Abstract
The baker's yeast Saccharomyces cerevisiae is generally classified as a non-xylose-utilizing organism. We found that S. cerevisiae can grow on d-xylose when only the endogenous genes GRE3 (YHR104w), coding for a nonspecific aldose reductase, and XYL2 (YLR070c, ScXYL2), coding for a xylitol dehydrogenase (XDH), are overexpressed under endogenous promoters. In nontransformed S. cerevisiae strains, XDH activity was significantly higher in the presence of xylose, but xylose reductase (XR) activity was not affected by the choice of carbon source. The expression of SOR1, encoding a sorbitol dehydrogenase, was elevated in the presence of xylose as were the genes encoding transketolase and transaldolase. An S. cerevisiae strain carrying the XR and XDH enzymes from the xylose-utilizing yeast Pichia stipitis grew more quickly and accumulated less xylitol than did the strain overexpressing the endogenous enzymes. Overexpression of the GRE3 and ScXYL2 genes in the S. cerevisiae CEN.PK2 strain resulted in a growth rate of 0.01 g of cell dry mass liter−1 h−1 and a xylitol yield of 55% when xylose was the main carbon source.
The pentose sugar xylose is a major constituent of lignocellulose. Saccharomyces cerevisiae cannot use xylose, instead converting it primarily to xylitol with only a small fraction going into biomass or ethanol (44, 45). Recombinant xylose-metabolizing S. cerevisiae strains contain genes from the xylose-utilizing yeast Pichia stipitis coding enzymes for the first two steps in xylose conversion (23, 38, 46). However, the potential of S. cerevisiae's own enzymes, if they are overexpressed, has not been evaluated.
In xylose-utilizing fungi, xylose reductase (XR) reduces xylose to xylitol, which is oxidized to xylulose by xylitol dehydrogenase (XDH). Xylulose is subsequently phosphorylated to xylulose 5-phosphate by xylulokinase and metabolized through the pentose phosphate pathway. S. cerevisiae cannot utilize xylose but can grow on xylulose (15, 43). Thus, the inability of S. cerevisiae to utilize xylose was attributed to its inability to convert xylose to xylulose (15), even though low XR and XDH activities are known in S. cerevisiae (4). A nonspecific aldose reductase, converting xylose to xylitol, was purified and characterized from S. cerevisiae (24); however, the genes coding for the putative XR and XDH enzymes remained unknown. The third enzyme in the xylose pathway, xylulokinase, is encoded by XKS1, a gene that has been cloned from, and probably is functional in, S. cerevisiae (19). Moderate increases in xylulokinase activity are beneficial in recombinant xylose-metabolizing S. cerevisiae strains (9, 18, 20, 21, 40).
Based on the S. cerevisiae genome sequence (14), the N-terminal amino acid sequence of the previously purified aldo-keto reductase corresponds to the open reading frame YHR104w (GRE3), which has 72% amino acid similarity to the XR enzyme of P. stipitis. This enzyme can reduce a wide variety of ketose substrates and requires a NADPH cofactor (24). The XR of P. stipitis can use either NADH or NADPH in the reduction reaction. GRE3 is induced under various stress conditions and may act on the toxic intermediates generated (2, 11, 30, 31). Under some conditions, deletion of GRE3 can decrease xylitol production (42). Two other aldose reductase gene homologs, YPR1 and YJR096w, also encode enzymes requiring NADPH and can utilize xylose as a substrate, but they have much higher Km values for xylose than does Gre3p (29, 41). Thus, several genes encoding aldose reductases that could utilize xylose are expressed in S. cerevisiae.
There are three genes in the S. cerevisiae genome that are similar to the gene encoding XDH, XYL2 of P. stipitis. Open reading frame YLR070c encodes an enzyme with XDH activity (referred to here as ScXYL2 and ScXDH, respectively) (32). The other two genes are SOR1, which encodes sorbitol dehydrogenase (SDH), and an open reading frame, YDL246c, that is almost identical to SOR1. The SDH enzyme also can use xylitol as a substrate (35).
The aim of this work was to study the endogenous xylose pathway in S. cerevisiae. The gene homologs needed for xylose metabolism exist in the S. cerevisiae genome, but it is not known how they are expressed or if the activities they encode form a functional pathway, collectively capable of metabolizing xylose. This study is the first attempt to use only endogenous genes for generating a xylose-metabolizing S. cerevisiae strain. The use of endogenous genes as alternatives in constructing recombinant xylose-utilizing strains is evaluated.
MATERIALS AND METHODS
Strains.
The yeast strain W303-1B (MATα leu2-3/112 his3-11/15 trp1-1 can1-100 ade2-1 ura31) (39) was used for cloning of the GRE3 and ScXYL2 genes. The yeast strains S150-2B (H308) (MATa his3Δ-1 leu2-3/112 trp1-289 ura3-52 cir+ gal+) (26) and CEN.PK2 (H1346) (MATa leu2-3/112 ura3-52 trp1-289 his3Δ1 MAL2-8c SUC2) (6) were used as host strains for expressing the XYL1 and XYL2 genes of P. stipitis and the GRE3 and XYL2 genes of S. cerevisiae. The strains CEN.PK2 and ENY.WA-1A (MATα ura3-52 leu2-3/112 trp1-289 his3Δ1 MAL2-8c MAL3 SUC3) (7) were used for gene expression analysis. The previously described CEN.PK2-derived strains expressing the XKS1 gene on a multicopy vector (strain H1695) and the corresponding control strain with an empty vector (strain H1697) were used as control strains in the XKS1 expression analyses (33). The genomic DNAs of strains S288C (34) or CEN.PK2 were used as the template for PCR probes. The Escherichia coli DH5α strain was used for the bacterial cloning steps.
