Abstract
OBJECTIVE
We investigated the prognostic performance of ST2 with respect to cardiovascular death (CVD) and heart failure (HF) in patients with non–ST-elevation acute coronary syndrome (NSTE-ACS) in a large multinational trial.
BACKGROUND
Myocytes that are subjected to mechanical stress secrete ST2, a soluble interleukin-1 receptor family member that is associated with HF after STE-ACS.
METHODS
We measured ST2 with a high-sensitivity assay in all available baseline samples (N = 4426) in patients enrolled in the Metabolic Efficiency With Ranolazine for Less Ischemia in the Non–ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocar-dial Infarction 36 (MERLIN-TIMI 36), a placebo-controlled trial of ranolazine in NSTE-ACS. All events, including cardiovascular death and new or worsening HF, were adjudicated by an independent events committee.
RESULTS
Patients with ST2 concentrations in the top quartile (>35 μg/L) were more likely to be older and male and have diabetes and renal dysfunction. ST2 was only weakly correlated with troponin and B-type natriuretic peptide. High ST2 was associated with increased risk for CVD/HF at 30 days (6.6% vs 1.6%, P <0.0001) and 1 year (12.2% vs 5.2%, P <0.0001). The risk associated with ST2 was significant after adjustment for clinical covariates and biomarkers (adjusted hazard ratio CVD/HF 1.90, 95% CI 1.15–3.13 at 30 days, P = 0.012; 1.51, 95% CI 1.15–1.98 at 1 year, P = 0.003), with a significant integrated discrimination improvement (P < 0.0001). No significant interaction was found between ST2 and ranolazine (Pinteraction = 0.15).
CONCLUSIONS
ST2 correlates weakly with biomarkers of acute injury and hemodynamic stress but is strongly associated with the risk of HF after NSTE-ACS. This biomarker and related pathway merit further investigation as potential therapeutic targets for patients with ACS at risk for cardiac remodeling.
Soluble ST2, a member of the interleukin (IL)-14 receptor family, is a novel biomarker in inflammatory conditions and cardiovascular disease (1–5). Originally identified in 1989 as an orphan receptor, the ST2 ligand was discovered to be IL-33 in 2005; its downstream effects include activation of T-helper type 2 (Th2) cells and production of Th2-associated cytokines (6, 7).
A role of ST2 in cardiovascular disease, including ventricular remodeling and heart failure (HF) progression has been suggested by both experimental and clinical studies. In patients with HF, ST2 is strongly associated with both disease severity and mortality. Expression of ST2 is markedly upregulated as early as 1 h following mechanical strain in cultured myocytes and in patients with acute myocardial infarction (MI) (8, 9). Intriguingly, ST2 has been associated with adverse remodeling in patients with acute MI, and we have shown an association with new or worsening HF in patients with ST-elevation MI (STEMI) (10). However, few studies have assessed the relationship between ST2 and outcomes in non–ST-elevation (NSTE) acute coronary syndrome (ACS). In the largest published study (n = 577), ST2 was independently associated with worse outcomes in a cohort of non–STEMI (NSTEMI) patients after multivariate adjustment (11). Moreover, the relationship between the ST2 signaling pathway and pathobiological and clinical determinants of outcomes in patients with ACS is incompletely defined.
Therefore, we investigated the relationship between ST2 and presenting clinical characteristics; markers of myocyte injury, inflammation, and hemodynamic stress; and cardiovascular outcomes in the large, multinational, randomized, placebo-controlled Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) trial (12). Our results indicate that ST2 provides unique prognostic information beyond the acute injury biomarkers currently in use.
Materials and Methods
PATIENT POPULATION
The design and primary results of the MERLIN-TIMI 36 trial have been published (12–14). Briefly, 6560 patients with NSTE-ACS were randomized 1:1 to placebo or ranolazine in addition to standardized medical therapy. The trial enrolled patients with moderate- to high-risk clinical features, including increased biomarkers of myonecrosis, ST depression ≥0.1 mV, history of diabetes mellitus, or an intermediate-to-high (≥3) TIMI risk score. Exclusion criteria included end-stage renal disease requiring dialysis, clinically significant hepatic disease, cardiogenic shock, or life expectancy <12 months. The protocol, including the biomarker sub-study, was approved by the relevant institutional review boards, and written consent was obtained from all patients.
