Abstract
Objective:
To commission a grid block for spatially fractionated grid radiation therapy (SFGRT) treatments and describe its clinical implementation and verification through the record and verify (R&V) system.
Methods:
SFGRT was developed as a treatment modality for bulky tumours that cannot be easily controlled with conventionally fractionated radiation. Treatment is delivered in the form of open–closed areas. Currently, SFGRT is performed by either using a commercially available grid block or a multileaf collimator (MLC) of a linear accelerator. In this work, 6-MV photon beam was used to study dosimetric characteristics of the grid block. We inserted the grid block into a commercially available treatment planning system (TPS), and the feasibility of delivering such treatment plans on a linear accelerator using a R&V system was verified. Dose measurements were performed using a miniature PinPointTM ion chamber (PTW, Freiburg, Germany) in a water phantom and radiochromic film within solid water slabs. PinPoint ion chamber was used to measure the output factors, percentage depth dose (PDD) curves and beam profiles at two depths, depth of maximum dose (zmax) and 10 cm. Film sheets were used to measure dose profiles at zmax and 10-cm depth.
Results:
The largest observed percentage difference between output factors for the grid block technique calculated by the TPS and measured with the PinPoint ion chamber was 3.6% for the 5 × 5-cm2 field size. Relatively significant discrepancies between measured and calculated PDD values appear only in the build-up region, which was found to amount to <4%, while a good agreement (differences <2%) at depths beyond zmax was observed. Dose verification comparisons performed between calculated and measured dose distributions were in clinically acceptable agreements. When comparing the MLC-based with the grid block technique, the advantage of treating large tumours with a single field reduces treatment time by at least 3–5 times, having significant impact on patient throughput.
Conclusion:
The proposed method supports and helps to standardize the clinical implementation of the grid block in a safer and more accurate way.
Advances in knowledge:
This work describes the method to implement treatment planning for the grid block technique in radiotherapy departments.
The treatments of large size tumours represent a challenge in radiation therapy. The increase in tumour size will raise the extent of the surrounding normal tissue damage, which will in turn limit the eventual dose escalation strategies. Based on the fact that small volumes of tissues can tolerate high radiation doses, it was postulated that the local control for the bulky tumour can be achieved by a combination of open–closed radiation areas. In the 1950s, this technique was used with orthovoltage units in order to minimize skin toxicity while delivering high doses to deeply seated tumours.1,2
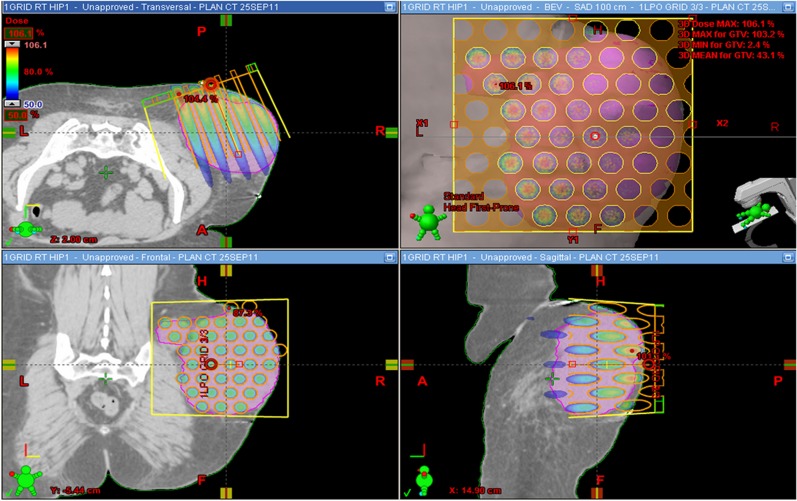
Spatially fractionated grid radiation therapy (SFGRT) was established in late 1980s, as a palliative treatment modality for bulky tumours.3 The SFGRT starts with a grid field, which represents a group of non-uniform pencil beams giving high radiation dose (usually, 15–18 Gy) in a single fraction, prescribed at depth of maximum dose (zmax) for a given beam size. This treatment is followed by a uniform 2 Gy per fraction dose coverage to the whole planning target volume using standard radiotherapy approaches (three-dimensional conformal or intensity-modulated radiotherapy/volumetric arc therapy). The beam delivered by the grid technique has a beam's eye view (BEV) pattern shown in Figure 1.
Figure 1.
