industrial conditions.
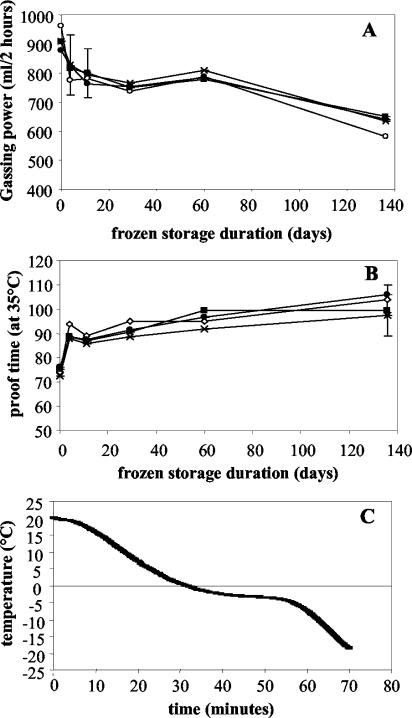
For large, industrial frozen doughs, no significant effect of aquaporin overexpression on the gassing power of the yeast (Fig. 1A) or the proofing time of the dough (Fig. 1B) was observed. This was the case for lean doughs (no saccharose) (Fig. 1), as well as for sugar-containing doughs (10% saccharose) (results not shown). These freezing conditions resulted in an average cooling rate of 0.6 to 0.8°C per min in the core of the dough (Fig. 1C).
FIG. 1.
(A and B) Evaluation of yeast gassing power (A) and dough proofing time (B) during frozen storage of large industrial doughs made with AT25 overexpressing AQY1-1 (•) or AQY2-1 (×) and with AT25 (▪) and a control strain containing an empty plasmid (○). The means and standard deviations for two independent tests are shown; the standard deviations for data points without error bars are less than 10% of the values of the points. (C) Temperature in the core of large, industrial doughs during freezing at −35°C with ventilation at Lesaffre Développement. The curve represents the average values for 64 measurements (two doughs, eight horizontal positions, and four vertical positions).
Importance of growth conditions.
The freeze tolerance of industrial strain AT25, as determined by calculating the RGC after the cells were frozen in an ethanol bath at −30°C, was significantly improved by overexpression of AQY1-1 or AQY2-1, both with the cells cultured under laboratory conditions and with the cells cultured under industrial conditions (results not shown). Essentially the same results were obtained when survival was determined for small doughs frozen in an ethanol bath at −30°C and then stored at −30°C in a freezer; the strain overexpressing AQY2-1 survived the initial freezing step and frozen storage better than the control strain, and it made no difference whether the strains were cultured under laboratory or industrial conditions (Fig. 2A).
FIG. 2.
Freeze tolerance of AT25 and AT25 overexpressing AQY2-1 in small frozen doughs, either cultured under laboratory conditions and harvested from liquid medium (◊ and ○, respectively) or produced at a pilot scale and resuspended from yeast cake (⧫ and •, respectively), frozen in an ethanol bath at −30°C (A) or in a freezer at −30°C (B). Survival is expressed as the percentage of CFU isolated from two frozen doughs based on the number of CFU in two nonfrozen control doughs. The standard deviations for replicate samples are indicated; the standard deviations for data points without error bars are less than 10% of the values of the points.
Importance of freezing conditions.
When survival was determined in small doughs that were slowly frozen by placing them in a freezer at −30°C, no difference was observed between the control strains and the strains overexpressing aquaporins (Fig. 2B). Thus, we evaluated the effect of the cooling rate in more detail. The cells generally remained highly viable under slow freezing conditions, whereas under rapid freezing conditions the RGC was significantly or even dramatically decreased (Table 1). Cells that were frozen in a small tube in liquid nitrogen had a low RGC rate that was significantly improved by overexpression of AQY1-1 or AQY2-1. Reduction of the cooling rate by use of a double plastic layer significantly improved the RGC of strains expressing AQY1-1 or AQY2-1 (Table 1). The improvement in the freeze tolerance due to aquaporin overexpression upon rapid freezing by immersion of a small tube directly in an ethanol bath at −30°C is consistent with the results obtained with other strain backgrounds (33). These freezing conditions resulted in an average cooling rate of 30 to 35°C per min (Fig. 3). Use of a double plastic layer and immersion in an ethanol bath at −30°C resulted in a lower cooling rate (10 to 15°C per min) (Fig. 3) and much higher RGC, especially in case of the control strains (Table 1). Although the RGC was still less than 100%, there was no significant difference between the controls and the strains overexpressing aquaporins. Similarly, when the cells were frozen by placing the vials in a freezer, the RGC was less than 100% and was not affected by aquaporin overexpression (Table 1).