ST2 TESTING
The protocol specified that at enrollment patient blood samples be obtained in EDTA-anticoagulated plastic tubes and that plasma be isolated within 60 min of sample acquisition. Some geographic regions were excluded from participation because of inability to comply with operational aspects of sample submission. Plasma samples were stored in plastic cryovials at −20 °C or colder at the enrolling site until being shipped to the TIMI Biomarker Core Laboratory, where they were maintained at −80 °C or colder. We measured soluble ST2 using the Presage ST2 assay (Critical Diagnostics). This assay has been validated with minimal variation (within-run CV <2.5% and total CV <4.0%) across the wide measurement range of the assay, and a cutpoint of 35 μg/L has been used to distinguish normal from increased values (15). We measured B-type natriuretic peptide (BNP) with the Advia Centaur (Siemens Healthcare Diagnostics) (16) and cardiac troponin I (cTnI) with the TnI-Ultra assay (Siemens). We used the 99th percentile decision limit (0.04 μg/L, <10% CV) for cTnI, consistent with current guidelines (17). All biomarker testing was performed at Brigham and Women’s Hospital by personnel blinded to clinical outcomes and treatment allocation.
END POINTS
Based on our previous work with ST2, the primary end point for this analysis was the composite of cardiovascular death (CVD) and new or worsening HF (CVD/HF) (14). All end points, including cardiovascular death, sudden cardiac death, recurrent ischemia, and new or worsening HF were adjudicated by a blinded clinical events committee. New or worsening HF was defined as rehospitalization or prolongation of the index hospitalization (≥24 h) in an acute-care facility primarily for the treatment of HF along with an objective sign of HF (14).
STATISTICAL ANALYSIS
We compared the baseline characteristics of patients with and without increased concentrations of ST2 using the Wilcoxon rank sum test for continuous variables and the χ2 test for categorical variables. ST2 >35 μg/L was defined as the threshold for increased values for the purpose of this analysis on the basis of previous work with the Presage ST2 assay in patients with HF (10, 18). This cutpoint defined the top quartile of our population. We evaluated the associations between ST2 and clinical outcomes using the log-rank test, stratified by intention for early invasive management. We estimated hazard ratios (HRs) and corresponding 95% CIs using a Cox proportional hazards regression model. Event rates are presented as Kaplan–Meier failure rates at 30 days and 12 months. Correlation with other cardiovascular biomarkers was performed with a nonparametric (Spearman) rank correlation coefficient.
We used a multivariable Cox proportional hazards regression model to adjust for (a) TIMI risk score covariates, as defined by age >65 years, known coronary artery disease, diabetes, hypertension, dyslipidemia, severe angina (≥2 episodes in 24h), ST changes ≥0.5 mm, and aspirin use; (b) other characteristics of interest: smoking, history of HF, creatinine clearance <60 mL/min (as defined by the Cockcroft–Gault equation); and (c) other biomarkers, including cTnI, BNP, myeloperoxidase (MPO), and high-sensitivity C-reactive protein (hsCRP). In addition, left ventricular ejection fraction (LVEF) was added to the model in the subset of patients for whom ventricular function was assessed locally as part of clinical practice. We tested for heterogeneity in the effect of ranolazine between patients with and without increased concentrations of ST2 using Cox regression with terms for the main effects and for the interaction of ST2 status with treatment allocation. We evaluated the performance of prediction models with and without ST2 using the net reclassification improvement (NRI) and the concordance (c) statistic, as described (19).
Analyses were performed using STATA v10.1 (STATA Corp). P values (2-tailed) <0.05 were considered to indicate statistical significance. The authors had full access to and take full responsibility for the integrity of the data.
Results
Soluble ST2 concentrations were determined in all available baseline samples (N = 4426). We have shown that there was no meaningful difference in the biomarker substudy population from those without biomarker samples (16). The median concentration of ST2 was 24.4 μg/L (25th, 75th percentile 17.6, 35.1 μg/L; range; 0.35–784.35 μg/L). Baseline characteristics in patients stratified by ST2 concentration are shown in Table 1. In general, patients with higher values of ST2 had a greater number of clinical risk factors: they were more likely to be older and male and have diabetes and renal dysfunction (each P < 0.001). There were no differences in history of HF or history of antecedent MI based on ST2 concentrations.
Table 1.
Baseline characteristics stratified by ST2.a
| Characteristics | ST2 <35 μg/L | ST2 >35 μg/L | P |
|---|---|---|---|
| n | 3320 | 1106 | |
| Age ≥75 years | 498 (15) | 247 (22) | <0.001 |
| Male | 2062 (62) | 800 (72) | <0.001 |
| Risk factors for atherosclerosis | |||
| Diabetes | 1032 (31) | 413 (37) | <0.001 |
| Smoking | 867 (26) | 238 (22) | 0.0022 |
| Hypertension | 2485 (75) | 818 (75) | 0.67 |
| Dyslipidemia | 2110 (70) | 620 (62) | <0.001 |
| Cardiac history | |||
| Prior MI | 1192 (36) | 389 (36) | 0.68 |
| Prior HF | 688 (21) | 243 (22) | 0.38 |
| Estimated creatinine clearance <60 mL/minb | 594 (18) | 300 (27) | <0.0001 |
| Presenting syndrome | |||
| Index NSTEMI | 1425 (43) | 717 (65) | <0.001 |
| cTnI ≥0.04 ng/mL | 1958 (60) | 860 (79) | <0.001 |
| BNP >80 pg/mL | 1196 (36) | 692 (63) | <0.001 |
Data are n (%).