Northern analysis.
Total RNA was isolated with a Trizol reagent kit (Invitrogen, Carlsbad, Calif.). The samples taken at 70 h were treated with 10 mg of Zymolyase (Seikagaku Corporation, Tokyo, Japan) per ml for 10 min at room temperature (22 to 24°C) prior to the RNA isolation. Probes for the Northern analysis (Table 1) were prepared by PCR for all genes except for XKS1 from the genomic DNA of strains S288C or CEN.PK2. To avoid cross-reactions with homologous genes, the probes for ScXYL2, TKL1, and TAL1 were chosen partly from the 3′ or 5′ noncoding regions. The probe for SOR1 also detects YDL246c. The PCR products were cloned into pCR2.1-TOPO (Invitrogen), excised from the vector by digestion with EcoRI, and verified by sequencing. XKS1 was amplified by PCR as described previously (33). The fragments were purified from agarose gels, and labeled with a random primed DNA labeling kit (Roche, Basel, Switzerland) and [α-32P]dCTP (Amersham Pharmacia Biotech, Uppsala, Sweden). Six identical gels were run and blotted to Hybond-N nylon filters (Amersham Pharmacia Biotech) and fixed with UV light (UV Stratalinker 2400; Stratagene, La Jolla, Calif.). Hybridized mRNAs were scanned with a Typhoon Phosphoimager and quantified with ImageQuant software (Amersham Pharmacia Biotech). The measured values were normalized by using the specified amount of pyrophosphate phosphohydrolase (IPP1) mRNA, coding for inorganic diphosphatase or actin (ACT1) mRNA, which codes for structural protein actin as a control for each sample.
TABLE 1.
Primers used for gene cloning and probe generation
| Gene | Primers |
|---|---|
| GRE3 (YHR104w) | fwd, AAATTGGATCCAGATGTCTTCACTGGTTA; rev, CATACGGATCCTGAGTATGGATTTTACTG |
| ScXYL2 (YLR070c) probe | fwd, GCCTTGAGGATAGAGAACACC; rev, GATTCATGTCCCAGCACCATT |
| SOR1 probe | fwd, ATGTCTCAAAATAGTAACCC; rev, TCATTCAGGACCAAAGATAATAGT |
| GRE3 (YHR104w) probe | fwd, AACCATCCAGGCAGTACCAC; rev, AGCATCGGAATGAGGGAAAT |
| TKL1 probe | fwd, TTTGAGTGTTGAAGCTGCTA; rev, CAAATCTGATGATCTACGATC |
| TAL1 probe | fwd, CGCTTAAGGAAGTATCTCGGA; rev, GCTTTGCTGCAAGGATTCAT |
| IPP1 probe | fwd, CCGATTGGAAAGTTATTGCC; rev, AGAACCGGAGATGAAGAACCA |
| ACT1 probe | fwd, AAGAAATGCAAACCGCTGCT; rev, TGGTGAACGATAGATGGACCA |
Strain construction.
GRE3 (YHR104w) was amplified from the genomic DNA of strain W303-1B (Table 1). The resulting fragment was cloned into the BglII site between the PGK promoter and terminator of the pMA91 vector (27), resulting in plasmid B1165. The ScXYL2 (YLR070c) gene was cloned into the pMA91 vector as described previously (32). The ScXYL2 gene with the PGK promoter and terminator was released from pMA91 by digestion with HindIII and the fragment ligated into the HindIII site of the YEp24H (1) vector (plasmid B1180). The PCR fragment containing ScXYL2 also was inserted into the BglII site of plasmid B1181, which contains the PGK promoter and terminator from the pMA91 vector as a HindIII fragment cloned into the corresponding site in the YEplac195 vector (13) (plasmid B1523). The XYL1 gene of P. stipitis was previously amplified by PCR and cloned between the PGK promoter and terminator in the pMA91 vector (plasmid B383) (16). The XYL2 gene of P. stipitis also was cloned into pMA91 (plasmid B731) (46). The XYL2 expression cassette was ligated into the HindIII site of YEplac195 (plasmid B1530) or blunt ended and cloned into the PvuII site of YEp24 vector (17) (plasmid B733).
To give a control strain, the yeast strain H308 (S150-2B) was transformed with the empty pMA91 and YEp24 vectors. The strain H308 was also transformed with corresponding vectors containing the P. stipitis XYL1 and XYL2 genes (B383 and B733) to give strain H1356 and with vectors containing the S. cerevisiae genes GRE3 and ScXYL2 (B1165 and B1180) to give strain MTen. The CEN.PK2 strain was transformed with GRE3- and ScXYL2-containing vectors (B1165 and B1523) or with GRE3- and P. stipitis XYL2 (B1165 and B1530)-expressing vectors, resulting in strains H2558 and H2560, respectively. Standard recombinant DNA methods were used (25). Yeast transformations were done as described by Gietz et al. (12).
Enzyme activities.