Dose distribution calculated using the Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA) for passive grid block technique. A, anterior; BEV, beam's eye view; F, feet; GTV, gross tumour volume; H, head; L, left; LPO, left posterior oblique; max, maximum; min, minimum; P, posterior; R, right; RT, radiation therapy; SAD, source axis distance.
Clinical experience in a large cohort of patients treated to different body sites (abdomen, lung) surrounded by sensitive tissues indicates that this approach is well tolerated both in terms of acute effects, and that it produces no significant long-term complication.4 Clinical experience with open field radiation indicates that doses >15 Gy would produce not only significant acute morbidity but also substantial late toxicity. By contrast, SFGRT doses of >15 Gy have been utilized in conjunction with definitive doses of conventionally fractionated radiation without adding to the morbidity or detracting from the tolerance of normal tissues.
While the existing clinical studies1,5 suggest improved tumour control especially for resistant tumours to conventional radiation, such as melanomas and soft tissue sarcomas, the radiobiological mechanisms of grid therapy are not fully explored. Many studies have been undertaken in order to understand the radiobiological effects of the grid treatment on tumour cells.6–8 It has been shown in tumour models that a key component of treatment efficacy is the induction of endothelial apoptosis;9 this requires doses of >12 Gy. Similarly, the cytokine release associated with bystander effects and autosensitization to subsequent conventional doses of radiation require minimum doses of >10 Gy.10
Tumour volume, hypoxia and intrinsic cellular resistance remain key factors in the inability to eradicate larger tumours with conventional radiation dose/time fractionation. Much of the recent improvements in treatment delivery systems have also focused on better targeting of tumour and normal tissue volumes, improving dose homogeneity and reduction of dose to critical surrounding organs. In some cases, this has enabled dose escalation strategies for better local control, and in small tumours, this has enabled the use of effective high-dose stereotactic treatment approaches. However, with larger tumours, especially those adjacent to critical organs, both our ability to target them and the dose that can be safely delivered could be severely compromised. The experience with megavoltage SFGRT1,4,5 shows that the treatment mimics the dose rate pattern of high-dose brachytherapy and allows safe delivery of doses similar to stereotactic body radiation therapy (15–20 Gy, Figure 1). The technique itself has been used extensively with orthovoltage radiation until the 1970s but was not utilized with the advent of megavoltage linear accelerators. However, the challenge of treating large tumours still persists. Clinical experience using megavoltage SFGRT indicates that dramatic tumour responses can be produced even in tumours with intrinsic resistance to radiation, such as large sarcomas and melanomas. Recent experience in pre-operative treatment of soft-tissue sarcoma for tumours ranging in size from 8 to 22 cm (median, 11.5 cm) utilizing a combination of SFGRT (18 Gy) and conventional radiation (50 Gy, study recently presented at American Society of Clinical Oncology 201411) shows that the treatment was safe with low operative morbidity (<5%) and resulted in >90% tumour necrosis in 65% of patients and 15% with a complete pathological response. SFGRT has been used to treat large intra-abdominal, thoracic, and head and neck cancers with similar success.3 There is an increasing interest in incorporating this technique into treatment strategies at several major centres with new information that shows that SFGRT may have not just a local effect on treated tumours but could potentially induce positive immune modulatory systemic effects and release of cytokines such as tumour necrosis factor-a, transforming growth factor-b and ceramide, the latter especially responsible for enhanced vascular apoptosis within tumour volumes that can accelerate the response and regression of treated tumours and also produce apscopal effects that can be harnessed with systemic therapies. Historically the dose profile with SFGRT had been an extrapolation from single-field prescriptions at depth of dose maximum, but with the incorporation into a robust clinical treatment planning system (TPS) and the ability to extend dose delivery to a multifield approach this technique has promising applications for a wide variety of cancers that have been especially frustrating for oncologists with traditional approaches of treatment.
Currently, SFGRT is performed by either using a commercially available grid block or a multileaf collimator (MLC) of a linear accelerator. More recently, a spatially fractionated radiation treatment on TomoTherapy® unit (Accuray Inc., Sunnyvale, CA) using a TOMOGRID template was described.12 The MLC-based grid approach is widely used, and its feasibility was demonstrated by many authors.13,14 The MLC-based grid technique has many advantages: (a) the ease of creating the MLC grid shape; (b) carrying and mounting of the heavy grid block is not needed; (c) using the MLC-based grid approach allows for dose calculation within TPS and hence radiation oncologists can examine dose distribution on CT images prior to treatment.