TABLE 1.
Freeze tolerance of laboratory strain BY4743 and industrial strain AT25, with and without AQY1-1 or AQY2-1 overexpression, grown under laboratory conditions and tested in different freezing conditionsa
| Strain | RGC (%)
|
|||||
|---|---|---|---|---|---|---|
| Liquid N2b
|
Ethanol (−30°C)c
|
Freezer (−30°C)d
|
||||
| Single plastic layer | Double plastic layer | Single plastic layer | Double plastic layer | Single plastic layer | Double plastic layer | |
| BY4743 + vector | 19 ± 17 | 27 ± 13 | 26 ± 12 | 83 ± 8.1 | 88 ± 3.2 | 95 ± 5.3 |
| BY4743 + pAQY1-1 | 30 ± 8.3 | 52 ± 8.1 | 72 ± 7.6 | 94 ± 2.7 | 94 ± 5.8 | 96 ± 0.4 |
| BY4743 + pAQY2-1 | 32 ± 2.0 | 74 ± 8.1 | 93 ± 7.2 | 96 ± 6.5 | 96 ± 5.5 | 99 ± 1.3 |
| AT25 + vector | 22 ± 6.0 | 20 ± 8.4 | 28 ± 9.6 | 89 ± 5.5 | 95 ± 4.6 | 97 ± 2.5 |
| AT25 + pAQY1-1 | 28 ± 12 | 58 ± 9.6 | 76 ± 10 | 94 ± 5.4 | 93 ± 8.1 | 100 ± 0.5 |
| AT25 + pAQY2-1 | 27 ± 2.0 | 75 ± 8.6 | 80 ± 4.7 | 94 ± 4.5 | 89 ± 6.3 | 99 ± 1.8 |
RGC was determined as described in Materials and Methods; the values are averages ± standard deviations calculated from three independent experiments.
Very rapid freezing in liquid nitrogen.
Rapid freezing in an ethanol bath at −30°C.
Slow freezing in a freezer at −30°C.
FIG. 3.
Temperature courses in cell suspensions during freezing in the ethanol bath (single plastic layer [⧫] or double plastic layer [▪]) and in the freezer at −30°C (single plastic layer [▴] or double plastic layer [×]) (average of two measurements). For comparison, the first 10 min of the temperature course in the core of large, industrial dough during freezing at −35°C with ventilation at Lesaffre Développement (Fig. 1C) also is shown.
After only one cycle of slow freezing the RGC was still very high, which made any improvement due to aquaporin overexpression difficult to detect. Successive slow freezing treatments (up to 30 treatments) were used to lower the overall RGC and to more accurately measure any protective effect of aquaporin overexpression in the slow freezing conditions (Table 2), and the results were compared to the RGC for successive rapid freeze treatments (up to 10 treatments) (Table 2). After 1, 5, and 10 cycles of rapid freezing, there was a two- to threefold increase in freeze tolerance in the aquaporin-overexpressing strains, whereas after the same number of slow freezing cycles no significant effect of aquaporin overexpression was observed (Table 2). Thus, in general, strains overexpressing aquaporin were more freeze tolerant than the control strains, but the degree of improvement depended on the freezing rate.
TABLE 2.