XR and XDH activities were measured in cell extracts made by disrupting cells with glass beads either in 100 mM sodium phosphate buffer (pH 7.0) or in 50 mM HEPES buffer (pH 7.0), containing 1 mM MgCl2, 0.1 mM EDTA, and 1 mM dithiothreitol. Both buffers were supplemented with the protease inhibitors phenylmethylsulfonyl fluoride and pepstatin A (final concentrations of 0.17 mg ml−1 and 0.01 mg ml−1, respectively). XDH activity was measured as described earlier (32). XR activity was measured in 100 mM sodium phosphate buffer (pH 7.0) containing 1 M xylose and 0.2 mM NADPH as a decrease in A340. Protein was measured with the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.). All enzymatic and protein analyses were carried out with the Cobas Mira automated analyzer (Roche).
Yeast cultures and metabolite analysis.
The recombinant strains were cultured in shake flasks containing synthetic complete (YSC) medium (modified from that of Sherman et al. [37]) either with or without glucose and without either leucine or uracil for plasmid selection. The ratio of xylose to glucose was 19:1 or 20:1, with either 19 or 20 g of xylose liter−1 and 1 g of glucose liter−1. Cultures were started from an initial optical density at 600 nm (OD600) of 0.2. Alternatively, no glucose was added and the cultures were started with a high biomass (OD600 of approximately 2). Growth was measured as OD600. Cells also were cultivated on 20 g of glucose liter−1 in YSC medium without leucine and uracil. For growth on xylulose, a mixture of xylose and xylulose (30 g of xylose liter−1 and 10 g of xylulose liter−1) was prepared by isomerization of d-xylose (28). Cultures were made in 50 ml of media and carried out in 250-ml Erlenmeyer flasks at 30°C and 250 rpm on an orbital shaker. Each strain was cultured in duplicate.
Anaerobic growth experiments were performed in a 1.8-liter Chemap CMF bioreactor (Chemap AG, Volketswil, Switzerland) with an initial working volume of 1.2 liters. Mass flow controllers (Bronkhorst High-Tech BV, Ruurlo, The Netherlands) regulated the air and nitrogen flow rates. Nitrogen was passed through an Oxisorb oxygen-absorbing device (Messer Griesheim GmbH, Krefeld, Germany) to reduce the residual oxygen level to >50 ppb. The dissolved oxygen concentration was monitored by an Ingold polarographic probe (Mettler-Toledo, Columbus, Ohio). The temperature was maintained at 30°C, and the pH was controlled at 5.5 by the addition of 2 M NaOH. The medium was YSC without leucine and uracil supplemented with 47.5 g of xylose liter−1 and 2.5 g of glucose liter−1. Cultures were started from an initial OD of 8 to 9. The total gas flow rate in this experiment was 0.5 standard liters per minute. Cell growth was measured as OD600 and cell dry mass (DM) as described previously (40).
Growth rates (Table 2) were calculated from time periods in which growth, xylose consumption, and xylitol production were linear, i.e., 63 to 121 h (Fig. 1) and 69 to 147 h (Fig. 2A). The growth rate was calculated as the change in DM per hour (gram liter−1 hour−1). The specific production and consumption rates were calculated as C-mmol of substrate consumed or product formed by 1 g of DM per h. The DM values for shake flask cultures were obtained by using a conversion of 0.3 g of DM liter−1 for an OD600 value of 1 (unpublished data).
TABLE 2.
Growth rates and specific xylose consumption and xylitol production rates for the experiments of Fig. 1 and 2
| Genotypea | Growth (g of DM liter−1 h−1) | Xylose consumption (C-mmol g of DM−1 h−1) | Xylitol production (C-mmol g of DM−1 h−1) |
|---|---|---|---|
| PsXYL1 PsXYL2b | 0.014 ± 0.001 | 2.7 ± 0.1 | 0.44 ± 0.04 |
| ScGRE3 ScXYL2b | 0.008 ± 0.001 | 2.5 ± 0.2 | 0.89 ± 0.13 |
| ScGRE3 ScXYL2c | 0.010 ± 0.001 | 3.5 ± 0.1 | 2.0 ± 0.1 |
| ScGRE3 PsXYL2c | 0.011 ± 0.000 | 3.4 ± 0.0 | 1.9 ± 0.2 |
FIG. 1.
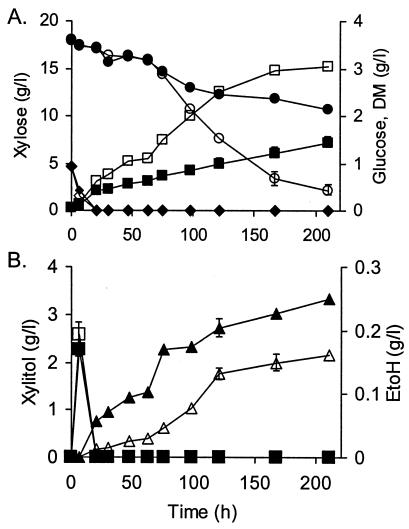
Comparison of S. cerevisiae S150-2B strains harboring XR and XDH either from P. stipitis or from S. cerevisiae in shake flask cultures. Growth and product formation on 20 g of xylose plus 1 g of glucose liter−1 as a carbon source. Filled symbols represent the strain overexpressing GRE3 and ScXYL2 genes, and open symbols represent the strain with P. stipitis XYL1 and XYL2 genes. (A) Shown are data for DM (▪), glucose (♦), and xylose (•). (B) Shown are data for xylitol (▴) and ethanol (EtOH) (▪). Results are based on two replications and are given in grams liter−1. Points without error bars have an associated error that is <10% of the value of the point.
FIG. 2.