Estimated by use of Cockcroft–Gault equation.
ST2 AND INDICATORS OF INJURY, INFLAMMATION, AND HEMODYNAMIC STRESS
Among patients with known LVEF (n = 2937), higher ST2 concentration was weakly but significantly associated with lower ejection fraction (ρ = −0.12, P < 0.0001). Although patients with a high ST2 concentration were more likely to have increased cTnI and BNP (Table 1), the correlation between ST2 and biomarkers of injury was weak [presenting cTnI ρ = 0.30; peak creatine kinase MB ρ = 0.25]. Similarly, ST2 showed a strong correlation with neither BNP nor the inflammatory biomarkers hsCRP or MPO (Table 2). In addition, after stratifying for BNP, ST2 was not correlated with the presence of severe (3-vessel or left-main) epicardial coronary disease among patients who underwent angiography (n = 2435).
Table 2.
Correlation between ST2 and other biomarkers.
| Biomarker | Spearman ρ | P |
|---|---|---|
| Troponin | 0.30 | <0.001 |
| CRP | 0.24 | <0.001 |
| MPO | 0.17 | <0.001 |
| BNP | 0.28 | <0.001 |
ST2 AND CLINICAL OUTCOMES
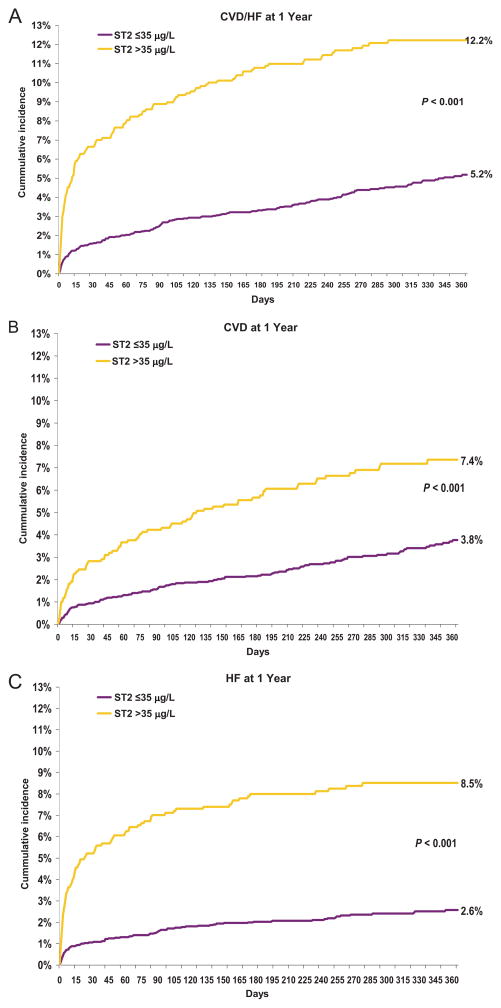
A high ST2 concentration (in the top quartile at presentation) was strongly associated with adverse outcomes at 30 days and at 1 year (Fig. 1). At 30 days, patients with ST2 >35 μg/L had a higher risk of CVD/HF, including significant relationships individually with CVD, HF, sudden cardiac death, and all-cause death. These relationships remained significant at 1 year (Table 3). The c statistic or area under the ROC curve for prediction of CVD/HF using ST2 increased modestly from 0.80 to 0.82 (P = 0.005) at 30 days and from 0.79 to 0.80 (P = 0.04) at 1 year. Similar results were observed for prediction of HF alone at both time points.
Fig. 1.
Baseline ST2 concentration and cardiovascular outcomes at 1 year for CVD/HF (A), CVD (B), and HF (C).
Table 3.