Freeze tolerance of laboratory strain BY4743 and industrial strain AT25, with and without AQY1-1 or AQY2-1 overexpression, grown under laboratory conditions and tested after successive rapid or slow freezing treatmentsa
| Strain | RGC (%)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rapid cyclesb
|
Slow cyclesc
|
|||||||||
| 1 cycle | 2 cycles | 3 cycles | 5 cycles | 10 cycles | 1 cycle | 5 cycles | 10 cycles | 20 cycles | 30 cycles | |
| BY4743 + vector | 27 ± 2.3 | 19 ± 8.2 | 17 ± 5.2 | 7.8 ± 7.1 | 9.8 ± 12 | 97 ± 9.8 | 91 ± 8.7 | 70 ± 5.8 | 45 ± 8.5 | 37 ± 7.9 |
| BY4743 + pAQY1-1 | 61 ± 5.0 | 46 ± 4.7 | 31 ± 8.1 | 15 ± 13 | 16 ± 7.6 | 97 ± 11 | 94 ± 2.7 | 85 ± 5.4 | 74 ± 4.7 | 52 ± 5.8 |
| BY4743 + pAQY2-1 | 74 ± 9.5 | 60 ± 21 | 40 ± 12 | 21 ± 15 | 19 ± 6.0 | 86 ± 11 | 85 ± 6.2 | 79 ± 9.0 | 68 ± 5.1 | 64 ± 13 |
| AT25 + vector | 28 ± 4.4 | 30 ± 10 | 18 ± 3.4 | 18 ± 6.3 | 15 ± 4.4 | 93 ± 4.7 | 88 ± 3.8 | 85 ± 4.6 | 71 ± 5.5 | 64 ± 3.5 |
| AT25 + pAQY1-1 | 70 ± 7.2 | 55 ± 3.9 | 53 ± 7.5 | 38 ± 7.1 | 28 ± 8.1 | 100 ± 15 | 91 ± 3.9 | 88 ± 14 | 77 ± 9.9 | 66 ± 11 |
| AT25 + pAQY2-1 | 81 ± 0.8 | 72 ± 5.1 | 67 ± 5.3 | 61 ± 6.3 | 38 ± 8.2 | 100 ± 6.8 | 93 ± 1.2 | 88 ± 2.6 | 82 ± 16 | 70 ± 14 |
RGC was determined as described in Materials and Methods; the values are averages ± standard deviations calculated from three independent experiments.
Rapid freezing in an ethanol bath at −30°C.
Slow freezing in a freezer at −30°C.
Importance of thawing conditions.
As described above, overexpression of aquaporin significantly improved freeze tolerance in all the conditions tested, but no significant differences in RGC were observed based on the thawing conditions. The RGC means ± standard deviations for the different conditions were as follows: BY4743 plus vector, 7.0% ± 3.0%; BY4743 plus pAQY1-1, 69% ± 4.8%; BY4743 plus pAQY2-1, 38% ± 5.8%; AT25 plus vector, 27% ± 2.0%; AT25 plus pAQY1-1, 90% ± 3.9%; and AT25 plus pAQY2-1, 100% ± 2.5%.
DISCUSSION
The starting point of this study was the observation that in contrast to results obtained in small, laboratory doughs (33), no effect of aquaporin overexpression on the leavening capacity of yeast cells could be observed in large, industrial frozen doughs (Fig. 1). Laboratory and industrial conditions differ in the yeast cultivation conditions, the thawing rate, and the freezing rate. Additional differences between laboratory tests and industrial frozen dough bread-making conditions include the addition of protective substances to the dough formulation, the methods used for mixing and kneading the dough, the temperature used for dough preparation, and the osmotic pressure of the dough. Our results show that laboratory growth conditions alone are not a prerequisite for improvement of freeze tolerance by aquaporin-overexpressing strains at the start of the fermentation (Fig. 2A), nor is the thawing process a significant factor in determining cell survival. These results are consistent with the observation that the main damage to cells caused by freeze-thaw stress results from freezing rather than from thawing (30).
Our results for the effect of different freezing rates on yeast survival in the range of cooling rates that we studied are consistent with the results of other workers (7, 8, 23, 24). The levels of survival (expressed as RGC) generally remained high at cooling rates of 10 to 15°C per min but decreased dramatically at cooling rates of 30 to 35°C per min or higher (Table 1 and Fig. 3). The cooling rate for samples frozen in liquid nitrogen could not be determined with the thermocouple used, although it was presumed to be very high (7, 8). Aquaporin overexpression was somewhat advantageous when small tubes were put directly in liquid nitrogen, but it was even more advantageous when the cooling rate was slowed by use of a double plastic layer (Table 1).