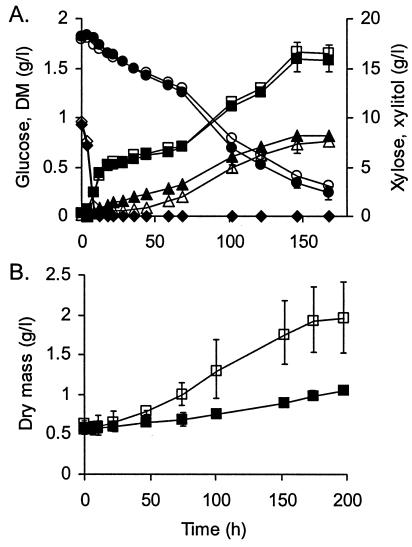
Xylose metabolism with the CEN.PK2 strains overexpressing GRE3 and ScXYL2 (filled symbols) or GRE3 and the P. stipitis XYL2 (open symbols) in shake flask cultures. Points without error bars have an associated error that is <10% of the value of the point. Results are based on two replications. (A) Growth and xylose consumption and xylitol formation on 19 g of xylose plus 1 g of glucose liter−1 as a carbon source. DM (▪), glucose (♦), xylose (•), and xylitol (▴), all in grams liter−1. (B) Growth on 20 g of xylose liter−1 as a carbon source.
The nontransformed strains CEN.PK2 and ENY.WA-1A were cultured in YP medium (10 g of yeast extract liter−1, 20 g of peptone liter−1) with either 20 g of glucose liter−1 or 20 g of glucose liter−1 and 100 g of xylose liter−1. Cells were harvested by centrifugation (3400 × g, 2 min at 20°C) and either frozen in liquid nitrogen (RNA samples) or moved directly to −70°C (samples for enzyme activities).
Metabolite production was analyzed from growth media by high-pressure liquid chromatography with an Aminex HPX-87H column (Bio-Rad Laboratories). The column was maintained at 35°C with an eluant of 5 mM H2SO4 at a constant flow rate of 0.6 ml min−1. Glucose, xylose, xylitol, glycerol, acetate, and ethanol were quantified by using a combination of a refractive index and UV (λ = 210 nm) detectors connected in series.
Plasmid stability was measured by plating cells from different time points on plates without selection, growing them for 2 days, and then replicating them on plates of selection media. All of the recombinant strains contained two expression vectors, so we studied possible recombination between the plasmids. Samples were taken when growth was complete, and plasmids were extracted and transformed into E. coli. Plasmid DNA was extracted from bacterial transformants and analyzed by restriction enzyme digestion.
RESULTS
Recombinant strains.
The XR activities of strains expressing XYL1, XYL2, GRE3, and ScXYL2 in glucose-grown cultures were similar, 15 to 22 nkat (per mg of total protein), for both GRE3- and XYL1-encoded enzymes. The XDH activity varied between about 10 nkat (per mg of total protein) for ScXDH and about 90 nkat (per mg of total protein) for the XDH of P. stipitis. Over 90% of the cells harbored both plasmids after cultivation for 2 days without auxotrophic selection, and no recombination between the plasmids was detected.
Growth on d-xylose.
When the S150-2B-derived strains containing XR and XDH either from P. stipitis or from S. cerevisiae were cultivated in shake flasks with 20 g of xylose liter−1 and 1 g of glucose liter−1 as the carbon source, glucose was consumed during the first 10 h, and then xylose consumption began. The strain with the S. cerevisiae XR and XDH consumed xylose slower and formed less biomass than did the strain containing the enzymes from P. stipitis (Fig. 1A). The control strain (data not shown) used practically no xylose, and growth on glucose and on ethanol derived from glucose resulted in 0.3 g of DM liter−1, compared to 1.5 and 3.0 g liter−1 with strains containing S. cerevisiae and P. stipitis enzymes, respectively. The biomass yield on xylose was 20% for both overexpressing strains.
Significantly more xylitol was formed by the strain with the S. cerevisiae enzymes (Fig. 1B). Equal amounts of glycerol were formed (0.1 g liter−1) and subsequently consumed by both strains (data not shown). Acetate (maximally 0.2 g liter−1) was detected only with the strain overexpressing the S. cerevisiae enzymes (data not shown).
The cultures grew in a biphasic manner with a period of slow xylose consumption (up to 60 h), followed by a faster metabolic phase (from 63 to 121 h) from which the specific rates (Table 2) were calculated. The strain overexpressing the S. cerevisiae genes grew much slower than did the strain with the P. stipitis genes. The specific xylose consumption rate differed by 15%, but the specific xylitol production rate was twice as high in the strain overexpressing the S. cerevisiae enzymes. The xylitol yield on xylose was about 45% for the strain overexpressing the S. cerevisiae enzymes and about 10% for the strain expressing the P. stipitis enzymes.
Effect of XDH activity on xylose metabolism of strains expressing GRE3.
XDH activity in the strains overexpressing the ScXDH-encoding gene was about 10 times lower than in the strains overexpressing the gene for P. stipitis XDH. Significantly higher amounts of xylitol were accumulated by the strain carrying the S. cerevisiae ScXDH. A CEN.PK2 strain overexpressing the S. cerevisiae GRE3 and P. stipitis XYL2 genes grew (Fig. 2A) and consumed xylose at the same rate as a strain carrying the GRE3 and ScXYL2 genes (Table 2). The strain expressing ScXDH initially produced xylitol slightly faster than did the strain with the P. stipitis XDH, but the final xylitol yield did not differ much, being 55 and 49%, respectively. Also, these strains showed a biphasic growth curve (Fig. 2A). Compared to the S150-2B-based strain, the growth rate of CEN.PK2-based strains was about 1.3 times higher, the specific xylose consumption rate was nearly 1.4-fold higher, and the specific xylitol production rate was over twofold higher, demonstrating the effects of different parental strains on the efficiency of xylose metabolism. Ethanol was produced only from glucose in the beginning of the cultivation, no glycerol was detected, and acetate accumulated in low quantities, maximally 0.6 g liter−1.