On the other hand, owing to the X1 and X2 jaws over travel distance limitation, the maximum field size for the MLC-based grid technique on Varian linear accelerators will be 3 cm wide. This in turn means that for a 9-cm diameter tumour, three segments will be required to cover the target fully. In addition, the MLC-based grid technique uses 1 × 1 cm2 square openings, arranged in a chess pattern and separated by 1 cm. In the case where tumour size is wider than 9 cm, more than a single isocentre will be required, which includes the isocentre shift during the course of treatment.
This article studies the grid block delivery technique that has not only better maximum to minimum dose distribution compared with MLC but also that the technique is much faster than the more convenient MLC-based approach. However, one disadvantage of SFGRT using a grid block has been the inability of the commercially available treatment planning software to tailor the dose to the target. In this work, the grid block was incorporated into a commercially available TPS, and the feasibility of delivering such treatment plan on a linear accelerator using a record and verify (R&V) system was also demonstrated. As a part of the commissioning process, we compared dose distributions calculated by the TPS with dose measurements performed using both edge detector (miniature diode) within a water phantom and radiochromic films within solid water phantom.
METHODS AND MATERIALS
A dual-energy Varian linear accelerator (23EX linac) was used in this study together with the ARIA v. 10 record and verify (R&V) system (Varian Medical Systems, Palo Alto, CA). The TPS used was Eclipse (v. 10) with analytic anisotropic algorithm (AAA v. 10.0.28; Varian Medical Systems) as dose calculation algorithm. In our calculations, we have set the dose calculation grid size to be 2.5 × 2.5 × 2.5 mm3. A 6-MV photon beam was commissioned for the grid treatments.
Grid treatment planning system commissioning
Figure 2 depicts the grid block (Radiation Products Design Inc., Albertville, MN), which is a 7.5-cm thick cerrobend block (lead alloy) specifically designed for Varian linacs, mounted in such a way that the bottom edge of the grid block is at 64.5 cm from the source and is considered a block accessory. It is fixed onto a metallic frame that is mounted on the linac's accessory mount. The maximum field size that the grid block can treat at 100 cm source-to-surface distance (SSD) is 25 × 25 cm2.
Figure 2.
Grid block design. (a) Actual design; (b) treatment planning system design; (c) geometrical parameters; and (d) experimental set-up used to determine size and distribution of openings at the isocentre level, with a source-to-surface distance (SSD) of 100 cm and source-to-grid distance (SGD) of 64.5 cm.
The grid block has diverging cylindrical holes arranged in a hexagonal pattern as shown in Figure 2. Grid holes have a diameter of 1.4 and 2.1 cm centre-to-centre spacing when projected at an orthogonal to the beam plane 100 cm from the source. The actual grid block design was based on the previous work of Meigooni et al15 and Stathakis et al.16 To measure the size of the holes and their spacing, we irradiated a piece of EBT3 model GAFCHROMICTM film (Ashland, Wayne, NJ) positioned at the isocentre (Figure 2d). The beam centre was first marked on the film piece prior to insertion of the grid block using the beam cross hair. The film piece was scanned 24 h after irradiation and the transmission signal from the red colour channel was converted into relative dose using radiochromic film dose–response linearization method.17 The size of the hole was measured at 50% of the maximum dose.
To incorporate the grid block into the Eclipse TPS, the option called “fit to structure” was used. This grid structure was composed of 149 spheres distributed within the grid block projected at plane at 100 cm from the source. The finalarrangement and size adjustment of these holes were achieved using the film measurement described above. Finally, the block was attached to the field that was fitted to the grid structure using the “fit to structure” option in the Eclipse. This way, the holes of the grid block could be inserted in the TPS and mimic the actual block design.
As a measure of safety, a metallic bar was added to the grid block so that the linac would recognize the grid block as both block and User-X accessory. The User-X accessory option is provided by Varian on clinical linear accelerators to add a user-defined accessory other than the default ones. The linear accelerator can recognize any accessory by distribution of the drilled holes in the accessory attached, which can be made by the user as well. A coded metallic bar was customized in our hospital workshop so that the grid block can be recognized as a User-X accessory. With this, the maximum field size for the Grid block treatments can be limited and not exceed 25 × 25 cm2.