When the survival after different consecutive slow freezing treatments was compared with the survival after the same number of rapid freezing treatments, it became clear that the increased freeze tolerance of aquaporin-overexpressing strains of S. cerevisiae seems to be restricted primarily to rapid freezing conditions (Table 2). Aquaporins mediate the rapid transport of water across the plasma membrane (2, 26). Our hypothesis is that higher levels of aquaporins in the plasma membrane allow rapid water efflux, especially at freezing temperatures, when water diffusion through the phospholipid layer of the membrane is limiting. Since the water permeability of the plasma membrane increases greatly at temperatures above the freezing point, the water flow probably is not limiting and the presence of excess aquaporins is not advantageous at low cooling rates.
This reasoning is consistent with the two-factor hypothesis of freezing injury. This hypothesis states that cells are mainly liable to cellular dehydration and exposure to solution effects at low freezing rates but that at high freezing rates mainly intracellular ice crystal formation occurs (23, 24). The observation that aquaporin-mediated improvement of freeze tolerance is restricted to rapid freezing conditions strengthens the hypothesis that improved freeze tolerance in baker's yeast overexpressing aquaporin implies that there is water export activity and results from a reduction in intracellular ice crystal formation (33). The results which we obtained with small doughs that were rapidly (Fig. 2A) or slowly (Fig. 2B) frozen and subsequently stored in the freezer also are consistent with this hypothesis. Dumont and coworkers relied on the same water permeability hypothesis that we relied on to explain the effects of cooling rate on yeast viability observed for the range of cooling rates that we studied (8).
If aquaporins effectively improve freeze tolerance only at high rates of freezing, then this limitation might help explain why aquaporin overexpression does not affect the leavening capacity of yeast cells in large, industrial frozen doughs (Fig. 1A and B). These industrial doughs usually are cooled at a rate of 0.6 to 0.8°C per min in the core of the dough (Fig. 1C and 3), which is much lower than the cooling rates used under laboratory conditions (Fig. 3). It is unlikely that the industrial freezing process could be adjusted in such a way that all cells in the dough undergo rapid enough freezing for aquaporins to be beneficial. Moreover, rapid freezing generally results in lower survival. Hence, the results described in this paper indicate that aquaporin overexpression is less useful for improvement of the maintenance of dough rising capacity in frozen doughs than originally thought (33).
Use of aquaporin overexpression for improvement of freeze tolerance might still have other potential applications (e.g., cryopreservation). The recent finding that artificial expression of an aquaporin improves the survival of mouse oocytes (5, 9) and fish embryos (11) after cryopreservation is particularly interesting in this respect. Finally, the fact that aquaporin overexpression is advantageous only for freeze tolerance in rapid freezing conditions also seems to limit the physiological importance of aquaporin-mediated freeze resistance of microorganisms under natural conditions.
Acknowledgments
This work was supported by a fellowship from the Institute for Scientific and Technological Research (IWT) and the Katholieke Universiteit Leuven (PDM/02/114) to A.T. and by grants from the Flemish Interuniversity Institute of Biotechnology (VIB/PRJ2), the Fund for Scientific Research-Flanders, and the Research Fund of the Katholieke Universiteit Leuven (Concerted Research Actions) to J.M.T.
We thank Renata Wicik and Kris Vandezande for technical assistance.