In shake flask cultures with xylose (20 g liter−1) as the sole carbon source, the biomass of the strain carrying the P. stipitis XDH increased more rapidly than did the biomass of the strain expressing ScXDH (Fig. 2B). With the latter, strain growth was almost negligible. The strain with P. stipitis XDH consumed more xylose than did the strain with S. cerevisiae XDH (16.2 versus 9.5 g in 200 h). The xylitol yield was 50% for the strain with P. stipitis XDH and 60% for the strain expressing the S. cerevisiae XDH-encoding gene. Thus, even 10-fold-higher XDH activity levels did not decrease the high xylitol yield with Gre3p. In bioreactor culture under anaerobic conditions, a small amount of xylitol (about 3.0 g liter−1) was formed from 47.5 g of xylose liter−1 and 2.5 g of glucose liter−1 in 140 h by both strains, and the xylose was not consumed further. The strain with P. stipitis XDH maintained more biomass under anaerobic conditions than did the strain with the S. cerevisiae XDH (data not shown).
Transcript pattern of the endogenous xylose pathway genes.
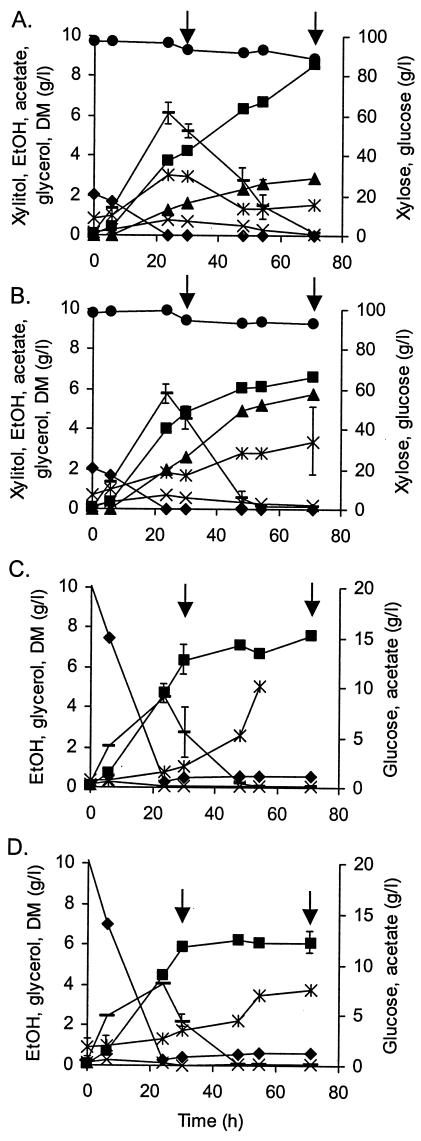
The glucose consumption and ethanol formation and subsequent consumption in cultures of nontransformed strains, CEN.PK2 and ENY.WA-1A, with and without xylose were similar (Fig. 3). Xylose metabolism and ethanol consumption began after glucose was exhausted. With the CEN.PK2 strain, biomass of the culture containing xylose and glucose continued to increase even after glucose, ethanol, and acetate consumption had stopped. The CEN.PK2 and ENY.WA-1A strains used about 9.0 and 6.0 g of xylose liter−1 and produced 3.0 and 5.5 g of xylitol liter−1, respectively. The ENY.WA-1A strain converted almost all of the xylose to xylitol, but the CEN.PK2 strain excreted only one-third of the xylose consumed as xylitol. This shows that S. cerevisiae strains differ in their ability to metabolize xylose.
FIG. 3.
Metabolism of nontransformed S. cerevisiae strains on glucose with and without xylose in shake flask cultures. Substrate consumption and main product formation in 100 g of xylose plus 20 g of glucose liter−1 (A, B) and 20 g of glucose liter−1 (C, D) media. Points without error bars have an associated error that is <10% of the value of the point. Results are based on two replications. Panels A and C, the CEN.PK2 strain; panels B and D, the ENY.WA-1A strain. DM (▪), xylitol (▴), xylose (•), glucose (♦), ethanol (EtOH) (-), glycerol (×), and acetic acid (*), all in grams liter−1. Time points for gene expression analysis are indicated by arrows.
RNA samples were taken at 29 h, after glucose depletion but while ethanol consumption and xylose metabolism were in progress, and at 70 h, when xylose alone was being slowly consumed. XR and XDH activities were measured at the same time.
ScXYL2 was not induced in the presence of xylose, but the expression in the presence of xylose remained two- to threefold higher than in the control culture at 70 h. The expression of SOR1 increased 12- and 220-fold (time point, 29 h) and 21- and 3-fold (70 h) compared to that of the nontransformed control cultures (data not shown). The XDH activity was 30- and 125-fold higher at both 29 and 70 h for the ENY.WA-1A and CEN.PK2 strains, respectively (data not shown). Therefore, the presence of xylose resulted in higher XDH activity and induced the expression of the SOR1 gene coding for SDH, which also has XDH activity.