Dose measurements
In order to validate the grid block design inserted into the TPS, dose measurements using PTW PinPoint™ ion chamber (PPIC; PTW, Freiburg, Germany) in the water phantom and EBT3 model GAFCHROMIC film within solid water slabs were performed. The PPIC was used to measure output factors, percentage depth dose (PDD) curves and beam profiles at two depths, at zmax and at a depth of 10 cm. Radiochromic film sheets were also used to measure dose profiles at the same two depths. All measurements were performed in a 100-cm SSD set-up. Both the results of ion chamber and film measurements were resampled to the size of the calculation grid of 2.5 mm used by the TPS prior to comparison.
PTW PinPoint ion chamber with 0.015 cm3 volume (model: 31014) was used to measure output factors for different field sizes: 5 × 5, 10 × 10, 15 × 15, 20 × 20 and 25 × 25 cm2. These measurements were performed with MP1 water phantom and UNIDOS E electrometer (PTW). The ion chamber was placed at depth of dose maximum at the centre of the central hole of the grid block. Output factors were calculated as the ratio of readings for different field sizes divided by the reading for 10 × 10-cm open field. The PDD along the central axis was also measured using the PPIC for 10 × 10 and 20 × 20 cm2 field sizes.
In-line and cross-line dose profiles were measured using two methods: (a) PPIC within a water phantom as described above and (b) EBT3 model GAFCHROMIC film sheets sandwiched in solid water phantom at depths of zmax (1.5 cm) and 10 cm. The radiochromic film reference dosimetry system was calibrated using the protocol described by Devic et al.18 An Epson® Expression® 10000XL document scanner (Epson America, Long Beach, CA) was used to scan both calibration film pieces and measuring film sheets to obtain dose profiles along both transverse and longitudinal directions. A red colour channel from scanned tiff images was used with a scanning resolution of 0.2 mm per pixel.
In order to determine the transmission factor of the grid block (ratio of the charge measured in air under blocked region with the block inserted to that measured without the block for the same number of monitor units), a Brilliance CT Big Bore (Philips Healthcare, Cleveland, OH) was used to acquire an image set of the solid water phantom. Dose distribution was subsequently calculated using the AAA algorithm in order to obtain the TPS-generated dose profiles. Because of the difficulty related to measurements of the grid block transmission factor, the factor was found by setting a range of different values within TPS and then by comparing it with the film measurements. The transmission factor for the 6-MV beam was found to be 7.5%. For comparison, the transmission factor of the solid cerrobend block of the same thickness for the 6-MV photon beam is 3.5%.
RESULTS
Table 1 summarizes the comparison between the output factors for the grid block technique calculated by the Eclipse TPS and measured by using an ion chamber. The maximum observed percentage difference was 3.6% for the 5 × 5 -cm2 field size, and within 2% for the rest of the field sizes.
Table 1.
Comparison between treatment planning system (TPS)-calculated and ion chamber-measured output factors for the 6-MV beam
| Output factor | Field size (cm2) |
||||
|---|---|---|---|---|---|
| 5 × 5 | 10 × 10 | 15 × 15 | 20 × 20 | 25 × 25 | |
| PinPointTM IC (measured) | 87.0 | 88.9 | 91.2 | 93.0 | 94.6 |
| TPS (Calc.) | 83.9 | 88.8 | 92.0 | 94.6 | 95.8 |
| % Diff. | 3.6 | 0.1 | −0.9 | −1.7 | −1.3 |
Calc., calculated; Diff., difference; Ic, ion chamber.
Pinpoint, PTW, Freiburg, Germany.
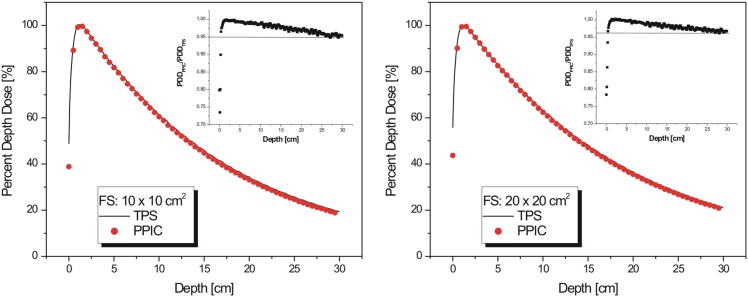
PDD measurements were performed by using the ionization chamber for 10 × 10 and 20 × 20 cm2 and compared with the PDD data calculated with the TPS. Figure 3 shows that a significant discrepancy between measured and calculated PDD appears in the build-up region. Such discrepancies are readily observed and attributed to inaccuracies of model calculations close to the surface of phantoms/patients. On the other hand, a good agreement (percentage difference <2%) at depths beyond zmax for both field sizes was observed, all the way down to 20-cm depth. The agreement between measured and calculated PDD data indicates that the beam quality calculated by Eclipse matches the actual beam quality generated by the attenuation of the radiation beam with small field-sized holes within the grid block.