REFERENCES
- 1.Attfield, P. V., A. Raman, and C. J. Northcott. 1992. Construction of Saccharomyces cerevisiae strains that accumulate relatively low concentrations of trehalose and their application in testing the contribution of the disaccharide to stress tolerance. FEMS Microbiol. Lett. 94:271-276. [DOI] [PubMed] [Google Scholar]
- 2.Bonhivers, M., J. M. Carbrey, S. J. Gould, and P. Agre. 1998. Aquaporins in Saccharomyces—genetic and functional distinctions between laboratory and wild-type strains. J. Biol. Chem. 273:27565-27572. [DOI] [PubMed] [Google Scholar]
- 3.Brachmann, C. B., A. Davies, C. J. Cost, E. Caputo, J. Li, P. Hieter, and J. D. Boeke. 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115-132. [DOI] [PubMed] [Google Scholar]
- 4.Calcott, P. H., and A. H. Rose. 1982. Freeze-thaw and cold-shock resistance of Saccharomyces cerevisiae as affected by plasma membrane lipid composition. J. Gen. Microbiol. 128:549-555. [Google Scholar]
- 5.Cho, Y. S., M. Svelto, and G. Calamita. 2003. Possible functional implications of aquaporin water channels in reproductive physiology and medically assisted procreation. Cell. Mol. Biol. 49:515-519. [PubMed] [Google Scholar]
- 6.Diniz-Mendez, L., E. Bernardes, P. S. de Araujo, A. D. Panek, and V. M. F. Paschoalin. 1999. Preservation of frozen yeast cells by trehalose. Biotechnol. Bioeng. 65:572-578. [DOI] [PubMed] [Google Scholar]
- 7.Dumont, F., P.-A. Marechal, and P. Gervais. 2003. Influence of cooling rate on Saccharomyces cerevisiae destruction during freezing: unexpected viability at ultra-rapid rates. Cryobiology 46:33-42. [DOI] [PubMed] [Google Scholar]
- 8.Dumont, F., P.-A. Marechal, and P. Gervais. 2004. Cell size and water permeability as determining factors for cell viability after freezing at different cooling rates. Appl. Environ. Microbiol. 70:268-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edashige, K., Y. Yamaji, F. W. Kleinhans, and M. Kasai. 2003. Artificial expression of aquaporin-3 improves the survival of mouse oocytes after cryopreservation. Biol. Reprod. 68:87-94. [DOI] [PubMed] [Google Scholar]
- 10.Gélinas, P., G. Fiset, A. LeDuy, and J. Goulet. 1989. Effect of growth conditions and trehalose content on cryotolerance of baker's yeast in frozen doughs. Appl. Environ. Microbiol. 55:2453-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagedorn, M., S. L. Lance, D. M. Fonseca, F. W. Kleinhans, D. Artimov, R. Fleischer, A. T. Hoque, M. B. Hamilton, and B. S. Pukazhenthi. 2002. Altering fish embryos with aquaporin-3: an essential step towards successful cryopreservation. Biol. Reprod. 67:961-966. [DOI] [PubMed] [Google Scholar]
- 12.Hino, A., K. Mihara, K. Nakashima, and H. Takano. 1990. Trehalose levels and survival ratio of freeze-tolerant versus freeze-sensitive yeasts. Appl. Environ. Microbiol. 56:1386-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirasawa, R., K. Yokoigawa, Y. Isobe, and H. Kawai. 2001. Improving the freeze tolerance of bakers' yeast by loading with trehalose. Biosci. Biotechnol. Biochem. 65:522-526. [DOI] [PubMed] [Google Scholar]
- 14.Honjoh, K., Y. Oda, R. Takata, T. Miyamoto, and S. Hatano. 1999. Introduction of the hiC6 gene, which encodes a homologue of a late embryogenesis abundant (LEA) protein, enhances freezing tolerance of yeast. J. Plant Physiol. 155:509-512. [Google Scholar]
- 15.Imai, R., L. Chang, A. Ohta, E. A. Bray, and M. Takagi. 1996. A lea-class gene of tomato confers salt and freezing tolerance when expressed in Saccharomyces cerevisiae. Gene 170:243-248. [DOI] [PubMed] [Google Scholar]
- 16.Kaul, S. C., K. Obuchi, and Y. Komatsu. 1992. Cold shock response of yeast cells: induction of a 33 kDa protein and protection against freezing injury. Cell. Mol. Biol. 38:553-559. [PubMed] [Google Scholar]
- 17.Komatsu, Y., S. C. Kaul, H. Iwahashi, and K. Obuchi. 1990. Do heat shock proteins provide protection against freezing? FEMS Microbiol. Lett. 72:159-162. [DOI] [PubMed] [Google Scholar]
- 18.Kondo, K., and M. Inouye. 1991. TIP1, a cold shock-inducible gene of Saccharomyces cerevisiae. J. Biol. Chem. 266:17537-17544. [PubMed] [Google Scholar]