Adjusted risk relationships for ST2 and cardiovascular outcomes.a
| Model and stepwise adjustment | 30-Day follow-up
|
1-Year follow-up
|
||
|---|---|---|---|---|
| ST2 >35 μg/L | P | ST2 >35 μg/L | P | |
| Unadjusted model | ||||
|
| ||||
| All-cause death | 3.21 (1.99–5.21) | <0.0001 | 2.50 (1.93–3.23) | <0.001 |
|
| ||||
| CVD | 3.03 (1.84–4.98) | <0.0001 | 2.24 (1.70–2.95) | <0.001 |
|
| ||||
| CHFb | 5.00 (3.28–7.62) | <0.0001 | 3.92 (2.91–5.27) | <0.001 |
|
| ||||
| CVD/CHF | 4.32 (3.03–6.16) | <0.0001 | 2.73 (2.18–3.41) | <0.001 |
|
| ||||
| Model adjusted for clinical factors and cTnI | ||||
|
| ||||
| All-cause death | 2.04 (1.25–3.36) | 0.005 | 1.70 (1.31–2.22) | <0.001 |
|
| ||||
| CVD | 1.88 (1.13–3.14) | 0.015 | 1.46 (1.10–1.94) | 0.010 |
|
| ||||
| CHF | 3.42 (2.22–5.28) | <0.001 | 2.73 (2.01–3.71) | <0.001 |
|
| ||||
| CVD/CHF | 2.91 (2.02–4.19) | <0.001 | 1.89 (1.50–2.38) | <0.001 |
|
| ||||
| Model adjusted for clinical factors, cTnI, BNP | ||||
|
| ||||
| All-cause death | 1.90 (1.15–3.13) | 0.012 | 1.51 (1.15–1.98) | 0.003 |
|
| ||||
| CVD | 1.75 (1.04–2.93) | 0.034 | 1.30 (0.97–1.73) | 0.078 |
|
| ||||
| CHF | 2.98 (1.92–4.61) | <0.001 | 2.37 (1.74–3.22) | 0.001 |
|
| ||||
| CVD/CHF | 2.56 (1.77–3.71) | <0.001 | 1.67 (1.32–2.11) | <0.001 |
Data are HR (95% CI).
CHF, congestive heart failure.
To examine whether there was a persistent late risk associated with an increased baseline ST2, we performed a landmark analysis starting at 30 days. Notably, patients with a baseline ST2 >35 μg/L had a higher risk of both new or worsening HF (HR 3.03, 95% CI 1.98–4.64, P < 0.001) and CVD (HR 1.96, 95% CI 1.40–2.74, P < 0.001) starting from 30 days onward.
In contrast to HF and CVD, we found no statistically significant association between ST2 and the risk of recurrent MI (4.0% vs 3.5%, P = 0.46) or recurrent ischemia (4.0% vs 4.1%, P = 0.99) at 30 days or 1 year (MI 8.7% vs 7.6%, P = 0.14; recurrent ischemia 13.5% vs 15.7%, P = 0.38).
MULTIVARIABLE ASSESSMENT
With adjustment for relevant clinical factors, the risks related to high ST2 were significant, including the risk of CVD or HF, which persisted when clinically established biomarkers (cTnI and BNP) were added to the model (adjusted HR 1.67, 95% CI 1.32–2.11, P < 0.0001) (Table 3). Adjusted HRs are summarized in Table 3. When markers of inflammation, hs-CRP, and MPO, an enzyme released from activated neutrophils, were further added to the model, ST2 remained associated with CVD or HF, as well as all-cause death. Similarly, when evaluated as a continuous variable, ST2 was significantly associated with CVD or HF, showing a 56% higher risk at 1 year per each log increase in ST2 (P < 0.001) (Table 4). Moreover, in an exploratory analysis in the subset of patients for whom LVEF was available (n = 2937), the results were consistent, with a significant relationship between ST2 and the risk of HF (HR 2.67, 95% CI 1.54–4.62, P <0.001 at 30 days; HR 2.00, 95% CI 1.36–2.94, P <0.001 at 1 year) and CVD or HF (HR 2.19, 95% 1.36–3.52, P = 0.001 at 30 days; HR 1.37, 95% CI 1.01–1.84, P = 0.04 at 1 year).
Table 4.
ST2 Concentration (continuous) and cardiovascular outcomes per log increase in ST2.
| 30-Day follow-up
|
1-Year follow-up
|
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| All-cause mortality | ||||
|
| ||||
| Unadjusted | 1.79 (1.50–2.13) | <0.001 | 1.56 (1.41–1.73) | <0.001 |
|
| ||||
| Adjusteda | 1.45 (1.19–1.76) | <0.001 | 1.26 (1.12–1.40) | <0.001 |
|
| ||||
| CVD | ||||
|
| ||||
| Unadjusted | 1.74 (1.45–2.10) | <0.001 | 1.51 (1.35–1.69) | <0.001 |
|
| ||||
| Adjusted | 1.39 (1.13–1.70) | 0.002 | 1.19 (1.06–1.34) | 0.004 |
|
| ||||
| New or worsening HF | ||||
|
| ||||
| Unadjusted | 1.83 (1.58–2.12) | <0.001 | 1.72 (1.54–1.92) | <0.001 |
|
| ||||
| Adjusted | 1.47 (1.25–1.74) | <0.001 | 1.37 (1.21–1.55) | <0.001 |
|
| ||||
| CVD/HF | ||||
|
| ||||
| Unadjusted | 1.80 (1.58–2.04) | <0.001 | 1.56 (1.43–1.71) | <0.001 |
|
| ||||
| Adjusted | 1.43 (1.24–1.65) | <0.001 | 1.25 (1.13–1.38) | <0.001 |
ST2 improved discrimination of the risk for CVD or HF at 30 days and 1 year when added to these established clinical factors and clinical biomarkers (cTnI and BNP) by integrated discrimination improvement (IDI) analysis (P < 0.0001 at 30 days, P = 0.013 at 1 year). However, there was not an improvement in net reclassification of risk at 30 days (NRI 0.028, P =0.32) above and beyond this robust model.