Figure 3.
Percentage depth dose for 10 × 10 cm2 (left) and 20 × 20 cm2 (right) field size: treatment planning system (TPS) (solid line) vs ion chamber measurements (dots). Insets show differences in percentage depth dose (PDD) values between TPS and measured values. PPIC, PinPointTM ion chamber (PTW, Freiburg, Germany).
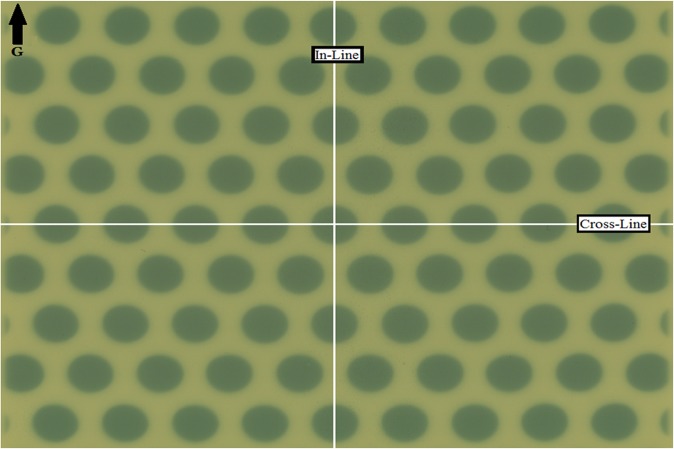
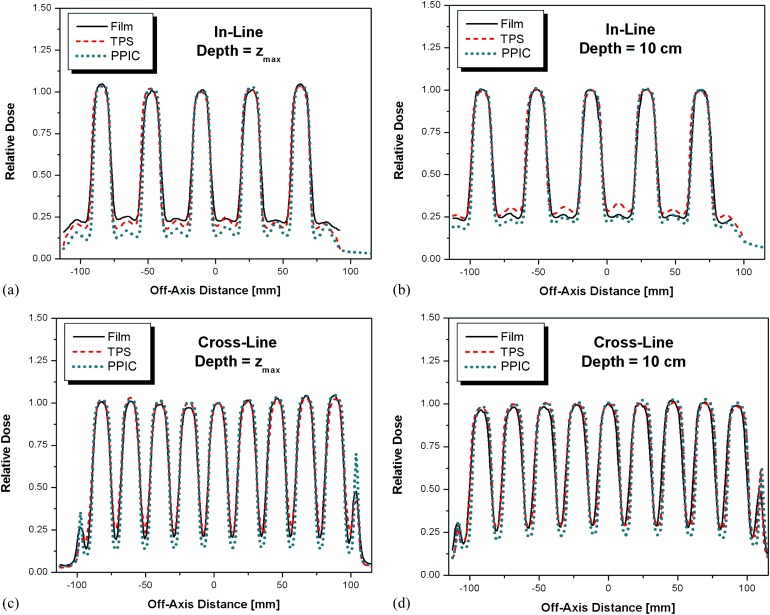
Transverse dose profiles were measured at a depth of zmax and depth of 10 cm using the ion chamber and sheets of EBT3 model GAFCHROMIC film. Figure 4 shows the irradiated piece of EBT3 model GAFCHROMIC film with indication of directions along which the dose profiles were obtained and subsequently compared with the corresponding dose profiles calculated by the TPS. Figure 5 shows a comparison between measured (PPIC and EBT3 films) and the calculated dose profiles along the in-line and cross-line dose profiles, respectively. Discrepancies were observed for PPIC in low-dose regions owing to the relatively low signal created within a relatively small ion chamber volume, and the percentage difference between the measured and calculated dose profiles were all within 5%. In the high-dose regions, discrepancies between the measured and calculated dose values were within 2%, in accordance to the increased precision of the radiochromic film dosimetry system at higher doses.15
Figure 4.