The expression of GRE3 was not affected by xylose in the ENY.WA-1A strain but was 2.6 times higher in the CEN.PK2 strain than in the control at 29 h (data not shown). XR activity was very similar in both cultures and was 4 and 20 times lower than the XDH activity for ENY.WA-1A and CEN.PK2, respectively (data not shown).
XKS1 was not induced in the presence of xylose, and its expression was 2 to 10 times lower than that in the control culture (data not shown). Furthermore, in the CEN.PK2 strain, the XKS1 expression on xylulose was 1.6 times lower than in glucose-grown cells at the early growth phase (0.7 g of DM liter−1, 5 h) and showed no increase during the later growth phase (2.1 g of DM liter−1, 21 h; data not shown). A multicopy expression construct in which XKS1 was controlled by an ADH1 promoter had over 60 times higher expression than cells grown on xylulose. Thus, neither xylulose nor xylose induced XKS1 expression.
The genes encoding transketolase (TKL1) and transaldolase (TAL1) were expressed at significantly higher levels in the presence of xylose than in the control culture. TKL1 expression increased about 10-fold in both strains at 29 h, while TAL1 expression increased by two- to eightfold (data not shown). At 72 h, TKL1 expression was not much higher than in the control, but TAL1 expression was still six- to eightfold higher than in the control.
DISCUSSION
Overexpression of the endogenous genes GRE3 and ScXYL2 enabled S. cerevisiae to grow on xylose in the presence of glucose in aerobic shake flask cultures. Relative to an S. cerevisiae strain expressing XR and XDH from P. stipitis, however, strains expressing the endogenous genes grew slower and accumulated more xylitol.
The accumulation of xylitol by the strain overexpressing ScXYL2 could be due to low XDH activity, but the xylitol yield decreased by <10% when ScXYL2 was replaced with the P. stipitis XYL2. A more likely explanation for the xylitol accumulation in the strain overexpressing the S. cerevisiae GRE3 and ScXYL2 is the strict NADPH specificity of Gre3p, since XRs accepting only NADPH as a cofactor cannot supply the NAD+ needed by the XDH reaction (8). In anaerobic conditions, where NAD+ regeneration is even lower due to lack of respiration, the strain overexpressing the endogenous enzymes was unable to utilize xylose. The NADPH specificity of Gre3p must create a severe redox imbalance, resulting in xylitol accumulation and the inability to metabolize xylose anaerobically.
The XR and XDH activities in xylose-utilizing yeasts (3, 5, 22, 36) and the XR activity in S. cerevisiae (4) increased when cells were grown on xylose. We detected an increase in only the XDH activity of S. cerevisiae. At the mRNA level, the SOR1 gene encoding SDH was induced on xylose. Thus, the increased SOR1 expression may lead to the higher SDH/XDH activity detected. The reason van Zyl and coworkers (44) did not detect XDH activity may be because they measured XDH activity in glucose-grown cells. Contradictory to Batt and coworkers (4) but in agreement with van Zyl and coworkers (44), we did not see an increase in the XR activity when S. cerevisiae was grown in the presence of xylose but detected a low level of activity with and without xylose. The differences between our results and the results of Batt and coworkers may be strain dependent.
Several putative aldose reductase (GRE3, YPR1, and YJR096w)- and polyol dehydrogenase (ScXYL2, SOR1, and YDL246c)-encoding genes exist in the S. cerevisiae genome, but no clear physiological functions have been attributed to the corresponding enzymes. It has been postulated that Ypr1p is involved in isoleucine catabolism and fusel alcohol formation, as it has activity with 2-methylbutyraldehyde (10). The GRE3 gene is up-regulated in stress conditions, such as osmotic and oxidative stress, high temperature, and carbon starvation (2, 11, 30, 31). It may have a role in detoxification of methylglyoxal synthesized in response to stress (2). The transcriptional analysis of xylose and glucose cultures performed in our laboratory did not show any xylose-specific responses for these genes, except for SOR1 (L. Salusjärvi, unpublished results).
The observation that the XDH activity and the TKL1 and TAL1 expression were induced in the presence of xylose, in addition to S. cerevisiae being able to grow on xylulose, suggests that S. cerevisiae has in its evolutionary past consumed xylose. On the other hand, the absence of induction on xylose and a wide substrate specificity of Gre3p indicate that the enzyme also has other roles. In the construction of recombinant, xylose-utilizing S. cerevisiae strains, the endogenous Gre3p and ScXDH cannot replace the P. stipitis XR and XDH enzymes before the redox constraints of the pathway are solved.
Acknowledgments
We thank John Londesborough and Peter Richard for critically reading the manuscript and Seija Rissanen, Pirjo Tähtinen, and Eila Leino for technical assistance.
This work is a part of the research program “VTT Industrial Biotechnology” (Academy of Finland; Finnish Center of Excellence program, 2000-2005, project no. 64330).