- 19.Kowalski, L. R. Z., K. Kondo, and M. Inouye. 1995. Cold-shock induction of a family of TIP1-related proteins associated with the membrane in Saccharomyces cerevisiae. Mol. Microbiol. 15:341-353. [DOI] [PubMed] [Google Scholar]
- 20.Kruuv, J., J. R. Lepock, and A. D. Keith. 1978. The effect of fluidity of membrane lipids on freeze-thaw survival of yeast. Cryobiology 15:73-79. [DOI] [PubMed] [Google Scholar]
- 21.Lewis, J. G., R. P. Learmonth, and K. Watson. 1994. Cryoprotection of yeast by alcohols during rapid freezing. Cryobiology 31:193-198. [DOI] [PubMed] [Google Scholar]
- 22.Lewis, J. G., R. P. Learmonth, and K. Watson. 1995. Induction of heat, freezing and salt tolerance by heat and salt shock in Saccharomyces cerevisiae. Microbiology 141:687-694. [DOI] [PubMed] [Google Scholar]
- 23.Mazur, P. 1970. Cryobiology: the freezing of biological systems. Science 168:939-949. [DOI] [PubMed] [Google Scholar]
- 24.Mazur, P., and J. J. Schmidt. 1968. Interactions of cooling velocity, temperature, and warming velocity on the survival of frozen and thawed yeast. Cryobiology 5:1-17. [DOI] [PubMed] [Google Scholar]
- 25.McKown, R. L., and G. J. Warren. 1991. Enhanced survival of yeast expressing an antifreeze gene analogue after freezing. Cryobiology 28:474-482. [DOI] [PubMed] [Google Scholar]
- 26.Meyrial, V., V. Laize, R. Gobin, P. Ripoche, S. Hohmann, and F. Tacnet. 2001. Existence of a tightly regulated water channel in Saccharomyces cerevisiae. Eur. J. Biochem. 268:334-343. [DOI] [PubMed] [Google Scholar]
- 27.Morris, G. J., G. E. Coulson, and K. J. Clarke. 1998. Freezing injury in Saccharomyces cerevisiae: the effect of growth conditions. Cryobiology 25:471-482. [Google Scholar]
- 28.Myers, D. K., and P. V. Attfield. 1999. Intracellular concentration of exogenous glycerol in Saccharomyces cerevisiae provides for improved leavening of frozen sweet doughs. Food Microbiol. 16:45-51. [Google Scholar]
- 29.Park, J., C. M. Grant, M. J. Davies, and I. W. Dawes. 1998. The cytoplasmatic Cu,Zn superoxide dismutase of Saccharomyces cerevisiae is required for resistance to freeze-thaw stress. J. Biol. Chem. 36:22921-22928. [DOI] [PubMed] [Google Scholar]
- 30.Park, J. I., C. M. Grant, P. V. Attfield, and I. W. Dawes. 1997. The freeze-thaw stress response of the yeast Saccharomyces cerevisiae is growth phase specific and is controlled by nutritional state via the RAS-cyclic AMP signal. Appl. Environ. Microbiol. 63:3818-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takagi, H., F. Iwamoto, and S. Nakamori. 1997. Isolation of freeze-tolerant laboratory strains of Saccharomyces cerevisiae from proline-analogue-resistant mutants. Appl. Microbiol. Biotechnol. 47:405-411. [DOI] [PubMed] [Google Scholar]
- 32.Takagi, H., K. Sakai, K. Morida, and S. Nakamori. 2000. Proline accumulation by mutation or disruption of the proline oxidase gene improves resistance to freezing and desiccation stresses in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 184:103-108. [DOI] [PubMed] [Google Scholar]
- 33.Tanghe, A., P. Van Dijck, F. Dumortier, A. Teunissen, S. Hohman, and J. M. Thevelein. 2002. Aquaporin expression correlates with freeze tolerance in yeast and overexpression improves freeze tolerance in industrial yeast. Appl. Environ. Microbiol. 68:5981-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanghe, A., P. Van Dijck, and J. Thevelein. Determinants of freeze tolerance in microorganisms, physiological importance, and biotechnological applications. Adv. Appl. Microbiol. 53:5981-5989. [DOI] [PubMed]
- 35.Teunissen, A., F. Dumortier, M.-F. Gorwa, J. Bauer, A. Tanghe, P. Smet, P. Van Dijck, and J. M. Thevelein. 2002. A freeze-tolerant diploid derivative of an industrial baker's yeast strain: isolation, characterization, and use in frozen doughs. Appl. Environ. Microbiol. 68:4780-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Dijck, P., D. Colavizza, P. Smet, and J. M. Thevelein. 1995. Differential importance of trehalose in stress resistance in fermenting and nonfermenting Saccharomyces cerevisiae cells. Appl. Environ. Microbiol. 61:109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]