Unlike our previous findings with BNP (16), we found no significant interaction between baseline ST2 concentration and the clinical efficacy of ranolazine on the trial primary end point of CVD, MI, or recurrent ischemia (Pinteraction = 0.15). Notably, different from BNP, ST2 was not associated with the composite of CVD, MI, or recurrent ischemia (HR 1.13; 95% CI 0.98–1.30, P = 0.10).
ST2 AND BNP
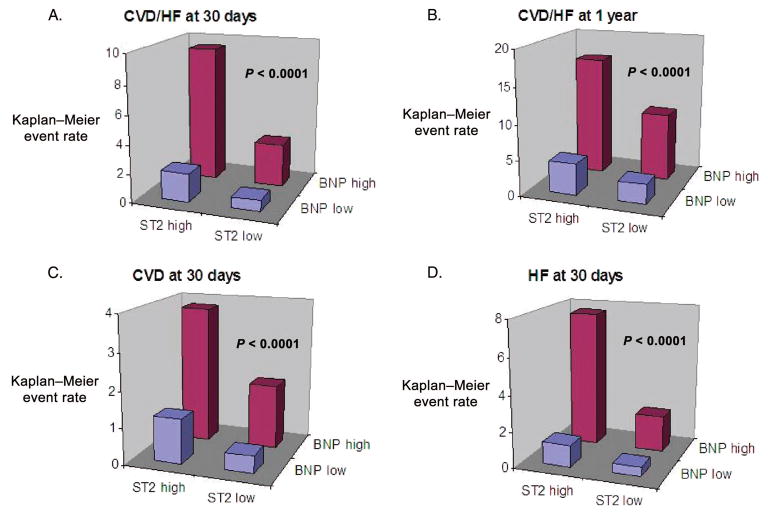
When ST2 was considered with BNP, strata of risk defined by both biomarkers revealed a markedly increased risk of CVD/HF in patients with high concentrations of both biomarkers (Fig. 2). Notably, formal interaction testing revealed that this risk was not multiplicative; rather, the relative increase in risk associated with ST2 was similar in patients with and without increased BNP (CVD/HF Pinteraction = 0.63).
Fig. 2. Kaplan–Meier event rates for CVD/HF (A, B) and CVD (C) and HF (D) alone are highest in patients with high ST2 and high BNP.
Formal testing for an interaction between ST2 and BNP was nonsignificant for each end point (P > 0.40 for each).
Examining the effect of ranolazine vs placebo in the 4 groups defined by ST2 and BNP, the incidence of CVD, MI, or recurrent ischemia was significantly lower with ranolazine in those patients with increased BNP and ST2 (HR 0.71; 95% CI 0.53–0.95, P =0.02) with a nonsignificant 15% lower event rate in patients allocated to ranolazine among those with high BNP and low ST2 (HR 0.85; 95% CI 0.68–1.07, P = 0.16). Among patients with high ST2 but low BNP, the primary end point did not differ between ranolazine and placebo (HR 0.92; 95% CI 0.59–1.44, P = 0.71).
Discussion
In this large trial of well-characterized patients with NSTE-ACS, we found that a high ST2 concentration at presentation identified patients with a >3-fold higher risk of CVD or HF early (30 days) and late (1 years) following presentation with unstable ischemic heart disease. Moreover, we observed that ST2 was independently associated with CVD or HF or their composite (CVD/HF). We found that the correlations of ST2 with indicators of myocardial injury (creatine kinase MB and troponin), inflammatory activation, and hemodynamic stress were, at most, moderate and that ST2 behaved differently from other inflammatory biomarkers associated with coronary artery disease, such as hsCRP. ST2 was only weakly correlated with extent of atherosclerotic disease or ejection fraction at presentation. These findings support a distinct and potentially relevant role of this signaling pathway in the pathogenesis of unstable ischemic heart disease and HF as a complication. Nevertheless, the incremental discriminatory information offered by ST2, while statistically significant, appears modest, highlighting the importance of investigating therapeutic interactions if ST2 is to be considered for clinical use in patients with ACS.