REFERENCES
- 1.Aalto, M. K., H. Ronne, and S. Keranen. 1993. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 12:4095-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilera, J., and J. A. Prieto. 2001. The Saccharomyces cerevisiae aldose reductase is implied in the metabolism of methylglyoxal in response to stress conditions. Curr. Genet. 39:273-283. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, M. A., V. W. Yang, and T. W. Jeffries. 1988. Levels of pentose phosphate pathway enzymes from Candida shehatae grown in continuous culture. Appl. Microbiol. Biotechnol. 29:282-288. [Google Scholar]
- 4.Batt, C. A., S. Caryallo, D. D. Easson, Jr., M. Akedo, and A. J. Sinskey. 1986. Direct evidence for a xylose metabolic pathway in Saccharomyces cerevisiae. Biotechnol. Bioeng. 28:549-553. [DOI] [PubMed] [Google Scholar]
- 5.Bolen, P. L., and R. W. Detroy. 1985. Induction of NADPH linked D-xylose reductase and NAD-linked xylitol dehydrogenase activities in Pachysolen tannophilus by D-xylose, L-arabinose, or D-galactose. Biotechnol. Bioeng. 27:302-307. [DOI] [PubMed] [Google Scholar]
- 6.Boles, E., H. W. Gohlmann, and F. K. Zimmermann. 1996. Cloning of a second gene encoding 5-phosphofructo-2-kinase in yeast, and characterization of mutant strains without fructose-2,6-bisphosphate. Mol. Microbiol. 20:65-76. [DOI] [PubMed] [Google Scholar]
- 7.Boles, E., W. Lehnert, and F. K. Zimmermann. 1993. The role of the NAD-dependent glutamate dehydrogenase in restoring growth on glucose of a Saccharomyces cerevisiae phosphoglucose isomerase mutant. Eur. J. Biochem. 217:469-477. [DOI] [PubMed] [Google Scholar]
- 8.Bruinenberg, P. M., P. H. M. De Bot, J. P. Van Dijken, and W. A. Scheffers. 1984. NADH-linked aldose reductase: the key to ethanolic fermentation of xylose by yeasts. Appl. Microbiol. Biotechnol. 19:256-260. [Google Scholar]
- 9.Eliasson, A., C. Christensson, C. F. Wahlbom, and B. Hahn-Hägerdal. 2000. Anaerobic xylose fermentation by recombinant Saccharomyces cerevisiae carrying XYL1, XYL2, and XKS1 in mineral medium chemostat cultures. Appl. Environ. Microbiol. 66:3381-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford, G., and E. M. Ellis. 2002. Characterization of Ypr1p from Saccharomyces cerevisiae as a 2-methylbutyraldehyde reductase. Yeast 19:1087-1096. [DOI] [PubMed] [Google Scholar]
- 11.Garay-Arroyo, A., and A. A. Covarrubias. 1999. Three genes whose expression is induced by stress in Saccharomyces cerevisiae. Yeast 15:879-892. [DOI] [PubMed] [Google Scholar]
- 12.Gietz, D., A. St. Jean, R. A. Woods, and R. H. Schiestl. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527-534. [DOI] [PubMed] [Google Scholar]
- 14.Goffeau, A., B. G. Barrel, H. Bussey, R. W. Davis, B. Dujon, H. Feldmann, F. Galibert, J. D. Hoheisel, C. Jacq, M. Johnston, E. J. Louis, H. W. Mewes, Y. Murakami, P. Philippsen, H. Tettelin, and S. G. Oliver. 1996. Life with 6000 genes. Science 274:546, 563-567. [DOI] [PubMed] [Google Scholar]
- 15.Gong, C.-S., L.-F. Chen, M. C. Flickinger, L.-C. Chiang, and G. T. Tsao. 1981. Production of ethanol from d-xylose by using d-xylose isomerase and yeasts. Appl. Environ. Microbiol. 41:430-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallborn, J., M. Walfridsson, U. Airaksinen, H. Ojamo, B. Hahn-Hägerdal, M. Penttilä, and S. Keränen. 1991. Xylitol production by recombinant Saccharomyces cerevisiae. Bio/Technology 9:1090-1105. [DOI] [PubMed] [Google Scholar]
- 17.Hartley, J. L., and J. E. Donelson. 1980. Nucleotide sequence of the yeast plasmid. Nature 286:860-865. [DOI] [PubMed] [Google Scholar]
- 18.Ho, N. W., Z. Chen, and A. P. Brainard. 1998. Genetically engineered Saccharomyces yeast capable of effective cofermentation of glucose and xylose. Appl. Environ. Microbiol. 64:1852-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho, N. W. Y., and S.-F. Chang. 1989. Cloning of yeast xylulokinase gene by complementation in Escherichia coli and yeast mutations. Enzyme Microb. Technol. 11:417-421. [Google Scholar]
- 20.Jin, Y.-S., H. Ni, J. M. Laplaza, and T. W. Jeffries. 2003. Optimal growth and ethanol production from xylose by recombinant Saccharomyces cerevisiae require moderate d-xylulokinase activity. Appl. Environ. Microbiol. 69:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson, B., C. Christensson, T. Hobley, and B. Hahn-Hägerdal. 2001. Xylulokinase overexpression in two strains of Saccharomyces cerevisiae also expressing xylose reductase and xylitol dehydrogenase and its effect on fermentation of xylose and lignocellulosic hydrolysate. Appl. Environ. Microbiol. 67:4249-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kern, M., D. Haltrich, B. Nidetzky, and K. D. Kulbe. 1997. Induction of aldose reductase and xylitol dehydrogenase activities in Candida tenuis CBS 4435. FEMS Microbiol. Lett. 149:31-37. [DOI] [PubMed] [Google Scholar]
- 23.Kötter, P., and M. Ciriacy. 1993. Xylose fermentation by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 38:776-783. [Google Scholar]