Exposed sheet of radiochromic film at zmax with 20 × 20 cm2 collimator setting. G, gantry direction.
Figure 5.
In-line profiles at depths of (a) depth of maximum dose (zmax) and (b) 10 cm, and the cross-line profiles at depths of (c) zmax and (d) 10 cm. PPIC, PinpointTM ion chamber (PTW, Freiburg, Germany); TPS, treatment planning system.
Results presented in Table 1 and Figures 3 and 5 indicate that the commissioning process for the grid block incorporation into the Eclipse TPS described in this work results in acceptable dosimetric accuracy. In addition, the incorporation of the grid block into the R&V system provides a safe way of treatment and prevents an adverse dose delivery that could be implicated by an attempt to deliver a plan with a field size larger than 25 × 25 cm at the isocentre plane.
DISCUSSION
The treatment of advanced, bulky malignant tumours continues to be a major challenge for the oncologist. Therapeutic options of fractionated radiation are often hampered by the bulk or the volume of disease and limited by normal tissue tolerance. SFGRT is an innovative concept in radiation therapy that has utilized spatially fractionated radiation delivery as a means of overcoming limitations of acute and late normal tissue tolerance. The MLC-based approach for treatment delivery of SFGRT appears to be attractive since the MLC is already incorporated into the linac head, and the dose calculation is readily available within the TPSs. However, from our clinical experience, we have found that the main drawback of the MLC-based approach is the relatively long delivery time when compared with that of the grid block approach. In addition to the actual prescription dose, delivery time with MLC-based approach is 2–3 times longer for smaller tumours (up to 9 cm in diameter), 3–6 times longer for medium size tumours (9–18 cm in diameter, using 2 isocentres) and 6–9 times longer for large tumours (18–27 cm in diameter, using 3 isocentres). The use of multiple isocentres do not only imply longer beam on time, they also require realignment of patients and, depending on the actual clinical protocol, the eventual reimaging of the patient in the new position. Additionally, the grid block approach provides better shielding-to-open beam ratio than the MLC-generated spatially fractionated dose distributions. To date, however, the use of the grid block for SFGRT is limited in ability to perform treatment planning and patient-specific dose assessment on a case-by-case basis. In addition, tabulated output factors for monitor units calculation (following more or less the same approach, as it was used for electron beam treatments in the past) were readily available only in cases whereby fixed collimator jaw settings were applicable. In such cases, the physicist would have to measure the corresponding output factors for almost every patient. The ability to calculate dose distribution for SFGRT within the TPS does not only provide a fast and accurate method for monitor unit calculation but it also allows the physician to visualize the actual dose distribution to the target and to the surrounding critical structures and prescribe a clinically relevant dose as well.
Commissioning method described in this work was applied to one commercially available clinical linear accelerator and its accompanying TPS. However, it can be easily extended to any other commercially available treatment modality.
CONCLUSION
Results presented in this work allow for implementing the grid block technique into the radiation therapy process in a safer and more accurate manner. Since the spatially fractionated grid therapy is not considered as a standard of care, all commercially available linear accelerators, as well as their accompanying TPSs are not customized to recognize the grid block as an accessory. Dose verification comparisons that we performed between the calculated and measured point doses as well as dose profiles were in clinically acceptable agreements. When comparing the MLC-based technique with the grid block technique, we should take into account the advantage of treating large tumours with a single field that would reduce treatment time by at least 3–5 times, which may have a significant impact on the patient throughput.
FUNDING
This work was supported in part by the Natural Sciences and Engineering Research Council of Canada, contracts number 386009. SD is a research scientist supported by the Fonds de Recherche en Santé du Québec (FRSQ) 26856.
Acknowledgments
ACKNOWLEDGMENTS
We thank Dr Rana Mahmood for useful discussions.