ST2 SIGNALING IN CARDIOVASCULAR DISEASE
ST2 is expressed basally by cardiac myocytes in 2 forms: the membrane-bound isoform (ST2L) and soluble isoform (sST2) (8, 20). Its ligand, IL-33, is released by cardiac cells (including fibroblasts) and normally protects the myocardium during states of pressure overload (10). Soluble ST2 counteracts this protective mechanism with the apparent mechanism of serving as a “decoy receptor” for IL-33. When bound to sST2, IL-33 is no longer able to interact with membrane-ST2L to prevent the activation of downstream signaling pathways that lead to myocyte hypertrophy and remodeling; this indirect antagonism of IL-33 activates adverse cardiac remodeling and hypertrophy, perhaps partially through Th2 lymphocyte function (21, 22). Interestingly, IL-33 has also been implicated in modulating atherogenesis (23).
On the basis of these experimental observations, ST2 has been investigated in a variety of cardiovascular settings, foremost in patients with HF in whom ST2 is strongly associated with adverse outcomes. In patients with HF, ST2 concentrations correlate inversely with LVEF (8) and New York Heart Association class (24) as well as echocardiographic predictors of HF (25). ST2 is predictive of a 2.5-fold higher risk of death in patients with acutely decompensated HF (24–28), whether in the presence of preserved or decreased LVEF (29). Moreover, in patients with chronic HF, serial ST2 concentrations predict risk of death, defibrillator firings, and sudden death (30). In addition, in patients with dyspnea, ST2 provides prognostic information complementary to that of natriuretic peptides (27, 30).
ST2 IN PATIENTS WITH MYOCARDIAL ISCHEMIA
Because ST2 expression is upregulated following myocardial ischemia or mechanical stress (8) and plays a role in cardiac remodeling after ischemic injury, the soluble protein has attracted interest as a biomarker to predict future clinical HF in patients with myocardial ischemia. Our group first investigated this application of ST2 in patients with large transmural STEMI in whom the likelihood of adverse remodeling is highest. In this setting, in 3 separate populations with STEMI treated with fibrinolysis, we found that ST2 concentration at presentation was associated with the risk of death or HF at 30 days, independently of traditional risk indicators (10, 21). In addition, in patients undergoing primary percutaneous coronary intervention for STEMI, patients with low serial ST2 concentrations had a lower short-term mortality than patients with a rise in ST2 early after admission (31).
Few data have been available in patients with NSTE-ACS. In a preliminary investigation in such a population (n =403), ST2 was associated with mortality at 1 year, but was no longer significant when adjusted for N-terminal pro-BNP (18). Another recent study (n = 577) found ST2 to be correlated with worse outcomes in patients with NSTEMI but found no improvement in the risk stratification and no relationship between adverse events and IL-33 (11). In the present, more robustly powered study (n = 4426), we found that higher concentrations of ST2 identified patients at higher risk of both CVD and HF early and late after presentation, which persisted after we adjusted for BNP, troponin, and major clinical risk indicators. In addition to the clear independent association demonstrated using multivariable modeling, we also found an increase in the IDI, a very sensitive metric of enhanced discrimination. Although statistically significant, this increase was modest and not detected by an increase in the c statistic or net reclassification. Therefore, our findings support pathobiological relevance of ST2-related pathways and indicate that prognostic assessment may be enhanced modestly by using ST2 in conjunction with standard tools. However, it is likely that demonstration of specific therapeutic interactions would be necessary for ST2 to be considered for routine clinical application in patients with ACS.
RESEARCH IMPLICATIONS
Although we observed a statistically significant negative correlation between ST2 and LVEF qualitatively, this correlation was weak. This finding is consistent with previous smaller studies (8) and indicates that ST2 is not merely a surrogate for the degree of ventricular dysfunction at the time of measurement. Similarly, although we and others have observed higher ST2 concentrations in patients with greater myocardial injury, we found that ST2 was only weakly correlated with both presenting and peak biomarkers as indicators of infarct size. Overall, our findings, integrated with those of others, suggest that ST2 is not purely a marker of hemodynamic stress but may also additionally reflect inflammation, fibrosis, and adverse myocardial remodeling, likely through pathways distinct from those detected by established biomarkers. We speculate that by integrating information regarding the extent of injury, the inflammatory response (supported by a correlation with inflammatory biomarkers), and mechanical stress on cardiac myocytes, ST2 may prove useful to target therapies aimed at adverse remodeling or that the ST2/IL-33 signaling pathway itself may be a useful target for intervention (8, 21, 32–34). Intriguingly, a small study (n = 100) in patients with STEMI studied with serial cardiac MRI found that increases of ST2 were associated with adverse remodeling over a period of 12–24 weeks and suggested that the aldosterone antagonist eplerenone may reduce medium-term adverse remodeling in high-risk patients with increased ST2 (32).