- 24.Kuhn, A., C. van Zyl, A. van Tonder, and B. A. Prior. 1995. Purification and partial characterization of an aldo-keto reductase from Saccharomyces cerevisiae. Appl. Environ. Microbiol. 61:1580-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Mellor, J., M. J. Dobson, N. A. Roberts, A. J. Kingsman, and S. M. Kingsman. 1985. Factors affecting heterologous gene expression in Saccharomyces cerevisiae. Gene 33:215-226. [DOI] [PubMed] [Google Scholar]
- 27.Mellor, J., M. J. Dobson, N. A. Roberts, M. F. Tuite, J. S. Emtage, S. White, P. A. Lowe, T. Patel, A. J. Kingsman, and S. M. Kingsman. 1983. Efficient synthesis of enzymatically active calf chymosin in Saccharomyces cerevisiae. Gene 24:1-14. [DOI] [PubMed] [Google Scholar]
- 28.Olsson, L., T. Linden, and B. Hahn-Hägerdahl. 1994. A rapid chromatographic method for the production of preparative amounts of xylulose. Enzyme Microb. Technol. 16:388-394. [Google Scholar]
- 29.Petrash, J. M., B. S. N. Murthy, M. Young, K. Morris, L. Rikimaru, T. A. Griest, and T. Harter. 2001. Functional genomic studies of aldo-keto reductases. Chem. Biol. Interact. 130-132:673-683. [DOI] [PubMed] [Google Scholar]
- 30.Posas, F., J. R. Chambers, J. A. Heyman, J. P. Hoeffler, E. de Nadal, and J. Arino. 2000. The transcriptional response of yeast to saline stress. J. Biol. Chem. 275:17249-17255. [DOI] [PubMed] [Google Scholar]
- 31.Rep, M., M. Krantz, J. M. Thevelein, and S. Hohmann. 2000. The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 275:8290-8300. [DOI] [PubMed] [Google Scholar]
- 32.Richard, P., M. H. Toivari, and M. Penttilä. 1999. Evidence that the gene YLR070c of Saccharomyces cerevisiae encodes a xylitol dehydrogenase. FEBS Lett. 457:135-138. [DOI] [PubMed] [Google Scholar]
- 33.Richard, P., M. H. Toivari, and M. Penttilä. 2000. The role of xylulokinase in Saccharomyces cerevisiae xylulose catabolism. FEMS Microbiol. Lett. 190:39-43. [DOI] [PubMed] [Google Scholar]
- 34.Riles, L., J. E. Dutchik, A. Baktha, B. K. McCauley, E. C. Thayer, M. P. Leckie, V. V. Braden, J. E. Depke, and M. V. Olson. 1993. Physical maps of the six smallest chromosomes of Saccharomyces cerevisiae at a resolution of 2.6 kilobase pairs. Genetics 134:81-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarthy, A. V., C. Schopp, and K. B. Idler. 1994. Cloning and sequence determination of the gene encoding sorbitol dehydrogenase from Saccharomyces cerevisiae. Gene 140:121-126. [DOI] [PubMed] [Google Scholar]
- 36.Sene, L., M. Vitolo, M. G. Felipe, and S. S. Silva. 2000. Effects of environmental conditions on xylose reductase and xylitol dehydrogenase production by Candida guilliermondii. Appl. Biochem. Biotechnol. 84-86:371-380. [DOI] [PubMed] [Google Scholar]
- 37.Sherman, F., G. Fink, and J. B. Hicks. 1983. Methods in yeast genetics. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 38.Tantirungkij, M., N. Nakashima, T. Seki, and T. Yoshida. 1993. Construction of xylose-assimilating Saccharomyces cerevisiae. J. Ferment. Bioeng. 75:83-88. [Google Scholar]
- 39.Thomas, B. J., and R. Rothstein. 1989. Elevated recombination rates in transcriptionally active DNA. Cell 56:619-630. [DOI] [PubMed] [Google Scholar]
- 40.Toivari, M. H., A. Aristidou, L. Ruohonen, and M. Penttilä. 2001. Conversion of xylose to ethanol by recombinant Saccharomyces cerevisiae: importance of xylulokinase (XKS1) and oxygen availability. Metab. Eng. 3:236-249. [DOI] [PubMed] [Google Scholar]
- 41.Träff, K. L., L. J. Jönsson, and B. Hahn-Hägerdal. 2002. Putative xylose and arabinose reductases in Saccharomyces cerevisiae. Yeast 19:1233-1241. [DOI] [PubMed] [Google Scholar]
- 42.Träff, K. L., R. R. Otero-Cordero, W. H. van Zyl, and B. Hahn-Hägerdal. 2001. Deletion of the GRE3 aldose reductase gene and its influence on xylose metabolism in recombinant strains of Saccharomyces cerevisiae expressing the xylA and XKS1 genes. Appl. Environ. Microbiol. 67:5668-5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueng, P. P., C. A. Hunter, C. S. Gong, and G. T. Tsao. 1981. D-Xylulose fermentation in yeasts. Biotechnol. Lett. 6:315-320. [Google Scholar]
- 44.van Zyl, C., B. A. Prior, S. G. Kilian, and E. V. Brandt. 1993. Role of d-ribose as a cometabolite in d-xylose metabolism by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 59:1487-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Zyl, C., B. A. Prior, S. G. Kilian, and J. L. Kock. 1989. D-xylose utilization by Saccharomyces cerevisiae. J. Gen. Microbiol. 135:2791-2798. [DOI] [PubMed] [Google Scholar]
- 46.Walfridsson, M., J. Hallborn, M. Penttilä, S. Keränen, and B. Hahn-Hägerdal. 1995. Xylose-metabolizing Saccharomyces cerevisiae strains overexpressing the TKL1 and TAL1 genes encoding the pentose phosphate pathway enzymes transketolase and transaldolase. Appl. Environ. Microbiol. 61:4184-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]