REFERENCES
- 1.Jolles B. The study of connective-tissue reaction to radiation; the sieve or chess method. Br J Cancer 1949; 3: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marks H. Clinical experience with irradiation through a grid. Radiology 1952; 58: 338–42. [DOI] [PubMed] [Google Scholar]
- 3.Mohiuddin M, Curtis DL, Grizos WT, Komarnicky L. Palliative treatment of advanced cancer using multiple nonconfluent pencil beam radiation. A pilot study. Cancer 1990; 66: 114–18. [DOI] [PubMed] [Google Scholar]
- 4.Mohiuddin M, Fujita M, Regine WF, Megooni AS, Ibbott GS, Ahmed MM. High-dose patially-fractionated radiation (GRID): a new paradigm in the management of advanced cancers. Int J Radiat Oncol Biol Phys 1999; 45: 721–7. [DOI] [PubMed] [Google Scholar]
- 5.Huhn JL, Regine WF, Valentino JP, Meigooni AS, Kudrimoti M, Mohiuddin M. Spatially fractionated GRID radiation treatment of advanced neck disease associated with head and neck cancer. Technol Cancer Res Treat 2006; 5: 607–12. [DOI] [PubMed] [Google Scholar]
- 6.Sathishkumar S, Dey S, Meigooni AS, Regine WF, Kudrioti M, Ahmed MM, et al. The impact of TNF-a induction on therapeutic efficacy following high dose spatially fractionated (GRID) radiation. Technol Cancer Res Treat 2002; 1: 141–7. [DOI] [PubMed] [Google Scholar]
- 7.Sathiskumar S, Boyanovsky B, Karakashian AA, Rozenova K, Giltiay NV, Kudrimoti M, et al. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol Ther 2005; 4: 979–86. [DOI] [PubMed] [Google Scholar]
- 8.Asur RS, Sharma S, Chang CW, Penagaricano J, Kommuru IM, Moros EG, et al. Spatially fractionated radiation induces cytotoxicity and changes in gene expression in bystander and radiation adjacent murine carcinoma cells. Radiat Res 2012; 177: 751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003; 300: 1155–9. [DOI] [PubMed] [Google Scholar]
- 10.Lyng FM, Seymour CB, Mothersill C. Early events in the apoptotic cascade initiated in cells treated with medium from the progeny of irradiated cells. Radiat Prot Dosimetry 2002; 99: 169–72. [DOI] [PubMed] [Google Scholar]
- 11.Mohiuddin M, Memon M, Nobah A, Elsebaie M, Al Suhaibani A, Pant R, et al. Locally advanced high-grade extremity soft tissue sarcoma: response with novel approach to neoadjuvant chemoradiation using induction spatially fractionated GRID radiotherapy (SFGRT). J Clin Oncol 2014; 32: 5s. [Google Scholar]
- 12.Zhang X, Penagaricano J, Yan Y, Sharma S, Griffin RJ, Hardee M, et al. Application of spatially fractionated radiation (GRID) to helical Tomotherapy using a novel TOMOGRID template. Technol Cancer Res Treat Aug 2013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Meigooni AS, Dou K, Meigooni NJ, Gnaster M, Awan S, Dini S, et al. Dosimetric characteristics of a newly designed grid block for megavoltage photon radiation and its therapeutic advantage using a linear quadratic model. Med Phys 2006; 33: 3165–73. [DOI] [PubMed] [Google Scholar]
- 14.Ha JK, Zhang GW, Naqvi SA, Regine WF, Yu CX. Feasibility of delivering grid therapy using a multileaf collimator. Med Phys 2006; 33: 76–82. [DOI] [PubMed] [Google Scholar]
- 15.Meigooni AS, Parker SA, Zheng J, Kalbaugh KJ, Regine WF, Mohiuddin M. Dosimetric characteristics with spatial fractionation using electron grid therapy. Med Dosim 2002; 27: 37–42. [DOI] [PubMed] [Google Scholar]
- 16.Stathakis S, Esquivel C, Gutiérrez AN, Shi C, Papanikolaou N. Dosimetric evaluation of multi-pattern spatially fractionated radiation therapy using a multi-leaf collimator and collapsed cone convolution superposition dose calculation algorithm. Appl Radiat Isot 2009; 67: 1939–44. doi: 10.1016/j.apradiso.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 17.Devic S, Tomic N, Aldelaijan S, Deblois F, Seuntjens J, Chan MF, et al. Linearization of dose-response curve of the radiochromic film dosimetry system. Med Phys 2012; 39: 4850–7. doi: 10.1118/1.4736800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devic S, Seuntjens J, Sham E, Podgorsak EB, Kirov AS, Schmidtlein CR, et al. Precise radiochromic film dosimetry using a flat-bed document scanner. Med Phys 2005; 32: 2245–53. [DOI] [PubMed] [Google Scholar]