In regard to the second hypothesis, that the ST2/IL-33 signaling pathway is a potential novel therapeutic target to mitigate cardiac fibrosis following ACS, animal experiments have confirmed that interruption of ST2/IL-33 signaling decreases Th2-mediated inflammation. Lohning and colleagues (35) found that administration of an antibody against a fusion protein containing the extracellular domain of ST2 significantly reduced eosinophilic infiltration. ST2-null mice have increased indicators of inflammation, which can be attenuated with gene transfer of sST2. Investigation of ST2 inhibitors has been underway, but because of their nonspecific effects, drug development and clinical testing for these agents is complex (22). Alternatively, agonists of the IL-33/ST2 signaling pathway may provide a cardioprotective effect, and preliminary data from our group show decreased cardiomyocyte apoptosis and improved outcomes after experimental MI (36).
LIMITATIONS
Our study has strengths and weaknesses. Overall, the trial was designed to assess the effect of ranolazine in patients with unstable ACS, and although the biomarker substudy is based on a nested, prospectively collected cohort, it was not designed to test specific aspects of the clinical course such as sophisticated measures of remodeling or serial assessments of LVEF. However, the large number of participants (n = 4513) and the extended clinical follow-up with independently adjudicated end points is a strength that is not replicated in most mechanistic studies. Moreover, in an exploratory analysis among a subset of nearly 3000 patients, we found that the relationship between ST2 and CVD or HF was independent of LVEF. Because LVEF was not available for the entire cohort, it is possible that there may have been some systematic bias with respect to obtaining ejection fraction that is unaccounted for in our multivariable model. Last, the trial patient population was enriched for high-risk NSTEMI patients, and results may not be applicable to low-risk patients with fewer cardiac risk factors.
Conclusions
In patients presenting with ACS, those with increased concentration of ST2, a novel biomarker of mechanical strain, are at >3-fold higher risk of CVD and HF over the following year. This information is independent of and additive to clinical risk factors, LVEF, and hemodynamic and inflammatory biomarkers. Because of their plausible role in ventricular remodeling, ST2 and its associated pathways are emerging as potential therapeutic targets for clinical stratification and treatment of high-risk NSTEMI patients.
Acknowledgments
Research Funding: CV Therapeutics (acquired by Gilead Pharmaceuticals) provided research grant support for the MERLIN-TIMI 36 trial. R.T. Lee, NIH; D.A. Morrow, Critical Diagnostics and National Heart, Lung, and Blood Institute (award number RC1HL099692); M.S. Sabatine, National Heart, Lung, and Blood Institute (award number RC1HL099692). Critical Diagnostics provided reagent for measurement of ST2.
Footnotes
Nonstandard abbreviations: IL, interleukin; CV, cardiovascular, Th2, T-helper type 2; HF, heart failure; MI, myocardial infarction; STEMI, ST-elevation MI; NSTE, non–ST-elevation; ACS, acute coronary syndrome; MERLIN-TIMI 36, Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36; BNP, B-type natriuretic peptide; CVD, cardiovascular death; cTnI, cardiac troponin I; HR, hazard ratio; MPO, myeloperoxidase; hsCRP, high-sensitivity C-reactive protein; LVEF, left ventricular ejection fraction; NRI, net reclassification improvement; IDI, integrated discrimination improvement; ST2L, ST2 membrane-bound isoform; sST2, ST2 soluble isoform.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: D.A. Morrow, Clinical Chemistry, AACC.
Consultant or Advisory Role: B.M. Scirica, Gilead; D.A. Morrow, Beckman Coulter, Critical Diagnostics, Instrumentation Laboratories, OrthoClinical Diagnostics, Siemens, Roche Diagnostics, Gilead, and Menarini.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
Role of Sponsor: The funding organizations played a direct role in review and interpretation of data.
References
- 1.Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164:277–81. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- 2.Leung BP, Xu D, Culshaw S, McInnes IB, Liew FY. A novel therapy of murine collagen-induced arthritis with soluble T1/ST2. J Immunol. 2004;173:145–50. doi: 10.4049/jimmunol.173.1.145. [DOI] [PubMed] [Google Scholar]
- 3.Kuroiwa K, Arai T, Okazaki H, Minota S, Tominaga S. Identification of human ST2 protein in the sera of patients with autoimmune diseases. Biochem Biophys Res Commun. 2001;284:1104–8. doi: 10.1006/bbrc.2001.5090. [DOI] [PubMed] [Google Scholar]
- 4.Brunner M, Krenn C, Roth G, Moser B, Dworschak M, Jensen-Jarolim E, et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med. 2004;30:1468–73. doi: 10.1007/s00134-004-2184-x. [DOI] [PubMed] [Google Scholar]
- 5.Barksby HE, Lea SR, Preshaw PM, Taylor JJ. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007;149:217–25. doi: 10.1111/j.1365-2249.2007.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trajkovic V, Sweet MJ, Xu D. T1/ST2: an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev. 2004;15:87–95. doi: 10.1016/j.cytogfr.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–6. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–90. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 10.Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, et al. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation. 2008;117:1936–44. doi: 10.1161/CIRCULATIONAHA.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhillon OS, Narayan HK, Quinn PA, Squire IB, Davies JE, Ng LL. Interleukin 33 and ST2 in non-ST-elevation myocardial infarction: comparison with Global Registry of Acute Coronary Events Risk Scoring and NT-proBNP. Am Heart J. 2011;161:1163–70. doi: 10.1016/j.ahj.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 12.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297:1775–83. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 13.Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–52. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 14.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Skene A, McCabe CH, Braunwald E. Evaluation of a novel anti-ischemic agent in acute coronary syndromes: design and rationale for the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-elevation acute coronary syndromes (MERLIN)-TIMI 36 trial. Am Heart J. 2006;151:1186 e1–9. doi: 10.1016/j.ahj.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Dieplinger B, Januzzi JL, Jr, Steinmair M, Gabriel C, Poelz W, Haltmayer M, Mueller T. Analytical and clinical evaluation of a novel high-sensitivity assay for measurement of soluble ST2 in human plasma: the Presage ST2 assay. Clin Chim Acta. 2009;409:33–40. doi: 10.1016/j.cca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Morrow DA, Scirica BM, Sabatine MS, de Lemos JA, Murphy SA, Jarolim P, et al. B-type natriuretic peptide and the effect of ranolazine in patients with non-ST-segment elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST Elevation Acute Coronary-Thrombolysis In Myocardial Infarction 36) trial. J Am Coll of Cardiol. 2010;55:1189–96. doi: 10.1016/j.jacc.2009.09.068. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–38. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 18.Eggers KM, Armstrong PW, Califf RM, Simoons ML, Venge P, Wallentin L, James SK. ST2 and mortality in non-ST-segment elevation acute coronary syndrome. Am Heart J. 2010;159:788–94. doi: 10.1016/j.ahj.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–38. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwahana H, Yanagisawa K, Ito-Kosaka A, Kuroiwa K, Tago K, Komatsu N, et al. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem. 1999;264:397–406. doi: 10.1046/j.1432-1327.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 21.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–49. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, et al. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–46. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehman SU, Mueller T, Januzzi JL., Jr Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458–65. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Serum levels of the interleukin-1 receptor family member ST2, cardiac structure and function, and long-term mortality in patients with acute dyspnea. Circ Heart Fail. 2009;2:311–9. doi: 10.1161/CIRCHEARTFAILURE.108.833707. [DOI] [PubMed] [Google Scholar]
- 26.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–6. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 27.Januzzi JL, Jr, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50:607–13. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Mueller T, Dieplinger B, Gegenhuber A, Poelz W, Pacher R, Haltmayer M. Increased plasma concentrations of soluble ST2 are predictive for 1-year mortality in patients with acute destabilized heart failure. Clin Chem. 2008;54:752–6. doi: 10.1373/clinchem.2007.096560. [DOI] [PubMed] [Google Scholar]
- 29.Manzano-Fernandez S, Mueller T, Pascual-Figal D, Truong QA, Januzzi JL. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am J Cardiol. 2011;107:259–67. doi: 10.1016/j.amjcard.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah RV, Januzzi JL., Jr ST2: a novel remodeling biomarker in acute and chronic heart failure. Curr Heart Fail Rep. 2010;7:9–14. doi: 10.1007/s11897-010-0005-9. [DOI] [PubMed] [Google Scholar]
- 31.Grabowski M, Szymanski FM, Januzzi J, Wu AH, Hrynkiewicz-Szymanska A, Cacko A, et al. Prognostic utility of serial measurements of a novel biomarker ST2 in STEMI patients treated with primary PCI. American Heart Association: Scientific Sessions, Orlando, FL. Circulation. 2009;120:S808. [Google Scholar]
- 32.Weir RA, Miller AM, Murphy GE, Clements S, Steedman T, Connell JM, et al. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol. 2010;55:243–50. doi: 10.1016/j.jacc.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 33.Dinarello CA. An IL-1 family member requires caspase-1 processing and signals through the ST2 receptor. Immunity. 2005;23:461–2. doi: 10.1016/j.immuni.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Rossler U, Thomassen E, Hultner L, Baier S, Danescu J, Werenskiold AK. Secreted and membrane-bound isoforms of T1, an orphan receptor related to IL-1-binding proteins, are differently expressed in vivo. Dev Biol. 1995;168:86–97. doi: 10.1006/dbio.1995.1063. [DOI] [PubMed] [Google Scholar]
- 35.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998;95:6930–5. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, Lee RT. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009;2:684–91. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]